Abstract
BACKGROUND AND PURPOSE:
The time from arterial puncture to successful recanalization is an important milestone toward timely recanalization. With the significant improvement in recanalization rates by using thrombectomy devices, procedural time to recanalization is becoming a determinant factor in choosing among available devices. We aimed to assess the impact of time to recanalization on the outcome of intra-arterial stroke therapies.
MATERIALS AND METHODS:
We conducted a meta-analysis of studies reporting procedural times in patients with stroke treated with the MD, PS, and RS.
RESULTS:
We identified 16 eligible studies: 4 on the MD (n = 357), 8 on the PS (n = 455), and 4 on RS (n = 113). Merci device studies described total procedural duration, while PS and RS studies described puncture-to-recanalization times. With a random-effects model, mean procedural duration for the MD was 120 minutes (95% CI, 105.7–134.2 minutes). Mean puncture to recanalization time for the PS was 64.6 minutes (95% CI, 44.4–84.8 minutes) and 54.7 minutes for RS (95% CI, 47.3–62.2 minutes). Successful recanalization was achieved in 211 of 357 patients (59.1%) in the MD studies (95% CI, 49.3–77.7), 394 of 455 (86.6%) in the PS studies (95% CI, 84.1–93.8), and 105 of 113 (92.9%) in the RS studies (95% CI, 90.9–99.9). Functional independence (mRS ≤2) was achieved in 31.5% of patients in the MD studies, 36.6% in the PS studies, and 46.9% in the RS studies.
CONCLUSIONS:
The use of the PS and RS was associated with comparable procedural time to recanalization. Available data did not allow this parameter to be determined for trials using the MD. Retrievable stents achieved the highest rate of successful recanalization and functional outcome and the lowest mortality.
Early complete recanalization is a strong predictor of good outcome in stroke,1 but the best means for achieving this remain uncertain.2,3 Fewer than half of MCA occlusions achieve recanalization with IV tPA,4,5 and even fewer in proximal occlusions.2,6 In addition, 12%–34% of those who achieve recanalization with IV tPA have early reocclusion.7,8
The only randomized trials of intra-arterial thrombolytic therapy are Prolyse in Acute Cerebral Thromboembolism Trial I and II,9,10 which randomized patients to prourokinase plus IV heparin versus IV heparin. Recanalization was achieved in 66% of the treatment group versus 18% in controls (P < .001).10 With a mean time to start treatment of 5.3 hours, Prolyse in Acute Cerebral Thromboembolism Trial II combined a relatively higher recanalization rate with a favorable outcome and validated the interest in intra-arterial stroke therapy.4,11
Research led to the development of mechanical devices that await evidence from randomized trials to support their efficacy over IV tPA. Physicians performing intra-arterial stroke therapies rely on their experience to choose from these devices. Among the commonly used devices are the MD12 (Concentric Medical, Mountain View, California), the PS13 (Penumbra, Alameda, California), and RS14 (Solitaire; ev3, Irvine, California; or Trevo; Concentric Medical). Many pooled analyses of recanalization rates with devices do not report procedural duration and intraprocedural complications.13,15,16 These technical aspects reflect the device safety, ease of use, and speed of recanalization. This meta-analysis aims to compare the procedural time to recanalization of the MD, PS, and RS in the acute stroke setting.
Materials and Methods
Search Strategy
We conducted a systematic review by using a predetermined protocol in accordance with the Meta-Analysis Of Observational Studies statement.17 We identified English language articles by searching the following electronic data bases from the year of the first Mechanical Embolus Removal in Cerebral Ischemia trial publication16: MEDLINE (January 2004 to February 2011) and EMBASE (January 2004 to February 2011). We also scanned the bibliographies of key articles to identify additional studies.
We combined 3 search themes by using the Boolean operator “AND.” These terms were searched in MEDLINE as both MeSH headings and text words:
-
Stroke or cerebrovascular accident
AND
-
Mechanical thrombolysis, thrombectomy, or endovascular intervention
AND
Device name (full and truncated)
Selection Criteria
The inclusion criteria were the following: 1) studies on humans; 2) published in full or as abstracts; 3) in the English language; 4) describing original data; 5) explicitly reporting the puncture-to-recanalization time or procedural duration; 6) using the MD, PS, or RS in the setting of acute ischemic stroke; and 7) in ≥10 patients. Studies exclusively reporting the use of devices other than the 3 devices of interest were excluded. Studies on nonretrievable stents were excluded.
Two of the authors (M.A.A., B.K.M.) screened the titles and abstracts and agreed on the included studies. Full text review of the articles retained from the primary screen was performed, and the data were extracted and summarized and the study quality was evaluated.
Data Extraction
Data were extracted by 1 author (M.A.A.). The data-extraction sheet included the following sections: 1) study characteristics, 2) baseline characteristics, 3) details of intra-arterial therapy, and 4) procedural and clinical outcome measures.
Study Characteristics
Data included single-institution or multicenter prospective or retrospective designs, year and journal of publication, and midyear of the study (median calendar year of treatment dates). The quality of included studies was judged on the basis of design, number of participating centers, presence of a nonhistorical comparative group, and adequacy of follow-up.
Baseline Characteristics of Patients
Baseline characteristics included number, mean age, stroke severity measured by the NIHSS score at presentation, time from stroke onset to presentation, extent of early ischemic changes on baseline imaging, treatment with IV tPA, and time from stroke onset to IV tPA administration.
Procedural Characteristics
Data included time from stroke onset to arterial puncture, site of occlusion, baseline TIMI/TICI scores, devices used, intra-arterial tPA use, procedural time to recanalization for the device studied, and final TIMI/TICI score. If the puncture-to-recanalization time was not stated, the reported “procedure duration” was used instead. Device-specific times were used if multiple devices were used. In only 1 study,13 the procedural time to recanalization was not reported in the main publication but was later reported in an abstract by the same authors.18 We have also investigated whether the procedural time-to-recanalization duration had changed during the years since 2004.
Outcomes
Outcomes included successful recanalization (TIMI grades 2 or 3, TICI grades 2b/3), procedural complications such as arterial perforation, device fracture or malfunction, and intracranial hemorrhage. Data on the duration of patient follow-up, mortality, and functional independence, defined as mRS ≤2 at 3 months, were collected. We assessed whether shorter procedural time to recanalization was associated with an mRS of ≤2.
Statistical Analysis
The mean procedural time to recanalization (in minutes) was recorded for each study along with a measure of variability. If the median time was reported, it was converted to the mean in studies reporting ≤25 patients by using the following formula: mean = (A+ 2 × median + B)/4, where A and B represent the low and high ends of the range respectively.19 In studies including >25 patients, the median was used as an estimate of the mean.19 Similarly, in studies not reporting the SD, we derived standard errors on the basis of the study by Hozo et al19 by using the range/4 for studies including ≤70 patients and range/6 for studies including >70 patients.
The proportion of patients experiencing adverse outcomes was recorded. Because the Q statistic indicated significant heterogeneity, a random-effects model was used to summarize the mean procedural time to recanalization across the studies stratified by the device used. For the test of trend, we used the Cuzick extension of the Wilcoxon rank sum test.20 For all tests, a 2-sided P value ≤.05 was deemed significant. Sensitivity analyses were conducted in which the meta-analysis was restricted to published studies or to prospective-design studies. Analyses were performed by using STATA 10.0 (StataCorp, College Station, Texas).
Results
Study Characteristics
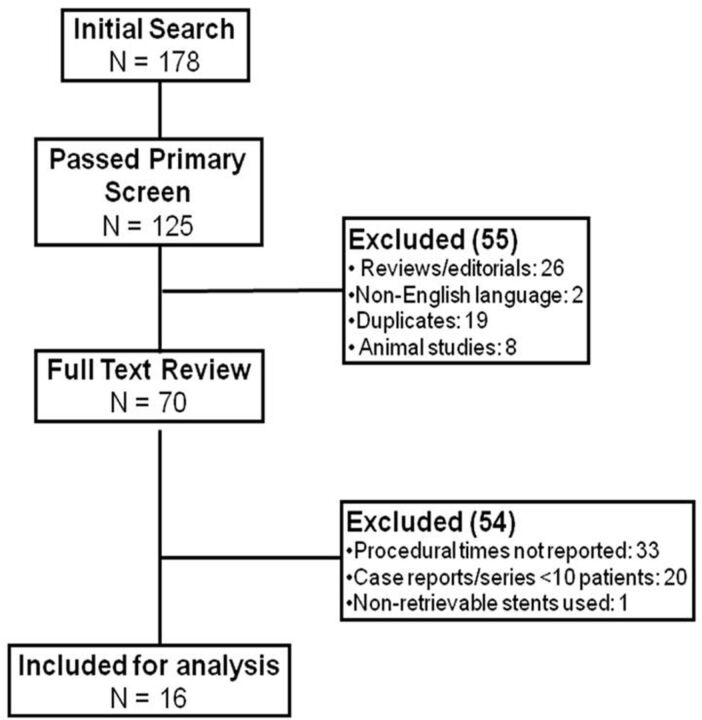
Figure 1 outlines the search and selection process. A total of 178 citations were retrieved, 125 of which passed the primary screen. A full-text review was performed for 70 studies, of which 16 were included in the final analysis. Those included 3 studies published in abstract form only.18,21,22 Table 1 shows some characteristics of these studies. The studies were published between 2005 and 2011 and recruited a total of 925 patients. None of the studies had a randomized treatment allocation, and 9 studies were prospective (607 patients). All studies except 1 reported their follow-up data.18 All studies reported 3-month follow-up data except 1 that reported outcomes at 1-month follow-up.5
Fig 1.
Outlines of the search and selection process.
Table 1:
Characteristics of the included studies
| Author | Year | Centers | Design | Device Used | Size (Patients) | Mean Age (yr) | Median NIHSS Score |
|---|---|---|---|---|---|---|---|
| Aleu et al21 | 2011 | Single | Retrospective | Ret stent | 54 | 68 | 19 |
| Castaño et al24 | 2010 | Single | Retrospective | Ret stent | 20 | 65.6 | 19 |
| Devlin et al37 | 2007 | Single | Retrospective | Merci | 25 | 63 | 18 |
| Frei et al18 | 2011 | Multi | Prospective | Penumbra | 53 | 63 | 18 |
| Grunwald et al38 | 2009 | Single | Retrospective | Penumbra | 29 | 58.4 | 20 |
| Kang et al25 | 2011 | Multi | Retrospective | Penumbra | 22 | 59 | 18.1 |
| Kulcsár et al26 | 2010 | Single | Prospective | Penumbra | 27 | 66 | 14 |
| Lee et al39 | 2009 | Single | Retrospective | Merci | 17 | 67 | 18 |
| Menon et al23 | 2011 | Single | Prospective | Penumbra | 27 | 61.5 | 18 |
| Menon et al40 | 2012 | Single | Prospective | Ret stent | 14 | 62.1 | 14 |
| Pereira et al22 | 2011 | Single | Prospective | Ret stent | 25 | 66.3 | 18.7 |
| Penumbra Pivotal Stroke Trial Investigators13 | 2009 | Multi | Retrospective | Penumbra | 125 | 63.5 | 20 |
| Smith et al12 | 2005 | Multi | Retrospective | Merci | 151 | 67 | 20 |
| Smith et al16 | 2008 | Multi | Retrospective | Merci | 164 | 68 | 19 |
| Struffert et al41 | 2009 | Single | Prospective | Penumbra | 15 | 60.3 | 14 |
| Tarr et al42 | 2010 | Multi | Prospective | Penumbra | 157 | 65 | 18.1 |
Note:—Multi indicates multiple; Ret stent, retrievable stent.
Baseline Characteristics
Table 1 shows demographic data. The mean age for each study ranged from 63 to 68 years for the MD trials, 58.4 to 66 years for the PS studies, and 62.1 to 68 years in RS studies. The median NIHSS score at the time of presentation for the MD- and PS-treated patients was 18 compared with 19 in RS studies. The median onset-to-groin puncture time was 4.3 hours for MD studies, 4.5 hours for PS studies, and 4.2 hours for RS studies.
The extent of early ischemic changes on the baseline CT scan was only reported in 1 study.23 Intravenous tPA was administered in 51 of 357 (14.3%) patients in the MD studies, 144 of 455 (31.6%) in the PS studies, and 56 of 113 (49.6%) in the RS studies. The most frequent occlusion site across the studies was the MCA, accounting for 58%, 55%, and 59% in MD, PS, and RS studies, respectively.
Procedural Details and Outcomes
All identified studies reporting RS exclusively used the Solitaire stent except 1 study21 that used both Solitaire and Trevo stents without indicating how many patients were treated with each stent. Intra-arterial tPA was used in 80 of 357 patients (22.4%) in MD studies compared with 140 of 387 (36.2%) in PS studies and 14 of 113 (20%) in the RS studies.
Use of other devices was reported in some studies. In studies on the MD, 2.8% of patients were treated with snares or other foreign-body retrieval devices, while 2.5% were treated by using balloon angioplasty at the site of occlusion. In PS studies, 1.3% of patients were treated with stent placement at the site of occlusion, while 1.3% of patients were treated with another device that was not specified. In RS studies, 2.7% were treated with the MD, 6.2% were treated with the PS, and 3.5% were treated with another device that was not specified.
The procedural time to recanalization was not always reported. All the included studies on the MD reported procedural durations, regardless of the recanalization outcome. With the random-effects model, mean procedural duration was 120 minutes for the MD (95% CI, 105.7–134.2 minutes). The procedural time to recanalization was 64.6 minutes in PS studies (95% CI, 44.4–84.8 minutes) and 54.7 minutes (95% CI, 47.3–62.2 minutes) in RS studies. There was a significant trend toward shorter mean procedural time to recanalization across the studies during the years 2004–2011 (P = .012).
Recanalization was reported according to TIMI in all studies except 324–26 that reported TICI scores. Successful recanalization (TIMI 2/3 or TICI 2b/3) was achieved in 59.1% (211 of 357) of patients in MD studies (95% CI, 49.3%–77.7%), in 86.6% (394 of 455) of patients in PS studies (95% CI, 84.1%–93.8%), and 92.9% (105 of 113) of patients in RS studies (95% CI, 90.9%–99.9%) (Table 2).
Table 2:
Procedural outcomes
| Author | Device Used | Mean/Median Puncture to Recanalization Time (min) | TIMI 2–3 Recanalization (No.) (%) |
|---|---|---|---|
| Aleu et al21 | RS | 51 | 50 of 54 (92.6) |
| Castaño et al24 | RS | 70 | 18 of 20 (90)a |
| Devlin et al37 | MD | 108b | 14 of 25 (56) |
| Frei et al18 | PS | 52 | 47 of 53 (88.7) |
| Grunwald et al38 | PS | 51 | 25 of 29 (86.2) |
| Kang et al25 | PS | 40 | 22 of 22 (100)a |
| Kulcsár et al26 | PS | 97 | 25 of 27 (92.6)a |
| Lee et al39 | MD | 129.6b | 13 of 17 (76.5) |
| Menon et al23 | PS | 80 | 23 of 27 (85.2) |
| Menon et al40 | RS | 84 | 12 of 14 (85.7) |
| Pereira et al22 | RS | 42c | 25 of 25 (100) |
| Penumbra Pivotal Stroke Trial Investigators13 | PS | 97 | 103 of 125 (82.4) |
| Smith et al12 | MD | 126b | 72 of 151 (47.7) |
| Smith et al16 | MD | 96b | 112 of 164 (68.3) |
| Struffert et al41 | PS | 60c | 12 of 15 (80) |
| Tarr et al42 | PS | 41 | 137 of 157 (87.3) |
TICI 2b/3.
Procedural duration.
Time from the first to last control series.
Procedural complications and device-related technical issues were not always reported. For the MD, all studies reported procedural complications, including vessel perforation in 6 of 357 cases (1.7%), dissection in 4 of 357 (1.1%), and device fractures in 13 of 357 (3.6%). In PS studies, procedural complications were reported in 6 of 8 studies. These included vessel perforations in 1.3% (5 of 380), dissection in 1.3% (5 of 380), and device fracture or malfunction in 1.1% (4 of 380). In studies of RS, 0.9% (5 of 113) of patients experienced in-stent thrombosis but no perforations, dissections, or other procedural complications or device malfunctions.
The mean incidence of postprocedure symptomatic intracranial hemorrhage was 8.7% with the MD (95% CI, 6.0–12.7), 2.5% (95% CI, 0.8–7.3) with the PS, and 9.9% with RS (95% CI, 5.1–19.2). The incidence of distal emboli was 1.1% in the MD trials, 4.4% in the PS studies, and 10.6% in RS studies.
Clinical Outcomes
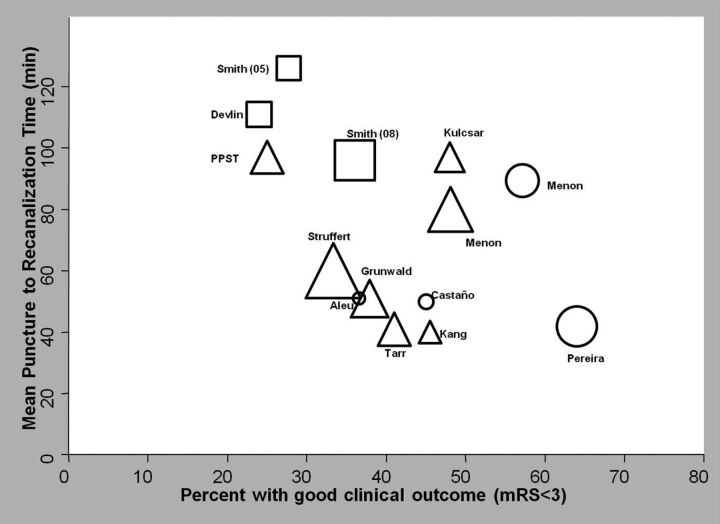
All studies except 1 (using the PS) reported follow-up data. Mean follow-up duration was 3 months in all the studies except 1 that followed patients for 1 month.5 The 3-month mortality rate was 37.8% in the MD studies, 20.7% in the PS studies, and 12.3% in RS studies. Functional independence (mRS ≤2) at 3 months was achieved in 31.5% of patients in the MD studies compared with 32% in the PS and 47.4% in RS studies. There was a suggestion of an inverse correlation between the procedural time to recanalization and the percentage of patients achieving functional independence in the included studies, irrespective of the device used (P = .06, Fig 2).
Fig 2.
The relation (with 95% confidence limits) between the mean procedural time to recanalization and independent functional outcome (mRS ≤2) in the different studies. P value for the Cuzick test of trend is .06. The square indicates the MD; the triangle, the PS; and the circle, the RS.
Sensitivity Analysis
A sensitivity analysis including only prospective studies did not significantly change the mean procedural time-to-recanalization results. For the MD, the mean time in the 4 prospective studies was 117 minutes (95% CI, 100.4–133.5 minutes), while in the 3 prospective studies using the PS, the mean time was 74.6 minutes (95% CI, 29.5–119.7 minutes), and in the 2 prospective studies using RS, the mean time was 57 minutes (95% CI, 39.7–74.3 minutes).
Analysis restricted to articles published in full yielded the same procedural time to recanalization for the MD. However, the mean time for the PS in the 7 studies published in full was 66.1 minutes (95% CI, 45.9–86.3 minutes), while for the 3 published studies on RS, the mean time was 71.5 minutes (95% CI, 53.9–89 minutes).
Discussion
In this meta-analysis, RS and PS studies reported equally short procedural times to recanalization, while RS had the best recanalization and favorable outcome among the 3 devices. Merci device studies reported only the total duration times for all patients, regardless of the recanalization result. While the importance of recanalization in acute stroke cannot be overemphasized, the speed of achieving recanalization is also important.27,28 Using this time interval to compare intra-arterial devices serves 2 goals. First, procedural time to recanalization reflects the technical feasibility of devices during the time constraints of stroke therapy. Second, shorter procedural time to recanalization results in shorter onset to recanalization times and thus higher chances of better outcome. Longer time in occlusion results in more tissue at risk of becoming infarcted core.29 Long procedural time-to-recanalization might partly explain the dissociation between the high rate of successful recanalization and the disappointing rate of good outcome in intra-arterial trials. In the Penumbra Pivotal Trial, in which only a quarter of recanalized patients achieved functional independence,13 a mean delay of 2 hours between emergency presentation and groin puncture30 was described.
The time-dependent treatment benefit in acute ischemic stroke has been shown in IV thrombolysis studies. In a pooled analysis of 3670 patients from 8 randomized IV tPA trials,31 the odds of good clinical outcome were 2.6 times higher in patients treated within 90 minutes of symptom onset compared with those treated with a placebo. Those treated within 91–180 minutes and 181–270 minutes had 1.64 and 1.34 odds ratios of achieving good clinical outcome, respectively. That analysis did not take into account successful recanalization, concluding that 5 patients need to be treated within 90 minutes of symptom onset for 1 of them to have an excellent outcome. If the high rates of successful recanalization with intra-arterial devices are combined with short procedural time-to-recanalization times, the number needed to treat to achieve good clinical outcome is likely to be lower.
Another important factor in deciding outcome is the extent of early ischemic changes on baseline brain imaging. Extensive changes on the Alberta Stroke Program Early CT Score32 have contributed to the lack of efficacy with intra-arterial therapy.33,34 In addition, the Alberta Stroke Program Early CT Score has been shown to reliably identify patients with stroke unlikely to make an independent recovery despite thrombolytic treatment.32 In this meta-analysis, only 1 of 16 studies reported the extent of early ischemic changes on baseline imaging. Tissue-based decision-making in acute stroke therapy regardless of stroke onset time has been shown, in a retrospective study, to be associated with safety comparable to that in patients treated within the conventional time window.35
In this analysis, RS had an equivalent time to recanalization compared with the PS. However, a unique feature of RS not accounted for is the ability to restore flow, even if temporarily, when the stent is deployed, bypassing the occluded segment. Whether this “resets the ischemia clock” for the tissue at risk and helps salvage more brain is still to be shown. In addition, RS use was associated with the lowest mortality and highest functional independence rates in this analysis. A number of factors have potentially accounted for this outcome, including the high rate of IV tPA use, shorter onset-to-puncture times, improved operator learning curves, availability of other mechanical devices, and improved stroke care.
None of the MD studies reported procedural time to recanalization, but instead they reported procedural duration. This is an overestimate of the actual procedural time to recanalization because procedural duration encompasses cases that failed to recanalize, which are likely to last longer than successful recanalization cases. Therefore, puncture-to-recanalization times with the MD might still be comparable with those of the RS and the PS. We elected not to exclude the MD studies from this analysis, to provide an historical perspective by using the first FDA-approved device for stroke thrombectomy and to emphasize the importance of reporting procedural time to recanalization in future studies. This analysis should not be interpreted as showing shorter procedural times with the PS or RS over those of the MD.
This analysis has limitations. Meta-analyses of observational studies may generate falsely precise results due to biases and confounding in component studies. These nonrandomized studies are subject to biases, including during selection, analysis, or reporting. All the studies identified by our search had a nonrandomized patient allocation. While the value of an intra-arterial approach in acute stroke is apparent to many, evidence supporting its safety and efficacy over IV tPA from randomized trials is lacking, though some are ongoing. If even a fraction of patients described in the studies of our analysis were randomized in such trials, evidence on the use of these devices would have been available.
We could not adjust for the impact of important factors, including age and stroke severity, because individual patient data were not available. Nonetheless, patient characteristics in these studies were comparable on average. Retrievable stent studies reported the highest rate of IV tPA use, which could have contributed to the high successful recanalization rates observed. We could not adjust for this effect or for the positive effect of improved stroke care. Different interpretations exist for the TICI score among different observers,36 which is a potential source of variability in defining recanalization. Some technical aspects that we attempted to extract were not always reported. It is difficult to hypothesize what impact this omission has on this analysis. We hope that this will draw attention to reporting these technical outcomes in future studies to enable a more generalizable comparison.
Conclusions
We aimed to summarize existing literature regarding the impact of procedural time to recanalization of 3 devices used in treating patients with ischemic stroke. The use of the PS and RS was associated with comparable procedural time to recanalization. Available data did not allow this parameter to be determined for trials using the Merci device. Retrievable stents achieved the highest rate of successful recanalization and functional outcome and the lowest mortality. The value of procedural time to recanalization and the effect it may have on functional outcome merit further exploration with ongoing prospective studies. One should interpret our findings, bearing in mind the limitations of available evidence.
ABBREVIATIONS:
- CI
confidence interval
- MD
Merci retriever device
- mRS
modified Rankin Scale
- PS
Penumbra system
- RS
retrievable stents
- TICI
Thrombolysis in Cerebral Ischemia
- TIMI
Thrombolysis in Myocardial Infarction
Footnotes
Disclosures: Mayank Goyal—RELATED: Grant: Penumbra,* Comments: partial support towards development of an educational Web site, Consulting Fee or Honorarium: Penumbra, Comments: less than $2000 honorarium for speaking engagement, Fees for Participation in Review Activities (such as data-monitoring boards, statistical analysis, end point committees, and so forth): ev3, Comments: part of a board to help design an acute stroke trial, Other: an Interventional Management of Stroke 3 executive, Comments: travel to Data and Safety Monitoring Board meetings: expenses paid by Interventional Management of Stroke 3, UNRELATED: Grants/Grans Pending: Bayer;* Stock/Stock Options: Calgary Scientific and NoNO, Comments: own shares. *Money paid to the institution.
References
- 1. Baker WL, Colby JA, Tongbram V, et al. Neurothrombectomy devices for the treatment of acute ischemic stroke: state of the evidence. Ann Intern Med 2011;154:243–52 [DOI] [PubMed] [Google Scholar]
- 2. Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 2010;41:2254–58 [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Menon BK, Coutts SB, et al. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke 2011;42:93–97 [DOI] [PubMed] [Google Scholar]
- 4. IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004, 35:904–11 [DOI] [PubMed] [Google Scholar]
- 5. Lee KY, Han SW, Kim SH, et al. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 2007;38:192–93 [DOI] [PubMed] [Google Scholar]
- 6. Intraarterial thrombolysis: ready for prime time? Executive Committee of the ASITN—American Society of Interventional and Therapeutic Neuroradiology. AJNR Am J Neuroradiol 2001;22:55–58 [PMC free article] [PubMed] [Google Scholar]
- 7. Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–67 [DOI] [PubMed] [Google Scholar]
- 8. Rubiera M, Alvarez-Sabin J, Ribo M, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 2005;36:1452–56 [DOI] [PubMed] [Google Scholar]
- 9. del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke—PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 10. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke; The PROACT II study—a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 11. Lee M, Hong KS, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke 2010;41:932–37 [DOI] [PubMed] [Google Scholar]
- 12. Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 13. Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–68 [DOI] [PubMed] [Google Scholar]
- 14. Castaño C, Serena J, Davalos A. Use of the new Solitaire AB Device for mechanical thrombectomy when Merci Clot Retriever has failed to remove the clot; a case report. Interv Neuroradiol 2009;15:209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fields JD, Lutsep HL, Smith WS. Higher degrees of recanalization after mechanical thrombectomy for acute stroke are associated with improved outcome and decreased mortality: pooled analysis of the MERCI and Multi MERCI Trials. AJNR Am J Neuroradiol 2011;32:2170–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–12 [DOI] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting—Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12 [DOI] [PubMed] [Google Scholar]
- 18. Frei D, Turk A, Heck D, et al.: The SPEED trial: A study of the Penumbra early evacuation device. In: Proceedings of the International Stroke Conference, Los Angeles, California. February 9–11, 2011:e232 [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985;4:87–90 [DOI] [PubMed] [Google Scholar]
- 21. Aleu A, Dorado L, Castao C, et al.: Endovascular therapy for acute ischemic stroke using retrievable stents-stent retrievers. In: Proceedings of the International Stroke Conference, Los Angeles, California. February 9–11, 2011:e64 [Google Scholar]
- 22. Pereira V, Narata A, Yilmaz H, et al. Solitaire device for immediate flow restoration and revascularization in acute stroke: Geneva experience. In: Proceedings of the International Stroke Conference, Los Angeles, California. February 9–11, 2011:e230 [Google Scholar]
- 23. Menon BK, Hill MD, Eesa M, et al. Initial experience with the Penumbra Stroke System for recanalization of large vessel occlusions in acute ischemic stroke. Neuroradiology 2011;53:261–66 [DOI] [PubMed] [Google Scholar]
- 24. Castaño C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010;41:1836–40 [DOI] [PubMed] [Google Scholar]
- 25. Kang DH, Hwang YH, Kim YS, et al. Direct thrombus retrieval using the reperfusion catheter of the Penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol 2011;32:283–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulcsár Z, Bonvin C, Pereira VM, et al. Penumbra system: a novel mechanical thrombectomy device for large-vessel occlusions in acute stroke. AJNR Am J Neuroradiol 2010;31:628–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–73 [DOI] [PubMed] [Google Scholar]
- 28. Goyal M. Poor clinical outcome despite successful arterial recanalization: What went wrong? How can we do better? Neuroradiology 2010;52:341–43 [DOI] [PubMed] [Google Scholar]
- 29. Becktepe JS, You SJ, Berkefeld J, et al. Clinical outcome after mechanical recanalization as mono- or adjunctive therapy in acute stroke: importance of time to recanalization. Cerebrovasc Dis 2011;32:211–18 [DOI] [PubMed] [Google Scholar]
- 30. Coutts SB, Goyal M. When recanalization does not improve clinical outcomes. Stroke 2009;40:2661. [DOI] [PubMed] [Google Scholar]
- 31. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703 [DOI] [PubMed] [Google Scholar]
- 32. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 [DOI] [PubMed] [Google Scholar]
- 33. Hill MD, Demchuk AM, Tomsick TA, et al. Using the baseline CT scan to select acute stroke patients for IV-IA therapy. AJNR Am J Neuroradiol 2006;27:1612–16 [PMC free article] [PubMed] [Google Scholar]
- 34. Hill MD, Rowley HA, Adler F, et al. Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke 2003;34:1925–31 [DOI] [PubMed] [Google Scholar]
- 35. Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke 2010;41:1996–2000 [DOI] [PubMed] [Google Scholar]
- 36. Saver JL, Liebeskind DS, Nogueira RG, et al. Need to clarify Thrombolysis In Myocardial Ischemia (TIMI) scale scoring method in the Penumbra Pivotal Stroke Trial. Stroke 2010;41:e115–116 [DOI] [PubMed] [Google Scholar]
- 37. Devlin TG, Baxter BW, Feintuch TA, et al. The Merci Retrieval System for acute stroke: the Southeast Regional Stroke Center experience. Neurocrit Care 2007;6:11–21 [DOI] [PubMed] [Google Scholar]
- 38. Grunwald IQ, Walter S, Papanagiotou P, et al. Revascularization in acute ischaemic stroke using the Penumbra system: the first single center experience. Eur J Neurol 2009;16:1210–16 [DOI] [PubMed] [Google Scholar]
- 39. Lee W, Sitoh YY, Lim CC, et al. The Merci Retrieval System for the management of acute ischaemic stroke: the NNI Singapore experience. Ann Acad Med Singapore 2009;38:749–55 [PubMed] [Google Scholar]
- 40. Menon BK, Kochar P, Ah-Seng A, et al. Initial experience with a self-expanding retrievable stent for recanalization of large vessel occlusions in acute ischemic stroke. Neuroradiology 2012;54:147–54 [DOI] [PubMed] [Google Scholar]
- 41. Struffert T, Kohrmann M, Engelhorn T, et al. Penumbra Stroke System as an “add-on” for the treatment of large vessel occlusive disease following thrombolysis: first results. Eur Radiol 2009;19:2286–93 [DOI] [PubMed] [Google Scholar]
- 42. Tarr R, Hsu D, Kulcsar Z, et al. The POST trial: initial post-market experience of the Penumbra system—revascularization of large vessel occlusion in acute ischemic stroke in the United States and Europe. J Neurointerv Surg 2010;2:341–44 [DOI] [PubMed] [Google Scholar]