Summary:
Dabigatran (Pradaxa) is a member of the relatively new class of antithrombotic drugs known as direct thrombin inhibitors (DTIs). It may supplant warfarin (Coumadin) in a number of applications as it may produce a more predictable, potent, and immediate anticoagulant effect, with fewer significant side effects and interactions, and requires less monitoring.
Thrombin catalyzes the conversion of factors V, VIII, and XI to their activated forms and the conversion of fibrinogen to fibrin and factor XIII to XIIIa (Fig 1).
Fig 1.
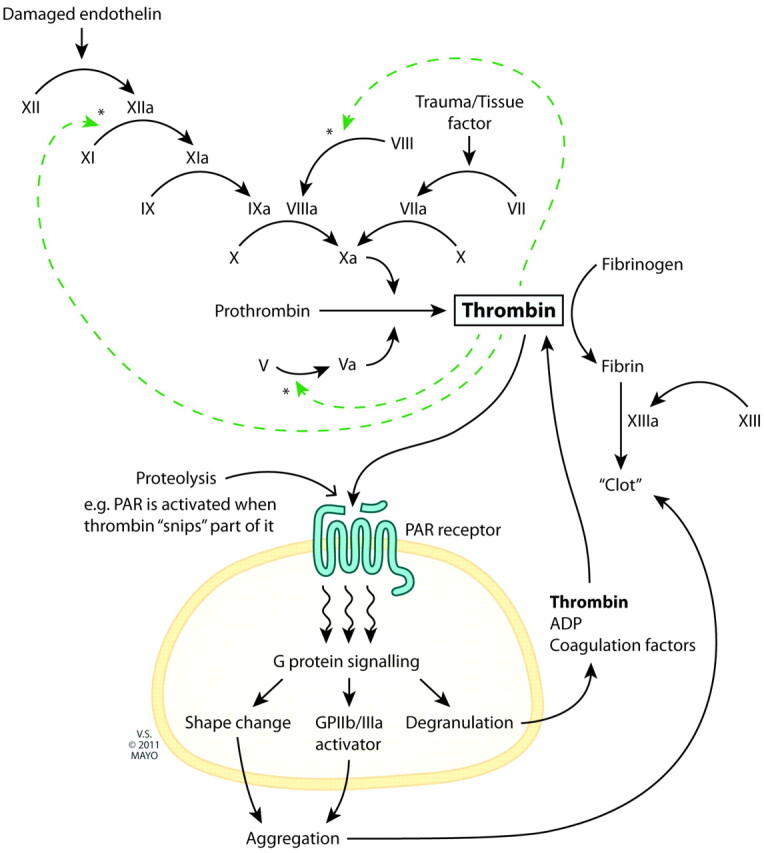
The central role of thrombin in clot formation.
Thrombin also activates transmembrane G-protein-coupled protease-activated receptors on the platelet cell membrane. Downstream signaling results in conformational change, in turn allowing platelet aggregation and release of more coagulation factors and generation of more thrombin (Fig 1). The multifactorial, central, and self-perpetuating role of thrombin in clot formation makes it an appealing theoretic target for the prevention of thrombosis.
Mechanism of Action
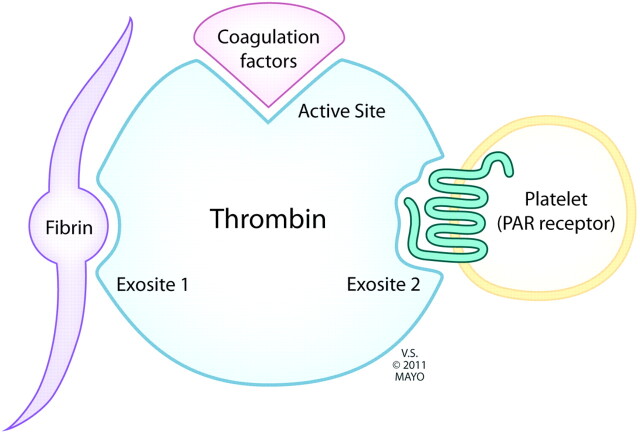
Dabigatran (Pradaxa) reversibly binds to the active site on the thrombin molecule, preventing thrombin-mediated activation of coagulation factors. It may have less of an antagonistic effect on thrombin-mediated platelet aggregation (see “Adverse Effects” section). Furthermore, Dabigatran can inactivate thrombin even when thrombin is fibrin-bound; it reduces thrombin-mediated inhibition of fibrinolysis and, therefore, may even enhance fibrinolysis (Fig 2).1
Fig 2.
Thrombin receptors. Dabigatran binds to the active site, independent of fibrin-binding, thereby reducing conversion of coagulation factors. Thrombin-mediated platelet aggregation is relatively less affected.
In contrast to direct thrombin inhibitors, warfarin (Coumadin, an “indirect” thrombin inhibitor) exerts its effect by reducing vitamin K–dependent synthesis of thrombin and of coagulation factors higher up the cascade. This results in a delayed onset of antithrombotic effect (already existent coagulation factors have half-lives of up to 3 days) and an initial prothrombotic effect (warfarin also inhibits synthesis of endogenous anticoagulants, which have far shorter half-lives); heparin (Lipo-Hepin and Liquaemin) usually has to be coadministered initially.
Clinical Indications
The indications for dabigatran are identical to those for warfarin: the prevention of arterial or venous thrombosis. Of interest to the neuroradiologist, the RE-LY trial compared dabigatran with warfarin in the setting of stroke prophylaxis for atrial fibrillation and showed it to be noninferior (at a dose of 110 mg twice daily) or superior (at 150 mg twice daily) in the prevention of ischemic stroke.2
Administration
Dabigatran etexilate is the bioavailable oral formulation and is converted by liver hydrolysis to the active form. It is not highly protein-bound in plasma, is not dependent on (highly genetically polymorphic) CYP P450 metabolism, and is formulated with tartaric acid to ensure reliable intestinal absorption independent of gastric pH. It, therefore, has generally predictable pharmacokinetics and dosing (in contrast to warfarin).
Time to maximal plasma concentration and maximal clinical effect is 2 hours or less.3 Plasma half-life is approximately 12 hours,4 and dosing is therefore twice daily. Clearance is predominantly (∼80%) renal,5 and the drug will accumulate in renal failure. Dosing modifications are straightforward given the linear metabolism.
Adverse Effects
As with all antithrombotic agents, the primary side effect is hemorrhage. The RE-LY trial demonstrated that dabigatran was associated with a lower incidence of major hemorrhage than warfarin; in particular, the incidence of hemorrhagic stroke was markedly reduced (P < .001).2
Furthermore, dabigatran has been shown to have no effect compared with a placebo on the volume of cerebral hematoma induced in mice; in comparison, mice pretreated with warfarin had significantly larger hematomas.6 This may be because dabigatran binds only to the active site on the thrombin molecule and not to the exosite involved in thrombin-mediated platelet aggregation. The relative preservation of this function with dabigatran compared with indirect thrombin inhibitors may explain the reduced incidence and volume of cerebral hematoma.7
RE-LY demonstrated dyspepsia as the most common reason for patients choosing to discontinue dabigatran.
Interactions
Dabigatran etexilate (but not its active metabolite) is a substrate for the pGP efflux transport system.8 There are numerous inhibitors (eg, ketoconazole, verapamil, amiodarone, quinidine) and inducers (eg, rifampin) of pGP. Many studies have been conducted demonstrating changes in cMax and area under the curve when these drugs are coadministered with dabigatran, but none have shown a significant effect on clotting function.9 There is not yet sufficient knowledge to allow the provision of strict guidelines for dose modification; these will undoubtedly emerge as experience with the drug increases.10
Measuring Response
Because of its predictable pharmacokinetics, dabigatran does not require routine close monitoring of clotting function, as warfarin does. If monitoring is required, prothrombin time/INR (a measure of extrinsic coagulation cascade) and aPTT (a measure of intrinsic coagulation cascade) are not quantitatively accurate for the effect of dabigatran on clotting function; these tests are instead replaced by TT and ECT.11
Reversal
In patients with normal renal function, dabigatran levels will fall to 25% of steady-state concentration after 24 hours and to 6.25% after 48 hours. Therefore, it may be reasonable to cease dabigatran 1 day before an elective procedure but earlier in higher-risk procedures (eg, spinal procedures, cardiac comorbidity, and so forth). The length of time the drug needs to be ceased obviously needs to be increased in patients with renal impairment, and aPTT/TT/ECT can be measured to ensure that they are within the normal range.
In the setting of bleeding complications or emergent surgery/procedures, no reversal agents have established efficacy in humans. Vitamin K and protamine are unlikely to have any significant effect. Hemodialysis/filtration is effective in rapidly reducing the concentration of the drug.
Economic Issues
Dabigatran twice daily costs approximately $13 per day,12 which far exceeds the cost of warfarin (probably <$1 per day). This does not, however, take into account the costs associated with INR monitoring; the overall Quality Adjusted Life Year costs are probably comparable.13
Summary
Dabigatran has a number of important advantages over warfarin and is likely to become increasingly frequently prescribed. Its potentially greater efficacy, simpler dosing, and better safety profile, particularly with regard to intracerebral hemorrhage, are all a direct result of its appealing pharmacokinetic and pharmacodynamic profiles. Its main comparative disadvantages relate to the monitoring of effect and reversal, and any proceduralist should be familiar with these.
Warfarin vs dabigatran comparison
| Onset | Side Effects | Monitoring | Interactions | Reversal | Cost | |
|---|---|---|---|---|---|---|
| Warfarin | Days | Osteoporosis | Intensive | Many | FFP | <$1/day |
| Necrosis | INR | Vit K | ||||
| Bleeding | ||||||
| Dabigatran | Hours | Dyspepsia | Rare | None | Dialysis | $13/day |
| Bleeding (less) | ECT, TT | rF VIIa, PCC |
Note:—FFP indicates fresh frozen plasma; Vit K, vitamin K; rF VIIa, recombinant Factor VIIa; PCC, prothrombin complex concentrate.
ABBREVIATIONS:
- aPTT
activated partial thromboplastin time
- ECT
Ecarin clotting time
- INR
international normalized ratio
- pGP
P-glycoprotein
- TT
thrombin clotting time
Footnotes
Disclosures: David F. Kallmes—UNRELATED: Grants/Grants Pending: MicroVention,* ev3*, Penumbra,* Micrus,* Payment for Lectures (including service on speakers bureaus): MicroVention,* Royalties: University of Virginia Patent Foundation; Payment for Development of Educational Presentations: ev3,* CareFusion,* Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention.* *Money paid to the institution.
References
- 1. Ammollo C, Semeraro F, Incampo F, et al. Dabigatran enhances clot susceptibility to fibrinolysis by mechanisms dependent on and independent of thrombin-activatable fibrinolysis inhibitor. J Thromb Haemost 2010; 8: 790– 98 [DOI] [PubMed] [Google Scholar]
- 2. Connolly S, Ezekowitz M, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139– 51 [DOI] [PubMed] [Google Scholar]
- 3. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64: 292– 303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stangier J, Stahle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008; 47: 47– 59 [DOI] [PubMed] [Google Scholar]
- 5. Stangier J, Rathgen K, Stahle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49: 259– 68 [DOI] [PubMed] [Google Scholar]
- 6. Lauer A, Cianchetti F, Van Cott E, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation 2011; 124: 1654– 62. Epub 2011 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celikel R, McClintock R, Roberts J, et al. Modulation of alpha-thrombin function by distinct interactions with platelet glycoprotein Ib alpha. Science 2003; 301: 218– 21 [DOI] [PubMed] [Google Scholar]
- 8. Eriksson B, Quinlan D, Weitz J. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 2009; 48: 1– 22 [DOI] [PubMed] [Google Scholar]
- 9. Liesenfeld K, Schafer H, Troconiz I, et al. Effects of the direct thrombin inhibitor dabigatran on ex vivo coagulation time in orthopedic surgery patients: a population model analysis. Br J Clin Pharmacol 2006; 62: 527– 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pradaxa [package insert]. Ridgefield, Connecticut: Boehringer Ingelheim Pharmaceuticals; 2003. [Google Scholar]
- 11. Van Ryn J, Stangier J, Naertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor—interpretation of coagulation assays and reversal of anticoagulant activity. Thrombo Haemost 2010; 103: 1116– 27 [DOI] [PubMed] [Google Scholar]
- 12. Flaker G, Weachter R. Pharmacologic strategies for the prevention of stroke in patients with atrial fibrillation. Curr Treat Options Cardiovasc Med 2011; 13: 361– 69 [DOI] [PubMed] [Google Scholar]
- 13. Freeman J, Zhu R, Owens D, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154: 1– 11. Epub 2010 Nov 1 [DOI] [PubMed] [Google Scholar]