Abstract
BACKGROUND AND PURPOSE:
Ischemic complications after coil embolization of the PcomA aneurysms are not thoroughly understood, especially in cases in which the PcomA is sacrificed. Our purpose was to examine the preoperative angiographic features and pattern of postoperative cerebral infarctions exhibited by patients who underwent embolization of ruptured PcomA aneurysms with PcomA sacrifice.
MATERIALS AND METHODS:
A retrospective review identified 14 patients with ruptured PcomA aneurysms who underwent embolization of the aneurysms in combination with PcomA sacrifice. Preoperative angiographic data, including the Allcock test, postoperative DWI, and neurologic status, were examined.
RESULTS:
Elimination of the aneurysm was complete in all cases. Postoperative DWI indicated 7 cases with infarctions (infarction group) and 7 cases without infarctions (noninfarction group). All patients in the infarction group developed infarctions in the vicinity of the tuberothalamic artery. In all 14 cases, a preoperative Allcock test demonstrated a retrograde filling of the PcomA through the P1 segment. The incidence of negative visualizations of the P1 segment on vertebral angiograms was significantly higher in the infarction group (100%) than in the noninfarction group (0%; P = .00058). The mean PcomA diameters, PcomA/P1 ratios, and aneurysm sizes observed in the infarction group were significantly greater than those in the noninfarction group (P < .05, P < .01, and P < .02, respectively). Tuberothalamic artery infarction caused hemiparesis and memory disturbance, which were associated with unfavorable outcomes.
CONCLUSIONS:
After the coil occlusion of ruptured PcomA aneurysms with PcomA sacrifice, tuberothalamic artery infarctions tended to occur in cases exhibiting negative visualization of the P1 segment, even when collateral flow was observed with the Allcock test.
Ischemic complications in the treatment of PcomA aneurysms have been demonstrated to occur when the PcomA is sacrificed.1–3 Both the elimination of the fetal variant PCA1,3 and the surgical division of the hypoplastic PcomA have been reported to potentially cause infarctions.2 However, Krayenbühl and Krisht4 demonstrated the safety and effectiveness of the surgical division of the PcomA while performing surgery within the interpeduncular fossa. In the course of surgical treatment, PcomA aneurysms presented the highest risk for postoperative infarctions, most of which were observed in the perforator regions.5 However, the ischemic complications occurring after coil embolization of the PcomA aneurysms are not thoroughly understood, especially in cases in which the PcomA is sacrificed.
Pelz and colleagues6 initially reported that the Allcock test (vertebral angiograms accompanied by carotid artery compression) is a useful method for evaluating PcomA morphology. The Allcock test reduces anteroposterior blood flow through the PcomA by compression of the carotid artery, which provides hemodynamics similar to those observed after the elimination of the PcomA. Thus, the Allcock test is considered useful in evaluating collateral blood flow and predicting postoperative ischemic complications encountered after the sacrifice of the PcomA.
In the present study, to clarify the relationship between preoperative angiographic features and ischemic complications, we investigated the pre- and postoperative neuroradiologic findings associated with the cases in which ruptured PcomA aneurysms were treated with coil embolization of the aneurysms in combination with PcomA sacrifice.
Materials and Methods
Patient Characteristics
A retrospective review of 94 consecutive patients presenting with PcomA aneurysms (ruptured/unruptured = 55/39) who underwent endovascular treatment at Kohnan Hospital between January 2007 and December 2010 identified 14 patients who were treated with intra-aneurysmal coil embolization with occlusion of the origin of the PcomA. All of the aneurysms were ruptured and were accompanied by extension of the PcomA from the sac. On the basis of the postoperative MR imaging findings, the patients were divided into 2 groups: an infarction group, consisting of patients exhibiting postoperative cerebral infarction, and a noninfarction group, consisting of patients without postoperative cerebral infarction. Clinical and radiographic data concerning these patients were abstracted from electronic and hard-copy records.
Neuroradiologic Assessment
Four-vessel cerebral angiography, including the Allcock test, was preoperatively performed for all cases. Conventional DSA and 3D DSA were performed with Innova 3131 technology (GE Healthcare, Buc, France). The usual contrast injection rate and total volume of 4 mL/s and 6 mL, respectively, were employed for the vertebral artery and the Allcock test. For the internal carotid artery, the rate and volume of 5 mL/s and 6 mL, respectively, were utilized. A positive result for the Allcock test was defined as the retrograde filling of the PcomA and the aneurysm through the P1 segment. A positive P1 result was defined as the apparent anterograde filling of the PCA through the P1 to P4 segments, as determined through vertebral angiography. A negative P1 result was defined as nonexistent or faint filling of the PCA, as measured with vertebral angiography. The diameters of the PcomA and the P1 segment were measured with the Allcock test, and the ratio of the diameters of the PcomA and the P1 segment (PcomA/P1 ratio) was calculated. Rotational spin angiography was performed. The 3D volume-rendering images were developed with an Advantage 4.4 workstation (GE Healthcare). The sizes of the aneurysms and the P1 diameters were measured using this workstation.
All MR imaging employed a Signa 3T system (GE Healthcare) with a standard head coil. DWI was performed the day after the endovascular treatment. High-intensity lesions that were detected by DWI performed on the first day after surgery were regarded as a treatment-related ischemic complications. MRA with gadolinium enhancement was performed on the following day, and 7 days after the treatment, to assess the recanalization of the PcomA and the extent of the occlusion of the aneurysms. Additional MR imaging or conventional angiography was conducted when symptomatic vasospasm was suspected.
All radiologic data were reviewed independently by 3 experienced neuroradiologists (K.S., R.K., and Y.M.).
Endovascular Treatment
Generally, endovascular treatment is performed under general anesthesia or under conscious sedation with local anesthesia, depending on the patient's condition. In this study, the bolus injection of heparin at the beginning of the procedure was 3000 U, with 1000 U given every hour. Coiling was performed to achieve attenuated packing of the aneurysms through the conventional single-catheter technique with various types of coils. The balloon remodeling technique was employed, if deemed necessary, by the operating interventionalist. The PcomA was sacrificed only when the aneurysms were accompanied by the PcomA arising from the sac, which was difficult to preserve, and the collateral circulation was observed on the preoperative angiography.
Clinical Assessment
The motor performances of the upper and the lower extremities were assessed by using the modified NIHSS on the day after surgery and 1 month after the treatment.7 Asymmetry in the NIHSS score was regarded as hemiparesis. The HDS-R was used to evaluate memory disturbance 1 month after the treatment.8 A score of 20 or below was regarded as indicative of memory disturbance. The functional outcome was assessed according to the mRS score 6 months after the treatment.9 The NIHSS, HDS-R, and mRS were administered by rehabilitation therapists and the authors (H.E., K.S., R.K., and Y.M.).
Statistical Analysis
The degrees of patency of the P1 segment in the preoperative vertebral angiography, the incidence of recanalization of the PcomA in the radiologic follow-up, and the proportion of patients with mRS scores of 3–6 were compared between the infarction group and the noninfarction group by using the Fisher exact test. Numeric data, such as the P1 diameters, the PcomA/P1 ratios, and the aneurysm sizes, are expressed as means ± SD, and comparisons between the 2 groups were performed by using the Mann-Whitney test. Differences were considered to be significant at P < .05.
Results
Baseline Characteristics and Neuroradiologic Findings
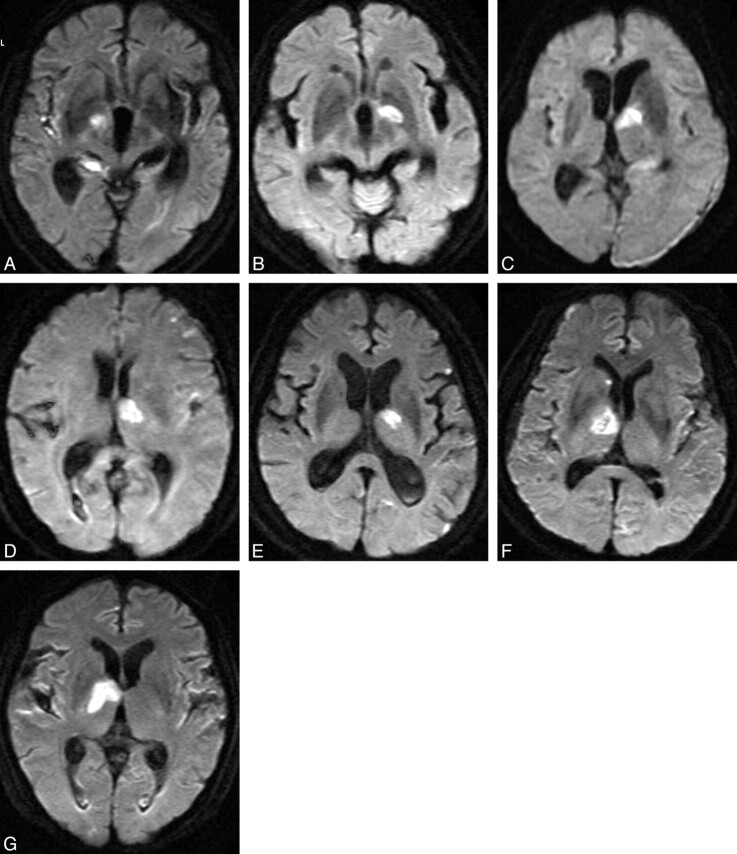
Fourteen patients (4 men and 10 women), ranging in age from 46–87 years (with a mean of 73 years), were treated with coil embolization of the aneurysms in combination with PcomA sacrifice. Elimination of the aneurysms was complete in all 14 cases. Seven patients developed cerebral infarctions postoperatively (infarction group, Fig 1; representative case, Fig 2), and the other 7 patients did not develop cerebral infarctions (noninfarction group, representative case, Fig 3). In the infarction group, cerebral infarctions appeared on postoperative DWI in the ventral part of the thalamus, ipsilateral to the treated aneurysm, which is supplied by the tuberothalamic artery originating from the PcomA (Fig 1). Five of 7 cases of the infarction group also showed small ischemic lesions in various ipsilateral cortical areas. None of the cases presented major cerebral infarctions at the cortical region of the PCA. The results of preoperative Allcock tests were positive in all 14 cases, which demonstrated apparent retrograde filling of the PcomA through the ipsilateral P1. The ipsilateral P1 segment was negative in the preoperative vertebral angiograms of all patients in the infarction group. In contrast, the ipsilateral P1 segment was positive in all patients of the noninfarction group. The balloon remodeling technique was employed for 3 of 7 patients in the infarction group, and for 4 of 7 patients in the noninfarction group. Postoperative MRA with gadolinium enhancement performed 7 days after the treatment indicated recanalization of the PcomA in 3 of 7 cases in the infarction group, and in all 7 cases in the noninfarction group. Rebleeding was not observed in any of the cases during the follow-up period.
Fig 1.
Diffusion-weighted imaging on the first day after surgery for 7 different patients of the infarction group, indicating tuberothalamic artery infarctions.
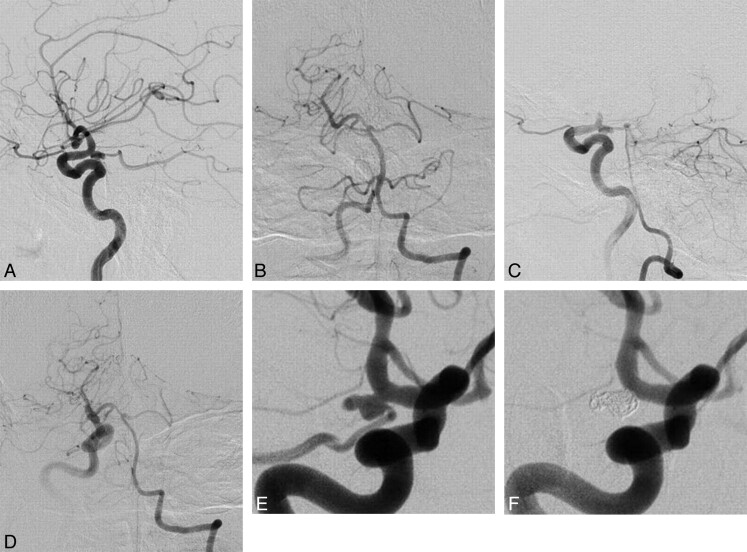
Fig 2.
A 69-year-old man without postoperative tuberothalamic infarction. A, Lateral view of the preoperative right internal carotid angiogram, indicating a posteriorly projecting right PcomA aneurysm with a maximum diameter of 6.9 mm. B, Anteroposterior view of the left vertebral angiography, demonstrating anterograde visualization of the right posterior cerebral artery (positive P1). C (lateral view) and D (anteroposterior view), The Allcock test revealed retrograde filling of the right PcomA and the aneurysm through the right P1. E, Preoperative right internal carotid angiography in a working projection depicted the right PcomA aneurysm with a PcomA arising from the aneurysmal sac. F, Right internal carotid angiography obtained immediately after coiling revealing the complete elimination of the aneurysm, along with the origin of the PcomA.
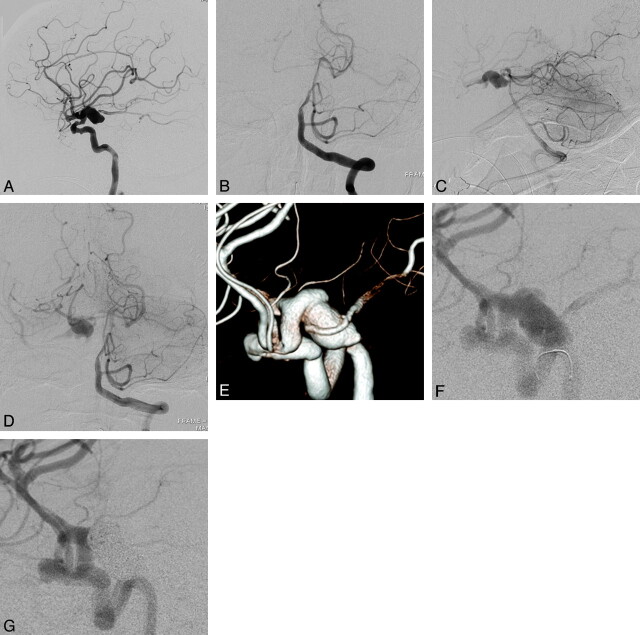
Fig 3.
A 70-year-old woman with postoperative tuberothalamic infarction. A, A lateral view of the preoperative right internal carotid angiogram, indicating posteriorly projecting large right PcomA aneurysm with a maximum diameter of 17.5 mm. B, An anteroposterior view of the preoperative left vertebral angiography, demonstrating a lack of visualization of the right posterior cerebral artery (negative P1). C (lateral view) and D (anteroposterior view), The Allcock test revealed retrograde filling of the right PcomA and the aneurysm through the right P1. E (3D angiography) and F (conventional angiography), Preoperative right internal carotid angiography in a working projection, depicting the right PcomA aneurysm with a PcomA arising from the aneurysmal sac. G, Right internal carotid angiography obtained immediately after coiling revealing the complete elimination of the aneurysm along with the origin of the PcomA.
A comparison of the factors associated with ischemic complication between the infarction group and the noninfarction group is depicted in the Table. The incidence of negative P1 visualization on the vertebral angiogram was significantly higher in the infarction group (7 of 7, 100%) than in the noninfarction group (0 of 7, 0%; P = .00058). The mean PcomA diameter in the infarction group (1.8 ± 0.4 mm) was significantly greater than that observed in the noninfarction group (1.2 ± 0.6 mm; P < .05). The mean PcomA/P1 ratio of the infarction group (1.6 ± 0.2) was significantly greater than that of the noninfarction group (0.7 ± 0.5; P < .01). The mean aneurysm size in the infarction group (11.6 ± 5.4 mm) was significantly greater than that observed in the noninfarction group (5.4 ± 1.0 mm; P < .02). The incidence of postoperative recanalization of the PcomA on MRA did not vary significantly between the infarction group (3 of 7, 43%) and the noninfarction group (7 of 7, 100%; P = .07).
Comparison of the factors
| Parameter | Infarction Group (n = 7) | Noninfarction Group (n = 7) | P |
|---|---|---|---|
| Negative P1 visualization | 7/7 | 0/7 | 0.00058a |
| PcomA diameter, mean ± SD (mm) | 1.8 ± 0.4 | 1.2 ± 0.6 | < 0.05b |
| PcomA/P1, mean ± SD | 1.6 ± 0.2 | 0.7 ± 0.5 | < 0.01b |
| Aneurysm size, mean ± SD (mm) | 11.6 ± 5.4 | 5.4 ± 1.0 | < 0.02b |
| Recanalization of PcomA | 3/7 | 7/7 | 0.07b |
Fisher exact test.
Mann-Whitney test.
Postoperative Course
Six of 7 patients in the infarction group postoperatively showed hemiparesis contralateral to the treated aneurysms. The degree of hemiparesis was moderate to severe on the first day after surgery and improved considerably within 1 month after the treatment. The motor performance of 1 case was not evaluated precisely because of the patient's moderate tetraparesis, which was caused by a previous stroke. Six of 7 patients in the infarction group manifested memory disturbances at 1 month after the treatment. The mRS scores after 6 months deteriorated in all 7 patients in the infarction group compared with the previous mRS. Instances of morbidity included remaining paresis, memory disturbances, and/or lack of spontaneity. One patient in the infarction group developed a left internal carotid artery occlusion caused by a cardiogenic cerebral embolism that resulted from atrial fibrillation on day 24 postsurgery, which resulted in massive brain edema. The brain edema was refractory to decompressive craniectomy, and the patient died 27 days after the treatment. Symptomatic vasospasm (transient aphasia) was observed in 1 case in the infarction group and did not result in permanent morbidity. A ventriculoperitoneal shunt for hydrocephalus was successfully deployed in 4 patients in the infarction group and in 1 patient in the noninfarction group. Four of 7 patients in the infarction group, and 1 of 7 patients in the noninfarction group, showed mRS scores of 3–6 at 6 months after the treatment. The proportion of patients with mRS scores of 3–6 at 6 months postsurgery did not differ significantly between the groups (P = .27).
Discussion
This study examined the preoperative angiographic features and the pattern of ischemic complications after the coil embolization of ruptured PcomA aneurysms in cases in which the PcomA was sacrificed during the procedure. Half of the patients developed tuberothalamic artery infarctions after the treatment, though collateral flow was observed with the preoperative Allcock test. The incidence of negative P1 visualization on the preoperative vertebral angiogram was significantly higher in the cases presenting postoperative tuberothalamic artery infarctions. The mean PcomA diameters, the PcomA/P1 ratios, and the sizes of the aneurysms were significantly higher in cases exhibiting postoperative tuberothalamic artery infarctions. Postoperative hemiparesis and memory disturbances caused by tuberothalamic artery infarctions affected the final outcome.
Ischemic complications associated with coil embolization of the aneurysms include embolism and hemodynamic ischemia with inadequate collateral reserves.10 Hemodynamic ischemia is possible mechanism for the ischemic complications associated with the perforator region in the present study. Ischemic lesions were homogeneously observed in the same perforator region arising from the PcomA. These perforators should be supplied by the retrograde blood flow from the vertebrobasilar system through the PcomA after the occlusion of the PcomA at its origin, which was confirmed by the preoperative Allcock test. However, actual blood flow to these perforators was considered insufficient, because hemodynamics under the Allcock test are not necessarily equal to physiologic condition. Insufficient collateral flow could induce flow stagnation and thrombus formation, which were also possible mechanisms for ischemic complications. Moreover, larger aneurysms are likely to have residual flow within the coil mass, which facilitates the thrombus formation and ischemic complications.10,11 In the present study, the mean aneurysm size in the infarction group was significantly greater than that observed in the noninfarction group. In contrast, recanalization of the PcomA could be associated with resolution of the flow stagnation and thrombus formation, resulting in avoidance of the ischemic complications, though the incidence of postoperative recanalization of the PcomA did not vary significantly between the 2 groups in our study.
From the results of this study, we propose the relationship between the preoperative angiographic features and the pattern of postoperative cerebral infarctions. A positive Allcock test and a positive P1 segment indicate a normal level of anatomic variation, which is not associated with postoperative infarctions. A negative P1 segment suggests the fetal variant PCA, which was roughly defined as a variant exhibiting primary perfusion of the PCA from the ICA and minimal perfusion of the PCA from the vertebrobasilar system, often with an absent or hypoplastic P1 segment.12 In these cases, the pattern of the postoperative infarctions depends on the degree of P1 patency, which can be measured with the Allcock test and vertebral angiography. A positive Allcock test with negative visualization of the P1 segment corresponds to the fetal variant of PCA with a hypoplastic P1 segment, which results in tuberothalamic artery infarctions without cortical lesions. The PcomA diameter and the PcomA/P1 ratio of patients in the infarction group were compatible with the anatomical features of the fetal variant PCA with a hypoplastic P1 segment. The exact mechanism by which the blood flow to the cortical region of the PCA was preferentially salvaged after the occlusion of the PcomA in the infarction group remains unknown. A negative Allcock test with negative visualization of the P1 segment corresponds to the fetal variant of PCA with an absent P1 segment, which might result in tuberothalamic artery infarctions with cortical lesions of the PCA territories, though it was not demonstrated in this study.
The number and the diameter of the perforating arteries are relatively constant, regardless of the size of the parent PcomA.13,14 Four to 12 branches (with an average of 7) with a diameter between 0.1 and 0.6 mm arise from the PcomA.13 The largest branch among these perforators has been called the tuberothalamic artery, which has also been referred to as the thalamotuberal artery, the premamillary artery, the polar artery, and the anterior thalamoperforating artery.15–17 The tuberothalamic artery commonly arises from the caudal part of the PcomA, which is close to the PCA, or from the border between the caudal and middle third of the PcomA.18 The tuberothalamic artery is considered to have an anatomically complementary relationship with the paramedian artery originating from the P1 segment.19 These anatomic features might be associated with the different infarct size associated with each case in the present study.
The tuberothalamic artery irrigates the ventral section of the thalamus, which includes the reticular nucleus, the ventral anterior nucleus, the rostral section of the ventrolateral nucleus, the ventral pole of the medial dorsal nucleus, the mamillothalamic tract, the ventral amygdalofugal pathway, the ventral section of the internal medullary lamina, and the anterior thalamic nuclei.20 A primary feature of tuberothalamic artery infarctions is the impairment of recent memory, which is more prominent with left-sided lesions.19 Amnestic syndrome is associated with a disconnection between the anterior thalamic nuclei and hippocampal formation, caused by the disruption of the mamillothalamic tract.21 In contrast, initial transient or mild contralateral hemiparesis is considered to be caused by the compression of the internal capsule by postischemic edema.19 The prognosis after tuberothalamic artery infarction is generally favorable in terms of a robust recovery from a motor deficit.20 However, cognitive deficits are more permanent and could potentially carry an unfavorable prognosis.22 In the present study, tuberothalamic artery infarctions were considered to exert an effect on the final outcome because vasospasm and chronic hydrocephalus did not affect the final outcome in the infarction group. Cognitive deficits arising from tuberothalamic artery infarctions decreased the activity of the patients, resulting in slower and more limited progress in rehabilitation. The contribution of the acute brain injury caused by SAH itself to the chronic cognitive deficits should be considered to be another factor contributing to an unfavorable outcome.23
Branch occlusion is justified only when sufficient collateral circulation is promised by the endovascular treatment of aneurysms with a branch arising from the sac.24 This study indicates that a positive Allcock test may not be a sufficient indicator of sufficient collateral flow after the PcomA sacrifice. The optimal treatment strategy should be chosen by comprehensively estimating the risks of the ischemic complications predicted by the preoperative 4-vessel angiography, including the Allcock test. Surgical clipping or coil embolization with a microcatheter protective technique are the optimal alternative treatment strategies to spare the PcomA, when sacrifice of the PcomA is considered risky.25
Conclusions
After the coil occlusion of ruptured PcomA aneurysms with PcomA sacrifice, tuberothalamic artery infarctions tend to occur in cases displaying negative visualization of the P1 segment through vertebral angiography, even when the collateral flow is observed with the Allcock test.
ABBREVIATIONS:
- HDS-R
Hasegawa Dementia Scale
- mRS
modified Rankin Scale
- PCA
posterior cerebral artery
- PcomA
posterior communicating artery
References
- 1. O'Shaughnessy BA, Getch CC, Bendok BR, et al. Surgical management of unruptured posterior carotid artery wall aneurysms. Neurosurg Focus 2003; 15: E9. [DOI] [PubMed] [Google Scholar]
- 2. Regli L, de Tribolet N. Tuberothalamic infarct after division of a hypoplastic posterior communicating artery for clipping of a basilar tip aneurysm: case report. Neurosurgery 1991; 28: 456– 59 [DOI] [PubMed] [Google Scholar]
- 3. Zada G, Breault J, Liu CY, et al. Internal carotid artery aneurysms occurring at the origin of fetal variant posterior cerebral arteries: surgical and endovascular experience. Neurosurgery 2008; 63: 55– 62 [DOI] [PubMed] [Google Scholar]
- 4. Krayenbühl N, Krisht AF. Dividing the posterior communicating artery in approaches to the interpeduncular fossa: technical aspects and safety. Neurosurgery 2007; 61: 392– 97 [DOI] [PubMed] [Google Scholar]
- 5. Umredkar A, Gupta SK, Khandelwal N, et al. Intracerebral infarcts following clipping of intracranial aneurysms: incidence, clinical correlation and outcome. Br J Neurosurg 2010; 24: 156– 62 [DOI] [PubMed] [Google Scholar]
- 6. Pelz DM, Viñuela F, Fox AJ, et al. Vertebrobasilar occlusion therapy of giant aneurysms. Significance of angiographic morphology of the posterior communicating arteries. J Neurosurg 1984; 60: 560– 66 [DOI] [PubMed] [Google Scholar]
- 7. Kusano Y, Seguchi T, Horiuchi T, et al. Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR Am J Neuroradiol 2009; 30: 1561– 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kato S, Shimogaki H, Onodera A, et al. Development of the revised version of Hasegawa's Demential Scale (HDS-R) [in Japanese]. Jpn J Geriatr Psychiatry 1991; 2: 1339– 47 [Google Scholar]
- 9. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604– 07 [DOI] [PubMed] [Google Scholar]
- 10. Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003; 24: 127– 32 [PMC free article] [PubMed] [Google Scholar]
- 11. Derdeyn CP, Cross DT, 3rd, Moran CJ, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg 2002; 96: 837– 43 [DOI] [PubMed] [Google Scholar]
- 12. van Raamt AF, Mali WP, van Laar PJ, et al. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis 2006; 22: 217– 24 [DOI] [PubMed] [Google Scholar]
- 13. Saeki N, Rhoton AL, Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg 1977; 46: 563– 78 [DOI] [PubMed] [Google Scholar]
- 14. Vincentelli F, Caruso G, Grisoli F, et al. Microsurgical anatomy of the cisternal course of the perforating branches of the posterior communicating artery. Neurosurgery 1990; 26: 824– 31 [DOI] [PubMed] [Google Scholar]
- 15. Foix C, Hillemand P. Les arteres de l'axe encéphalique jusqu' au diencéphale inclusivement. Rev Neurol (Part 2) 1925; 32: 705– 39 [Google Scholar]
- 16. Percheron G. The anatomy of the arterial supply of the human thalamus and its use for the interpretation of the thalamic vascular pathology. Z Neurol 1973; 205: 1– 13 [DOI] [PubMed] [Google Scholar]
- 17. Westberg G. Arteries of the basal ganglia. Acta Radiol Diagn (Stockh) 1966; 5: 581– 96 [DOI] [PubMed] [Google Scholar]
- 18. Gibo H, Mrinkovic, Brigante L. The microsurgical anatomy of the premamillary artery. J Clin Neurosci 2001; 8: 256– 60 [DOI] [PubMed] [Google Scholar]
- 19. Bogousslavsky J, Regli F, Assal G. The syndrome of unilateral tuberothalamic artery territory infarction. Stroke 1986; 17: 434– 41 [DOI] [PubMed] [Google Scholar]
- 20. Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003; 34: 2264– 78 [DOI] [PubMed] [Google Scholar]
- 21. von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain 1988; 108: 993– 1008 [DOI] [PubMed] [Google Scholar]
- 22. Kaurussis D, Leker RR, Abramsky O. Cognitive dysfunction following thalamic stroke: a study of 16 cases and review of the literature. J Neurol Sci 2000; 172: 25– 29 [DOI] [PubMed] [Google Scholar]
- 23. Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010; 41: e519– 36 [DOI] [PubMed] [Google Scholar]
- 24. Lubicz B, Lefranc F, Lecicier M, et al. Endovascular treatment of intracranial aneurysms with a branch arising from the sac. AJNR Am J Neuroradiol 2006; 27: 142– 47 [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JY, Seo JH, Cho YD, et al. Endovascular treatment of wide-neck intracranial aneurysms using a microcatheter protective technique: results and outcomes in 75 aneurysms. AJNR Am J Neuroradiol 2011; 32: 917– 22 [DOI] [PMC free article] [PubMed] [Google Scholar]