Abstract
This paper describes benthic coral reef community composition point-based field data sets derived from georeferenced photoquadrats using machine learning. Annually over a 17 year period (2002–2018), data were collected using downward-looking photoquadrats that capture an approximately 1 m2 footprint along 100 m–1500 m transect surveys distributed along the reef slope and across the reef flat of Heron Reef (28 km2), Southern Great Barrier Reef, Australia. Benthic community composition for the photoquadrats was automatically interpreted through deep learning, following initial manual calibration of the algorithm. The resulting data sets support understanding of coral reef biology, ecology, mapping and dynamics. Similar methods to derive the benthic data have been published for seagrass habitats, however here we have adapted the methods for application to coral reef habitats, with the integration of automatic photoquadrat analysis. The approach presented is globally applicable for various submerged and benthic community ecological applications, and provides the basis for further studies at this site, regional to global comparative studies, and for the design of similar monitoring programs elsewhere.
Subject terms: Environmental impact, Biodiversity, Marine biology
| Measurement(s) | marine benthic feature |
| Technology Type(s) | photoquadrat transect surveys |
| Factor Type(s) | benthic composition |
| Sample Characteristic - Organism | benthic communities |
| Sample Characteristic - Environment | coral reef • marine coral reef flat zone • marine coral reef crest • marine coral reef back reef • marine coral reef fore reef |
| Sample Characteristic - Location | Heron Island Reef, 23–052 |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.14034320
Background & Summary
This study describes a unique point-based data set for coral reef environments, collected using a photoquadrat survey method published for seagrass environments1. The data set describes the spatial and temporal distribution of benthic community abundance and composition for Heron Reef, a 28 km2 shallow platform reef located in the Capricorn Bunker Group, Southern Great Barrier Reef (GBR), Australia. On average, 3,600 coral reef data points were collected annually over the period 2002 to 2018. Annual data sets were acquired for independent research projects, but the collection methods were consistent. The initial field data collection design was planned to acquire detailed field data to describe the spatial distribution and variability of benthic composition across the study site to assist with calibration and validation of earth observation-based mapping products.
To create a map based on earth observation imagery, it is common to use training or calibration data to transform the imagery into a map of surface properties using a supervised algorithm (e.g. multivariate statistical clustering, random forest)2. To report on the accuracy measures of the maps, reference or validation data are contrasted with the output maps3. Hence for calibration and validation purposes, georeferenced field data must be representative of all the features to be mapped and collection should ideally coincide with satellite image acquisition. Many earth observation approaches have been implemented for mapping the benthic communities of Heron Reef4–12 and several of these maps are now accessible online6,13,14.
Several studies have utilised time series benthic data to analyse changes in benthic community and coral type trends, supporting broad ecological knowledge of coral reef ecosystems such as the Caribbean reef degradation15 and coral cover decline on the GBR16. Similarly, benthic community and coral cover data sets have been identified as important indicators of coral reef health providing the backbone for monitoring and management initiatives around the world17,18.
Articles and data sets have been published that describe the benthic community properties of Heron Reef, however, their spatial coverage, number of georeferenced data points, and revisit times are limited19. The time series photoquadrat data sets presented in this paper could be used for further understanding of benthic community distribution, including statistical analysis of trends in coral cover, analysis of changes in benthic community and coral type, or used for testing of other earth observation-based mapping and modelling approaches. Additionally, as our methodology describes machine annotation of the field photoquadrats, it would be possible to reanalyse the photoquadrats with new categories not previously considered important from a biological perspective (e.g. unknown disease or impact, or a specific benthic community type), or for other features (e.g. the counting of sea cucumbers (Holothuroidea sp.)).
Detailed analyses of our complete data set may permit a greater understanding of the persistence and/or dynamics of the benthic community at Heron Reef. As such, our ongoing analyses include evaluation of changes in community composition following major impacts such as cyclones, coral bleaching, crown of thorns predation, etc., and additionally, statistical analyses of coral recovery after such impacts. To this degree, these benthic community data sets are invaluable.
Methods
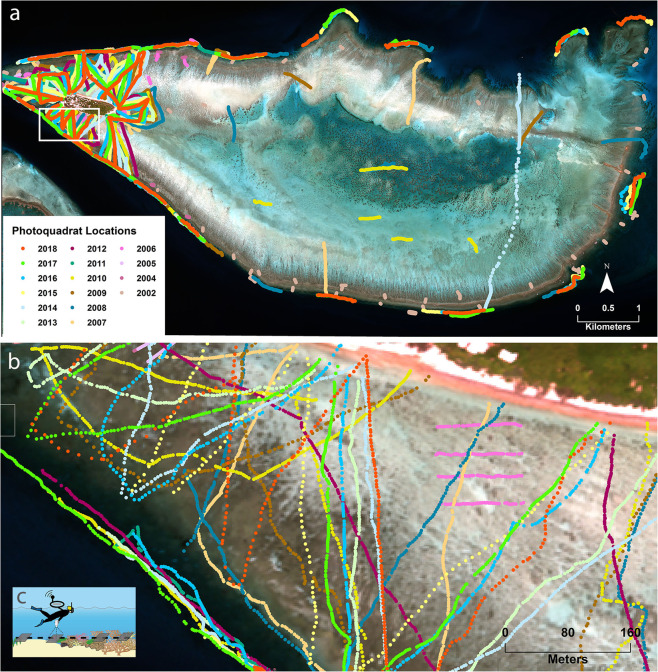
The photoquadrat-based data in this study was collected for Heron Reef, Southern Great Barrier Reef, Australia (Fig. 1). Here we provide a short overview of the collection methods, however a detailed description can be found in11. These methods are applicable to any habitat. Photoquadrats were analysed for substrate and/or benthic community types known to be present on the reef (Fig. 1). The benthic community classes included in the analysis are shown in Table 1.
Fig. 1.
Heron Reef, southern Great Barrier Reef, Australia. (a) Location of photoquadrat transect surveys on Heron Reef collected over a period of 17 years, (b) example of the individual photoquadrat locations along the transect survey where each individual point represents a photoquadrat, and (c) conceptualisation of snorkeler-based georeferenced photoquadrat transect surveys.
Table 1.
Benthic community and coral type descriptions and their class codes used for photoquadrat annotation.
| Class Code | Description | Group | Simplified Group |
|---|---|---|---|
| ACR_BRA | Acropora formosa, branching Montipera | Branching | Hard Coral |
| ACR_BRA_B_ | Acropora formosa, branching Montipera - Bleached | Branching | Hard Coral |
| ACR_HIP | Acroporidae Hispidoes; thick branches, predominantly hispidose | Branching | Hard Coral |
| ACR_HIP_B_ | Acroporidae Hispidoes; thick branches, predominantly hispidose - Bleached | Branching | Hard Coral |
| ACR_OTH | Acroporids with indeterminate shape, predominantly cuneiform | Branching | Hard Coral |
| ACR_OTH_B_ | Acroporids with indeterminate shape, predominantly cuneiform - Bleached | Branching | Hard Coral |
| ACR_PE | Encrusting Monipora | Plate | Hard Coral |
| ACR_PE_B_ | Encrusting Monipora - Bleached | Plate | Hard Coral |
| BRA_TAB_Ac | Acropora tabular/corymbose/plate | Plate | Hard Coral |
| BRA_TAB_B_ | Acropora tabular/corymbose/plate - Bleached | Plate | Hard Coral |
| BRA_DIG_Ac | Acropora digitate, branches resembling fingers | Branching | Hard Coral |
| BRA_DIG_B_ | Acropora digitate, branches resembling fingers - Bleached | Branching | Hard Coral |
| FAV_MUS | Favia, Favites, Platygyra, Goniastrea, Diploastrea, Lobophyllia | Massive | Hard Coral |
| FAV_MUS_B_ | Favia, Favites, Platygyra, Goniastrea, Diploastrea, Lobophyllia - Bleached | Massive | Hard Coral |
| MASE_OTH | Massive, submassive, encrusting colonies of undetermined taxonomic group | Massive | Hard Coral |
| MASEoth_B_ | Massive, submassive, encrusting colonies of undetermined taxonomic group - Bleached | Massive | Hard Coral |
| TFP_RDG_Al | Thin, foliose or plating colonies with visible relief structures on the plates | Plate | Hard Coral |
| TFP_RDG_B_ | Thin, foliose or plating colonies with visible relief structures on the plates -Bleached | Plate | Hard Coral |
| TFP_RND_Al | Thin, foliose or plating colonies with visible rounded corallites on the plates | Plate | Hard Coral |
| TFP_RND_B_ | Thin, foliose or plating colonies with visible rounded corallites on the plates - Bleached | Plate | Hard Coral |
| BRA_OTH | Branching other | Branching | Hard Coral |
| BRA_OTH_B_ | Branching other - Bleached | Branching | Hard Coral |
| OTH_HC | Other HC not assigned to any other category | HC Other | Hard Coral |
| OTH_HC_B_ | Other HC not assigned to any other category - Bleached | HC Other | Hard Coral |
| POCI | Pocilloporidae sp. (includes Seriatopora and Stylophora) | Branching | Hard Coral |
| POCI_B_ | Pocilloporidae sp. (includes Seriatopora and Stylophora) - Bleached | Branching | Hard Coral |
| POR_BRA | Porites cylindrica, Goniopora (Poritidae branching) | Branching | Hard Coral |
| POR_BRA_B_ | Porites cylindrica, Goniopora (Poritidae branching) - Bleached | Branching | Hard Coral |
| POR_ENC | Porites lichen (Poritidae encrusting) | Massive | Hard Coral |
| POR_ENC_B_ | Porites lichen (Poritidae encrusting) - Bleached | Massive | Hard Coral |
| POR_MASS | Porites lobata, P. lutea (Poritidae massive) | Massive | Hard Coral |
| POR_MASS_B_ | Porites lobata, P. lutea (Poritidae massive) - Bleached | Massive | Hard Coral |
| GORG | Sea Fans/Plumes; Gorgonia, Pseudopterogorgia | Soft | Other |
| GORG_B_ | Sea Fans/Plumes; Gorgonia, Pseudopterogorgia - Bleached | Soft | Other |
| ALC_SF | Common large fleshy Alcyoniidae representatives | Soft | Other |
| ALC_SF_B_ | Common large fleshy Alcyoniidae representatives - Bleached | Soft | Other |
| OTH_SF | Other soft coral (not sea fans) | Soft | Other |
| OTH_SF_B_ | Other soft coral (not sea fans) - Bleached | Soft | Other |
| Other | All other | All other | Other |
| MINV_COTS | Crown of thorns sea star, Acanthaster planci | Invertebrates | Other |
| MOB_INV | Mobile invertebrates 1 (sea cucumber, urchin) | Invertebrates | Other |
| OTH_SINV | Other sessile invertebrates (zoanthids, anemones, corallimorphs, sponges, clams, etc) | Invertebrates | Other |
| Lobph | Lobophora; fleshy algae | Algae | Algae |
| Turbin | Turbinaria sp. | Algae | Algae |
| MAECBS | Erect Course Branching Brown: Sargassum sp. | Algae | Algae |
| Pad | Padina sp. (pencil shavings) | Algae | Algae |
| Dicsp | Dictyota sp. | Algae | Algae |
| Chlor | Chlorodesmis sp (turtle weed); green filamentous | Algae | Algae |
| MACR_Cal_H | Calicifying algae: Halimeda | Algae | Algae |
| Caul | Caulerpa sp., green algae | Algae | Algae |
| Cya_spe | Cyanobacterium sp. | Algae | Algae |
| ALG_OTH | Other algae | Algae | Algae |
| CAL_CCA_DC | Crustose Coralline Algae on dead coral | Rock | Rock |
| CAL_CCA_RB | Crustose Coralline Algae on rubble | Rubble | Rubble |
| EAM_DHC | Epithelial algal matrix smothering dead hard coral (Turf on Rock) | Rock | Rock |
| EAM_RB | Epithelial algal matrix smothering rubble (Turf on Rubble) | Rubble | Rubble |
| Sand | Sand | Sand | Sand |
| BMA_sand | Benthic microalgae on sand | Sand | Sand |
| Seagrass | Seagrass, any type | Other | Other |
| TAPE | Line or hardware | Other | Other |
| Unk | Unknown, but represents something (annotator doesn’t know what it is) | Other | Other |
| Unc | Unclear; point falls in a shadowy, blurry, dark area | Other | Other |
| WATE | Blue background | Other | Other |
Manual and automated (machine) annotation utilized the full labelset (63 class codes). Following machine annotation, these 63 class codes were aggregated via broad groups into six simplified groups for validation of the machine learning.
Georeferenced photoquadrat data collection
Detailed information on benthic community composition was gathered at Heron Reef on the reef flat (0–2 m depth) and at the 5 m contour on the reef slope using a repeatable and fine spatial scale (sampling every 2–4 m) technique for surveying benthic cover11. The technique required a snorkeler or diver manually capture georeferenced photoquadrats along defined transect surveys using a standard digital camera in a waterproof housing (e.g. Sony Cyber shot, Canon AA540, Lumix, or Olympus T4). A plumb-line attached to the camera, ensured that the footprint of each photoquadrat approximated 1 m2 of the benthos.
From 2002–2004, a 100 m transect tape was deployed at each defined survey start site at a maximum depth of 3 m, or on scuba at 5 m depth. From 2005 onwards, instead of deploying a tape, the surveyor towed a standard handheld GPS (e.g. Garmin eTrex, Garmin 72) at the surface in a waterproof bag for all surveys. This enabled accurate registration of the location of the acquisition of each photoquadrat, which was subsequently assigned via time synchronization, with the track log from the towed GPS. Once this method was established transect survey lengths were extended to distances of 500 m–1500 m. The start and end point of each transect was defined by GPS waypoints, permitting accurate revisits in subsequent years. The distance between successive photoquadrats was estimated by the surveyor’s kick cycle. However this was not considered a problem as the exact location of each photograph was known through the GPS synchronisation.
All surveys were performed during the day, and derivation of sunlight and sun angle can be ascertained through the timestamp of each photoquadrat and its corresponding GPS location. Reef Flat surveys were collected at high tide to provide sufficient water depth for the snorkeler to safely traverse the reef. Reef Slope surveys were collected at low tide. No water quality information was recorded.
The locations of the transect surveys were chosen to ensure they traversed gradients or edge features to detect any change in benthic cover over these features. This was done initially through visual assessment of existing satellite imagery in combination with expert knowledge of the study area. The aim was to produce data that provided an adequate representation of the variation in benthic community cover across Heron Reef. Limited transect surveys were located within the deep lagoonal area of the reef, as this area is hard to access by boat due to tidal range restrictions permitting short working times in the lagoon. Transect surveys were revisited in subsequent years, and additional transect surveys were included on subsequent trips based on increased knowledge of the environment. The benthic data sets and photoquadrat images are available at20.
Automated photoquadrat analysis for benthic community composition
Percentage cover of the benthic communities for each photoquadrat was determined through a machine-learning (ML) approach which assessed benthic community composition. A previously devised category scheme consisting of 63 class codes that differentiated all major GBR-specific coral morphologies and other bottom types was used21 which, following machine annotation, were collapsed first into broad groups and subsequently into six simplified groups for validation purposes (Table 1).
Initial training of the ML platform was achieved via manual annotation of approximately 5% of the total number of photoquadrats (equivalent to 108,700 annotated points; based on21), to achieve a machine annotation accuracy of >70% as determined by the classifier21. A unique source was created for each camera used. To give a default and uniform image annotation area, boundaries of 5% were used for the top and left sides of the photoquadrat, whilst a boundary of 95% was used for the right and bottom sides of the photoquadrat. Annotation points (50) were generated randomly over the entire annotation area per photoquadrat. For manual annotation of photoquadrat sets, the level of confidence was set to 100%. A further approximately 2.5% of photoquadrats were manually annotated in an identical manner to provide a validation data set to calculate the accuracy of the machine annotation. Automated annotation of the remaining 92.5% of the photoquadrats was achieved subsequently22.
Data Records
Detailed information regarding the output benthic cover percentages and the number of benthic photoquadrats acquired for each field campaign are documented in Table 2. The benthic data sets and photoquadrat images are available at20, with the photoquadrats and benthic cover analysis for individual survey years accessible online through the campaign specific DOIs listed in the table, from where the data can be downloaded directly.
Table 2.
Overview of the data files that represent the 58,941 georeferenced photoquadrats captured during the field campaigns, in addition to links to the percentage benthic cover data sets generated via machine learning for each year.
| Year-Month | Photoquadrats | Length of survey (m) | Benthic DOI (pangaea.de) | Photoquadrat DOI (pangaea.de) |
|---|---|---|---|---|
| 2002–11 | 1965 | 100 | 10.1594/PANGAEA.907025 | 10.1594/PANGAEA.895556 |
| 2004–03; 2004–05 | 1588 | 100 | 10.1594/PANGAEA.903850 | 10.1594/PANGAEA.895557 |
| 2005–05 | 1004 | 100 | 10.1594/PANGAEA.903851 | 10.1594/PANGAEA.894796 |
| 2006–06 | 1941 | 300–1500 | 10.1594/PANGAEA.903847 | 10.1594/PANGAEA.895558 |
| 2007–09 | 2923 | 300–1500 | 10.1594/PANGAEA.903779 | 10.1594/PANGAEA.895563 |
| 2008–10 | 3608 | 300–1500 | 10.1594/PANGAEA.903788 | 10.1594/PANGAEA.895569 |
| 2009–11 | 4400 | 300–1500 | 10.1594/PANGAEA.90378 | 10.1594/PANGAEA.895570 |
| 2010–11 | 4701 | 300–1500 | 10.1594/PANGAEA.903784 | 10.1594/PANGAEA.894797 |
| 2011–11 | 3602 | 300–1500 | 10.1594/PANGAEA.904704 | 10.1594/PANGAEA.895157 |
| 2012–07 | 3903 | 300–1500 | 10.1594/PANGAEA.904706 | 10.1594/PANGAEA.895121 |
| 2013–11 | 3589 | 300–1500 | 10.1594/PANGAEA.904710 | 10.1594/PANGAEA.895160 |
| 2014–11 | 4194 | 300–1500 | 10.1594/PANGAEA.904715 | 10.1594/PANGAEA.895124 |
| 2015–11 | 4277 | 300–1500 | 10.1594/PANGAEA.904716 | 10.1594/PANGAEA.895147 |
| 2016–09 | 4197 | 300–1500 | 10.1594/PANGAEA.907013 | 10.1594/PANGAEA.894800 |
| 2017–11 | 6499 | 300–1500 | 10.1594/PANGAEA.903766 | 10.1594/PANGAEA.895154 |
| 2018–11 | 5545 | 300–1500 | 10.1594/PANGAEA.903767 | 10.1594/PANGAEA.899670 |
The complete data set is available at20.
Technical Validation
To understand the validation technique applied to these data sets, it is important to reiterate the purpose of collecting the data set itself, which was a fast field method to gather benthic community information over a large spatial extent, whilst accurately representing variability. Validation of the data set was conducted on various levels, and included: standardisation of photoquadrat capture method and conditions, and a quantitative accuracy assessment.
Standardisation of photoquadrat image capture
To standardise photoquadrat image capture, the camera and lens setup used was calibrated prior to annual survey, so as to capture a footprint that covered the same extent of the benthos. This was accomplished by attaching a plumb-line to the camera system such that when it touched the bottom, the captured photoquadrats represented ~1 m2 of the benthos. To do this standardisation, the camera was moved vertically over a marked 1 m2 until the field of view enveloped the area, and the plumb-line was fixed. During the survey the operator used the plumb-line to determine the camera height above the ground. When held vertically with the weight touching the substrate this permitted reproducible capture of photoquadrats that covered the same area for all surveys. Light conditions were generally the same for each expedition, the data collected over a consecutive 4–5 day period, with stable weather, water clarity conditions and tidal range. Ideally light conditions would have been standardised using a strobe, however this would slow down the speed of the transect surveys.
Quantitative accuracy assessment
To determine the accuracy of the machine annotation we constructed a confusion matrix that compared, for a select set of validation photoquadrats, the benthic composition output from the machine learning annotation (modelled data), with the equivalent manual annotations (reference data). Using the confusion matrix we calculated the overall accuracy and the individual benthic label user and producer accuracy following a well-documented method3. All cameras demonstrated an overall accuracy of between 74% and 82% (Table 3;3). To provide a validation data set, ~2.5% of photoquadrats were manually annotated in an identical manner to the training data (36,950 annotated points; see Methods Section).
Table 3.
Quantitative assessment of the machine annotation stevia construction of a confusion matrix.
| Camera | SONY | Canon | Lumix | Olympus | |
|---|---|---|---|---|---|
| Years | 2002–2006 | 2007–2010 | 2011–2016 | 2017–2018 | |
| Overall Accuracy (%) | 79.1 | 81.8 | 73.9 | 79.8 | |
| User’s Accuracy (%) | Hard Coral | 79.9 | 83.6 | 83.2 | 88.2 |
| Rock | 77.2 | 79.3 | 71.2 | 74.4 | |
| Rubble | 68.0 | 68.8 | 61.5 | 25.0 | |
| Sand | 85.7 | 90.3 | 87.2 | 93.9 | |
| Algae | 85.7 | 79.4 | 74.4 | 71.4 | |
| Other | 52.4 | 33.3 | 57.3 | 61.7 | |
| Producer’s Accuracy (%) | Hard Coral | 76.0 | 72.7 | 72.5 | 70.2 |
| Rock | 89.2 | 92.6 | 90.5 | 94.8 | |
| Rubble | 5.3 | 15.6 | 4.7 | 10.2 | |
| Sand | 92.1 | 94.5 | 89.8 | 91.8 | |
| Algae | 6.8 | 42.8 | 19.4 | 24.2 | |
| Other | 23.7 | 18.7 | 24.0 | 33.5 | |
| # Points | 8,000 | 7,150 | 18,500 | 3,300 | |
For each camera used, machine annotation (modelled data) of 2.5% of all the photoquadrats captured was compared with manual annotation (reference data) of the same validation data set in a using standard confusion matrix3. From this, the overall accuracy and individual class accuracies were calculated following a well-documented approach3.
Acknowledgements
Funding provided by: University of Queensland; CSIRO; Cooperative Research Centre Coastal Zone, Estuaries and Waterways Management; ARC Linkage Grant to Prof. S Phinn; and World Bank Global Environment Facility Coral Reef Remote Sensing, ARC linkage innovative Coral Reef Monitoring. Fieldwork support was provided by: Coral and Reef Check Volunteers, Staff and students at University of Queensland, Heron Island Research Station. Field assistance: Rodney Borrego, Ian Leiper, Douglas Stetner, Josh Passenger, Megan Saunders, Robert Canto, Peran Bray, Emma Kennedy.
Author contributions
Chris M. Roelfsema, design (50%), methods (55%), collection (55%), analysis (15%), writing (30%). Eva M. Kovacs, design (25%), methods (25%), collection (30%), analysis (20%), writing (30%). Kathryn Markey, design (0%), methods (5%), collection (0%), analysis (25%), writing (4%). Julie Vercelloni, design (5%), methods (5%), collection (0%), analysis (10%), writing (10%). Alberto Rodriguez- Ramirez, design (0%), methods (0%), collection (0%), analysis (10%), writing (4%). Sebastian Lopez-Marcano, design (0%), methods (0%), collection (0%), analysis (5%), writing (5%). Manuel Gonzalez-Rivero, design (0%), methods (5%), collection (0%), analysis (5%), writing (5%). Ove Hoegh-Guldberg, design (0%), methods (0%), collection (0%), analysis (2%), writing (4%). Stuart R. Phinn, design (20%), methods (10%), collection (15%), analysis (0%), writing (5%).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roelfsema CM, Kovacs EM, Phinn SR. Field data sets for seagrass biophysical properties for the Eastern Banks, Moreton Bay, Australia, 2004–2014. Scientific Data. 2015;2:150040. doi: 10.1038/sdata.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons, M. et al. Mapping the world’s coral reefs using a global multiscale earth observation framework. Remote Sensing in Ecology and Conservation6, 10.1002/rse2.157 (2020).
- 3.Congalton, R. G. & Green, K. Assessing the accuracy of remotely sensed data: Principles and practices. Mapping Science. 2 edn, 200 (CRC Press, 2008).
- 4.Andréfouët S, et al. Multi-site evaluation of IKONOS data for classification of tropical coral reef environments. Remote Sensing of Environment. 2003;88:128–143. doi: 10.1016/j.rse.2003.04.005. [DOI] [Google Scholar]
- 5.González-Rivero M, et al. Scaling up Ecological Measurements of Coral Reefs Using Semi-Automated Field Image Collection and Analysis. Remote Sensing. 2016;8:30. doi: 10.3390/rs8010030. [DOI] [Google Scholar]
- 6.Hedley J, Roelfsema CM, Phinn SR. Supplement to: Hedley, J. et al. (2009): Efficient radiative transfer model inversion for remote sensing applications. Remote Sensing of Environment. 2012;113(11):2527–2532. doi: 10.1016/j.rse.2009.07.008. [DOI] [Google Scholar]
- 7.Joyce K, Phinn S, Roelfsema C, Neil D, Dennison W. Combining Landsat ETM+ and Reef Check classifications for mapping coral reefs: a critical assessment from the southern Great Barrier Reef, Australia. Coral Reefs. 2004;23:21–25. doi: 10.1007/s00338-003-0357-7. [DOI] [Google Scholar]
- 8.Joyce KE, Phinn SR, Roelfsema CM. Live coral cover index testing and application with hyperspectral airborne image data. Remote Sensing. 2013;5:6116–6137. doi: 10.3390/rs5116116. [DOI] [Google Scholar]
- 9.Ortiz, J. C. et al. The effect of wave exposure and competition for space on the community composition of coral reefs. Coral Reefs (in press).
- 10.Phinn SR, Roelfsema CM, Mumby PJ. Multi-scale image segmentation for mapping coral reef geomorphic and benthic community zone. International Journal of Remote Sensing. 2012;33:3768–3797. doi: 10.1080/01431161.2011.633122. [DOI] [Google Scholar]
- 11.Roelfsema C, Phinn S. Integrating field data with high spatial resolution multispectral satellite imagery for calibration and validation of coral reef benthic community maps. J Appl Remote Sens. 2010;4:043527-043527–043528. doi: 10.1117/1.3430107. [DOI] [Google Scholar]
- 12.Purkis, S. & Roelfsema, C. M. In Remote Sensing of Wetlands: Applications and Advances(pp. (eds Tiner, R. W., Lang, M. W. & Klemas, V. V.) 223–242 (CRC Press, 2015).
- 13.Borrego-Acevedo, R., Roelfsema, C. M., Phinn, S. R. & Grinham, A. In Supplement to: Borrego-Acevedo, R. et al. (2014): Predicting distribution of microphytobenthos abundance on a reef platform by combining in-situ underwater spectrometry and pigment analysis. Limnology and Oceanography, 5(5), 461–470, 10.1080/2150704X.2014.922723 (2013).
- 14.Phinn, S. R., Roelfsema, C. M. & Mumby, P. J. In Supplement to: Phinn, S. R. et al. (2012): Multi-scale, object-based image analysis for mapping geomorphic and ecological zones on coral reefs. International Journal of Remote Sensing, 33(12), 3768–3797, 10.1080/01431161.2011.633122 (2012).
- 15.Hughes TP. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 16.De’ath G, Fabricius KE, Sweatman H, Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proceedings of the National Academy of Sciences. 2012;109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obura, D. O. et al. Coral Reef Monitoring, Reef Assessment Technologies, and Ecosystem-Based Management. Frontiers in Marine Science6, 10.3389/fmars.2019.00580 (2019).
- 18.Flower J, et al. Interpreting coral reef monitoring data: A guide for improved management decisions. Ecological Indicators. 2017;72:848–869. doi: 10.1016/j.ecolind.2016.09.003. [DOI] [Google Scholar]
- 19.Connell JH. Disturbance and recovery of coral assemblages. Coral Reefs. 1997;16:S101–S113. doi: 10.1007/s003380050246. [DOI] [Google Scholar]
- 20.Roelfsema CM, Kovacs E, Stetner D, Phinn SR. 2018. Georeferenced benthic photoquadrats captured annually from 2002-2017, distributed over Heron Reef flat and slope areas. PANGAEA. [DOI]
- 21.González-Rivero M, et al. The Catlin Seaview Survey – kilometre-scale seascape assessment, and monitoring of coral reef ecosystems. Aquatic Conservation: Marine and Freshwater Ecosystems. 2014;24:184–198. doi: 10.1002/aqc.2505. [DOI] [Google Scholar]
- 22.González-Rivero, M. et al. Monitoring of Coral Reefs Using Artificial Intelligence: A Feasible and Cost-Effective Approach. Remote Sensing12, 10.3390/rs12030489 (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Roelfsema CM, Kovacs E, Stetner D, Phinn SR. 2018. Georeferenced benthic photoquadrats captured annually from 2002-2017, distributed over Heron Reef flat and slope areas. PANGAEA. [DOI]