Abstract
Purpose
Mastitis is one of the major global problems severely affecting the dairy sector. Staphylococcus species are the primary bacteria consistently identified from mastitic milk. This study was aimed to estimate the prevalence of mastitis, isolate Staphylococcus species, determine risk factors, and the antimicrobial susceptibility profile of Staphylococcus species from mastitic Zebu cows in West Shewa Zone, Ethiopia.
Materials and Methods
A total of 404 lactating Zebu cows were tested for mastitis. Isolation and identification of Staphylococcus from mastitis positive samples were done by bacteriological culture and biochemical tests. Further identification of coagulase-negative Staphylococcus (CNS) species and antimicrobial susceptibility test of the Staphylococcus aureus and the CNS was done by the Phoenix machine. Descriptive statistics was used to summarize the prevalence of mastitis while the Chi-square test and logistic regression were used to determine the association between the prevalence of mastitis and the risk factors and the magnitude of association, respectively.
Results
The present study showed an overall cow and quarter level mastitis prevalence of 30.5% (95% confidence interval [CI]:26.0–35.2) and 8.3% (95% CI 7.0–9.8), respectively. The quarter level isolation rate of Staphylococcus species was 38.6% (95% CI: 30.1–47.6). Five Staphylococcus species namely S. intermedius, S. hyicus, S. aureus, S. lentus, and S. sciuri were identified. The latter two are CNS and were identified for the first time in Ethiopia. Antimicrobial susceptibility testing showed none of the isolates of S. aureus, 100% of S. sciuri, and 87.5% of S. lentus species were multidrug-resistant. The independent predictors of mastitis (p<0.05) were the age of the cows, stage of lactation, type of housing, the interval of bedding cleaning, and previous history of mastitis.
Conclusion
The study showed a high prevalence of mastitis, Staphylococcus species, and multidrug resistant S. lentus, and S. sciuri in Zebu cows.
Keywords: antimicrobial resistance, mastitis, prevalence, risk factor, Staphylococcus, Zebu cows
Introduction
Ethiopia holds large potential for dairy development due to its huge cattle population and favorable climate for improved high-yielding animal breeds for milk production.1 However, milk production in the country often does not satisfy the country’s requirements. Accordingly, the per capita milk availability was estimated at 17 to18 kg per year compared to the global average of 100 kg per year. Animal diseases are among several factors that contribute to these limitations in milk production and productivity.2 Mastitis is one of the major diseases severely affecting animal health, milk yield, quality of milk and milk products, and the economics of the dairy sector in the globe.3
Mastitis is an inflammation of the udder caused by a variety of microorganisms, mostly bacteria that gain access to the interior of the mammary glands through the teat canal. Bovine mastitis is one of the most frequently encountered and important diseases of dairy cows in Ethiopia.4 Higher prevalence of the disease has been reported by different authors.5–8 The absence of post milking teat dipping, lack of culling of chronically infected cows, absence of dry cow therapy, and the invariable hand milking practice among the dairy herds6 and the optimum temperature in most parts of Ethiopia are the predisposing factors of mastitis. Parity, stage of lactation, previous history of mastitis, floor type, milking hygiene, and udder injury are the potential risk factors of mastitis.2
It can be clinical mastitis, which is characterized by visible signs on the udder and milk, and subclinical mastitis, which lacks any clinical manifestation. It is one of the diseases of livestock that incurs serious economic losses to the dairy industry.9 Staphylococcus aureus is also an important cause of mastitis in dairy animals.10 More than 50 species and sub-species of Staphylococcus have been characterized to cause Staphylococcal mastitis. Based on the ability to coagulate rabbit plasma, staphylococci are categorized as coagulase-positive Staphylococcus (CPS) and coagulase-negative Staphylococcus (CNS). Among CPS, S. aureus is the frequent causative agent of clinical or subclinical mastitis in cattle.11 It is also a foodborne pathogen and the leading cause of foodborne intoxications around the globe.10 The CNS is of great importance due to its multidrug resistance nature.12 In addition, studies showing the specific species of CNS in Ethiopia are scarce. Therefore, the current study was conducted with the objectives of estimating the prevalence of mastitis, assessment of potential risk factors associated with Zebu (Bos indicus) cows’ mastitis, isolation, and identification of Staphylococcus species, and determination of the antimicrobial susceptibility of the Staphylococcus aureus and CNS isolates.
Materials and Methods
Study Area
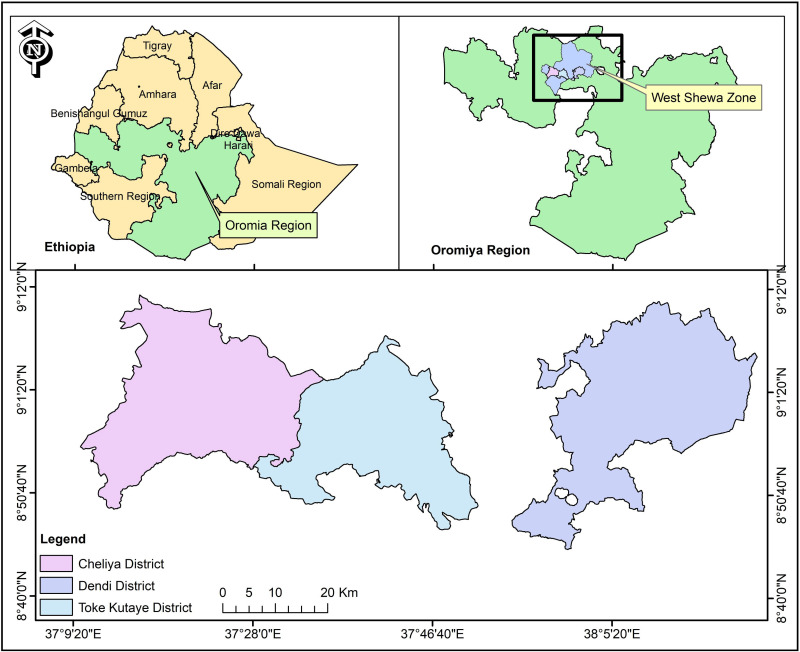
The study was conducted in three purposively selected districts of West Shewa Zone of Oromia regional state of Ethiopia namely: Toke Kutaye (08° 59ʹ01.1ʹ N and 37° 46ʹ 27.6ʹ E), Cheliya (9° 0′ 0″ N and 37° 30′ 0″ E), and Dendi districts (8° 54ʹ 59.99” N and 38° 09ʹ 60.00” E) (Figure 1). The study areas have an agro-ecological zone of high land to midland. The total cattle population in Cheliya, Toke Kutaye and Dendi districts were 111,020,13 174,79914 and 191,333,15 respectively.
Figure 1.
Map of Ethiopia showing the study areas (Dendi, Cheliya and Toke Kutaye districts) where milk samples were collected from Zebu cows. The map was sketched using ArcGIS 9 software (ArcGIS™ version 10.7, California, USA).
Study Populations
The study populations were those lactating Zebu cows (indigenous cattle) found in the Toke Kutaye, Cheliya, and Dendi districts. These cows were found in three accessible kebeles found in each district and managed under an extensive system. All age categories of cows and the number of parities were included.
Study Design and Sampling Technique
A cross-sectional study type was carried out from November 2016 to December 2017. The sample size was calculated based on the formula given by Thrusfield16 considering 50% expected prevalence and 95% confidence interval and 5% level of precision. The total sample size using the formula given here below was 384.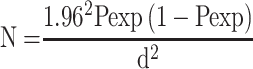
Where n= required sample size, p= expected prevalence, and d=desired absolute precision. Additionally, 5% of the above sample size was considered to reduce the risk of sample loss during the laboratory test, which makes the total sample size 404.
The number of study animals from each district was determined based on the proportion of the cattle population in each district. Initially, three kebeles (Kebele refers to the smallest administrative unit under district) were purposively selected from each district on basis of accessibility. Using simple random techniques, the households were chosen from each of the selected kebeles. Also, a simple random sampling technique was used to select the animals within the households. Accordingly, 94,148 and 162 lactating Zebu cows were sampled from Cheliya, Toke Kutaye, and Dendi districts, respectively.
Study Methodology
Questionnaires Survey
A structured questionnaire containing close-ended questions was used to collect data regarding the different potential risk factors for mastitis (age, parity, stage of lactation, district, floor type, udder washing, cleaning bedding, using towels, tick infestation, and previous history of mastitis) by interviewing 121 animal owners/attendants. The classification of the potential risk factors for mastitis was the age of lactating cows’ (young ≤4 years, adult 5–6 years and old ≥ 7 years), parity (few: ≤2calves, moderate: 3 to 5 calves, many: ≥6 calves) and lactation (early=3 to 5 months, mid=4 to7 months, late= ≥ 7 months).17–19
Detection of Mastitis
Clinical Mastitis: Using a physical examination, those lactating cows that had developed signs of mastitis were categorized into clinical mastitis according to Quinn et al.20 Signs of clinical mastitis diagnosed during the study period were: swelling, pain, heat, and abnormal secretion in the mammary gland (the presence of clots or flakes in milk or watery consistency) are accompanied by signs of systemic disturbance such as fever, depression, anorexia, and weakness.
Subclinical Mastitis: cows were considered to be affected by subclinical mastitis when they had no visible signs either in the udder or in the milk, but the milk production decreases.9 Teats were examined to check whether they are patent (those being milked by owners) or blocked. The milk samples from the patent teats were checked for subclinical mastitis using California Mastitis Test (CMT) as per Quinn et al.21 Accordingly, a squirt of milk, about 2 mL from each quarter was placed in each of four cups in the CMT paddle and an equal amount of the commercial CMT reagent was added to each cup. The mixture was mixed by a gentle circular motion in a horizontal plane for 15 seconds. The CMT results were scored as zero (negative), trace, one (weakly positive), two (distinct positive), and three (strongly positive) based on gel formation. All CMT scores of 0 and trace were considered as negative while CMT scores of one, two, and three were considered indicators of subclinical mastitis. Positive cows were defined as having at least one quarter with a CMT score of 1 +. The examination of udder and milk and sample collection was performed by veterinarian.
For bacteriological culture, 10 mL of mastitis milk (clinical and subclinical) were collected aseptically from affected teats separately and transported within 2 to 3 hrs using an icebox to Ambo University, Veterinary Microbiology Laboratory.
Isolation and Identification of Staphylococcus Species
The samples were inoculated aseptically onto sterile blood agar plates (BAP) enriched with 7% heparinized sheep blood, incubated at 37°C for 24–48 hrs under aerobic culture conditions, and examined for the presence of Staphylococcus. Colonies of Staphylococcus species were identified based on their morphological aspects (creamy, greyish, white, or yellow colonies) and hemolytic pattern on the surface of BAP. Presumed staphylococcal colonies were sub-cultured on nutrient agar plates (NAP) and incubated at 37°C for 24–48 hrs to get pure culture. Pure cultures of a single colony type from the NAP were inoculated into nutrient slants and incubated at 37°C for 24–48 hrs under aerobic culture conditions; the pure isolates in the nutrient slant were preserved and maintained at 4°C for further analysis.9
The isolation and identification of Staphylococcus species were performed from pure isolates grown on NAP. The shape and arrangement of these colonies were detected after performing Gram staining. The catalase tests were then conducted and the Staphylococcus species were assumed to be the colonies that produced gas bubbles.21
The colonies that were identified by Gram staining and catalase tests were sub-cultured on Mannitol Salt Agar (MSA) plates and incubated at 37°C examined after 24–48hrs for growth and change in the color of the medium. The presence of growth and change of pH in the media (red to yellow color) were regarded as confirmative identification of the salt-tolerant staphylococci. The fermentation of mannitol by S. aureus causes yellow discoloration of the medium. Colonies that develop weak or delayed yellow color after 24 hrs of incubation were regarded as S. intermedius and colonies that failed to produce any change on the medium were considered as S. hyicus and CNS.22 Colonies that were grown on the MSA plate were sub-cultured on nutrient medium broth and incubated at 37 °C for 24 hrs. Then, 0.5 mL of rabbit plasma and a drop of the 24 hrs old colonies taken from nutrient Broth (NB) were mixed and incubated for 4–24 hrs at 37°C. The clotting of suspension was evaluated at 30 minutes intervals for the first 4hrs of the test and then after 24hrs of incubation. The reaction was considered as coagulase-positive if any degree of clotting was visible.23
Identification of CNS Species and Antimicrobial Susceptibility Testing
Species identification for CNS and antimicrobial susceptibility of CNS and S. aureus were performed using the Phoenix machine,24 which is automated identification and susceptibility testing system found at the International Clinical Laboratory, Addis Ababa, Ethiopia. The S. aureus were selected purposively for antimicrobial susceptibility test due to their relatively higher public health and economic importance whereas the CNS were chosen due to their emerging causes of mastitis. Accordingly, of the total 49 Staphylococcus isolates, 17 Staphylococcus isolates belonging to three species namely S. aureus (n=7), S.lentus (n=8) and S.sciuri (n=2) were subjected to antimicrobial testing. Antimicrobial susceptibility testing was performed against 21 antimicrobials following the manufacturer’s instruction.24,25 Isolates were recorded as multiple drug resistance when they show a resistance pattern to at least one agent in three or more antimicrobial classes.26,27
Data Management and Statistical Analysis
Data were analyzed using STATA software version 11.0.28 Descriptive statistics was used to summarize the data and compute ratios and percentages. Chi-square test and logistic regression were used to assess the association of risk factors with the prevalence of mastitis. Dummy variables were created for those explanatory variables with more than two categories. For all risk factors, the level with the lowest prevalence was used as a reference category. Those variables with a p-value of less than 0.25 in the univariable analysis were further analyzed by multivariable logistic regression after checking for confounders. In all tested cases, 95% confidence intervals and p < 0.05 were set for significance.
Results
Prevalence of Mastitis
The overall prevalence of mastitis in lactating Zebu cows of the study area was found to be 30.5% (95% CI: 26.0–35.2%). The prevalence of clinical and subclinical mastitis were 3.2% (95% CI: 1.7 −5.4%) and 27.23% (95% CI: 22.9–31.9%), respectively. On the other hand, from the total of 1616 quarters examined, 1528 teats were found to be patent and sampled for this study. The quarter level overall prevalence of mastitis was 8.3% (95% CI: 7.0–9.8%) while quarter level clinical and subclinical mastitis were 1.1% (95% CI: 0.6–1.7) and 7.3% (95% CI: 6.0–8.7%), respectively. There was a higher prevalence of subclinical mastitis than clinical mastitis both at cow and quarter levels (Table 1).
Table 1.
Prevalence of Mastitis at the Cow and Quarter Level
| Categories | Type of Mastitis | ||
|---|---|---|---|
| Overall Prevalence of Mastitis (95% CI) | Prevalence of Clinical Mastitis (95% CI) | Prevalence of Subclinical Mastitis (95% CI) | |
| Cow level (N1=404) | 30.4 (26.0−35.2) | 3.2 (1.7–5.4) | 27.2 (22.9–31.9) |
| Quarter level (N2=1528) | 8.4 (7.0–9.8) | 1.1 (0.6–1.7) | 7.3 (6.0–8.7) |
Abbreviations: N1, number of cows; N2, number of patent teats; CI, confidence interval.
Identification of Staphylococcus Species
The overall isolation rate of the different species of the Staphylococcus species from quarter-level mastitic milk samples was 38.6% (95% CI: 30.1–47.6). The isolation rate from the subclinical mastitic case 38.7% (95% CI: 29.6–48.5) was slightly higher than that of clinical one 37.5% (95% CI: 15.2–64.6%). The prevalence of S. aureus, S. intermedius, S. hyicus, S. lentus, and S. sciuri from mastitic cows were 5.5, 10.2, 15.0, 6.3, and 1.6% respectively. S. intermedius and S. hyicus were not isolated from cases of clinical mastitis (Table 2).
Table 2.
Isolation Rate of Staphylococcus Species from Mastitic Quarters of Zebu Cows in the Study Areas
| Type of Staphylococcus Species (No. of Isolates in CM, SCM, M) | % Isolation (95% CI) from Clinical Mastitis (N=16) | % Isolation (95% CI) from Subclinical Mastitis (N=111) | % Isolation (95% CI) from Overall Mastitis (N=127) |
|---|---|---|---|
| S. aureus (3,4,7) | 18.75(4.1–45.6) | 3.6 (1.0–9.0) | 5.5(2.2–11.0) |
| S. intermedius (0,13,13) | 0(0.00) | 11.7(6.4–19.2) | 10.2(5.6–16.9) |
| S. hyicus (0,19,19) | 0(0.00) | 17.1(10.6–25.4) | 15.0(9.3–22.4) |
| CPS (3,36,39) | 18.75 (4.1–45.6) | 32.4(23.9–42.0) | 30.7(22.8–39.5) |
| S. lentus (3,5,8) | 18.75(4.1–4.7) | 4.5 (1.5–10.2) | 6.3(2.8–12.0) |
| S. sciuri (0,2,2) | 0(0.00) | 1.8(0.2–6.4) | 1.6(0.2–5.6) |
| CNS(3,7,10) | 18.75(4.1–4.7) | 6.3(2.8–12.6) | 7.9(3.8–14.0) |
| All species (6,43, 49) | 37.5(15.2–64.6) | 38.7(29.6–48.5) | 38.6(30.1–47.6) |
Abbreviations: CNS, coagulase negative Staphylococcus; CPS, coagulase positive Staphylococcus; CI, confidence interval; CM, clinical mastitis; M, overall mastitis cases; N, number of quarters; SCM, subclinical mastitis.
Risk Factors Associated with Mastitis
Among the risk factors, district, age, number of parities, frequency of cleaning bedding, udder washing, and previous history of mastitis were significantly associated (p< 0.05) with the prevalence of mastitis. Univariable logistic regression analysis showed that the risk of mastitis in Cheliya districts is 2.19 times higher than in the Toke Kutaye districts. Zebu cows with ages ranging from 5 to 6 years old have a higher probability of being affected by mastitis (OR=10.31) than cows with ages less than 4 years old. Similarly, results on bedding cleaning showed that Zebu cows living in the house cleaned at the interval of greater than one week (poor hygiene) have a higher probability to be infected with mastitis (OR=7.11) as compared to Zebu cows living in the house cleaned less than one week (good hygienic). The probability of being affected by mastitis was also significantly higher in cows at the mid-lactation stage and compared to the early stage of lactation as well as in cows with a history of the previous mastitis than its counterpart (Table 3)
Table 3.
Logistic Regression Analysis of Potential Risk Factors for the Occurrence of Mastitis in the Study Area
| Risk Factors | Categories | No. of Animals Exam. | No. Positive (%) | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | p value | ||||
| District | Toke Kutaye | 148 | 35 (23.65) | 1.0 | – | – | 1.0 | – | – |
| Dendi | 162 | 45 (27.78) | 1.24 | 0.74–2.07 | 0.407 | 1.55 | 0.68–3.49 | 0.295 | |
| Cheliya | 94 | 43 (45.74) | 2.19 | 1.56–4.73 | 0.004 | 1.68 | 0.73–3.85 | 0.224 | |
| Age | ≤ 4 years | 234 | 41 (17.52) | 1.0 | – | – | 1.0 | – | – |
| ≥ 7 years | 103 | 36 (34.95) | 2.53 | 1.49–4.28 | 0.001 | 1.88 | 0.91–3.91 | 0.089 | |
| 5–6 years | 67 | 46 (68.66) | 10.31 | 5.57–19.10 | ≤0.001 | 8.68 | 3.89–19.38 | ≤0.001 | |
| Stage of lactation | > 7 months (late) | 48 | 9 (18.75) | 1.0 | – | – | 1.0 | – | – |
| < 3 months (early) | 297 | 92 (30.98) | 1.94 | 0.90–4.18 | 0.089 | 3.51 | 1.29–9.51 | 0.014 | |
| 4–7 months (mid) | 59 | 22 (37.29) | 2.58 | 1.05–6.32 | 0.039 | 4.01 | 1.23–13.12 | 0.022 | |
| Number of parities | ≤ 2 calves | 119 | 18 (15.13) | 1.0 | – | – | |||
| 3 to 5 calves | 211 | 52 (24.64)) | 1.84 | 1.02–3.31 | 0.044 | ||||
| ≥ 6 calves | 74 | 53 (71.62) | 14.16 | 6.95–28.86 | ≤0.001 | ||||
| Type of Housing | Separate pen | 52 | 12 (23.08) | 1.0 | – | – | 1.0 | – | – |
| Shared barn | 352 | 111 (31.53) | 1.53 | 0.78–3.04 | 0.219 | 2.61 | 1.01–6.74 | 0.047 | |
| Interval of cleaning bedding | Once a week | 209 | 32 (15.31) | 1.0 | – | – | 1.0 | – | – |
| < once a week | 51 | 10 (19.61) | 1.35 | 0.61–2.96 | 0.456 | 1.30 | 0.45–3.75 | 0.622 | |
| > a week | 144 | 81 (56.25) | 7.11 | 4.31–11.73 | ≤0.001 | 7.96 | 3.73–17.00 | ≤0.001 | |
| Udder washing | Yes | 101 | 11 (10.89) | 1.0 | – | – | |||
| No | 303 | 112 (36.96) | 4.80 | 2.46–9.36 | ≤0.001 | ||||
| Type of floor | Concrete | 326 | 98 (30.06) | 1.0 | – | – | |||
| Muddy | 78 | 25 (32.05) | 1.10 | 0.65–1.87 | 0.732 | ||||
| Using towel | No | 313 | 95 (30.35) | 1.0 | – | – | |||
| Yes | 91 | 28 (30.77) | 1.02 | 0.61–1.69 | 0.939 | ||||
| Tick infestation | Yes | 264 | 76 (28.79) | 1.0 | – | – | |||
| No | 140 | 47 (33.57) | 1.25 | 0.80–1.94 | 0.321 | ||||
| History of the previous mastitis | No | 221 | 21 (9.50) | 1.0 | – | – | 1.0 | – | – |
| Yes | 183 | 102 (55.74) | 11.99 | 7.02–20.50 | ≤0.001 | 9.35 | 4.92–17.78 | ≤0.001 | |
Abbreviations: CI, confidence interval; OR, odds ratio; No., number; Exam, examined.
Results of the multicollinearity matrix revealed that all independent variables were not collinear with each other (r<0.5) except the use of towel versus the type of floor (r=0.50) and use of towel versus udder washing (r=0.69). The independent variables entered into the multivariable model were district, age, stage of lactation, parity, type of housing, cleaning of bedding, udder washing, and previous history of mastitis. However, parity and udder washing were removed from the model due to confounding (the change in % OR between univariable and multivariable analysis is 30%). Thus, considering univariable p-value < 0.2, non-collinearity, and frequency of variable categories, the following variables were selected for entry into the multivariable model: district, age, lactation, type of housing, the interval of cleaning of bedding, and history of the previous mastitis. The final multivariable logistic regression model of risk factors analysis revealed that age (p<0.001), stage of lactation (p< 0.05), type of housing (P<0.05), the interval of bedding cleaning (p<0.001), and previous history of mastitis (p<0.001) had a significant association with mastitis and hence are independent predictors (Table 3).
Antimicrobial Susceptibility of Staphylococcus Species
The antimicrobial study showed that 100% (7/7) of the S. aureus isolates were susceptible to 16 antimicrobials and 100% (8/8) of S. lentus and 100% (2/2) of the S. sciuri were susceptible to 13 antimicrobials. However, 87.5% (7/8) of S. lentus and 100% (2/2) of S. sciuri showed resistance against 7 antimicrobials (Table 4). Six out of the 7 resistant S.lentus isolates and both the resistant S.sciuri isolates were from the Toke Kutaye district.
Table 4.
Antimicrobial Susceptibility Pattern of S. aureus, S. lentus, and S. sciuri Isolates
| Antimicrobials | Antimicrobial Susceptibility Pattern of Staphylococcus Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (n=7) | S. lentus (n=8) | S. sciuri (n=2) | ||||||||||
| S | R | ND | I | S | R | ND | I | S | R | ND | I | |
| Amoxicillin - Clavulanate | 100 | 0 | 0 | 0 | 12.5 | 87.5 | 0 | 0 | 0 | 100 | 0 | 0 |
| Ampicillin | 85.7 | 14.3 | 0 | 0 | 0 | 87.5 | 12.5 | 0 | 0 | 100 | 0 | 0 |
| Cefotaxime | 100 | 0 | 0 | 0 | 12.50 | 87.5 | 0 | 0 | 0 | 100 | 0 | 0 |
| Cefoxitin | 0 | 0 | 100 | 0 | 0 | 87.5 | 12.5 | 0 | 0 | 100 | 0 | 0 |
| Ciprofloxacin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Clindamycin | 85.7 | 0 | 0 | 14.3 | 12.5 | 12.5 | 0 | 75 | 0 | 0 | 0 | 100 |
| Daptomycin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Erythromycin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Gentamycin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Imipenem | 100 | 0 | 0 | 0 | 12.5 | 87.5 | 0 | 0 | 0 | 100 | 0 | 0 |
| Linezolid | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Moxifloxacin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Mupirocin high level | 85.7 | 14.3 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Nitrofurantoin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Oxacillin | 100 | 0 | 0 | 0 | 12.5 | 87.5 | 0 | 0 | 0 | 100 | 0 | 0 |
| Penicillin-G | 71.4 | 28.6 | 0 | 0 | 0 | 87.5 | 12.5 | 0 | 0 | 100 | 0 | 0 |
| Rifampin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Teicoplanin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Tetracycline | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Trimethoprim-sulfamethoxazole | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Vancomycin | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
Abbreviations: S, susceptible; R, resistant; I, intermediate; ND, not determined.
Multidrug Resistance of Staphylococcus Species
The present study indicated an overall of 52.9% (9/17) of the Staphylococcus species isolated were multidrug-resistant (MDR). Of these, the newly isolated Staphylococcus species; namely S. sciuri (100%) and S. lentus (87.5%) were MDR whereas none of the S. aureus isolates showed MDR. The drugs against which resistances detected were amoxicillin-clavulanate, ampicillin, oxacillin, penicillin-G, cefotaxime, cefoxitin, clindamycin, and imipenem.
Discussion
The overall prevalence of mastitis in Zebu cows recorded in the present study (30.5%) was closely in agreement with Bitew et al29 who reported 28.2% prevalence in Bahir Dar town from Zebu cows. However, the current result was lower than what has been reported in exotic and crossbred cows by Zeryehun et al5 from Addis Ababa, Abebe et al6 from Hawassa, Derib et al7 from in and around Wolaita Sodo, Southern Ethiopia and Zeryehun and Abera8 from Eastern Hararghe Zone with the respective prevalence of 74.7%, 64.3%, 50%, and 62.6%. The relatively lower prevalence in the current study may be due to differences in breeds, agroecology, and management systems practiced by farmers in different parts of the country. Most of the dairy farms in Ethiopia have cross-breed cows with Holstein Frisian with local cows and they manage their cows under intensive or semi-intensive systems. However, in the current study, the farmers were managing their cows under a fully extensive system and the cows were kept the whole day in communal grazing areas. Such practice is known to decrease the chance of transmission of contagious mastitis between cows.9 Moreover, it has also been documented that Zebu cows are known to have low milk production and relatively resistant to mastitis.30
The prevalence of clinical mastitis in this study (3.2%) was in agreement with the finding of Bitew et al,29 who reported a 3% prevalence in Zebu cows and crossbreed dairy farms in Bahir Dar town, Ethiopia. However, the present finding was higher than that of Belachew18 who reported 0.7% prevalence around Bishoftu town in Zebu cows but much lower than 39.8% and 12.5% prevalence reported by Abebe et al6 from Hawassa and Zeryehun and Abera8 from Eastern Hararghe Zone of Ethiopia, respectively. The variation in the prevalence might be attributed to the differences in the management practices of clinical mastitis at different places and the availability of veterinary services. Besides, the lack of awareness of the farmers about mastitis, the high cost, and the shortage of intermammary infusion observed in the current study areas might contribute to the variation in the reported prevalence. The overwhelming cases of subclinical mastitis compared to clinical mastitis in the present study are also in agreement with studies by Workineh et al,31 Dego and Tareke.32 The prevalence of subclinical mastitis in the present study is higher than the findings by Girma et al,17 who reported a 15.89% prevalence in Doba districts, East Hararghe in local Zebu lactating cows, and Belachew,18 who reported 5.3% prevalence around Bishoftu town in local cows. Due to the difficulty of detecting subclinical mastitis as compared to the easily detectable clinical cases, the subclinical form of mastitis still received little attention in Ethiopia and efforts have been concentrated on the treatment of clinical cases while the high economic loss could come from subclinical mastitis. Moreover, Zebu cows are owned by the majority of smallholder farmers, who lack awareness about the disease and lives in the rural part of the country where there are limited veterinary services.
The overall quarter level prevalence (8.3%) recorded in this study is nearly in agreement with Girma et al,17 who reported a 10.12% prevalence in Doba District, East Hararghe zone. However, the present finding is lower than the findings of Bitew et al,29 who reported 12.3% prevalence in and around Bahir Dar town and higher than that of Belachew,18 who reported a 6% prevalence around Bishoftu town, and Zeryehun et al,5 who reported a 5.2% prevalence from in and around Addis Ababa, Ethiopia. The difference in the results might be related to the variation in veterinary services, animal and barn hygienic practices at different places. In Ethiopia, a large number of farmers treat their sick animals themselves or get the services from Para-veterinarians. Such practices are particularly very common in areas where there is limited veterinary service like the current study areas as compared to areas near major towns like Addis Ababa and Bishoftu.
The present study revealed that the prevalence of mastitis was higher in older cows than younger ones, in cow kept in the shared house than a separate house, in cows kept in a house cleaned less frequently than frequently cleaned ones, in cows at mid-stage of lactation than the late stage of lactation, in cows with the previous history of mastitis than those cows without the previous history of mastitis and absence of udder washing before milking cows. It has been well documented that older cows have the largest teats and more relaxed sphincter muscles, which increase the accessibility of infectious agents in the cows’ udder.9 The increased prevalence of mastitis with parity reported in the current study is in line with the previous reports by Zeryehun et al,5 Abunna et al33 and Belayneh et al.34 This could be because primiparous cows have a more effective defense mechanism than multiparous cows35 and there is an increased chance of infection with time and the prolonged duration of infection in multiparous cows.9 Confining of several lactating cows in a barn with a poor hygienic condition, poor bedding increases the chance of animals getting contaminated by microorganisms from urine and feces. The microorganisms thus can contaminate the exterior of the teats followed by entry to the teat canal to cause mastitis.36 Particularly the absence of the practice of udder washing before milking will further spread contagious mastitis.
In the current study, the overall isolation rate of CPS from Zebu cow mastitic milk was 30.7% of which the prevalence for S. aureus, S. intermedius, and S. hyicus accounted for 5.5%, 10.2%, and 15.0%, respectively (Table 2). This prevalence was nearly equivalent with 33.4% CPS isolation rate reported by Belachew,18 but he reported a higher isolation rate for S. aureus (16.7%) and lower S. hyicus (5.6%) from 300 samples collected from Zebu cow’s mastitic milk in and around Bishoftu town, Central Ethiopia. An even greater prevalence than the current study was also reported for overall Staphylococcus species (47.11%) by Girma et al,17 S. aureus (24.2%) by Zeryehun and Abera,8 and S. aureus (51.2%) by Abebe et al.6 The variation in the prevalence of CPS reported from mastitic milk samples from different districts of the country might be due to differences in the agroecological zone and management practices of the milking cows at different places.
The present study showed a 7.9% overall isolation rate of CNS from mastitic zebu cow milk. This value is much less than the 34.2% isolation rate from Holstein-Zebu cross-breeds and local Zebu cows reported by Zeryehun and Abera.8 Studies that have shown CNS isolation from mastitis milk in Zebu Cows in Ethiopia are generally scarce. In the current study, S. lentus and S. sciuri were identified as newly emerging CNS isolated from mastitic milk from Zebu cows in the study areas. The 7.9% overall prevalence of CNS in the current study was lower than the 13.7% prevalence of S. lentus and S. sciuri out of 255 samples collected from mastitic milk samples of dairy cattle in Timisoara, Romania.37 This variation in the prevalence of CNS reported from mastitic milk might be attributed to the variation in the breed of the dairy cows in the different countries, management systems and the difference in methods of laboratory analysis.
In the present study, the prevalence of Staphylococcus species in subclinical mastitis (38.7%) was slightly higher than clinical mastitis (37.5%). This difference might be explained by the already documented pieces of evidence that Staphylococcus species are adapted to survive in the udder and usually establishes chronic subclinical infection of long duration from which it is shaded through milk serving as sources of infection for other healthy cows and transmitted during the milking process. Transmission among cows increases whenever there is the absence of dry cow therapy and post milking teat dipping, the invariable hold milking practice, and the low culling rate of chronically infected cows.9 The prevalence and degree of antimicrobial resistance in veterinary medicine are increasing worldwide. The dissemination of antimicrobial-resistant staphylococci is presenting a challenge to both human and animal health professionals.38 The CNS (S. lentus and S. sciuri), one of the causes of subclinical mastitis in the present study, is reported for the first time in Ethiopia. These isolates were resistant to amoxicillin-clavulanate, ampicillin, cefotaxime, cefoxitin, imipenem, oxacillin, and penicillin-G. This finding is in line with Turutoglu et al,39 who identified that the CNS from mastitic milk in Turkey had 100% resistance to penicillin-G and ampicillin, and 96% to oxacillin. The high degree of resistance to the beta-lactam class of antimicrobials might be related to their prolonged usage in the public health sector and a limited extent in veterinary medicine. This result is also similar to previous findings conducted in Brazil by Soares et al40 who showed the CNS isolates from mastitic milk were resistant to penicillin and ampicillin. This may be due to oxacillin-resistant Staphylococcus are resistant to all beta-lactam antibiotics which carry an altered penicillin-binding protein, which is encoded by the mecA gene.41
In the current study Staphylococcus aureus was 100% susceptible to amoxicillin-clavulanate, cefotaxime, ciprofloxacin, nitrofurantoin, oxacillin, daptomycin, erythromycin, gentamycin, rifampin, teicoplanin, tetracycline, trimethoprim-sulfamethoxazole, imipenem, linezolid, and moxifloxacin. The high degree of susceptibility of Staphylococcus aureus to several drugs may be due to the rare use of these drugs in the treatment of Zebu cows in the study area. However, the absence of resistance for tetracycline, trimethoprim-sulfamethoxazole, and oxacillin was not to our expectation since these drugs have been used in the country for many years both in the veterinary and public health sectors. Further research is required to determine the reason for the absence of resistance to these drugs. In line with this finding, Tassew et al19 reported 100% susceptible S. aureus to gentamycin in Kombolcha town, Ethiopia from mastitic milk of crossbreed and local dairy cows. In this study, the relative lowest resistance of S. aureus was found to Penicillin G, ampicillin, and mupirocin. Antonov et al42 have also been reported that 31.3% of S. aureus isolated from human samples were resistant to mupirocin. Werckenthin et al43 have reported that antibiotic resistance is carried on plasmids and transposons that can pass from one Staphylococcal species to another.
S. sciuri and S. lentus are known to be common in animals, humans, and the environment. It has been previously isolated from infections in veterinary and human medicine.44 These CNS are important causes of subclinical mastitis. In the current study, S. lentus was isolated from both subclinical (15.8%) and clinical mastitis (4.6%) cases while S. sciuri was isolated only from subclinical mastitis cases (1.9%). The high level of MDR observed in S. sciuri and S. lentus in the present study might suggest the possession and spread of multiple virulences and resistance genes44 or mutation. S. sciuri could acquire diverse virulence factors from other staphylococci through horizontal gene transfer, hence augmenting its pathogenic potential.44 The MDR observed in these CNS is of great concern since these bacteria might lead to serious economic losses and serve as reservoirs for resistance and virulence genes.45
The limitations of this study include failure to perform molecular studies such as genotypic analysis to accurately identify Staphylococcus species, detection of genes responsible for antimicrobial resistance and conduct the antibiogram study for all the Staphylococcus isolates.
Conclusion
The present study showed a high prevalence of bovine mastitis and a higher isolation rate of Staphylococcus species in Zebu cows in the study areas. Five Staphylococcus species namely S. intermedius, S. hyicus, S. aureus, S. lentus, and S. sciuri were identified, of which the later two were isolated for the first time in Ethiopia. The S. lentus, and S. sciuri were highly multidrug resistant. The age of cows, stage of lactation, type of housing, the interval of bedding cleaning, and previous antimicrobial treatments were predictors of mastitis. Therefore, there is a need to create awareness among farmers on proper hygienic practices, and the selection of appropriate antimicrobials for the treatment of mastitis. Moreover, continuous surveillance of the emerging and antimicrobial-resistant species using molecular studies is warranted.
Acknowledgments
We thank Ambo University for providing funds to conduct this study, Holeta Agricultural Research Center, Asella Regional Veterinary Laboratory, and Sebata National Animal Diagnostic and Disease Investigation Center for providing laboratory reagents. Many thanks to the Department of Veterinary Laboratory Technology staff members for their kind and unreserved laboratory assistance. We also appreciate and thank animal owners and animal health assistants of the different districts for their cooperation during sample collection.
Funding Statement
The funding source for the current study was Ambo University, Ethiopia.
Data Sharing Statement
The data generated and analyzed during the current study are available in the [Raw data.xls] Open Science Framework, [https://osf.io/k3spm/].
Ethics Approval and Informed Consent
The animal owners were informed about the purpose of the study and consent of each animal owner was obtained before the physical examination of cows for clinical mastitis and the collection of milk samples. Only volunteer animal owners have participated in the study. The questionnaire and methodology for this study were approved by the research ethics review and clearance committee of the University of Ambo (Date 25/10/2016./Ref. No: ARCEC 004).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.CSA. Central Statistical Authority, Federal Democratic Republic of Ethiopia, Statistical Abstract. 2010. [Google Scholar]
- 2.FAO. Milking, Milk Production Hygiene, and Udder Health. Animal Production and Health Paper. 2003. [Google Scholar]
- 3.Sharma N, Maiti S, Sharma KK. Prevalence, etiology, and antibiogram of microorganisms associated with sub-clinical mastitis in buffaloes in durg, Chhattisgarh State (India). Int J Dairy Sci. 2007;2(2):145–151. doi: 10.3923/ijds.2007.145.151 [DOI] [Google Scholar]
- 4.Getaneh AM, Gebremedhin EZ. Meta-analysis of the prevalence of mastitis and associated risk factors in dairy cattle in Ethiopia. Trop Anim Health Prod. 2017;49(4):697–705. doi: 10.1007/s11250-017-1246-3 [DOI] [PubMed] [Google Scholar]
- 5.Zeryehun T, Aya T, Bayecha R. Study on prevalence, bacterial pathogens and associated risk factors of bovine mastitis in smallholder dairy farms in and around Addis Ababa, Ethiopia. J Anim Plant Sci. 2013;23(1):50–55. [Google Scholar]
- 6.Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12(1):270. doi: 10.1186/s12917-016-0905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derib BT, Birhanu BT, Sisay T. Isolation and identification of methicilin-resistant Staphylococcus aureus from bovine mastitic milk in and around Wolaita Sodo, Southern Ethiopia. J Vet Sci Res. 2017;2(3):1–11. [Google Scholar]
- 8.Zeryehun T, Abera G. Prevalence and bacterial isolates of mastitis in dairy farms in selected districts of Eastern Hararghe Zone, Eastern Ethiopia. J Vet Med. 2017;2017:6498618. doi: 10.1155/2017/6498618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Mastitis in Veterinary Medicine: Text Textbook of the Disease of Cattle, Horse, Sheep, Pigs, and Goats. 10th ed. London: Elsevier; 2007. [Google Scholar]
- 10.Fetsch A, Johler S. Staphylococcus aureus as a foodborne pathogen. Curr Clin Microbiol Rep. 2018;5(2):88–96. doi: 10.1016/B978-0-12-809671-0.00001-2 [DOI] [Google Scholar]
- 11.Simojoki H, Salomäki T, Taponen S, Iivanainen A, Pyörälä S. Innate immune response in experimentally induced bovine intramammary infection with Staphylococcus simulans and S. epidermidis. Vet Res. 2011;42(1):49. doi: 10.1186/1297-9716-42-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gizaw F, Kekeba T, Teshome F, et al. Distribution and antimicrobial resistance profile of coagulase-negative staphylococci from cattle, equipment, and personnel on a dairy farm and abattoir settings. Heliyon. 2020;6(3):e03606. doi: 10.1016/j.heliyon.2020.e03606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLFDO. Cheliya Districts Livestock and Fishery Development Office; the Annual Report. Cheliya, Ethiopia; 2016. [Google Scholar]
- 14.TLFDO. Toke Kutaye Districts Livestock and Fishery Development Office, the Annual Report. Toke Kutaye, Ethiopia; 2016. [Google Scholar]
- 15.DLFDO. Dendi Districts Livestock and Fishery Development Office; the Annual Report. Dendi, Ethiopia; 2016. [Google Scholar]
- 16.Thrusfield M. Veterinary Epidemiology; Describing Disease Occurrence. 3rd ed. Blackwell Publishing; 2005. [Google Scholar]
- 17.Girma S, Mammo A, Bogele K, Sori T, Tadesse F, Jibat T. Study on the prevalence of bovine mastitis and its major causative agents in West Hararghe zone, Doba district, Ethiopia. J Vet Med Anim Health. 2012;4(8):116–123. doi: 10.5897/JVMAH12.016 [DOI] [Google Scholar]
- 18.Belachew T. Bovine mastitis: prevalence, isolation of bacterial species involved and its antimicrobial susceptibility test around Debrezeit, Ethiopia. J Vet Sci Technol. 2016;7(06):2. doi: 10.4172/2157-7579.1000396 [DOI] [Google Scholar]
- 19.Tassew A, Negash M, Demeke A, Feleke A, Tesfaye B, Sisay T. Isolation, identification, and drug resistance patterns of methicillin-resistant Staphylococcus aureus from mastitic cow’s milk from selected dairy farms in and around Kombolcha, Ethiopia. J Vet Med Anim Health. 2015;8(11):1–10. doi: 10.5897/JVMAH2015.0422 [DOI] [Google Scholar]
- 20.Quinn PJ, Carter ME, Markey B, Carter GR. Clinical Veterinary Microbiology. Spain: Mosby Elsevier Limited; 1999. [Google Scholar]
- 21.Quinn PJ, Carter ME, Markey BK, Carter GR. Veterinary Microbiology Microbial Diseases- Bacterial Causes of Bovine Mastitis. 8th ed. London: Mosby International Limited; 2002. [Google Scholar]
- 22.Quinn PJ, Markey B, Donnelly WJ, Leonard FC, Maghire D. Veterinary Microbiology and Microbial Disease. London: Blackwell Science, Ltd; 2005. [Google Scholar]
- 23.Tallent S, Hait J, Bennett RW, Lancette GA. Bacteriological Analytical Manual. USA; 2001. [Google Scholar]
- 24.BD-Phoenix™, Becton D. Phoenix company TM system user manual, laboratory procedure. USA2006–2008:20–25.
- 25.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI; 2015:64–70. [Google Scholar]
- 26.Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(12):1619–1629. doi: 10.1099/jmm.0.46747-0 [DOI] [PubMed] [Google Scholar]
- 27.Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug‐resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 28.Stata statistical software [computer program]. Version 11. USA; 2009. [Google Scholar]
- 29.Bitew M, Tafere A, Tolosa T. Study on bovine mastitis in dairy farms of Bahir Dar and its environs. J Anim Vet Adv. 2010;9(23):2912–2917. doi: 10.3923/javaa.2010.2912.2917 [DOI] [Google Scholar]
- 30.Beriso K, Tamir B, Feyera T. Characterization of smallholder cattle milk production system in Aleta Chukko District, southern Ethiopia. J Adv Dairy Res. 2015;1–8. doi: 10.4172/2329-888X.1000132 [DOI] [Google Scholar]
- 31.Workineh S, Bayleyegn M, Mekonnen H, Potgieter L. Prevalence and etiology of mastitis in cows from two major Ethiopian dairies. Trop Anim Health Prod. 2002;34(1):19–25. doi: 10.1023/a:1013729626377 [DOI] [PubMed] [Google Scholar]
- 32.Dego OK, Tareke F. Bovine mastitis in selected areas of southern Ethiopia. Trop Anim Health Prod. 2003;35(3):197–205. doi: 10.1023/A:1023352811751 [DOI] [PubMed] [Google Scholar]
- 33.Abunna F, Fufa G, Megersa B, Regassa A. Bovine mastitis: prevalence, risk factors and bacterial isolation in small-holder dairy farms in Addis Ababa City, Ethiopia. Glob Vet. 2013;10(6):647–652. doi: 10.5829/idosi.gv.2013.10.6.7349 [DOI] [Google Scholar]
- 34.Belayneh R, Belihu K, Tesfaye A. Microbiological study on bacterial causes of bovine mastitis and its antibiotics susceptibility patterns in East Showa Zone, Akaki District, Ethiopia. J Vet Med Anim Health. 2014;6(4):116–122. doi: 10.5897/JVMAH2013.0272 [DOI] [Google Scholar]
- 35.Eriskine R. Intramuscular administration of ceftiofur sodium versus intramammary infusion of penicillin/novobiocin for treatment of Streptococcus agalactiae mastitis in dairy cows. J Am Vet Med Assoc. 2001;208:258–260. [PubMed] [Google Scholar]
- 36.Tamime AY. Milk Processing and Quality Management. Blackwell Publishing Ltd; 2009. [Google Scholar]
- 37.Brinda M, Herman V, Fodor I. Phenotypic characterization of coagulase-negative staphylococci isolated from mastitic milk in cows. Lucrari Stiintifice Medicine Veterinara. 2010;43:97–101. [Google Scholar]
- 38.Al-Thani RF, Al-Ali F. Incidences and antimicrobial susceptibility profile of Staphylococcus species isolated from animals in different Qatari farms. Afr J Microbiol Res. 2012;6(48):7454–7458. doi: 10.5897/AJMR12.1270 [DOI] [Google Scholar]
- 39.Turutoglu H, Ercelik S, Ozturk D. Antibiotic resistance of Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine mastitis. Bull Vet Inst Pulawy. 2006;50(1):41. [Google Scholar]
- 40.Soares LC, Pereira IA, Pribul BR, Oliva MS, Coelho SMO, Souza MMS. Antimicrobial resistance and detection of mecA and blaZ genes in coagulase-negative Staphylococcus isolated from bovine mastitis. Pesqui Vet Bras. 2012;32(8):692–696. doi: 10.1590/S0100-736X2012000800002 [DOI] [Google Scholar]
- 41.Miragaia M. Factors contributing to the evolution of mecA-mediated β-lactam resistance in staphylococci: update and new insights from whole genome sequencing (WGS). Front Microbiol. 2018;9:2723. doi: 10.3389/fmicb.2018.02723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonov NK, Garzon MC, Morel KD, Whittier S, Planet PJ, Lauren CT. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob Agents Chemother. 2015;59(6):3350–3356. doi: 10.1128/aac.00079-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werckenthin C, Cardoso M, Martel J-L, Schwarz S. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus, and canine Staphylococcus intermedius. Vet Res. 2001;32(3–4):341–362. doi: 10.1051/vetres:2001129 [DOI] [PubMed] [Google Scholar]
- 44.Nemeghaire S, Argudín MA, Fessler AT, Hauschild T, Schwarz S, Butaye P. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet Microbiol. 2014;171(3–4):342–356. doi: 10.1016/j.vetmic.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 45.Frey Y, Rodriguez JP, Thomann A, Schwendener S, Perreten V. Genetic characterization of antimicrobial resistance in coagulase-negative staphylococci from bovine mastitis milk. J Dairy Sci. 2013;96(4):2247–2257. doi: 10.3168/jds.2012-6091 [DOI] [PubMed] [Google Scholar]