Abstract
Peripheral nerve stimulation (PNS) is an effective tool for the treatment of chronic pain, although its efficacy and utilization have previously been significantly limited by technology. In recent years, purpose-built percutaneous PNS devices have been developed to overcome the limitations of conventional permanently implanted neurostimulation devices. Recent clinical evidence suggests clinically significant and sustained reductions in pain can persist well beyond the PNS treatment period, outcomes that have not previously been observed with conventional permanently implanted neurostimulation devices. This narrative review summarizes mechanistic processes that contribute to chronic pain, and the potential mechanisms by which selective large diameter afferent fiber activation may reverse these changes to induce a prolonged reduction in pain. The interplay of these mechanisms, supported by data in chronic pain states that have been effectively treated with percutaneous PNS, will also be discussed in support of a new theory of pain management in neuromodulation: Peripherally Induced Reconditioning of the Central Nervous System (CNS).
Keywords: chronic pain, neuromodulation, peripheral nerve stimulation, cortical plasticity, peripherally induced reconditioning, mechanism of action
Introduction
Modern understanding of the relationship between electrical stimulation and pain dates back to 1965 with Melzack and Wall’s seminal paper outlining their theory of the “gate control” system of pain.1 It proposed that there is a gating mechanism in the spinal cord that relies on the relative firing of small (nociceptive) and large (sensory) diameter neurons. Increased firing of the large diameter neurons would “close” the gate, reducing transmission of painful stimuli to the brain, while firing of small diameter neurons would “open” it. Although the first therapeutic application of this theory involved stimulation of peripheral nerves following neurosurgical lead implantation,2 the field was quickly dominated by widespread adoption of implanted leads delivering dorsal column or spinal cord stimulation (SCS) for the treatment of chronic pain.
SCS has been the leading force in the neuromodulation market for the last 50 years, with many advances in device technology during that time.3–7 Due to the market dominance of SCS, the electrode technology available for researchers in PNS has historically been limited to adaptation of SCS devices for use in the periphery.8–10 Nonetheless, continued research over the decades has shown that PNS can be successfully delivered by conventional (ie, permanently implanted) PNS systems with a variety of nerve targets, lead designs, waveforms, frequencies, and stimulation paradigms to treat a wide range of chronic pain conditions, such as intractable neuropathic pain, post-traumatic nerve pain, causalgia, chronic axial back pain, post-operative pain, joint pain, postherpetic neuralgia, chronic migraine, and orofacial pain (available PNS systems are not currently approved for use in the craniofacial region).11–32
Despite this efficacy, PNS has historically been conceptualized as a treatment of last resort.33 One major limitation has been the lack of systems specifically designed for use in the periphery. Physicians often used devices designed for SCS, including percutaneous cylindrical leads or surgically placed paddle-type leads, both placed immediately adjacent to or in contact with the targeted nerve.8,9,13–17,19,34,35 However, the periphery induces greater mechanical stresses on the lead than those experienced in the epidural space,10 historically resulting in frequent lead migration (9–25% of PNS cases36), and limiting placement to locations that did not require the tunneled leads to cross joints with high degrees of flexion or extension that could cause stress-related lead migration or fracture.37
Recent years have seen the advancement of various PNS features and techniques intended to enable the development and adoption of improved neurostimulation systems designed specifically for use in the periphery, including: 1) The development of methods for the minimally invasive, percutaneous implantation of conventional PNS leads,35 eliminating the need for invasive techniques to expose the nerve; 2) Advancements in and growing prevalence of ultrasound imaging to guide lead placement,17,23 enabling visualization and targeting of an increasing number of peripheral nerves;38 3) Increases in the number and quality of interventionally trained pain physicians, especially with regard to ultrasound guided procedures; 4) Improvement in reimbursement for PNS; 5) Renewed focus on the development and implementation of non-opioid treatment alternatives for acute and chronic pain; 6) Improvement in long-term efficacy when a percutaneously implanted lead is employed without an implanted pulse generator or receiver;39,40 7) The incorporation of open coil leads with axial flexibility designed to enable tissue ingrowth within the coils to secure the electrode in place with lower rates of infection, as seen throughout a long history of use in electrical stimulation applications.41–52
Recently, based on these advancements, percutaneous PNS with temporary (eg, up to 60 days) treatment through open coil leads has been used to treat a wide variety of pain conditions via two different implementations. The first method (Figure 1A) has demonstrated effectiveness in acute and chronic pain conditions such as neuropathic and non-neuropathic pain following amputation,39,53–56 post-surgical pain following total knee arthroplasty,57,58 and ambulatory foot, knee, and rotator cuff surgeries.59–61 This method targets mixed or sensory nerve(s) innervating the painful region with the goal of activating large diameter primary afferent sensory fibers at frequencies (eg, ~100 Hz) that induce comfortable sensations in the region of pain. In the second method (Figure 1B), efferent fibers are targeted at a lower frequency (eg, ~12 Hz) and an intensity that induces comfortable contractions in muscle(s) in the region of pain innervated by the targeted nerve, as demonstrated for chronic musculoskeletal pain including chronic shoulder pain,32,62–66 and axial low back pain.40,67,68 Recent studies using these two implementations reported that 77% (75/98) of subjects experienced substantial (≥50%) reductions in pain intensity and/or pain interference during treatment, with 90% (88/98) of patients experiencing clinically meaningful (≥30%) reductions.32,53–55,57,62–69 Of note, many of those studies reported significant pain relief that may be maintained long after the end of the short-term PNS treatment, with some reports of sustained pain relief through one year of follow-up.39,40
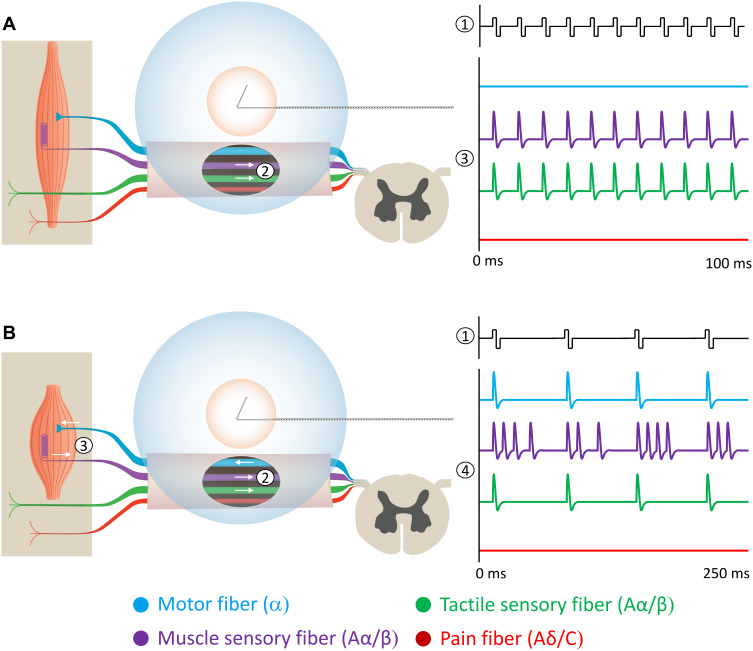
Figure 1.
Two percutaneous PNS approaches have demonstrated sustained relief of chronic pain. Stimulation is delivered from a system with open-coiled leads designed to be placed remote from the nerve to selectively activate Aα/β fibers while avoiding Aδ/C fiber activation (ie, remote selective targeting). The activation zones are shown for Aα/β fibers (blue) and Aδ/C fibers (orange). (A) Stimulation of mixed nerves at 100 Hz (1) can selectively activate the largest sensory afferents (many of which are larger than muscle efferents147). (2) Stimulation activates the large diameter muscle and tactile afferents while avoiding activation of muscle efferents and nociceptive afferents. (3) Directly induced large diameter afferent action potentials enter the spinal dorsal horn at the rate of the stimulation frequency (100 Hz) to engage the gating mechanism, typically producing comfortable sensations in the innervated region. (B) Stimulation of mixed nerves at 12 Hz (1) at a sufficient intensity can also activate muscle efferent fibers. (2) Stimulation activates large diameter fibers, including cutaneous afferents, muscle afferents, and muscle efferents while avoiding nociceptive afferents. (3) Orthodromic firing of muscle efferents causes muscle contraction, generating physiological activation of muscle afferent fibers. (4) Large diameter afferent action potentials (directly induced by stimulation and indirectly through muscle contraction) enter the spinal dorsal horn to collectively engage the gating mechanism.
Conventional forms of neuromodulation for chronic pain, such as PNS, SCS, and dorsal root ganglion stimulation (DRGS), have not typically provided prolonged pain relief after cessation of stimulation, with preclinical studies reporting a short-term carryover effect on the order of minutes to a few days and very little clinical data on the matter.2,70–74 Reports of sustained analgesia across multiple pain indications following a short-term PNS treatment are therefore a unique observation that merits further examination from a mechanistic perspective. While the clinical evidence for PNS has been reviewed elsewhere,75,76 the primary goal of this narrative review is to explore potential theories and mechanisms by which percutaneous PNS may produce sustained pain relief. A secondary goal is to generate discussion in the clinical and scientific communities that may lead to studies that further explore the possibility of modulating a centrally maintained pain state by providing peripheral input through PNS.
Chronic Pain is Associated with Peripheral and Central Sensitization
Under basal conditions, noxious thermal, chemical, and mechanical stimuli activate nociceptive receptors in the skin. These noxious signals are then carried to the spinal cord by small diameter first order afferents with slower conduction velocities, typically unmyelinated C or myelinated Aδ fibers. The Aδ fibers are thought to carry the sharp, “first pain,” while C fibers carry “second pain” signals, characterized by more prolonged aching or burning.77 In the spinal cord, nociceptive fibers generally synapse in the dorsal horn with nociceptive-specific (NS) or wide dynamic range (WDR) second-order neurons (Figure 2A) that then project to the brainstem or thalamus.77 Within the brain, pain signals are processed in a number of different regions collectively referred to as the “pain matrix,” including the thalamus, somatosensory cortex, insular cortex, anterior cingulate cortex (ACC), prefrontal cortex, amygdala, and hippocampus.77–79 In the case of chronic pain, persistent nociceptive input induces multilevel changes from the periphery to the brain that result in abnormal pain processing and hypersensitivity, including hyperalgesia (increased sensitivity to noxious stimuli), secondary hyperalgesia (painful sensitivity at sites adjacent to or removed from the injured site), allodynia (painful sensitivity to non-noxious stimuli), and spontaneous pain.78,80
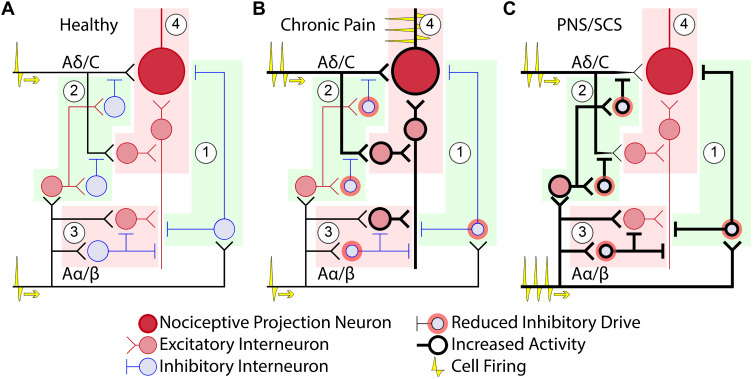
Figure 2.
Pain circuitry in the spinal dorsal horn. Four primary sub-circuits are represented: (1) post-synaptic inhibition of nociceptive projection neurons, (2) pre-synaptic inhibition of nociceptive projection neurons, (3) basally inhibited PKCγ excitatory interneurons, and (4) polysynaptically excited nociceptive projection neurons. (A) In a healthy case there is a balance between nociceptive and non-nociceptive afferent input and dorsal horn circuit strengths, resulting in minimal activation of nociceptive projection neurons. (B) In the case of chronic pain, peripheral nerve damage/inflammation elevates firing of nociceptive afferent fibers. Additionally, GABAergic and glycinergic drive from inhibitory interneurons are reduced, resulting in: (1) reduction in post-synaptic inhibition, (2) reduction in pre-synaptic inhibition, (3) disinhibition of PKCγ interneurons, enabling allodynia-producing circuits, and (4) sensitization of nociceptive projection neurons, characterized by increased excitability and decreased inhibition. (C) Neurostimulation is believed to cause elevated firing of Aα/β afferent fibers, counteracting many of the circuit-level effects of chronic pain. Specifically, high rates of Aα/β firing induce: (1) elevated post-synaptic inhibition, (2) elevated pre-synaptic inhibition (3) return of inhibition to the PKCγ cells, reducing allodynia, and (4) elevated inhibition and reduction of nociceptive drive to the nociceptive projection neurons.
In the periphery, damage to nerves can induce peripheral sensitization. Peripheral sensitization is mediated by the release of a wide variety of pro-inflammatory cytokines and neuropeptides whose net result is a drastic reduction in nociceptive thresholds that causes hyperexcitability of nociceptive afferents, spontaneous discharge, and plays a crucial role in the onset and maintenance of hyperalgesia and spontaneous pain.81–84
Increased nociceptive activity due to injury and/or sensitization in the periphery also triggers a complex series of changes in the central nervous system collectively referred to as central sensitization.85,86 Sustained firing of nociceptive afferents leads to sensitization of NS and WDR neurons in the dorsal horn.79,86 The influx of Ca2+ triggered by persistent nociceptive input causes phosphorylation of ion channels and receptors, trafficking of more excitatory channels to the surface, increases in dendritic spine density, and transcriptional changes, all of which promote and maintain a state of increased excitability and decreased inhibition in the dorsal horn (Figure 2B).79,80,86,87 Neuronal hyperexcitability is further exacerbated by the activation of glial cells and their subsequent release of pro-inflammatory signaling molecules. The role of glial activation in chronic pain is reviewed elsewhere,79,80,88,89 and spinal glial involvement in neurostimulation-induced analgesia is only recently being explored.90,91
In addition to the increased excitability of nociceptive pathways in the spinal cord, nerve injury typically results in a reduction in inhibitory GABAergic and glycinergic drive in the spinal dorsal horn (Figure 2B).78–80,86,87,92 This disinhibition further amplifies nociceptive signaling directly and also engages excitatory PKCγ interneurons that are driven by activity in large diameter Aβ fibers and are typically held in check by glycinergic inhibition (Figure 2B).79,92 Loss of GABAergic and glycinergic inhibition, therefore, perpetuates pain hypersensitivity and causes tactile sensations, which are not typically perceived as painful, to activate nociceptive pathways (one of the key mechanisms believed to contribute to allodynia).93–96
Supraspinal circuits also play a major role in the processing of pain and have been implicated in centrally mediated chronic pain, including changes in descending modulation from the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM)78,97,98 and major structural and functional cortical changes such as alterations in cell spiking dynamics, microglia activation, brain connectivity, gray matter volume, and cortical representation (see87,99 for review). Specifically, in the somatosensory cortex, which encodes the sensory-discriminative aspects of pain,100 the nociceptive representational zones exhibit a sensitized state characterized by expansion and/or shifting of pain representations, reduced GABAergic inhibition, and stronger response to activation, while non-nociceptive representational zones may diminish in size and response to activation.101–106 These maladaptive shifts in the balance of sensory processing are likely due to activity-dependent cortical remapping caused by the increase in nociceptive and relative decrease in non-nociceptive information coming from the region of pain.104,107 Maladaptive cortical plasticity, coupled with spinal and peripheral sensitization, presents a challenge to treatments’ intent on producing long-term pain relief. It is theorized, therefore, that sustained analgesia may be produced by neurostimulation that acts at multiple levels, beginning with spinal modulation of the nociceptive barrage from the periphery.
Activation of Large Diameter Fibers Has the Potential to Attenuate Nociceptive Signaling in the Spinal Dorsal Horn
Neurostimulation systems delivering stimulation at conventional frequencies (eg, 5–150 Hz), including conventional SCS, DRGS, PNS, and even peripheral nerve field stimulation (PNFS), have long been theorized to produce analgesia by modulating pain signals in the spinal dorsal horn via spinal segmental mechanisms that were first described in the well-known gate control theory.1 Spinal segmental mechanisms of analgesia, including the putative gating mechanism, rely on the activation of large diameter fibers, which are typically myelinated Aα and Aβ fibers (often either grouped together as Aα/β or simply referred to as Aβ due to their highly overlapping morphologies and fiber diameters).108–116 Since Aα/β fibers generally transmit signals from low-threshold mechanoreceptors and proprioceptors, successful activation often elicits non-painful sensations in the innervated region. The colocalization of these sensations with the region of pain can be used as a marker for focal (ie, specifically targeting the region of pain) activation of large diameter fibers.54,117 Experimental studies have demonstrated the profound control that Aα/β fibers exert over the transmission of nociceptive signals in the spinal dorsal horn (Figure 2C). Conventional PNS, DRGS, and dorsal column stimulation of large diameter fibers can inhibit the firing of WDR neurons in response to painful stimuli through the inhibition of long-term potentiation and induction of long-term depression of C fiber activity.70,118–123
These effects are mediated by a variety of post-synaptic and pre-synaptic circuits in the dorsal horn. Post-synaptically, Aα/β fibers play a primary role in the activation of GABA- and glycinergic inhibitory interneurons in the dorsal horn, which polysynaptically reduce the firing of both superficial and deep dorsal horn projection neurons, subsequently reducing the transmission of nociceptive signals through the spinal dorsal horn (Figure 2C).92,124,125 Pre-synaptically, early recordings of extracellular potentials in the dorsal root (the dorsal root potential, DRP) found that activation of Aα/β fibers induces widespread subthreshold depolarization of primary afferents (primary afferent depolarization, PAD) in the dorsal root.126,127 This depolarization is mediated by GABAergic interneurons that synapse on the pre-synaptic terminals of primary afferents and can cause pre-synaptic inhibition.128 Although fibers most commonly inhibit others of the same type (eg, Aα/β fibers inhibit other Aα/β fibers),129 some studies have shown that activation of Aα/β fibers can cause pre-synaptic inhibition of nociceptive primary afferents,124,128,130,131 suggesting a pre-synaptic gating mechanism by Aα/β fiber activation (Figure 2C).
Although gate control has provided the long-standing framework for how many neurostimulation systems may modulate pain, the original 1965 theory has been critically reviewed and supplemented over time to better explain phenomena observed experimentally.92,125 For example, additional proposed mechanisms of action for conventional stimulation include both peripheral (eg, altering nerve fiber excitability or conduction), and central factors (eg, inducing or depleting excitatory and/or inhibitory neurotransmitters, modulating expression of neuronal signaling proteins, altering activity in central pain matrix regions or descending inhibitory pathways).9,132–134 These additional mechanisms highlight the overall complexity of the chronic pain state, though spinal segmental mechanisms remain the predominate mechanistic theory for pain relief with conventional neurostimulation.
Novel Approaches to Selective Activation of Large Diameter Fibers
Nerve fibers with larger diameters are activated by electrical stimulation at a lower intensity compared to smaller diameter fibers,135,136 so the gating mechanism may be engaged by titrating stimulation intensities to maximally activate large diameter Aα/β fibers while avoiding activation of small diameter nociceptive fibers. Preclinical and clinical evoked compound action potential (eCAP) recordings and computational modeling indicate that conventional SCS at therapeutic intensities activates only a small proportion of the Aα/β fibers in the dorsal columns (estimates range from 0.25% to 8.7% of targeted fibers137,138) before reaching discomfort thresholds, purportedly due to activation of the adjacent dorsal roots.137,139–141 Meanwhile, PNS and DRGS have the potential to activate Aα/β fibers in a more focal, targeted fashion by stimulating the specific nerve(s) or ganglia innervating the region of pain. However, conventional PNS and DRGS utilize small electrodes placed on or immediately adjacent to a nerve that are likely to produce intense electric fields that rapidly decay across short distances such that fibers nearer the electrode may be activated (including small diameter fibers) while fibers slightly more distant from the electrode (eg, deeper in or across the nerve) may experience little to no stimulation (Figure 3).
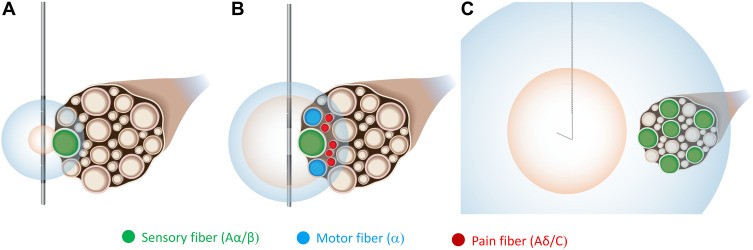
Figure 3.
Remote selective targeting promotes activation of large diameter fibers while avoiding activation of small diameter fibers using PNS systems and open coil leads designed for placement distant to the nerve. Large diameter fibers have lower activation thresholds than smaller diameter fibers, and thresholds also increase with electrode-to-fiber distance. The activation zones are shown for Aα/β fibers (blue) and Aδ/C fibers (orange). (A) For a conventional PNS electrode placed intimate to the nerve, a limited number of Aα/β fibers may be activated. (B) Increasing the intensity to activate a larger proportion of Aα/β fibers begins to concurrently activate Aδ/C fibers or motor fibers, causing unintended discomfort. (C) A system using a percutaneous open-coil electrode placed remotely from the nerve (eg, 0.5–3 cm) is designed to selectively activate a larger proportion of Aα/β fibers without concomitant activation of Aδ/C fibers.
In contrast to conventional “intimate” electrode placement, it has been hypothesized that percutaneous PNS systems designed to enable remote selective targeting may activate a greater proportion of large diameter fibers while avoiding the unwanted activation of nociceptive afferents (Figure 3).54 Remote selective targeting describes a PNS system and leads designed to optimize the relationships between stimulation strength, electrode characteristics, electrode-fiber distance, and fiber diameter to create a greater separation of activation thresholds between large and small diameter fibers and enable stimulation from electrodes placed up to several centimeters away (eg, 0.5–3 cm) at therapeutic intensities more selective for large diameter fibers.54,135,136,142–144 Leads designed for remote selective targeting have multiple features that may enable activation of larger-diameter fibers and avoidance of smaller-diameter fibers while delivering stimulation from such distances. For example, these leads have large monopolar electrodes such that the generated electric fields, which decay exponentially across distance, are broad and relatively homogeneous at remote distances and have the potential to activate large diameter fibers throughout the entire cross-section of a nerve before reaching activation thresholds of smaller fibers. Remote selective targeting may therefore enable more robust activation of large diameter fibers (ie, a larger proportion of targeted fibers) while avoiding unintended discomfort by optimizing the strength-distance and strength-diameter relationships that govern the activation of nerve fibers by electrical stimulation (Figure 3).54,69,135,136,142
In addition to activation of Aα/β fibers, percutaneous PNS studies have demonstrated prolonged pain relief using stimulation parameters and electrode locations specifically targeting the activation of efferent fibers in mixed nerves that result in strong, physiological muscle contractions without discomfort (Figure 1B).64,67,145 Remote selective targeting can enable a wider therapeutic window that aids in the activation of motor efferent fibers while avoiding activation of small nociceptive fibers (Figure 1B). Muscle afferents, including proprioceptive Aα/β fibers linked to muscle spindles and Golgi tendon organs, have similar diameter, morphology, and functional connections in the dorsal horn compared to tactile Aα/β fibers.129,146 Proprioceptive afferents secondarily activated by physiological muscle contractions therefore likely contribute to the gate control mechanism of pain relief in the same way as tactile afferent fibers that innervate the skin.124 In addition to secondary activation of proprioceptive afferents, the stimulation approach that activates efferent fibers in mixed nerves also likely produces primary activation of Aα/β sensory afferents, which tend to be larger in diameter147 and are recruited at lower stimulation intensities than efferent fibers (Figure 1B).142 Notably, this strategy contrasts with conventional stimulation therapies for the treatment of chronic pain, which have historically attempted to avoid efferent activation and consequent motor activity.14,140,148,149
Improving the selectivity and robustness of large diameter afferent fiber activation may enhance the transient reduction in pain via spinal segmental mechanisms, such as the gating mechanism. However, these mechanisms rely on active stimulation and are likely insufficient to produce sustained analgesia following the end of treatment, as evidenced by the lack of significant sustained relief following the cessation of stimulation provided by conventional approaches. As the next section will explore, sustained pain relief is theorized instead to be enabled by reconditioning of the central nervous system by robust activation of large diameter fibers in the periphery.
Stimulation of Afferents Can Result in Peripherally Induced Plasticity to Reverse Central Features of Chronic Pain
In addition to spinal segmental mechanisms of pain relief, stimulation of large diameter fibers is believed to induce supraspinal analgesic effects. On a macro scale, studies have identified changes in the magnitude and latency of cortical evoked potentials during PNS, which may relate to changes in the sensory and affective components of pain processing.150–152 Additionally, electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) studies have revealed that dorsal column stimulation induces changes in cortical activation throughout many of the regions making up the pain matrix, and is hypothesized to activate the descending pain inhibitory system through modulation of the pregenual ACC.153,154 Given the significant role that cortical processes play in producing, and potentially reducing, chronic pain,87,99,155 the new theory of Peripherally Induced Reconditioning of the Central Nervous System may help to explain sustained relief following PNS.
The somatotopic representation map in the primary somatosensory cortex (S1) is dynamic and can substantially change as a result of shifts in afferent input, with expansion of regions that experience stronger and more frequent input than those around them and contraction of regions that have reduced inputs.156,157 In cases of chronic pain, sensory imbalance in the form of elevated nociceptive input from the painful region and/or, importantly, decreased non-nociceptive input can result in drastic shifts in cortical organization and function. Magnetoencephalography (MEG) studies show a correlation between severity of pain and sensitization of the nociceptive region in S1, characterized by reduced intracortical inhibition, expansion and/or shifting of the representational zone, and stronger response to nociceptive stimuli.101–104 Expansion of the nociceptive response may be coupled with a decrease in the non-nociceptive representational zone and an attenuated response to non-nociceptive stimuli.102,105,106,158–160 For some patients, blocking nociceptive afferent input is sufficient to transiently alter the cortical reorganization and reduce chronic pain.103,161 However, for others, nerve block has no effect or only a transient effect,103 indicating that although some cases of cortical sensitization rely on continued peripheral input, others appear to be centrally maintained.
Robust non-nociceptive afferent input to the cortical areas representing the focal painful region may reduce the severity of pain by actively reconditioning the CNS from the periphery (Figure 4), as opposed to the passive deprivation of nociceptive input that may occur as the result of nerve blocks or ablation.104,107,162,163 This process has been termed “reconditioning” because it remains unclear whether the cortex reverses or returns to its exact pre-injury architecture as opposed to achieving a new homeostatic state.
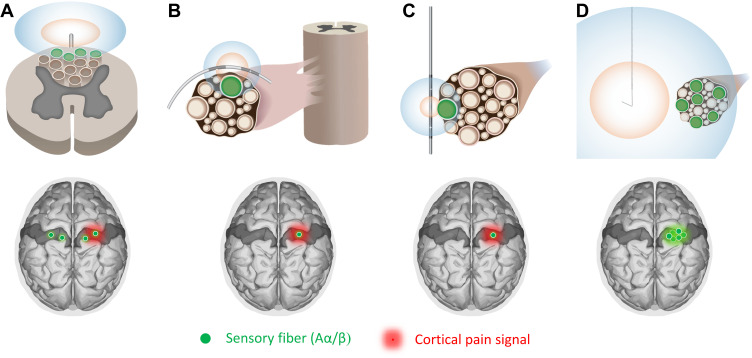
Figure 4.
Varying degrees of cortical activation using different stimulation methods. Optimal induction of cortical remapping requires selective activation of a large number of afferent fibers (ie, robust activation) that is generated focally (ie, from the region of pain). The activation zones are shown for Aα/β fibers (blue) and Aδ/C fibers (orange). (A) SCS activates a small number of fibers in the superficial dorsal column before reaching discomfort thresholds due to dorsal root activation, and the dorsal column fibers it does activate are commonly spread across multiple dermatomes. The afferent input to S1 is thus neither robust nor focal. Conventional DRGS (B) or PNS (C) can more focally target the dermatome and/or nerve innervating the specific region of pain, though DRGS often involves multi-level stimulation, but limitations with conventional systems and stimulation strategies curb the degree of large diameter fiber activation before reaching discomfort thresholds due to small diameter nociceptor activation. The afferent input to S1 is thus more focal than SCS but not robust. (D) Percutaneous PNS with remote selective targeting enables both focal and robust activation of the target nerves, potentially resulting in optimal cortical input to induce activity-dependent remapping and sustained analgesia, facilitating reconditioning of the CNS.
Activity-dependent cortical remapping requires that the peripheral conditioning input to the cortex be robust, since sufficient signal strength is needed to drive the plasticity, and focal from a specific region, since functional plasticity relies on low relative activity in surrounding cortical regions.107,156,157,162–165 Analysis of conventional stimulation techniques suggests that they are unlikely to achieve these conditions, potentially informing why they can produce excellent pain relief but have not been reported to produce significant sustained outcomes without permanent implantation.2,70–74 For example, conventional SCS activates only a small proportion of Aα/β fibers before reaching discomfort thresholds, likely spread non-focally across multiple dermatomes due to the lack of somatotopically targeted stimulation (Figure 4A).139–141 Paresthesia-based DRGS may act via similar mechanisms as other conventional stimulation modalities (ie, activation of large diameter sensory afferents) by placing electrodes in the compact intraforaminal space in close proximity to the DRG to target large diameter axons in the ganglia (Figure 4B).166–168 Although more focal than SCS, recent computational modeling of DRGS suggests that the percentage of activated Aα/β fibers at clinically relevant stimulation amplitudes varies significantly with lead location, stimulation polarity, and stimulation parameters, indicating that electrode placement in close contact with the DRG may, much like conventional PNS, amplify the deleterious effects of lead migration and limit the scope of activation before discomfort thresholds are reached.167,169 Lastly, conventional PNS can provide focal stimulation by targeting individual nerve(s) that innervate a region of pain, but a large proportion of Aα/β fibers in the nerve are not typically activated before discomfort thresholds are reached (Figure 4C).70,112
Percutaneous PNS with remote selective targeting is theorized to enable more selective activation of non-nociceptive, large diameter afferent fibers, generating peripheral signals that are both focal and robust to optimally recondition the S1 cortex (Figure 4D). Unlike SCS, stimulating individual nerves in a distribution-specific pattern to target a defined region of pain may provide a focal signal from the periphery that is well suited for cortical reconditioning. And, in contrast to conventional DRGS and PNS, remote selective targeting is theorized to widen the gap in activation thresholds between Aα/β and small diameter pain fibers to permit more robust activation of the targeted fiber populations. Furthermore, cortical reorganization can occur on the time course of weeks,157,170 suggesting that prolonged pain relief may be produced from short-term (weeks-long) treatments without requiring a permanent implant if the peripheral signals are sufficiently robust and focal to drive beneficial plastic changes.
Applications in Treating Chronic Pain
Percutaneous PNS with remote selective targeting has been successfully used to treat a variety of chronic pain conditions, including chronic pain following amputation, chronic shoulder pain, and axial low back pain. The following section will explore how the proposed mechanisms are theorized to occur in specific cases in which sustained pain relief has been reported following up to 60 days of PNS.
Post-Amputation Pain
Amputation of a limb is incredibly traumatic and induces chronic pain in the residual limb (RLP) and phantom limb (PLP) that can last for many years in up to 80% of patients.171,172 RLP and PLP have neuropathic features and are associated with peripheral and central sensitization, including functional reorganization of nociceptive pathways in the spinal cord and brain, sensory remapping, expansion of receptive fields, and altered cortical representation of the limb.172–175 Historically, conventional neurostimulation has been used to treat RLP and PLP with permanently implanted systems that require continuous treatment and tend to lose efficacy over time.12,176,177 A recent randomized, double-blind, placebo-controlled study delivered percutaneous PNS to the femoral and sciatic nerves for up to 60 days in lower extremity amputees (n=28 total enrollment, n=12 in treatment group). Despite attrition of 25% during follow-up in the treatment group, significant reductions in both RLP and PLP were maintained through 12 months from the start of the 60-day treatment in a majority of subjects (67%, 6/9 at 12 months in treatment group, 70% average pain reduction in responders).39,55 Activation of large diameter sensory afferents at frequencies that evoke comfortable sensations in the region of pain (eg, 100 Hz) may activate spinal gating mechanisms during the 60-day treatment period to modulate peripheral nociceptive signals (eg, ectopic firing of nociceptive afferents from neuromas or dorsal root ganglia). This attenuated spinal transmission of nociceptive signals, coupled with the robust selective activation of tactile and proprioceptive afferents that innervate the painful region, may also help recondition the maladaptive cortical plasticity that occurs following amputation and restore balance between non-nociceptive and nociceptive representations in S1 to produce the observed sustained pain relief.
Chronic Shoulder Pain
Chronic shoulder pain is a common and complex complication following stroke, with recent studies reporting a prevalence ranging from 19% to 63% in stroke survivors.178 Shoulder pain may impede rehabilitation from stroke by interfering with self-care activities, reducing ambulation, limiting ability and desire to participate socially, and leading to withdrawal from rehabilitation programs.179,180 Persistent shoulder pain has characteristics of peripheral and central sensitization, such as allodynia, hyperalgesia, central hypersensitivity, and altered cortical somatosensory processing.181–185 Multiple randomized controlled trials (RCTs) and case series (n=8–2832,63,64,66) using percutaneous PNS with remote selective targeting of the axillary nerve branches innervating the shoulder (Figure 1B) have shown effective long-term pain relief through 6 months in patients with chronic shoulder pain. Stimulation of the terminal branches of the axillary nerve with a lower frequency (eg, 12 Hz) pulse train likely has a dual effect, activating both sensory afferents and muscle efferent fibers. Efferent fiber activation in the terminal branches of the nerve causes contraction of the middle and posterior deltoid muscles,63 producing proprioceptive signals in large diameter fibers that convergently, along with directly activated sensory afferents, engage the gating mechanism in the spinal cord. Supraspinally, the non-noxious proprioceptive and cutaneous afferent barrage may facilitate cortical neuroplasticity and representational remapping, potentially reversing the cortical contribution to the chronic pain state and enabling patients to achieve sustained relief of their shoulder pain.
Chronic Low Back Pain
Chronic low back pain (LBP) is a leading cause of disability among adults and is both prevalent and challenging to treat.186,187 In many cases (up to 85%), chronic LBP may be nonspecific or have an unidentified cause.40 A recent case series (n=9) suggested that low frequency (eg, 12 Hz) stimulation of efferent fibers in the lumbar region may produce sustained relief of chronic low back pain (67%, 6/9 with ≥50% pain relief at 12 months, 80% average pain reduction in responders).40 Stimulation of the medial branch nerves of the dorsal ramus in the lumbar region may act by similar mechanisms as described above for chronic shoulder pain, specifically through lower-frequency pulse train activation of efferent fibers, producing secondary isolated contractions of the lumbar multifidus (Figure 1B).67 A combination of proprioceptive signals from the multifidus and sensory input from direct activation of afferents in the targeted nerve may engage spinal segmental mechanisms of pain relief during stimulation while also providing focal, robust physiological input to drive beneficial central plasticity and produce sustained relief.
Summary and Conclusions
Advancements in imaging and neurostimulation technology have enabled a resurgence of PNS for pain relief in recent years. Studies of percutaneous PNS systems utilizing remote selective targeting have suggested the ability to produce clinically meaningful sustained reductions in pain following temporary (eg, up to 60 days) treatment periods across a variety of chronic pain conditions. Mechanistically, it is theorized that these results may be the result of a widened therapeutic window for stimulation that enables robust and selective activation of Aα/β fibers at frequencies (such as 5–150 Hz) that produce comfortable sensations in the region of pain, leading to multiple analgesic mechanisms from the periphery to the dorsal horn and cortex. These diverse effects may be explained in a new theory of pain management, Peripherally Induced Reconditioning of the CNS, involving stimulation-evoked reversal of the central sensitized state that contributes to chronic pain.
The goal of this narrative review is to propose a mechanism of action theory based on observations in the clinical literature and novel technological advancements in the field of PNS and to generate discussion in the clinical and scientific communicates that may encourage future studies to further explore the observed clinical phenomena. Although the purpose of the present review is not to systematically review the clinical evidence, sustained relief following a short-term percutaneous PNS treatment has emerged in small studies across multiple pain indications, and additional studies that address the limitations of existing evidence would help support the proposed mechanistic theories, including independent investigations, larger cohorts, more active or sham controlled studies, and more consistent periods of long-term follow-up. Direct evidence supporting the mechanistic theory proposed here, such as the reversal of maladaptive cortical plasticity driven by robust and focal inputs from stimulation of peripheral nerves with remote selective targeting, is also needed to confirm the phenomena that may underlie the observed clinical evidence. Future research efforts should therefore endeavor to continue to evaluate this proposed mechanistic theory and explore its clinical utility in a wide range of chronic pain conditions.
The development of neurostimulation systems specifically designed for use in the periphery and the growing volume of clinical data supporting the utilization of PNS across a wide range of pain indications is an encouraging development that offers interventionally trained physicians and neuromodulators new effective tools to treat chronic pain. The demonstrated ability to potentially provide sustained relief from a temporary system that does not require a permanent implant may enable the further adoption of percutaneous PNS earlier in the treatment continuum and avoid the potential costs and/or risks of more invasive or neurodestructive procedures. Future research efforts should continue to evaluate the validity of the theories proposed in the present work, including the role of central plasticity in chronic pain conditions and the potential role for treatments that peripherally target and reverse centrally mediated pain.
Acknowledgments
Portions of the abstract of this work were presented at the 2020 ASRA, 2020 Pain Week, and 2021 NANS Conferences as poster presentations.
Abbreviations
PNS, peripheral nerve stimulation; CNS, central nervous system; SCS, spinal cord stimulation; DRGS, dorsal root ganglion stimulation; NS, nociceptive-specific; WDR, wide dynamic range; ACC, anterior cingulate cortex; PAG, periaqueductal gray; RVM, rostral ventromedial medulla; PNFS, peripheral nerve field stimulation; GABA, gamma aminobutyric acid; DRP, dorsal root potential; PAD, primary afferent depolarization; eCAP, evoked compound action potential; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; MEG, magnetoencephalography; RLP, residual limb pain; PLP, phantom limb pain; RCT, randomized controlled trial; LBP, low back pain.
Disclosure
TRD has consulted for Abbott, Axonics, Cornerloc, Ethos, Flowonix, Nalu, Saluda, SpineThera, Stimgenics, SI Bone, Medtronic, PainTeq, Vertiflex (Boston Scientific), Nevro, Vertos, and SPR Therapeutics. He has received research support from Vertiflex, Vertos, Abbott, Mainstay, Saluda, SPR Therapeutics. He holds minor equity in Bioness, Cornerloc, Ethos, Vertiflex, Vertos, Nalu, SpineThera, Saluda, and SPR Therapeutics. SE has consulted for Medtronic, Inc, Mainstay Medical, Boston Scientific Corp, Saluda, and Abbott. He has received research support from the National Institute of Health Research, Medtronic, Inc, and Nevro Corp. SMF has consulted for Abbott, Medtronic, Nevro, Saluda Medical, SPR Therapeutics, and Vertiflex (Boston Scientific). He has received research support from Abbott, Biotronik, Medtronic, Nuvectra, and Saluda Medical. He holds stock options in SpineThera, SPR Therapeutics, Stimgenics, Cornerloc, and Thermaquil, and has equity interest in Saluda Medical. MAH has consulted for SPR, Saluda and Mainstay Medical. PSS has consulted for Abbott, Nevro, Medtronic, and SPR Therapeutics. He has received research support from Boston Scientific, Abbott, Vertos, Nevro, Saluda and Grunenthal Halyard. He is in the Clinical Advisory Board for AIS Therapeutics. He has stock or equity interest in SPR Therapeutics, Nalu and electroCore. IRC, NDC, and JWB are employees of SPR Therapeutics. NDC and JWB report systems and methods for sustained relief of chronic pain patent pending. The authors report no other conflicts of interest in this work.
References
- 1.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971 [DOI] [PubMed] [Google Scholar]
- 2.Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108–109. doi: 10.1126/science.155.3758.108 [DOI] [PubMed] [Google Scholar]
- 3.Gildenberg PL. History of electrical neuromodulation for chronic pain. Pain Med. 2006;7:S7–S13. doi: 10.1111/j.1526-4637.2006.00118.x [DOI] [Google Scholar]
- 4.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–491. doi: 10.1213/00000539-196707000-00025 [DOI] [PubMed] [Google Scholar]
- 5.Kumar K, Rizvi S. Historical and present state of neuromodulation in chronic pain. Curr Pain Headache Rep. 2013;18:387. doi: 10.1007/s11916-013-0387-y [DOI] [PubMed] [Google Scholar]
- 6.Cavuoto J. The birth of an industry. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. San Diego: Academic Press; 2018:1665–1674. [Google Scholar]
- 7.North RB, Prager JP. History of spinal cord stimulation. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. San Diego: Academic Press; 2018:587–596. [Google Scholar]
- 8.Stanton-Hicks M. Peripheral nerve stimulation for pain peripheral neuralgia and complex regional pain syndrome. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. San Diego: Academic Press; 2009:397–407. [Google Scholar]
- 9.Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100–106. doi: 10.1016/j.nurt.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boggs JW, Chae J, Bennett ME. Peripheral nerve stimulation for pain suppression. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. San Diego: Academic Press; 2018:729–740. [Google Scholar]
- 11.Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg. 1976;45:692–699. doi: 10.3171/jns.1976.45.6.0692 [DOI] [PubMed] [Google Scholar]
- 12.Nashold BS, Goldner JL, Mullen JB, et al. Long-term pain control by direct peripheral-nerve stimulation. J Bone Joint Surg Am. 1982;64:1–10. doi: 10.2106/00004623-198264010-00001 [DOI] [PubMed] [Google Scholar]
- 13.Hassenbusch SJ, Stanton-Hicks M, Schoppa D, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415. doi: 10.3171/jns.1996.84.3.0415 [DOI] [PubMed] [Google Scholar]
- 14.Novak CB, Mackinnon SE. Outcome following implantation of a peripheral nerve stimulator in patients with chronic nerve pain. Plast Reconstr Surg. 2000;105:1967–1972. doi: 10.1097/00006534-200005000-00008 [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg E, Waisbrod H, Gerbershagen HU. Long-term peripheral nerve stimulation for painful nerve injuries. Clin J Pain. 2004;20:143–146. doi: 10.1097/00002508-200405000-00003 [DOI] [PubMed] [Google Scholar]
- 16.Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14:216–221. doi: 10.1016/j.jocn.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369–1377. doi: 10.1111/j.1526-4637.2009.00745.x [DOI] [PubMed] [Google Scholar]
- 18.Deer T, Pope J, Benyamin R, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation. 2016;19:91–100. doi: 10.1111/ner.12381 [DOI] [PubMed] [Google Scholar]
- 19.Mirone G, Natale M, Rotondo M. Peripheral median nerve stimulation for the treatment of iatrogenic complex regional pain syndrome (CRPS) type II after carpal tunnel surgery. J Clin Neurosci. 2009;16:825–827. doi: 10.1016/j.jocn.2008.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Dunteman E. Peripheral nerve stimulation for unremitting ophthalmic postherpetic neuralgia. Neuromodulation. 2002;5:32–37. doi: 10.1046/j.1525-1403.2002._2006.x [DOI] [PubMed] [Google Scholar]
- 21.Johnson MD, Burchiel KJ. Peripheral stimulation for treatment of trigeminal postherpetic neuralgia and trigeminal posttraumatic neuropathic pain: a pilot study. Neurosurgery. 2004;55:135–142. doi: 10.1227/01.NEU.0000126874.08468.89 [DOI] [PubMed] [Google Scholar]
- 22.Ordia J, Vaisman J. Subcutaneous peripheral nerve stimulation with paddle lead for treatment of low back pain: case report. Neuromodulation. 2009;12:205–209. doi: 10.1111/j.1525-1403.2009.00215.x [DOI] [PubMed] [Google Scholar]
- 23.Burgher AH, Huntoon MA, Turley TW, et al. Subcutaneous peripheral nerve stimulation with inter-lead stimulation for axial neck and low back pain: case series and review of the literature. Neuromodulation. 2012;15:100–107. doi: 10.1111/j.1525-1403.2011.00388.x [DOI] [PubMed] [Google Scholar]
- 24.Hegarty D, Goroszeniuk T. Peripheral nerve stimulation of the thoracic paravertebral plexus for chronic neuropathic pain. Pain Physician. 2011;14:295–300. [PubMed] [Google Scholar]
- 25.Stidd DA, Wuollet A, Kirk Bowden D, et al. Peripheral nerve stimulation for trigeminal neuropathic pain. Pain Physician. 2012;15:27–33. doi: 10.36076/ppj.2012/15/27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giorgio Lambru M, Paul Shanahan M. Occipital nerve stimulation in the treatment of medically intractable SUNCT and SUNA. Pain Physician. 2014;17:29–41. [PubMed] [Google Scholar]
- 27.Guentchev M, Preuss C, Rink R, et al. Long-term reduction of sacroiliac joint pain with peripheral nerve stimulation. Oper Neurosurg. 2017;13:634–639. doi: 10.1093/ons/opx017 [DOI] [PubMed] [Google Scholar]
- 28.Goroszeniuk T. The effect of peripheral neuromodulation on pain from the sacroiliac joint: a retrospective cohort study. Neuromodulation. 2018. doi: 10.1111/ner.12803 [DOI] [PubMed] [Google Scholar]
- 29.Silberstein SD, Dodick DW, Saper J, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32:1165–1179. doi: 10.1177/0333102412462642 [DOI] [PubMed] [Google Scholar]
- 30.Yakovlev AE, Peterson AT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation. 2007;10:373–375. doi: 10.1111/j.1525-1403.2007.00126.x [DOI] [PubMed] [Google Scholar]
- 31.Ilfeld BM, Ball ST, Gabriel RA, et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation. 2019;22:653–660. doi: 10.1111/ner.12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RD, Harris MA, Gunzler DD, et al. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation. 2014;17:771–776; discussion 776. doi: 10.1111/ner.12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen EA, Slavin KV. Peripheral nerve/field stimulation for chronic pain. Neurosurg Clin. 2014;25:789–797. doi: 10.1016/j.nec.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 34.Mobbs RJ, Blum P, Rossato R. Mesh electrode for peripheral nerve stimulation. J Clin Neurosci. 2003;10:476–477. doi: 10.1016/S0967-5868(03)00090-0 [DOI] [PubMed] [Google Scholar]
- 35.Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2:217–221. doi: 10.1046/j.1525-1403.1999.00217.x [DOI] [PubMed] [Google Scholar]
- 36.Corriveau M, Lake W, Hanna A. Nerve Stimulation for Pain. Neurosurg Clin N Am. 2019;30:257–264. doi: 10.1016/j.nec.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 37.de Leon-casasola OA. Spinal cord and peripheral nerve stimulation techniques for neuropathic pain. J Pain Symptom Manage. 2009;38:S28–S38. doi: 10.1016/j.jpainsymman.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 38.Kele H. Ultrasonography of the peripheral nervous system. Perspect Med. 2012;1:417–421. doi: 10.1016/j.permed.2012.02.047 [DOI] [Google Scholar]
- 39.Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2020;45:44–51. doi: 10.1136/rapm-2019-100937 [DOI] [PubMed] [Google Scholar]
- 40.Gilmore CA, Kapural L, McGee MJ, et al. Percutaneous peripheral nerve stimulation for chronic low back pain: prospective case series with 1 year of sustained relief following short-term implant. Pain Pract. 2020;20:310–320. doi: 10.1111/papr.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract. 2017;17:753–762. doi: 10.1111/papr.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldwell CW, Reswick JB. A percutaneous wire electrode for chronic research use. IEEE Trans Biomed Eng. 1975;BME-22:429–432. doi: 10.1109/TBME.1975.324516 [DOI] [PubMed] [Google Scholar]
- 43.Marsolais E, Kobetic R. Implantation techniques and experience with percutaneous intramuscular electrodes in the lower extremities. J Rehabil Res Dev. 1986;23:1–8. [PubMed] [Google Scholar]
- 44.Memberg WD, Peckham PH, Thrope G, et al. An analysis of the reliability of percutaneous intramuscular electrodes in upper extremity FNS applications. IEEE Trans Rehabil Eng. 1993;1:126–132. doi: 10.1109/86.242426 [DOI] [Google Scholar]
- 45.Shimada Y, Sato K, Kagaya H, et al. Clinical use of percutaneous intramuscular electrodes for functional electrical stimulation. Arch Phys Med Rehabil. 1996;77:1014–1018. doi: 10.1016/S0003-9993(96)90061-1 [DOI] [PubMed] [Google Scholar]
- 46.Knutson J, Naples G, Peckham PH, et al. Electrode fracture rates and occurrences of infection and granuloma associated with percutaneous intramuscular electrodes in upper-limb functional electrical stimulation applications. J Rehabil R D. 2002;39:671. [PubMed] [Google Scholar]
- 47.Onders RP, Elmo M, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23:1433–1440. doi: 10.1007/s00464-008-0223-3 [DOI] [PubMed] [Google Scholar]
- 48.Peckham PH, Knutson JS. Functional Electrical Stimulation for Neuromuscular Applications. Annu Rev Biomed Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103 [DOI] [PubMed] [Google Scholar]
- 49.Mortimer JT, Bhadra N. Peripheral nerve and muscle stimulation. In: Horch KW and Dhillon GS, eds. Neuroprosthetics: Theory and Practice. World Scientific; 2004:638–682. [Google Scholar]
- 50.Bhadra N, Mortimer JT. Extraction forces and tissue changes during explant of CWRU-type intramuscular electrodes from rat gastrocnemius. Ann Biomed Eng. 1997;25:1017–1025. doi: 10.1007/BF02684137 [DOI] [PubMed] [Google Scholar]
- 51.Bhadra N, Mortimer JT. Extraction force and tissue change during removal of a tined intramuscular electrode from rat gastrocnemius. Ann Biomed Eng. 2006;34:1042–1050. doi: 10.1007/s10439-006-9125-5 [DOI] [PubMed] [Google Scholar]
- 52.Akers JM, Peckham PH, Keith MW, et al. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1997;5:207–220. doi: 10.1109/86.593301 [DOI] [PubMed] [Google Scholar]
- 53.Rauck RL, Kapural L, Cohen SP, et al. Peripheral nerve stimulation for the treatment of postamputation pain–a case report. Pain Pract. 2012;12:649–655. doi: 10.1111/j.1533-2500.2012.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation. 2013;17:188–197. doi: 10.1111/ner.12102 [DOI] [PubMed] [Google Scholar]
- 55.Gilmore CA, Ilfeld BM, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med. 2019;44:637–645. doi: 10.1136/rapm-2018-100109 [DOI] [PubMed] [Google Scholar]
- 56.Rosenow JM, Gilmore C, Ilfeld BM, et al. One year follow-up of a randomized, double-blind, placebo-controlled trial of percutaneous peripheral nerve stimulation for chronic neuropathic pain following amputation. Neurosurgery. 2019;66. doi: 10.1093/neuros/nyz310_144 [DOI] [Google Scholar]
- 57.Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract. 2016;17:892–901. doi: 10.1111/papr.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res. 2017;12:4. doi: 10.1186/s13018-016-0506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilfeld BM, Finneran JJ, Gabriel RA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg Anesth Pain Med. 2019;44:310–318. doi: 10.1136/rapm-2018-100121 [DOI] [PubMed] [Google Scholar]
- 60.Ilfeld BM, Gabriel RA, Said ET, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med. 2018;43:580–589. doi: 10.1097/AAP.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ilfeld BM, Said ET, Finneran J, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the femoral nerve for postoperative analgesia following ambulatory anterior cruciate ligament reconstruction: a proof of concept study. Neuromodulation. 2019;22:621–629. doi: 10.1111/ner.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson RD, Bennett ME, Lechman TE, et al. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil. 2011;92:837–840. doi: 10.1016/j.apmr.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chae J, Wilson RD, Bennett ME, et al. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract. 2013;13:59–67. doi: 10.1111/j.1533-2500.2012.00541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson RD, Gunzler DD, Bennett ME, et al. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:17–28. doi: 10.1097/PHM.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen VQ, Bock WC, Groves CC, et al. Fully implantable peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Am J Phys Med Rehabil. 2015;94:146–153. doi: 10.1097/PHM.0000000000000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson RD, Bennett ME, Nguyen VQC, et al. Fully implantable peripheral nerve stimulation for hemiplegic shoulder pain: a multi-site case series with two-year follow-up. Neuromodulation. 2018;21:290–295. doi: 10.1111/ner.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilmore CA, Kapural L, McGee MJ, et al. Percutaneous peripheral nerve stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation. 2018. [DOI] [PubMed] [Google Scholar]
- 68.Kapural L, Gilmore CA, Chae J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain: two clinical case reports of sustained pain relief. Pain Pract. 2018;18:94–103. doi: 10.1111/papr.12571 [DOI] [PubMed] [Google Scholar]
- 69.Ilfeld BM, Grant SA. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks? Reg Anesth Pain Med. 2016;41:720–722. doi: 10.1097/AAP.0000000000000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan Y, Wacnik PW, Yang F, et al. Spinal cord stimulation-induced analgesiaelectrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c [DOI] [PubMed] [Google Scholar]
- 71.Sun L, Tai L, Qiu Q, et al. Endocannabinoid activation of CB1 receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur J Pain. 2017;21:804–814. doi: 10.1002/ejp.983 [DOI] [PubMed] [Google Scholar]
- 72.Meyerson B, Ren B, Herregodts P, et al. Spinal cord stimulation in animal models of mononeuropathy: effects on the withdrawal response and the flexor reflex. Pain. 1995;61:229–243. doi: 10.1016/0304-3959(94)00171-A [DOI] [PubMed] [Google Scholar]
- 73.Lindblom U, Meyerson B. Influence on touch, vibration and cutaneous pain of dorsal column stimulation in man. Pain. 1975;1:257–270. doi: 10.1016/0304-3959(75)90042-1 [DOI] [PubMed] [Google Scholar]
- 74.Wolter T, Winkelmuller M. Continuous versus intermittent spinal cord stimulation: an analysis of factors influencing clinical efficacy. Neuromodulation. 2012;15:13–19; discussion 20. doi: 10.1111/j.1525-1403.2011.00410.x [DOI] [PubMed] [Google Scholar]
- 75.Deer TR, Naidu R, Strand N, et al. A review of the bioelectronic implications of stimulation of the peripheral nervous system for chronic pain conditions. Bioelectron Med. 2020;6:9. doi: 10.1186/s42234-020-00045-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deer TR, Esposito MF, McRoberts WP, et al. A systematic literature review of peripheral nerve stimulation therapies for the treatment of pain. Pain Med. 2020;21:1590–1603. doi: 10.1093/pm/pnaa030 [DOI] [PubMed] [Google Scholar]
- 77.Basbaum AI, Kandel ER, Schwartz JH, et al. Pain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science (Ed 5). New York: McGraw-hill; 2000. [Google Scholar]
- 78.Bentley N, Awad AJ, Patil PG. Physiology and pathophysiology of chronic pain. In: Krames ES, Peckham PH, Rezai AR, editors. Neuromodulation. San Diego: Academic Press; 2018:565–573. [Google Scholar]
- 79.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E [DOI] [PubMed] [Google Scholar]
- 82.Haroutounian S, Nikolajsen L, Bendtsen TF, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155:1272–1279. doi: 10.1016/j.pain.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 83.Treede RD. Gain control mechanisms in the nociceptive system. Pain. 2016;157:1199–1204. doi: 10.1097/j.pain.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 84.Meacham K, Shepherd A, Mohapatra DP, et al. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21:28. doi: 10.1007/s11916-017-0629-5 [DOI] [PubMed] [Google Scholar]
- 85.Cervero F. Spinal cord hyperexcitability and its role in pain and hyperalgesia. Exp Brain Res. 2009;196:129–137. doi: 10.1007/s00221-009-1789-2 [DOI] [PubMed] [Google Scholar]
- 86.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18:20–30. doi: 10.1038/nrn.2016.162 [DOI] [PubMed] [Google Scholar]
- 88.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1 [DOI] [PubMed] [Google Scholar]
- 90.Ruiz-Sauri A, Orduña‐Valls JM, Blasco-Serra A, et al. Glia to neuron ratio in the posterior aspect of the human spinal cord at thoracic segments relevant to spinal cord stimulation. J Anat. 2019;235:997–1006. doi: 10.1111/joa.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallejo R, Kelley CA, Gupta A, et al. Modulation of neuroglial interactions using differential target multiplexed spinal cord stimulation in an animal model of neuropathic pain. Mol Pain. 2020;16:1744806920918057. doi: 10.1177/1744806920918057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braz J, Solorzano C, Wang X, et al. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neumann S, Braz JM, Skinner K, et al. Innocuous, not noxious, input activates PKCγ interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takazawa T, MacDermott AB. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann N Y Acad Sci. 2010;1198:153–158. doi: 10.1111/j.1749-6632.2010.05501.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malmberg AB, Chen C, Tonegawa S, et al. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279 [DOI] [PubMed] [Google Scholar]
- 96.Lu Y, Dong H, Gao Y, et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest. 2013;123:4050–4062. doi: 10.1172/JCI70026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Q, Heinricher MM. Descending control mechanisms and chronic pain. Curr Rheumatol Rep. 2019;21:13. doi: 10.1007/s11926-019-0813-1 [DOI] [PubMed] [Google Scholar]
- 98.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008 [DOI] [PubMed] [Google Scholar]
- 99.Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci. 2012;35:629–637. doi: 10.1016/j.tins.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 100.Treede R-D, Kenshalo DR, Gracely RH, et al. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/S0304-3959(98)00184-5 [DOI] [PubMed] [Google Scholar]
- 101.Flor H, Braun C, Elbert T, et al. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224:5–8. doi: 10.1016/S0304-3940(97)13441-3 [DOI] [PubMed] [Google Scholar]
- 102.Maihöfner C, Handwerker HO, Neundörfer B, et al. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715. doi: 10.1212/01.WNL.0000098939.02752.8E [DOI] [PubMed] [Google Scholar]
- 103.Birbaumer N, Lutzenberger W, Montoya P, et al. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flor H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl Psychophysiol Biofeedback. 2002;27:215–227. doi: 10.1023/A:1016204029162 [DOI] [PubMed] [Google Scholar]
- 105.Pleger B, Tegenthoff M, Schwenkreis P, et al. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome I. Exp Brain Res. 2004;155:115–119. doi: 10.1007/s00221-003-1738-4 [DOI] [PubMed] [Google Scholar]
- 106.Vartiainen N, Kirveskari E, Kallio-Laine K, et al. Cortical reorganization in primary somatosensory cortex in patients with unilateral chronic pain. J Pain. 2009;10:854–859. doi: 10.1016/j.jpain.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 107.Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair. 2012;26:646–652. doi: 10.1177/1545968311433209 [DOI] [PubMed] [Google Scholar]
- 108.Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 110.Seburn KL, Catlin PA, Dixon JF, et al. Decline in spontaneous activity of group Aαβ sensory afferents after sciatic nerve axotomy in rat. Neurosci Lett. 1999;274:41–44. doi: 10.1016/S0304-3940(99)00667-9 [DOI] [PubMed] [Google Scholar]
- 111.Burgess P, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–848. doi: 10.1152/jn.1968.31.6.833 [DOI] [PubMed] [Google Scholar]
- 112.Yang F, Xu Q, Cheong YK, et al. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur J Pain. 2014;18:978–988. doi: 10.1002/j.1532-2149.2013.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gilman S. Joint position sense and vibration sense: anatomical organisation and assessment. J Neurol Neurosurg Psychiatry. 2002;73:473–477. doi: 10.1136/jnnp.73.5.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gardner E, Johnson K. The somatosensory system: receptors and central pathways. Principles Neural Sci. 2013;5:475–495. [Google Scholar]
- 115.Parker JL, Shariati NH, Karantonis DM. Electrically evoked compound action potential recording in peripheral nerves. Bioelectron Med. 2018;1:71–83. doi: 10.2217/bem-2017-0005 [DOI] [Google Scholar]
- 116.Li CL, Bak A. Excitability characteristics of the A- and C-fibers in a peripheral nerve. Exp Neurol. 1976;50:67–79. doi: 10.1016/0014-4886(76)90236-3 [DOI] [PubMed] [Google Scholar]
- 117.Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x [DOI] [PubMed] [Google Scholar]
- 118.Hillman P, Wall P. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9:284–306. doi: 10.1007/BF00235240 [DOI] [PubMed] [Google Scholar]
- 119.Foreman R, Beall J, Coulter J, et al. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39:534–546. doi: 10.1152/jn.1976.39.3.534 [DOI] [PubMed] [Google Scholar]
- 120.Chung J, Fang Z, Hori Y, et al. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain. 1984;19:259–275. doi: 10.1016/0304-3959(84)90004-6 [DOI] [PubMed] [Google Scholar]
- 121.Guan Y, Bradley K, Parker JL, et al. Spinal cord stimulation: mechamisms of action. In: Krames ES, Peckham PH, Rezai AR, eds. Neuromodulation. Cambridge, MA: Academic Press; 2018:161–178. [Google Scholar]
- 122.Yang F, Xu Q, Shu B, et al. Activation of cannabinoid CB1 receptor contributes to suppression of spinal nociceptive transmission and inhibition of mechanical hypersensitivity by Abeta-fiber stimulation. Pain. 2016;157:2582–2593. doi: 10.1097/j.pain.0000000000000680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sdrulla AD, Xu Q, He SQ, et al. Electrical stimulation of low-threshold afferent fibers induces a prolonged synaptic depression in lamina II dorsal horn neurons to high-threshold afferent inputs in mice. Pain. 2015;156:1008–1017. doi: 10.1097/01.j.pain.0000460353.15460.a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience. 2014;283:95–106. doi: 10.1016/j.neuroscience.2014.09.032 [DOI] [PubMed] [Google Scholar]
- 125.Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210–216. doi: 10.1016/j.pain.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wall PD. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958;142:1–21. doi: 10.1113/jphysiol.1958.sp005997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eccles JC, Krnjević K. Potential changes recorded inside primary afferent fibres within the spinal cord. J Physiol. 1959;149:250–273. doi: 10.1113/jphysiol.1959.sp006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Willis WD Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637 [DOI] [PubMed] [Google Scholar]
- 129.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933 [DOI] [PubMed] [Google Scholar]
- 130.Cervero F, Laird JMA, García-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–351. doi: 10.1016/S1090-3801(03)00047-8 [DOI] [PubMed] [Google Scholar]
- 131.Calvillo O. Primary afferent depolarization of C fibres in the spinal cord of the cat. Can J Physiol Pharmacol. 1978;56:154–157. doi: 10.1139/y78-020 [DOI] [PubMed] [Google Scholar]
- 132.Lin T, Gargya A, Singh H, et al. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020;21:S6–S12. doi: 10.1093/pm/pnaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakravarthy K, Nava A, Christo PJ, et al. Review of recent advances in peripheral nerve stimulation (PNS). Curr Pain Headache Rep. 2016;20:60. doi: 10.1007/s11916-016-0590-8 [DOI] [PubMed] [Google Scholar]
- 134.Bartsch T, Goadsby PJ. Central mechanisms of peripheral nerve stimulation in headache disorders. In: Slavin KV, editor. Peripheral Nerve Stimulation. Karger Publishers; 2011:16–26. [DOI] [PubMed] [Google Scholar]
- 135.Gielen F, Molnar G. Basic principles of deep brain stimulation. In: Denys D, Feenstra M, Schuurman R, eds. Deep Brain Stimulation. Springer; 2012:1–10. [Google Scholar]
- 136.Brocker DT, Grill WM. Principles of electrical stimulation of neural tissue. In: Lozano AM, Hallett M, eds. Handbook of Clinical Neurology. Elsevier; 2013:3–18. [DOI] [PubMed] [Google Scholar]
- 137.Parker JL, Karantonis DM, Single PS, et al. Compound action potentials recorded in the human spinal cord during neurostimulation for pain relief. Pain. 2012;153:593–601. doi: 10.1016/j.pain.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 138.Feirabend H, Choufoer H, Ploeger S, et al. Morphometry of human superficial dorsal and dorsolateral column fibres: significance to spinal cord stimulation. Brain. 2002;125:1137–1149. doi: 10.1093/brain/awf111 [DOI] [PubMed] [Google Scholar]
- 139.Yang F, Carteret AF, Wacnik PW, et al. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–480. doi: 10.1016/j.neuroscience.2011.09.049 [DOI] [PubMed] [Google Scholar]
- 140.Molnar G, Barolat G. Principles of cord activation during spinal cord stimulation. Neuromodulation. 2014;17(Suppl 1):12–21. doi: 10.1111/ner.12171 [DOI] [PubMed] [Google Scholar]
- 141.Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation. 2002;5:25–31. doi: 10.1046/j.1525-1403.2002._2005.x [DOI] [PubMed] [Google Scholar]
- 142.Gaines JL, Finn KE, Slopsema JP, et al. A model of motor and sensory axon activation in the median nerve using surface electrical stimulation. J Comput Neurosci. 2018;45:29–43. doi: 10.1007/s10827-018-0689-5 [DOI] [PubMed] [Google Scholar]
- 143.Rattay F, Paredes L, Leao R. Strength–duration relationship for intra-versus extracellular stimulation with microelectrodes. Neuroscience. 2012;214:1–13. doi: 10.1016/j.neuroscience.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J, Kong X, Gozani SN, et al. Current-distance relationships for peripheral nerve stimulation localization. Anesth Analg. 2011;112:236–241. doi: 10.1213/ANE.0b013e3181fca16b [DOI] [PubMed] [Google Scholar]
- 145.Wilson RD, Knutson JS, Bennett ME, et al. The effect of peripheral nerve stimulation on shoulder biomechanics: a randomized controlled trial in comparison to physical therapy. Am J Phys Med Rehabil. 2017;96:191–198. doi: 10.1097/PHM.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lidierth M. Local and diffuse mechanisms of primary afferent depolarization and presynaptic inhibition in the rat spinal cord. J Physiol. 2006;576:309–327. doi: 10.1113/jphysiol.2006.110577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dorfman LJ. The distribution of conduction velocities (DCV) in peripheral nerves: a review. Muscle Nerve. 1984;7:2–11. doi: 10.1002/mus.880070103 [DOI] [PubMed] [Google Scholar]
- 148.Huntoon MA, Burgher AH. Review of ultrasound-guided peripheral nerve stimulation. Tech Reg Anesth Pain Manag. 2009;13:121–127. doi: 10.1053/j.trap.2009.06.018 [DOI] [Google Scholar]
- 149.Law JD, Swett J, Kirsch WM. Retrospective analysis of 22 patients with chronic pain treated by peripheral nerve stimulation. J Neurosurg. 1980;52:482. doi: 10.3171/jns.1980.52.4.0482 [DOI] [PubMed] [Google Scholar]
- 150.Ellrich J, Lamp S. Peripheral nerve stimulation inhibits nociceptive processing: an electrophysiological study in healthy volunteers. Neuromodulation. 2005;8:225–232. doi: 10.1111/j.1525-1403.2005.00029.x [DOI] [PubMed] [Google Scholar]
- 151.Ristic D, Spangenberg P, Ellrich J. Analgesic and antinociceptive effects of peripheral nerve neurostimulation in an advanced human experimental model. Eur J Pain. 2008;12:480–490. doi: 10.1016/j.ejpain.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 152.Ristic D, Ellrich J. Innocuous peripheral nerve stimulation shifts stimulus-response function of painful laser stimulation in man. Neuromodulation. 2014;17:686–694; discussion 694–685. doi: 10.1111/ner.12133 [DOI] [PubMed] [Google Scholar]
- 153.De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: different and common brain mechanisms. Neuromodulation. 2016;19:47–59. doi: 10.1111/ner.12368 [DOI] [PubMed] [Google Scholar]
- 154.Stancak A, Kozak J, Vrba I, et al. Functional magnetic resonance imaging of cerebral activation during spinal cord stimulation in failed back surgery syndrome patients. Eur J Pain. 2008;12:137–148. doi: 10.1016/j.ejpain.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 155.Urien L, Wang J. Top-down cortical control of acute and chronic pain. Psychosom Med. 2019;81:851–858. doi: 10.1097/PSY.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149 [DOI] [PubMed] [Google Scholar]
- 157.Jenkins WM, Merzenich MM, Ochs MT, et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82 [DOI] [PubMed] [Google Scholar]
- 158.Byl NN, Merzenich MM, Cheung S, et al. A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther. 1997;77:269–284. doi: 10.1093/ptj/77.3.269 [DOI] [PubMed] [Google Scholar]
- 159.Gustin SM, Peck CC, Cheney LB, et al. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32:14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tecchio F, Padua L, Aprile I, et al. Carpal tunnel syndrome modifies sensory hand cortical somatotopy: a MEG study. Hum Brain Mapp. 2002;17:28–36. doi: 10.1002/hbm.10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Preißler S, Dietrich C, Meissner W, et al. Brachial plexus block in phantom limb pain: a case report. Pain Med. 2011;12:1649–1654. doi: 10.1111/j.1526-4637.2011.01247.x [DOI] [PubMed] [Google Scholar]
- 162.De Nunzio AM, Schweisfurth MA, Ge N, et al. Relieving phantom limb pain with multimodal sensory-motor training. J Neural Eng. 2018;15:066022. doi: 10.1088/1741-2552/aae271 [DOI] [PubMed] [Google Scholar]
- 163.Flor H, Denke C, Schaefer M, et al. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357:1763–1764. doi: 10.1016/S0140-6736(00)04890-X [DOI] [PubMed] [Google Scholar]
- 164.Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol. 2005;18:667–674. doi: 10.1097/01.wco.0000189876.37475.42 [DOI] [PubMed] [Google Scholar]
- 165.Wu CW, van Gelderen P, Hanakawa T, et al. Enduring representational plasticity after somatosensory stimulation. Neuroimage. 2005;27:872–884. doi: 10.1016/j.neuroimage.2005.05.055 [DOI] [PubMed] [Google Scholar]
- 166.Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158:669–681. doi: 10.1097/j.pain.0000000000000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Graham RD, Bruns TM, Duan B, et al. Dorsal root ganglion stimulation for chronic pain modulates Abeta-fiber activity but not C-fiber activity: a computational modeling study. Clin Neurophysiol. 2019;130:941–951. doi: 10.1016/j.clinph.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sperry ZJ, Graham RD, Peck-Dimit N, et al. Spatial models of cell distribution in human lumbar dorsal root ganglia. J Comp Neurol. 2020;528(10):1644–1659. doi: 10.1002/cne.24848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kent AR, Min X, Hogan QH, et al. Mechanisms of dorsal root ganglion stimulation in pain suppression: a computational modeling analysis. Neuromodulation. 2018;21:234–246. doi: 10.1111/ner.12754 [DOI] [PubMed] [Google Scholar]
- 170.Wang X, Merzenich MM, Sameshima K, et al. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71. doi: 10.1038/378071a0 [DOI] [PubMed] [Google Scholar]
- 171.Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anaesth. 2001;87:107–116. doi: 10.1093/bja/87.1.107 [DOI] [PubMed] [Google Scholar]
- 172.Neil M. Pain after amputation. BJA Educ. 2016;16:107–112. doi: 10.1093/bjaed/mkv028 [DOI] [Google Scholar]
- 173.Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. doi: 10.2147/JPR.S32299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Flor H, Nikolajsen L, Staehelin jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991 [DOI] [PubMed] [Google Scholar]
- 175.Nikolajsen L. Postamputation pain: studies on mechanisms. Dan Med J. 2012;59. [PubMed] [Google Scholar]
- 176.Van Calenbergh F, Gybels J, Van Laere K, et al. Long term clinical outcome of peripheral nerve stimulation in patients with chronic peripheral neuropathic pain. Surg Neurol. 2009;72:330–335. doi: 10.1016/j.surneu.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 177.Krainick J-U, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation: long-term follow-up study over 5 years. J Neurosurg. 1980;52:346–350. doi: 10.3171/jns.1980.52.3.0346 [DOI] [PubMed] [Google Scholar]
- 178.Kalichman L, Ratmansky M. Underlying pathology and associated factors of hemiplegic shoulder pain. Am J Phys Med Rehabil. 2011;90:768–780. doi: 10.1097/PHM.0b013e318214e976 [DOI] [PubMed] [Google Scholar]
- 179.Griffin JW. Hemiplegic shoulder pain. Phys Ther. 1986;66:1884–1893. doi: 10.1093/ptj/66.12.1884 [DOI] [PubMed] [Google Scholar]
- 180.Walsh K. Management of shoulder pain in patients with stroke. Postgrad Med J. 2001;77:645–649. doi: 10.1136/pmj.77.912.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Roosink M, Renzenbrink GJ, Buitenweg JR, et al. Somatosensory symptoms and signs and conditioned pain modulation in chronic post-stroke shoulder pain. J Pain. 2011;12:476–485. doi: 10.1016/j.jpain.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 182.Soo Hoo J, Paul T, Chae J, et al. Central hypersensitivity in chronic hemiplegic shoulder pain. Am J Phys Med Rehabil. 2013;92:1–9; quiz 10–13. doi: 10.1097/PHM.0b013e31827df862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roosink M, Renzenbrink GJ, Buitenweg JR, et al. Persistent shoulder pain in the first 6 months after stroke: results of a prospective cohort study. Arch Phys Med Rehabil. 2011;92:1139–1145. doi: 10.1016/j.apmr.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 184.Roosink M, van Dongen RT, Renzenbrink GJ, et al. Classifying post-stroke shoulder pain: can the DN4 be helpful? Eur J Pain. 2011;15:99–102. doi: 10.1016/j.ejpain.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 185.Roosink M, Buitenweg JR, Renzenbrink GJ, et al. Altered cortical somatosensory processing in chronic stroke: a relationship with post-stroke shoulder pain. Neurorehabil. 2011;28:331–344. doi: 10.3233/NRE-2011-0661 [DOI] [PubMed] [Google Scholar]
- 186.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002 [DOI] [PubMed] [Google Scholar]