Abstract
BACKGROUND AND PURPOSE:
Two injectable materials for the treatment of vertebral compression fractures, Cortoss and PMMA, were compared in a prospective, randomized study. Our purpose was to analyze the incidence and nature of subsequent fractures following treatment, one of the secondary outcomes.
MATERIALS AND METHODS:
A prospective study was conducted at 21 US sites by 38 investigators by using Cortoss randomized 2:1 to PMMA in 256 patients blinded to treatment assignment. Inclusion criteria were 1–2 osteoporotic fractures causing significant pain or worsening vertebral collapse on radiographs and visual analog scale pain measuring ≥50 mm. Assessments were conducted pretreatment, on treatment day, and at 7 posttreatment intervals. Imaging studies underwent independent blinded review. Internal and independent monitors, including the FDA, verified data.
RESULTS:
Of the 256 patients, 45/162 Cortoss-treated (27.8%) and 30/94 PMMA-treated (31.9%) patients experienced new fractures, most within 30–365 days. In patients with 1 acute or subacute fracture and no previous fractures, subsequent fracture incidence was less in patients treated with Cortoss (17.6%) than with PMMA (27.3%). In this subgroup, adjacent fractures occurred in 10.3% of patients treated with Cortoss and 18.2%, with PMMA, a 43.4% lower incidence in the Cortoss group.
CONCLUSIONS:
Compared with PMMA, Cortoss use resulted in fewer subsequent fractures, especially in patients with first fractures. In patients without previous fractures, the subsequent fracture rate was also lower in Cortoss-treated versus conservatively treated patients in other studies. This reduced subsequent fracture rate may be due to differences in the material and mechanical properties of Cortoss compared with PMMA. As finite-element analysis modeling demonstrated, Cortoss restores a more physiologic load transfer through the treated vertebra. Patients treated with Cortoss were less likely to be hospitalized for new fractures.
In the United States, the lifetime risk of vertebral fracture at age 50 or older is 16% for white women and 5.0% for white men.1 One in 5 women will develop VCFs,2 with 1 in 10 developing a moderate-to-severe fracture,3 constituting a significant health issue and health care challenge.4 Painful VCFs refractory to conservative therapy are candidates for treatment with percutaneous vertebroplasty. The material most widely used in vertebral augmentation is PMMA. While considered acceptable, PMMA has shortcomings, including viscosity that changes during delivery time,5 high exothermic reaction,5,6 tissue toxicity,7 biomechanical stressing of an adjacent vertebra from maximum filling,8 and stress concentrations directly above and below the treated vertebra due to its compact distribution pattern.9–12 Jasper et al13 noted decreased biomechanical strength as a result of the addition of radio-opacifying agents used to enable visibility and of altering the monomer-to-polymer ratio to lower viscosity and extend the working time of the material.
A study by Lindsay et al14 revealed a 19.2% incidence of subsequent fracture in the year following osteoporotic vertebral fracture treated conservatively. They also reported that the presence of ≥1 previous osteoporotic vertebral fracture at the time of the index fracture increased the risk of subsequent vertebral fracture 5-fold over the course of 1 year compared with patients without prevalent vertebral fractures at baseline. The effect of vertebroplasty on the risk of subsequent fractures is not known. Studies that assessed subsequent fracture in patients treated with vertebroplasty by using PMMA have reported rates of fracture ranging from 19.9% to as high as 44%.15–19
The limitations of PMMA have spurred the search for alternative materials for use in vertebral augmentation. One of these is Cortoss (Orthovita, Malvern, Pennsylvania), an FDA-cleared bioactive, injectable, nonresorbable composite consisting of highly cross-linked resins and reinforcing bioactive glass fillers. These fillers cause surface deposition of natural hydroxyapatite and promote direct apposition and interdigitation between the material and host bone with time.20 In recent fractures, the flow characteristics and hydrophilic nature of the composite material lead to a dispersed fill, which coats and reinforces bony trabeculae. This is in contrast to the doughy consistency and hydrophobicity of PMMA, which leads to a more bolus-like fill. FEA has shown that the combination of the mechanical and fill properties of Cortoss leads to a more physiologic load transfer through the treated vertebra than is seen with PMMA.12 The question raised was whether this difference in load transfer patterns could lead to a difference in the risk of subsequent fractures.
This study examines the incidence, location, timing, subsequent treatment of, and hospitalization for subsequent fractures in a large, randomized, prospective, long-term clinical study of Cortoss and PMMA bone cement (SpinePlex; Stryker, Kalamazoo, Michigan) used in the treatment of osteoporotic VCFs.
Materials and Methods
This is an analysis of data from an FDA-approved IDE pivotal trial (Clinical Trial Registry #NCT 00290862; http://clinicaltrials.gov) intended to evaluate the safety and effectiveness of Cortoss compared with PMMA.21 The study was conducted at 21 US sites by 38 investigators selected on the basis of their experience in using PMMA in vertebroplasty. Sample size was calculated by using the Farrington and Manning Maximum Likelihood Method,22 which yielded a size requirement of 207 patients—138 in treatment and 69 controls. This was adjusted to 256 for an expected 15% lost to follow-up and withdrawals.
Institutional review board approvals were obtained from each investigational site. Patients were screened for eligibility and provided informed consent, and 256 patients were randomly assigned by computer to treatment groups. Patients were blinded to treatment assignment except for 2 patients who were unintentionally unblinded (1 in each group). All imaging studies underwent independent blinded review by a board-certified radiologist (Bio-Imaging Technologies, Newtown, Pennsylvania). Both internal and third-party study monitors were used for 100% verification of adherence to the protocol and study procedures and to ensure accurate and timely data collection. In addition, the FDA performed audits at 3 sites, verifying data for 44% of patients.
The main inclusion criteria were the presence of 1 or 2 osteoporotic VCFs between T6 and L5 causing significant pain for at least 4 weeks but no longer than 1 year or radiographic evidence of at least a 5% acute worsening of vertebral collapse compared with previous radiographs, and pain measuring ≥50 mm on the visual analog scale. Acuteness of the fracture was confirmed by MR imaging or bone scanning. Patients had to have central pain over the spinous process at the planned treatment level confirmed by palpation/percussion on physical examination. The pain had to result in the regular use of analgesics or had to cause a substantially altered lifestyle as evidenced by an Oswestry Disability Index score of at least 30% (moderate disability).23
Exclusion criteria were vertebral collapse of >70% of the original vertebral height, a burst or pedicle fracture with posterior wall disruption, >20% narrowing of the spinal canal, neurologic symptoms or deficits, a herniated nucleus pulposus, bone tumor, bleeding disorders, severe cardiopulmonary deficiencies, active infection, and current cancer or HIV treatment.
The first patient was enrolled and treated in February 2004 and the last, in February 2007. Data were collected until the last study patient reached the 24-month visit. Twenty-four-month follow-up was completed for 83% of participants in February 2009. Study evaluations and assessments were conducted pretreatment; on treatment day and post-treatment at 72 hours; at 1 week; and at 1, 3, 6, 12, and 24 months. Assessments included AP and lateral radiographs, obtained pretreatment; postoperatively; and at 1, 3, 6, 12, and 24 months.
Participants
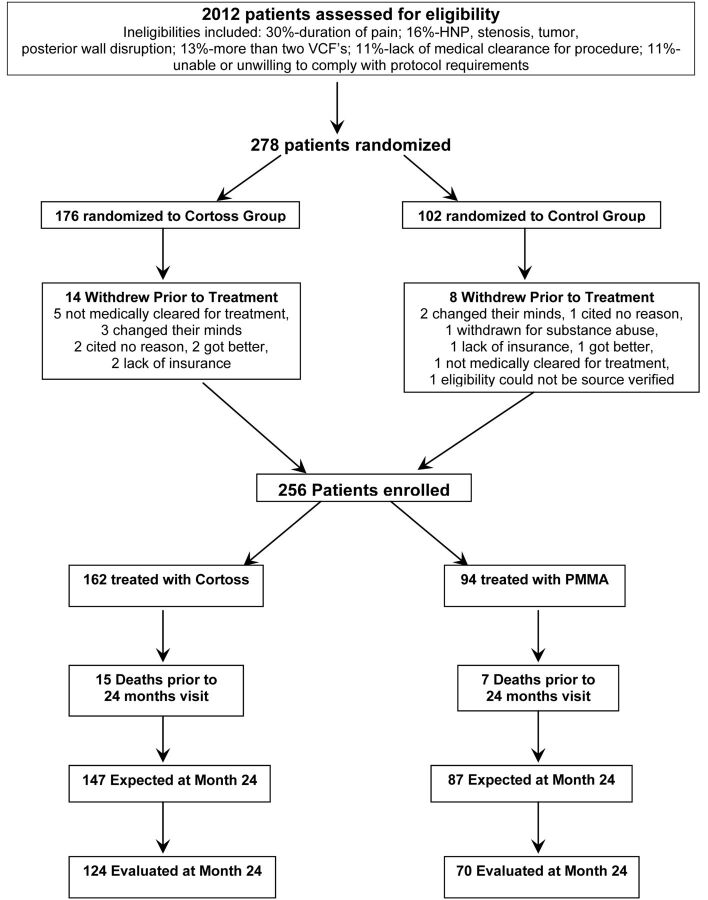
A total of 256 patients with osteoporotic VCFs were randomized in a 2:1 ratio: 162 were treated with Cortoss, and 94, with PMMA (Fig 1). Enrollment was spread across 21 participating centers; 12 centers enrolled ≥8 patients. Participating investigators included interventional radiologists as well as orthopedic surgeons and neurosurgeons.
Fig 1.
Participant flow. HNP indicates herniated nucleus pulposus.
Interventions
Patients were either fully anesthetized or underwent conscious sedation. Depending on the location and preference of the operator, either a transpedicular or parapedicular approach under fluoroscopic guidance was used to place the delivery needles. Following needle placement, the material was injected. Cortoss was delivered by using a coaxial catheter-based system; PMMA was delivered with the system the investigator routinely used. For either material, the goal was to achieve a fill which, on the lateral view, extended from superior to inferior endplate and from the anterior wall to a point about 1 cm from the posterior wall of the vertebral body. On the anteroposterior view, material should have crossed the midline. Unipedicular or bipedicular approaches were both permitted. In case a unipedicular approach did not result in the material crossing the midline, a second injection from the contralateral side was necessary.
Hypothesis
The focus of this analysis was to explore the incidence, location, timing, subsequent treatment of, and hospitalization for subsequent fractures in patients who received PMMA versus Cortoss for treatment of osteoporotic vertebral fractures. The hypothesis was that the dispersed fill pattern and load-bearing properties of the bioactive composite would result in fewer subsequent fractures, particularly adjacent fractures, where the load-transfer characteristics of each material have a more direct potential impact on the development of subsequent fractures.12
Statistical Methods
All statistical testing was 2-sided and performed at the .05 significance level. Tests were declared statistically significant if the calculated P value was ≤.05. All analyses and tabulations were performed by using SAS, Version 8.2 (SAS Institute, Cary, North Carolina) and StatXact 7 (http://www.scientificcomputing.com/statxact-7.aspx) PROCs on a PC platform. Continuous variables were summarized with means, SDs, medians, minimums, and maximums. Categoric variables were summarized by counts and by the percentage of patients in corresponding categories. Correction for multiple comparisons was not performed for the analysis of subsequent fractures.
Assessments
Assessments relevant to the scope of this analysis are all imaging studies performed during the course of the study. These included lateral and AP radiographs, MR imaging or bone scanning, and CT scans obtained at baseline and before discharge. Postprocedural AP and lateral radiographs of the spine were obtained postoperatively and at 1, 3, 6, 12, and 24 months for all participants and, in a small number of cases, at 36 months. A blinded independent radiologist determined the presence of subsequent fractures on review of radiographs compared with baseline. Additionally, imaging was performed if patients presented with new pain between scheduled follow-up visits. All subsequent fractures were recorded as adverse events.
Results
Participant flow is provided in Fig 1. The 38 investigators at their respective sites performed recruitment. At 24 months, 84.3% of patients treated with Cortoss and 80.5% of those treated with PMMA were seen for follow-up. Reasons for withdrawal among the 17% of participants who were not seen at 24 months included lack of insurance coverage, no pain or persistent pain, development of other nonvertebral fracture–related health issues, health decline of the participant's spouse or family illness, relocation, transportation or mobility issues, and other logistical limitations.
One hundred sixty-two patients were randomly assigned to the Cortoss group; 94 patients, to the PMMA group. Patients were blinded to their treatment throughout the study, except for 1 patient in each group who was unintentionally unblinded. The presence of symptomatic fractures was confirmed by radiographs, MR imaging, or bone scanning in all participants, as well as by physical examination that included palpation of the spinous processes.
CT scans of a subset of 90 patients (52 Cortoss, 38 PMMA) were of sufficient quality to perform a post hoc calculation of bone attenuation. For this, FDA-approved PC-based QCT bone mineral attenuation software (Bone Density Measurement International, Frederick, Maryland) was used. The results showed an average t-score of −3.14255 for the Cortoss-treated patients and −2.84857 for the PMMA group. Both groups, therefore, were confirmed to be osteoporotic (≤2.5), and there was no difference between the groups.
Approximately one-third of patients entered the study with previous fractures (34.6% Cortoss and 33.0% PMMA; Table 1). In the 56 patients treated with Cortoss with a previous fracture, a total of 106 levels had been previously fractured. Similarly, there were 60 previously fractured levels in the 31 PMMA-treated patients with previous fracture. Twenty of these Cortoss-treated patients (35.7%) and 13 of the PMMA-treated patients (41.9%) had undergone a previous vertebral augmentation. A total of 112 patients, 68 treated with Cortoss and 44 with PMMA, entered the study with a single-level fractured and no previous fracture.
Table 1:
Previous fractures
| Cortoss (n = 162) | PMMA (n = 94) | Total (n = 256) | |
|---|---|---|---|
| No. of patients with any previous fracturesa | 56 (34.6%) | 31 (33.0%) | 87 (34.0%) |
| Total no. of levels with previous fracturesb | 106 | 60 | 166 |
| No. of patients with any previous fractures (treated)c | 20 (12.3%) | 13 (13.8%) | 33 (12.9%) |
| Total no. of levels with previous fractures (treated) | 33 | 25 | 58 |
| No. of patients with any previous fractures (untreated)d | 39 (24.1%) | 21 (22.3%) | 60 (23.4%) |
| Total no. of levels with previous fractures (untreated)e | 73 | 35 | 108 |
Excludes pre-existing untreated fractures that were treated along with the acute fracture in the current study. Includes treated and untreated fractures other than the index treated levels.
Includes all levels with previously treated fractures and all levels with previous untreated fractures, excluding those levels treated in the current study.
No. and percentage of patients with any previous fractures treated with vertebral augmentation.
Excludes untreated levels treated in the current study.
Excludes levels treated in the current study.
On average, per level treated, one-third less volume of Cortoss was used (2.30 mL) than PMMA (3.49 mL). Leakage was determined by using both fluoroscopy and postprocedural CT scans, and 63.8% of levels treated with either material demonstrated leaks. The incidence of leaks was the same for both Cortoss and PMMA. Although the exact measurement of small leak volumes is difficult, the average Cortoss leak was ∼25% smaller than the average PMMA leak, in parallel with the difference seen for the injection volumes. All except 3 leaks were asymptomatic. Intradiskal leaks occurred in 28.8% of levels treated in the Cortoss group and 33.1% of levels treated in the PMMA group.
Outcomes
Of the 256 study patients, 45/162 of the Cortoss-treated patients (27.8%) and 30/94 PMMA-treated patients (31.9%) had new fractures, with most occurring between 30 and 365 days (Table 2). Overall, 12.9% fewer Cortoss-treated than PMMA-treated patients developed subsequent fractures during the course of the study.
Table 2:
New fracture classification
| Time Period | Cortoss |
PMMA |
||||||
|---|---|---|---|---|---|---|---|---|
| Adjacent Fracture | Non-Adjacent Fracture | Treatment-Level Fracturea | Total | Adjacent Fracture | Non-Adjacent Fracture | Treatment-Level Fracturea | Total | |
| No. (%) of patients with ≥1 additional fractureb | 30 (18.5%) | 26 (16.0%) | 3 (1.9%) | 45 (27.8%) | 19 (20.2%) | 16 (17.0%) | 2 (2.1%) | 30 (31.9%) |
| No. of fractures | 43 | 32 | 4 | 79 | 21 | 24 | 2 | 47 |
| <30 days | 13 (30.2%) | 7 (21.9%) | 1 (25.0%) | 21 (26.6%) | 6 (28.6%) | 4 (16.7%) | 2 (100.0%) | 12 (25.5%) |
| 30 days to 1 yr (day 365) | 28 (65.1%) | 21 (65.6%) | 3 (75.0%) | 52 (65.8%) | 12 (57.1%) | 12 (50.0%) | 0 (0.0%) | 24 (51.1%) |
| 1 yr (day 366) to 2 yr (day 730) | 1 (2.3%) | 2 (6.3%) | 0 (0.0%) | 3 (3.8%) | 3 (14.3%) | 7 (29.2%) | 0 (0.0%) | 10 (21.3%) |
| >2 yr | 1 (2.3%) | 2 (6.3%) | 0 (0.0%) | 3 (3.8%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | 1 (2.1%) |
| No. (%) of first-time patients with ≥1 additional fracturec | 7 (10.3%) | 6 (8.8%) | 2 (2.9%) | 12 (17.6%) | 8 (18.2%) | 6 (13.6%) | 1 (2.3%) | 12 (27.3%) |
Further collapse of treated fracture.
Patients experiencing ≥1 additional fracture are counted once within each fracture category.
Patients originally treated at 1 level who had not had previous fractures (Cortoss, n = 68; PMMA, n = 44).
When we correlated the number of previous fractures at baseline to the risk of experiencing new fractures, 34 of the 136 patients (25.0%) entering the study with 1 previous fracture experienced a new subsequent fracture in the first year postvertebroplasty. The highest rate of subsequent fractures was seen in 7 patients: Each entered the study with 5 previous fractures and 4 experienced new fractures (57.1%). In general, as the number of previous fractures at study entry rose, so did the rate of subsequent fractures. In the homogeneous subgroup of 112 patients with 1 acute or subacute fracture and no previous fractures, the difference in outcomes between the materials used was more pronounced (Tables 2 and 3), with 17.6% of Cortoss-treated and 27.3% of PMMA-treated patients experiencing subsequent fractures.
Table 3:
Subsequent fracture incidence stratified
| Subsequent Fractures | Cortoss |
PMMA |
||
|---|---|---|---|---|
| Adjacent | Non-Adjacent | Adjacent | Non-Adjacent | |
| No. (%) of patients with ≥1 Fx | 30 (18.5%) | 26 (16.0%) | 19 (20.2%) | 16 (17.0%) |
| No. (%) of first-time patients with Fxa | 7 (10.3%) | 6 (8.8%) | 8 (18.2%) | 6 (13.6%) |
| % Difference C < P | 43.4% | 35.3% | ||
Note:—Fx indicates fracture; C, Cortoss; P, PMMA.
Patients originally treated at 1 level with no previous fractures: C = 68, P = 44.
Regarding the timing of the subsequent fractures, we found a slight trend for adjacent fractures to occur earlier than nonadjacent fractures in both groups and a trend for nonadjacent fractures in PMMA-treated patients to occur somewhat later than in those treated with Cortoss (Table 2). More than 80% of patients who experienced a new fracture did so within 1 year after treatment.
Half of the patients with subsequent fractures in both the Cortoss group and the PMMA group experienced pain. In the Cortoss group, 22/45 (49%) patients had painful subsequent fractures; similarly, 15/30 (50%) in the PMMA group experienced subsequent fractures with pain.
Regarding the clinical consequences of subsequent fractures, 10/44 PMMA-treated patients (22.7%) underwent a subsequent vertebral augmentation for osteoporotic vertebral compression fractures versus 5/68 of Cortoss-treated patients (7.4%). Similarly, 2/68 Cortoss-treated patients (2.9%) were re-hospitalized for causes related to the subsequent fracture versus 5/44 in PMMA-treated patients (11.4%) (Table 4). Three-to-four times more PMMA than Cortoss patients underwent a subsequent vertebral augmentation or were hospitalized because of subsequent fractures.
Table 4:
Consequences of subsequent fractures
| Subsequent Vertebral Augmentation | Re-hospitalized for VCF-Related Reasons | |
|---|---|---|
| Cortoss | 7.4% | 2.9% |
| PMMA | 22.7% | 11.4% |
In the statistical plan for this study, tests were declared significant if the calculated P value was ≤.05. There were no P values for these comparisons <.2.
Discussion
Trout et al15 performed a large retrospective analysis of the risk and timing of subsequent vertebral fractures in patients who had undergone vertebroplasty with PMMA. Of the 432 patients, 86 (19.9%) developed subsequent vertebral fractures; 41.4% of those fractures were in adjacent vertebrae. This incidence of new fractures is approximately the same as that in patients with osteoporosis without prior vertebroplasty or kyphoplasty.4,14 The mean time to adjacent fracture was 55 days; for nonadjacent fractures, which are thought less likely to be related to the index procedure than to underlying disease progression, the mean time to fracture was 127 days.15
Other studies also noted a trend for adjacent fractures to occur earlier than nonadjacent fractures.18,24 Additional literature describing patients treated with PMMA reports ranges for subsequent fractures from 21.7% to as high as 44%.16–19 In patients treated conservatively, Lindsay et al14 found a 19.2% new fracture incidence within 1 year after a first fracture in postmenopausal women with osteoporosis. The trend for subsequent adjacent fractures to occur sooner than nonadjacent fractures15,25 is mirrored in the current analysis. Studies suggest that the compact distribution of PMMA concentrates stress in the bone tissue directly above and below the treated vertebra, which may lead to fractures in both the adjacent vertebra and the already treated vertebra.9–12,18
In this analysis, there was a 12.9% overall reduction in the incidence of subsequent fractures in Cortoss-treated patients (27.8%) compared with those treated with PMMA (31.9%), which could not be explained by a difference in the degree of osteoporosis. The difference becomes more pronounced when comparing a more homogeneous population of “virgin back” patients with no previous fracture at study outset and only 1 level treated. Although this was not a prospectively defined subset analysis, examination of this group of patients eliminates the variables of previous fractures and treatment type from affecting the comparison. Such factors may affect the risk of subsequent fractures. In this group, 12/68 (17.6%) Cortoss-treated patients and 12/44 (27.3%) PMMA-treated patients experienced a subsequent fracture. For adjacent fractures, the rate in this subgroup was 10.3% for Cortoss-treated patients and 18.2% for PMMA-treated patients (Table 3). The reduction in incidence of Cortoss-treated patients with any subsequent fracture in this subgroup was 35.5% (Table 2). The reduction in incidence of adjacent fractures was 43.4% (P = .2460) less for Cortoss-treated than for PMMA-treated patients (Table 3).
Intradiskal cement leaking has been associated with the induction of new adjacent osteoporotic vertebral compression fractures.26–28 In our study, Cortoss-treated patients had fewer intradiskal leaks and fewer adjacent fractures than PMMA-treated patients.
Lindsay et al14 found that the combination of low lumbar spine bone mineral attenuation and previous fracture history is the best predictor of increased fracture risk in the year following a first fracture. Our results confirm that previous fracture history increases the risk of subsequent fractures. In addition, our results suggest that the distribution pattern and mechanical properties of the material used have a measurable effect on the risk of subsequent fractures, especially in patients who undergo treatment for their first fracture. Although the differences in incidence observed do not reach statistical significance, their occurrence appears to point to the clinical effects of differences in the manner in which load-bearing and load transfer are being restored by different materials, as was demonstrated in the laboratory.12
Limitations
There were several limitations in this study. First, this was an analysis of data from an FDA-approved IDE pivotal study conducted to evaluate the safety and effectiveness of Cortoss compared with PMMA in the treatment of VCFs. The rate of subsequent fractures was a secondary and not a primary end point of this study. While the results suggest that there is a correlation between treatment material and the risk of subsequent fractures, the study was not powered for this end point and the results do not reach statistical significance. More research is needed to elucidate whether there is a true association between these factors. Because no statistical significance in subsequent fractures was found, correction for multiple comparisons was not performed. Finally, bone mineral attenuation measurements were calculated post hoc in a subset of patients, and not on the entire population.
Conclusions
In our analysis, the use of Cortoss instead of PMMA resulted in a lower subsequent fracture rate, especially in patients treated for a single first fracture. The subsequent fracture rate for Cortoss in these patients was lower than that for PMMA and lower than the rate reported for new vertebral fractures in such patients treated conservatively.14 Cortoss-treated patients were less likely to be hospitalized or treated for new fractures, suggesting an overall cost benefit of using the composite material. Based on the results of FEAs, the reduction seen in subsequent fractures may be due to the difference in fill patterns and mechanical properties between the materials, and the resulting differences in the restoration of load-bearing and load transfer of the treated vertebra. These findings will need to be confirmed in future clinical studies and registries.
Acknowledgments
We acknowledge Carolyn Cott and Stacey Ewing for their assistance in preparation of this manuscript and the Cortoss Study Group. This body of work is a result of the contributions to the Pivotal IDE Study by the following clinicians: Hyun Bae, MDA, Pierce Nunley, MDB, H. Paul Hatten, Jr, MDC, Raymond Linovitz, MDD, Erik Westerlund, MDD, Timothy Peppers, MDD, A. David Tahernia, MDE, Armen Khachatryan, MDE, Michael K. Schaufele, MDF, Doug Beall, MDG, Vance McCollom, MDH, Louis Gilula, MDI, Hani Haykal, MDJ, Philip Maurer, MDK, Ramsin Benyamin, MDL, William Marx, MDM, William Horsley, MDN, Federico Girardi, MDO, Frank Cammisa, MDO, Joseph Lane, MDO, Mubin I. Syed, MDP, Bernard Bendok, MDQ, Lee Guterman, MDR, Allan L. Brook, MDS, Matthew Hannibal, MDT, Richard D. Fessler, MDU, and Ben Pradhan, MD.V
ASt. John's Spine Institute, Santa Monica, California; BSpine Institute of Louisiana, Shreveport, Louisiana; CIndian River Radiology, Vero Beach, Florida; DCore Orthopedic Medical Center, P.C., Encinitas, California; EDesert Orthopedics Center, Rancho Mirage, California; FEmory Orthopaedics and Spine Center, Atlanta, Georgia; GEdmond Medical Center, Edmond, Oklahoma; HMercy Health Center, Oklahoma City, Oklahoma; IWashington University Medical Center, St Louis, Missouri; JThe Methodist Hospital, Houston, Texas; KPennsylvania Hospital, Philadelphia, Pennsylvania; LMillennium Pain Center, Bloomington, Illinois; MMission Hospitals, Asheville, North Carolina; NScottsdale Medical Imaging, Scottsdale, Arizona; OHospital for Special Surgery, New York; PRadiology Physicians of Springfield, Kettering, Ohio; QNorthwestern University, Chicago, Illinois; RUniversity of Buffalo Neurosurgery, Buffalo, New York; SMontefiore Medical Center, Bronx, New York; TSt. Mary's Spine Center, San Francisco, California; UHarper University Hospital, Detroit, Michigan; and VRisser Orthopaedic Group Inc, Pasadena, California.
ABBREVIATIONS:
- AP
anteroposterior
- FEA
finite-element analysis
- IDE
investigation device exemption
- PMMA
polymethylmethacrylate
- VCF
vertebral compression fracture
Footnotes
Disclosures: Louis Gilula—RELATED: Grant: Orthovita,* Comments: The company provided financial support for the research coordinator to manage patients and collect data during the study, Provision of Writing Assistance, Medicines, Equipment, or Administrative Support: There was some help with data analysis from an independent data company (indicated in the article); some manuscript preparation was provided, but I did the manuscript editing, final checking, and so forth. No money was provided, OTHER RELATIONSHIPS: I was a participant in the study performing vertebroplasty on patients entered into the study. Maarten Persenaire—RELATED: Orthovita, Comments: I am the Chief Medical Officer of Orthovita, the company that initiated and conducted the IDE study on the basis of which this article was written. The full report of the study was used in support of a 510(k) filing with the FDA, UNRELATED: Employment: Orthovita, Stryker Orthobiologics, Comments: employed (Orthovita) since 1999 as Chief Medical Officer. *Money paid to the institution.
The IDE Study was sponsored by Orthovita Inc in a revenue-neutral manner.
Paper previously presented at: American Society of Spine Radiology and Educational Research Foundation Symposium, May 15–20, 2010; Boston, Massachusetts.
References
- 1. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–67 [DOI] [PubMed] [Google Scholar]
- 2. Melton LJ, 3rd. Epidemiology of spinal osteoporosis. Spine 1997;22(24 suppl):2S–11S [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services. The frequency of bone disease. In: Bone Health and Osteoporosis: A Report of the Surgeon General. http://www.surgeongeneral.gov/library/bonehealth/content.html. January 4, 2007. Accessed March 24, 2011
- 4. Sanfélix-Genovés J, Reig-Molla B, Sanfélix-Gimeno G, et al. The population-based prevalence of osteoporotic vertebral fracture and densitometric osteoporosis in postmenopausal women over 50 in Valencia, Spain (the FRAVO study). Bone 2010;47:610–16 [DOI] [PubMed] [Google Scholar]
- 5. Heini PF, Berlemann U. Bone substitutes in vertebroplasty. Eur Spine J 2001;10:S205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. San Millán Ruíz D, Burkhardt K, Jean B, et al. Pathology findings with acrylic implants. Bone 1999;25:85S–90S [DOI] [PubMed] [Google Scholar]
- 7. Kalteis T, Lüring C, Gugler G, et al. Acute tissue toxicity of PMMA bone cements [in German]. Z Orthop Ihre Grenzgeb 2004;142:666–72 [DOI] [PubMed] [Google Scholar]
- 8. Berlemann U, Ferguson SJ, Nolte LP, et al. Adjacent vertebral failure after vertebroplasty: a biomechanical evaluation. J Bone Joint Surg Br 2002;84:748–52 [DOI] [PubMed] [Google Scholar]
- 9. Fribourg D, Tang C, Sra P, et al. Incidence of subsequent vertebral fracture after kyphoplasty. Spine 2004;29:2270–76 [DOI] [PubMed] [Google Scholar]
- 10. Baroud G, Heini P, Nemes J, et al. Biomechanical explanation of adjacent fractures following vertebroplasty. Radiology 2003;229:606–07. Author reply 607–08 [DOI] [PubMed] [Google Scholar]
- 11. Polikeit A, Nolte LP, Ferguson SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine 2003;28:991–96 [DOI] [PubMed] [Google Scholar]
- 12. Sun K, Mendel E, Rhines L, et al. Cement filling pattern has a significant effect on biomechanics of vertebroplasty. In: Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, Illinois. March 19–22, 2006 [Google Scholar]
- 13. Jasper LE, Deramond H, Mathis JM, et al. Material properties of various cements for use with vertebroplasty. J Mater Sci Mater Med 2002;13:1–5 [DOI] [PubMed] [Google Scholar]
- 14. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320–23 [DOI] [PubMed] [Google Scholar]
- 15. Trout A, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: Adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27:217–23 [PMC free article] [PubMed] [Google Scholar]
- 16. Li YA, Lin CL, Chang MC, et al. Subsequent vertebral fracture after vertebroplasty: incidence and analysis of risk factors. Spine (Phila Pa 1976) 2011;37:179–83 [DOI] [PubMed] [Google Scholar]
- 17. Syed MI, Patel NA, Jan S, et al. New symptomatic vertebral compression fractures within a year following vertebroplasty in osteoporotic women. AJNR Am J Neuroradiol 2005;26:1601–04 [PMC free article] [PubMed] [Google Scholar]
- 18. Voormolen MH, Lohle PN, Juttmann JR, et al. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol 2006;17:71–76 [DOI] [PubMed] [Google Scholar]
- 19. Legroux-Gérot I, Lormeau C, Boutry N, et al. Long-term follow-up of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Clin Rheumatol 2004;23:310–17 [DOI] [PubMed] [Google Scholar]
- 20. Hench LL. The story of Bioglass. J Mater Sci Mater Med 2006;17:967–78 [DOI] [PubMed] [Google Scholar]
- 21. Bae H, Hatten HP, Jr, Tahernia AD, et al. A prospective randomized FDE-TDE trial comparing Cortoss to PMMA for vertebroplasty. Spine 2012;37:544–50. [DOI] [PubMed] [Google Scholar]
- 22. Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 2002;21:1958–60 [DOI] [PubMed] [Google Scholar]
- 23. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940–52 [DOI] [PubMed] [Google Scholar]
- 24. Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 2000;39:1410–14 [DOI] [PubMed] [Google Scholar]
- 25. Ploeg WT, Veldhuizen AG, The B, et al. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 2006;15:1749–58 [DOI] [PubMed] [Google Scholar]
- 26. Komemushi A, Tanigawa N, Kariya S, et al. Percutaneous vertebroplasty for osteoporotic compression fracture: multivariate study of predictors of new vertebral body fracture. Cardiovasc Intervent Radiol 2006;29:580–85 [DOI] [PubMed] [Google Scholar]
- 27. Ahn Y, Lee JH, Lee HY, et al. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine 2008;9:129–36 [DOI] [PubMed] [Google Scholar]
- 28. Chen WJ, Karo YH, Yang SC, et al. Impact of cement leakage into disks on the development of vertebral compression fractures. J Spinal Disord Tech 2010;23:35–39 [DOI] [PubMed] [Google Scholar]