Abstract
BACKGROUND AND PURPOSE:
Non-EPI DWI is a promising alternative to second-look surgery for the detection of residual and/or recurrent cholesteatoma. We evaluated the diagnostic accuracy, expressed as a positive predictive value, of MR imaging for the detection of residual and/or recurrent cholesteatoma in our hospital.
MATERIALS AND METHODS:
Fifty-six MR imaging studies were performed from 2005 to 2010 in patients having previously undergone surgery for cholesteatoma. Pre- and postgadolinium T1-weighted, T2-weighted, and non-EPI DWI sequences were performed and correlated with clinical and intraoperative findings. Twenty-seven patients underwent second-look surgery; 7 were under close clinical follow-up. Twenty-two patients without evidence of cholesteatoma were under regular follow-up (range, 14–44 months).
RESULTS:
Non-EPI DWI sequences showed increased DW signal intensity in 36 patients. Of those, 27 had second-look surgery, confirming cholesteatoma in 25 patients; in 1 patient, an empyema was diagnosed, and in the other patient, no cholesteatoma was found at surgery. In 2 patients who had not undergone surgery, increased DW signal intensity was accompanied by hyperintense signal intensity on T1-weighted images, consistent with transplanted fat in the postoperative cavity. The positive predictive value for detection of cholesteatoma was 93% (25/27).
CONCLUSIONS:
Residual and/or recurrent cholesteatomas after primary cholesteatoma surgery can be accurately detected by increased DW signal intensity on non-EPI DWI. However, DWI without conventional sequences increased the risk of misdiagnosis in our patient setting because transplanted fat within the postoperative cavity may show increased DW signal intensity.
Cholesteatoma is a collection of keratinous debris lined by stratified squamous epithelium trapped in the middle ear.1,2 If the cholesteatoma becomes large enough, it will displace and eventually destroy the ossicles and erode the wall of the middle ear cavity (typically the lateral wall and the scutum). In children, even a small cholesteatoma can cause ossicular chain erosions and, consequently, conductive hearing loss. Other complications of cholesteatoma include vertigo, cranial nerve palsies, and intracranial infection.3,4 Cholesteatomas that are not surgically removed usually continue to enlarge and can cause gross osseous destruction or become secondarily infected.
The surgical treatment of cholesteatoma consists of complete excision of the lesion with tympanoplasty. In case of mastoid involvement, a mastoidectomy is performed. Different surgical techniques are implemented to perform cholesteatoma surgery, depending on the location and extension of the cholesteatoma. The canal-wall-up procedure consists of a mastoidectomy combined with a posterior tympanoplasty (CAT); the posterior meatal wall is preserved. For the canal-wall-down approach, a modified radical mastoidectomy is performed with resection of the posterior meatal wall. In addition to general risks of surgery (bleeding, infection), complications of middle ear surgery include further hearing loss, facial nerve palsy, and CSF leak.5–8
Recurrence rates for cholesteatomas after primary cholesteatoma surgery vary widely, depending upon the method of surgery and the duration of follow-up. Canal-wall-up mastoidectomy is generally accompanied by a high rate of recurrent disease; some studies report a 5-year recurrence rate as high as 57%.7–9 The more invasive canal-wall-down approach is reported to have lower recurrence rates.5 If recurrence is suspected clinically, on the basis of otoscopy and audiometry, until now second-look surgery was indicated.
Because of the invasive character of second-look surgery and the risk of complications, the need for less invasive methods for diagnosing residual and/or recurrent cholesteatoma is needed. A cholesteatoma has specific signal-intensity characteristics on MR imaging. The lesion causes high signal intensity on T2-weighted images, low signal intensity on unenhanced and postcontrast T1-weighted images with a thin rim enhancement on the late gadolinium-enhanced images, and a very high signal intensity on non-EPI DWI.
In previous studies, delayed contrast-enhanced MR imaging could discriminate between the nonenhancing cholesteatoma and other postoperative findings, which all will show enhancement, such as mucosal edema, inflammation, and scar or granulation tissue.10–13
At the present time, non-EPI DWI is becoming an alternative to invasive second-look surgery. Recent studies showed that residual and/or recurrent cholesteatomas after primary cholesteatoma surgery are very accurately detected by increased DW signal intensity on non-EPI DWI.14–18 Middle ear cholesteatomas as small as 2 mm can be detected by using this technique.14,16 The non-EPI DWI sequence is particularly important in diagnosing cholesteatomas because the use of this technique is reported to hardly ever produce false-positive results.14–18 The signal intensity of postoperative findings other than cholesteatoma is demonstrated to be much lower than that of residual and/or recurrent cholesteatoma on non-EPI DWI.14,15,17 Because using increased DW signal intensity in non-EPI DWI sequences as a diagnostic criterion for cholesteatoma produces practically no false-positive results, non-EPI DWI is a very promising technique to be used in screening for residual and/or recurrent cholesteatoma.14–21 Recent reports even discussed the use of a non-EPI DWI sequence as the only sequence in screening for pre-second-look residual and/or recurrent cholesteatomas.14–16,19,20
In 2005, we introduced MR imaging with non-EPI DWI sequences for the detection of residual and/or recurrent cholesteatoma after primary cholesteatoma surgery. The aim of this study was to evaluate the diagnostic accuracy, expressed as a positive predictive value, of non-EPI DWI in our hospital as an alternative for second-look surgery in detecting residual and/or recurrent cholesteatoma. Furthermore, we investigated whether the use of only a non-EPI DWI sequence without additional conventional sequences is accurate enough in screening for residual and/or recurrent cholesteatoma.
Materials and Methods
Research Design
A retrospective cohort study was conducted at Maastricht University Medical Centre.
In 2005, the non-EPI DWI sequence was introduced in our hospital for screening for residual and/or recurrent cholesteatoma in the postoperative patient. Data were obtained from a data base of all MR imaging examinations of the temporal bone, which included non-EPI DWI sequences performed from 2005 to 2010. The indications of these examinations were reviewed for screening for cholesteatoma, and only the imaging studies of patients with a history of primary cholesteatoma surgery were included in the analysis.
As part of the regular follow-up or in case of clinical and/or otoscopic findings suggestive of residual and/or recurrent disease, all patients had MR imaging evaluation before undergoing probable second-look surgery.
The decision as to whether to perform second-look surgery was made by a specialized ENT surgeon. In adults, non-EPI DWI served as an alternative for second-look surgery in our hospital; in cases of negative non-EPI DWI findings, no second-look surgery was performed and those patients are under close clinical and MR imaging follow-up. Because until 2005 in our hospital, we did not have any experience with the non-EPI DWI sequence for screening for residual and/or recurrent cholesteatoma, the ENT surgeons decided that children who were suspected of having residual and/or recurrent cholesteatoma after primary surgery would always undergo second-look surgery, irrespective of the results of routine non-EPI DWI.
Cholesteatoma surgery was performed by 2 experienced ENT surgeons. Positive imaging findings were correlated with intraoperative findings in the patients undergoing second-look surgery, to confirm the diagnosis of residual and/or recurrent cholesteatoma.
Patients
The study group consisted of 56 consecutive non-EPI DWI studies in 51 patients in the period from 2005 to 2010; 26 males and 25 females with a mean age of 43 years (ranging from 9 to 85 years). Five children were included in the study group. All patients had previously undergone cholesteatoma surgery; primary cholesteatoma surgery was performed by the canal-wall-up technique in 34 patients, and 17 patients underwent the more invasive canal-wall-down procedure.
Patients underwent regular clinical follow-up by otoscopy, audiometry, and imaging studies once a year. Only patients suspected of cholesteatoma clinically and on imaging or children underwent second-look surgery. Patients with positive clinical and imaging findings for residual and/or recurrent cholesteatoma along with important comorbidity, which should be treated first before undergoing second-look cholesteatoma surgery, or patients with stable clinical, otoscopic, and audiometric findings are under close clinical follow-up, which is individually tapered.
Imaging Technique
MR imaging was performed with a 1.5T MR imaging scanner (Intera; Philips Healthcare, Best, the Netherlands). A 6-channel sensitivity encoding head coil was used. The imaging protocol consisted of a 3-mm-thick multishot turbo spin-echo DWI sequence in the transverse plane (TR, 2500 ms; TE, 80 ms; matrix, 128 × 128; FOV, 200 × 150 mm; b factors of 0 and 800 s/mm2; 14 sections; acquisition time, 2 minutes 12 seconds) and a 3-mm-thick multi-turbo spin-echo DWI sequence in the coronal plane (TR, 2500 ms; TE, 80 ms; matrix, 128 × 128; FOV, 200 × 150 mm; b factors of 0 and 800 s/mm2; 9 sections; cardiac-gated; mean acquisition time, 4 minutes 30 seconds). ADC cartography was systematically performed. Transverse 3-mm-thick spin-echo T1-weighted sequences (TR, 550 ms; TE, 15 ms; matrix, 256 × 256; FOV, 180 × 180 mm; 20 sections; acquisition time, 5 minutes 33 seconds) were performed. Transverse 3-mm-thick turbo spin-echo T2-weighted sequences (TR, 5000 ms; TE, 120 ms; matrix, 320 × 320; FOV, 180 × 180 mm; 20 sections; acquisition time, 5 minutes 9 seconds) and coronal 3-mm-thick turbo spin-echo T2-weighted sequences (TR, 5000 ms; TE, 120 ms; matrix, 320 × 320; FOV, 180 × 180 mm; 20 sections; acquisition time, 5 minutes 23 seconds) were also obtained. In all patients postgadolinium T1-weighted images were acquired in the axial plane (1 mm) 5 minutes after intravenous contrast injection of 0.1 mmol/kg of body weight of gadobutrol (Gadovist; Bayer-Schering, Berlin, Germany). No delayed enhancement was performed.
Imaging Evaluation
The MR images were evaluated by 2 head and neck radiologists. The diagnoses made by the radiologists in the original reports were used in this study. No reassessment of the included imaging studies was performed.
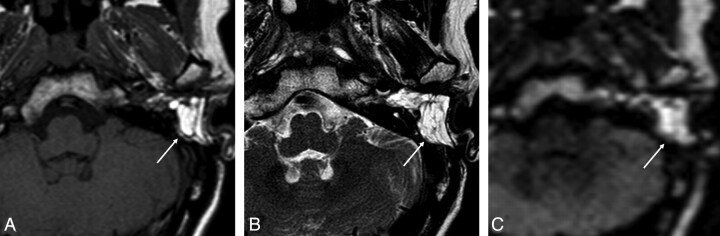
The diagnosis of cholesteatoma on imaging studies was based on the presence of increased DW signal intensity on non-EPI DWI without evidence of other pathology on the conventional MR imaging sequences (Fig 1). Furthermore, on the MR imaging studies showing hyperintense signal intensity on the non-EPI DWI sequence, we retrospectively measured the corresponding ADC values in a selected region of interest.
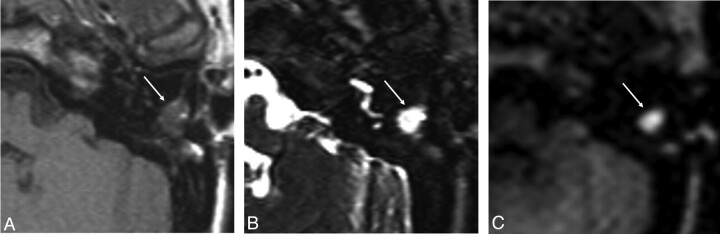
Fig 1.
MR images of recurrent cholesteatoma in the left middle ear. Transverse spin-echo T1-weighted image (A) shows a lesion of low signal intensity, which is shown as a hyperintense lesion on the transverse turbo spin-echo T2-weighted image (B). C, At this location, increased DW signal intensity is shown on the transverse non-EPI DWI sequence, indicative of cholesteatoma.
At the time we started performing non-EPI DWI for detection of residual and/or recurrent cholesteatoma in our hospital in 2005, the head and neck radiologists did not have any experience in evaluating this specific sequence for the presence of residual and/or recurrent cholesteatoma after primary cholesteatoma surgery. The results of the interpretation of these very first imaging studies, the learning curve, were also included in the analysis.
Statistical Analysis
The diagnostic accuracy of the detection of residual and/or recurrent cholesteatoma after primary cholesteatoma surgery by non-EPI DWI with and without additional conventional MR imaging sequences was described by means of positive predictive values.
Results
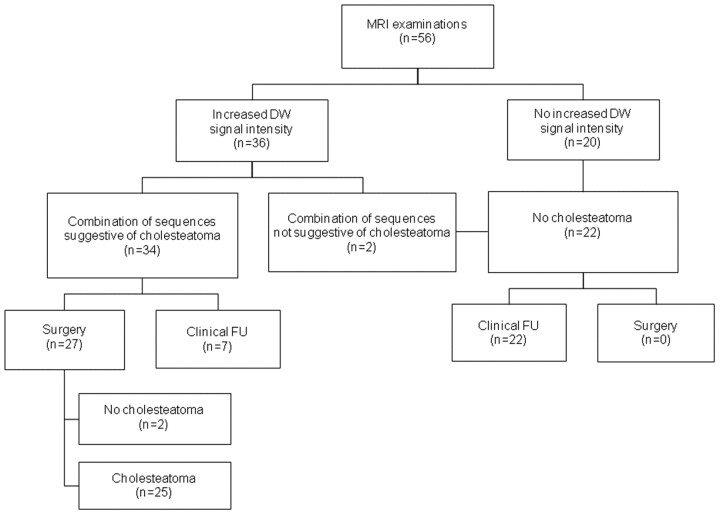
Figure 2 gives a schematic overview of the results of our patient group. A total of 56 MR imaging examinations was included in our study. The non-EPI DWI sequence showed increased DW signal intensity in 36 cases. The mean calculated ADC value was 1.041 × 10−3 mm2/s (range: 1.368–0.743 × 10−3 mm2/s).
Fig 2.
Schematic overview of the results of our patient group.
In 27 of the cases showing increased DW signal intensity, second-look surgery was performed. Two patients who had not undergone surgery with increased DW signal intensity on the non-EPI DWI sequences did not show evidence of cholesteatoma on the conventional T1- and T2-weighted images, as will be discussed later in this section. Seven patients with increased DW signal intensity on non-EPI DWI studies were under close clinical and MR imaging follow-up because of comorbidity or stable clinical, otoscopic, and audiometric findings.
Non-EPI DWI sequences detected and located 25 cholesteatomas, confirmed by second-look surgery. In 2 patients, MR imaging findings were shown to be false-positive after correlation with the intraoperative second-look surgery findings. One patient was diagnosed with an empyema in the postoperative cavity (which was suggested in the differential diagnosis of the MR imaging report). The corresponding calculated ADC value in this case was 0.932 × 10−3 mm2/s; therefore, the empyema could not definitely be differentiated from a cholesteatoma by imaging findings alone (Fig 3).
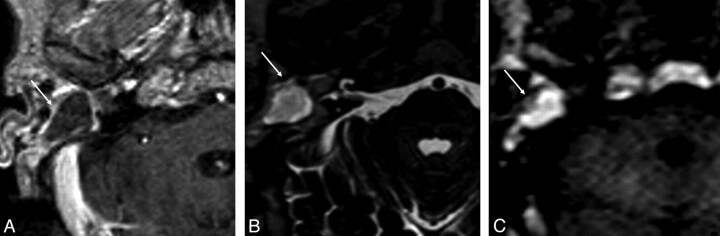
Fig 3.
MR images of an empyema in the postoperative cavity of the right middle ear. A, On the transverse postgadolinium spin-echo T1-weighted image, a lesion with low signal intensity surrounded by a rim of enhancing tissue is shown. B, At this location, a high signal intensity lesion is shown on the axial turbo spin-echo T2-weighted image. C, Transverse non-EPI DWI sequence shows the increased DW signal intensity of the lesion. The calculated ADC value of the lesion (0.932 × 10−3 mm2/s) was not typical for an empyema. Imaging findings alone cannot definitely differentiate an empyema from a cholesteatoma.
The second patient had increased DW signal intensity located very anteriorly in the middle ear cavity on MR imaging (Fig 4); no evidence of cholesteatoma was detected at second-look surgery in this surgically unclear area. This patient is currently under close clinical and imaging follow-up.
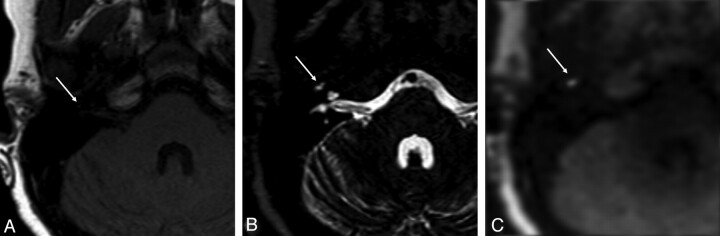
Fig 4.
MR images of a lesion suspicious for residual and/or recurrent cholesteatoma in the right middle ear. Transverse spin-echo T1-weighted image (A) shows a very small hypointensity located very anteriorly in the middle ear cavity, which can be correlated with small hyperintense signal intensity on the transverse turbo spin-echo T2-weighted image (B). C, At this location, increased DW signal intensity is shown on the transverse non-EPI DWI sequence suspicious for residual and/or recurrent cholesteatoma located very anteriorly in the middle ear cavity.
As described in the beginning of this section, in the subgroup of patients with increased DW signal intensity on non-EPI DWI sequences (36 patients), 2 patients who had not undergone surgery showed suggestion of cholesteatoma on the basis of non-EPI DWI alone. After interpretation of the DWI in combination with the conventional T1-weighted images, hyperintense signal intensity on the conventional T1-weighted images was found, which could be correlated to the location of hyperintense signal intensity on the DWI (Fig 5). These imaging findings were suggestive of fat in the mastoidectomy cavity; at primary cholesteatoma surgery, fat of the abdominal wall had been placed in the cavity. No fat-saturation images were included in the standard imaging protocol.
Fig 5.
MR images of transplanted fat in the mastoidectomy cavity of the left middle ear. On the transverse spin-echo T1-weighted image (A) and on the transverse turbo spin-echo T2-weighted image (B), a lesion with high signal intensity and multiple strands is shown. C, This could be correlated to the location of hyperintense signal intensity on the transverse non-EPI DWI sequence. The high signal intensity on the DWI was caused by the use of fat from the abdominal wall placed in the mastoidectomy cavity during surgery.
Twenty patients did not show hyperintense signal intensity on the non-EPI DWI sequences. Clinical follow-up (ranging from 14 to 44 months, with a mean follow-up period of 26 months), including otoscopy and audiometry, was without suspicion of residual and/or recurrent cholesteatoma. Findings in all of these patients were no residual and/or recurrent disease after primary cholesteatoma surgery.
The positive predictive value of non-EPI DWI sequences in depicting pathologically confirmed residual and/or recurrent cholesteatomas was 93% (25/27).
Discussion
After primary cholesteatoma surgery by means of the canal-wall-up technique or the canal-wall-down approach as performed in our hospital, there is a risk of leaving some diseased tissue behind at first-stage surgery (residual cholesteatoma) or a new cholesteatoma can develop after surgery (recurrent cholesteatoma). Therefore, these techniques, especially the canal-wall-up technique, often require second-look surgery. In the past, all patients had to undergo routine second-look surgery several months after their primary cholesteatoma surgery to rule out residual and/or recurrent disease.
The use of non-EPI DWI has been shown to be a promising alternative for invasive second-look surgery in screening for residual and/or recurrent cholesteatoma in previous studies, with very high sensitivity and specificity and high positive and negative predictive values as reported by De Foer et al14,16,19 and Vercruysse et al.18
There are hardly any false-positive findings reported in the literature by using non-EPI DWI sequences in screening for residual and/or recurrent disease and only sparse false-negative findings. False-negative findings are almost exclusively a consequence of examinations degraded by motion artifacts or empty retraction pockets. Non-EPI DWI can detect residual and/or recurrent cholesteatomas with a size limit as small as 2 mm, because of a high imaging matrix, thin section thickness, and a complete lack of susceptibility artifacts.14,16,21
The findings of our study concerning the predictive value of current MR imaging techniques for detecting residual and/or recurrent cholesteatoma are in agreement with recent literature; we reported a positive predictive value for the non-EPI DWI sequence of 93% in our study population. Because the learning curve of the 2 head and neck radiologists was incorporated in the final outcome of our study, the results of this study can be reliably translated to daily practice.
The sensitivity and specificity of non-EPI DWI sequences combined with conventional sequences in depicting residual and/or recurrent cholesteatomas was respectively 100% and 92%. This is also in agreement with recent literature.14–21
There are some limitations to our study. First, the study included only a small number of 56 MR imaging studies. Second, the study design was retrospective. Third, the high sensitivity reported in our study is, to some extent, a consequence of the fact that our hospital patients who did not show increased DW signal intensity on pre-second-look non-EPI DWI studies did not undergo second-look surgery. Therefore, in 22 patients with negative findings on non-DWI, we were not able to correlate the radiologic findings with intraoperative findings to confirm the absence of residual and/or recurrent cholesteatoma. In our study, an empty retraction pocket on imaging was considered as a true-negative. This is in agreement with very recently published studies regarding the use of non-EPI DWI in relation to second-look cholesteatoma surgery.19,25 Finally, we used the reported diagnoses based on the initial assessment by the head and neck radiologists in the analysis of our study. No reassessment of the included imaging studies took place. Although this can be considered as a limitation of the study design, it also implies that the results of our study are adaptable to everyday practice.
Recent studies investigated the possibility of screening for residual and/or recurrent cholesteatoma by use of the non-EPI DWI sequence as the only sequence in the imaging protocol.14–16,19,20 The DWI sequence needs very little scanning time, and intravenous contrast injection of gadolinium can be avoided by use of a DWI-only sequence. Furthermore, the correct interpretation of non-EPI DWI is not particularly dependent on the observer's experience. The results of these studies were positive regarding the use of non-EPI DWI alone in screening for residual and/or recurrent cholesteatoma.
A very interesting finding of our study was that, in contrast to the literature, we encountered 4 patients who showed increased DW signal intensity on non-EPI DWI. Two of these patients underwent surgery and showed no evidence of cholesteatoma at second-look surgery. One patient who showed increased DW signal intensity on imaging turned out to have an empyema in the postoperative cavity. This was suggested in the differential diagnosis by the use of conventional MR imaging sequences in combination with non-EPI DWI. Recent studies reported that calculating the ADC value of a middle ear lesion can allow greater specificity to differentiate cholesteatoma from empyema.22,23 The increased signal intensity of an empyema on non-EPI DWI is primarily caused by restricted diffusion and results less from T2 shine through effect.23 However, the calculated ADC value of the lesion of the above-mentioned patient (0.932 × 10−3 mm2/s) was not typical for an empyema (reported mean ADC value of 0.650 × 10−3 mm2/s)23; therefore, in this case, the precision of the diagnosis could not be improved by measuring the ADC value of the lesion. The second patient with hyperintense signal intensity on DWI showed this increased DW signal intensity located very anteriorly in the middle ear cavity. No evidence of cholesteatoma could be found at second-look surgery. Because the increased DW signal intensity in this patient was located in an area very difficult to explore by surgery, there is a chance that a possible cholesteatoma in this region was overlooked during second-look surgery. Therefore, the patient is under a close individual follow-up regimen.
In the 2 patients who did not undergo surgery, the high signal intensity on the DWI was caused by the use of fat from the abdominal wall placed in the mastoidectomy cavity during surgery. Without the availability of conventional MR imaging sequences, we would have misdiagnosed these patients as having a large residual and/or recurrent cholesteatoma. On T1-weighted images, theoretically, a residual and/or recurrent cholesteatoma in transplanted fat would be seen as a hypointense lesion within the hyperintense zone. Confirmation of the presence of fat can be obtained with the use of fat-saturation images. Cholesterol granuloma typically shows high signal intensity on T1- and T2-weighted images, similar to that in the 2 cases with fat transplant after primary cholesteatoma surgery. On DWI, cholesterol granuloma usually does not show diffusion restriction, though Kösling and Bootz24 published 1 case of cholesterol granuloma with diffusion restriction on DWI. The high signal intensity of cholesterol granuloma on T1-weighted images is an overall homogeneous high signal intensity (cholesterol component) possibly in combination with a low signal intensity rim (hemosiderin). The predominantly homogeneous high signal intensity of a cholesterol granuloma on T1-weighted images is distinct from the hyperintense signal intensity caused by fat transplant on T1-weighted images because in case of a fat transplant, the strands in the fat are visualized on the T1-weighted images (no homogeneous high signal intensity).
If we take the above-mentioned considerations into account, the positive predictive value of non-EPI DWI in combination with conventional imaging can reach 100% for detecting residual and/or recurrent cholesteatoma. In contrast to the current literature, in our patients, setting the use of non-EPI DWI as the only sequence would increase the number of misdiagnoses due to transplanted fat in the cavity.
Recently, De Foer et al25 retrospectively investigated the diagnostic accuracy of non-EPI DWI combined with delayed postgadolinium T1-weighted imaging compared with the diagnostic accuracy of non-EPI DWI alone. No significance difference was found in comparing these 2 imaging protocols. The non-EPI DWI sequences were proved to have significantly higher sensitivity, specificity, positive predictive value, and negative predictive value than delayed postgadolinium T1-weighted imaging sequences, and the results were less dependent on the observer's experience.25 At this time, we have adjusted the imaging protocol for the screening of residual and/or recurrent cholesteatoma in our hospital; we excluded the routine administration of gadolinium. As a consequence, the imaging time and the cost of the imaging protocol are reduced.
Conclusions
Residual and/or recurrent cholesteatomas after primary cholesteatoma surgery are very accurately detected by increased DW signal intensity on non-EPI DWI. This technique can be safely used in screening for residual and/or recurrent cholesteatoma as an alternative for invasive second-look surgery and also in a daily practice routine where the learning curve is taken into account.
In contrast to the literature, in which false-positive non-EPI DWI studies are hardly ever described, we encountered 4 patients with increased DW signal intensity on imaging. Non-EPI DWI without conventional MR imaging sequences will increase the risk of misdiagnosis in our patient setting. Therefore, non-EPI DWI should be completed with conventional T1- and T2-weighted sequences.
ABBREVIATIONS:
- CAT
combined-approached tympanoplasty
- DW
diffusion-weighted
- ENT
ear, nose, and throat
Footnotes
Paper previously presented in part at: Annual Meeting of the European Congress of Radiology, March 3–7, 2011; Vienna, Austria.
References
- 1. Semaan MT, Megerian CA. The pathophysiology of cholesteatoma. Otolaryngol Clin North Am 2006; 39: 1143– 59 [DOI] [PubMed] [Google Scholar]
- 2. Persaud R, Hajioff D, Trinidade A, et al. Evidence-based review of aetiopathogenic theories of congenital and acquired cholesteatoma. J Laryngol Otol 2007; 121: 1013– 19. Epub 2007 Aug 15 [DOI] [PubMed] [Google Scholar]
- 3. Mustafa A, Heta A, Kastrati B, et al. Complications of chronic otitis media with cholesteatoma during a 10-year period in Kosovo. Eur Arch Otorhinolaryngol 2008; 265: 1477– 82 [DOI] [PubMed] [Google Scholar]
- 4. Smith A, Danner CJ. Complications of chronic otitis media and cholesteatoma. Otolaryngol Clin North Am 2006; 39: 1237– 55 [DOI] [PubMed] [Google Scholar]
- 5. Ajalloueyan M. Experience with surgical management of cholesteatomas. Arch Otolaryngol Head Neck Surg 2006; 132: 931– 33 [DOI] [PubMed] [Google Scholar]
- 6. Chadha NK, Jardine A, Owens D, et al. A multivariate analysis of the factors predicting hearing outcome after surgery for cholesteatoma in children. J Laryngol Otol 2006; 120: 908– 13 [DOI] [PubMed] [Google Scholar]
- 7. Stangerup SE, Drozdziewics D, Tos M, et al. Surgery for acquired cholesteatoma in children: long-term results and recurrence of cholesteatoma. J Laryngol Otol 1998; 112: 742– 49 [DOI] [PubMed] [Google Scholar]
- 8. Sie KC. Cholesteatoma in children. Pediatr Clin North Am 1996; 43: 1245– 52 [DOI] [PubMed] [Google Scholar]
- 9. Roger G, Denoyelle F, Chauvin P, et al. Predictive risk factors of residual cholesteatoma in children: a study of 256 cases. Am J Otol 1997; 18: 550– 58 [PubMed] [Google Scholar]
- 10. Lemmerling MM, De Foer B, VandeVyver V, et al. Imaging of the opacified middle ear. Eur J Radiol 2008; 66: 363– 71. Epub 2008 Mar 12 [DOI] [PubMed] [Google Scholar]
- 11. Williams MT, Ayache D, Alberti C, et al. Detection of postoperative residual cholesteatoma with delayed contrast-enhanced MR imaging: initial findings. Eur Radiol 2003; 13: 169– 74 [DOI] [PubMed] [Google Scholar]
- 12. Pisaneschi MJ, Langer B. Congenital cholesteatoma and cholesterol granuloma of the temporal bone: role of magnetic resonance imaging. Top Magn Reson Imaging 2000; 11: 87– 97 [DOI] [PubMed] [Google Scholar]
- 13. Martin N, Sterkers O, Nahum H. Chronic inflammatory disease of the middle ear cavities: Gd-DTPA-enhanced MR imaging. Radiology 1999; 212: 333– 39 [DOI] [PubMed] [Google Scholar]
- 14. De Foer B, Vercruysse JP, Bernaerts A, et al. Detection of postoperative residual cholesteatoma with non-echo-planar diffusion-weighted magnetic resonance imaging. Otol Neurotol 2008; 29: 513– 17 [DOI] [PubMed] [Google Scholar]
- 15. Dhepnorrarat RC, Wood B, Rajan GP. Postoperative non-echo-planar diffusion-weighted magnetic resonance imaging changes after cholesteatoma surgery: implications for cholesteatoma screening. Otol Neurotol 2008; 30: 54– 58 [DOI] [PubMed] [Google Scholar]
- 16. De Foer B, Vercruysse JP, Bernaerts A, et al. The value of single-shot turbo spin-echo diffusion-weighted MR imaging in the detection of middle ear cholesteatoma. Neuroradiology 2007; 49: 841– 48 [DOI] [PubMed] [Google Scholar]
- 17. Dubrulle F, Souillard R, Chechin D, et al. Diffusion-weighted MR imaging sequence in the detection of postoperative recurrent cholesteatoma. Radiology 2006; 238: 604– 10 [DOI] [PubMed] [Google Scholar]
- 18. Vercruysse JP, De Foer B, Pouillon M, et al. The value of diffusion-weighted MR imaging in the diagnosis of primary acquired and residual cholesteatoma: a surgical verified study of 100 patients. Eur Radiol 2006; 16: 1461– 67 [DOI] [PubMed] [Google Scholar]
- 19. De Foer B, Vercruysse JP, Spaepen M, et al. Diffusion-weighted magnetic resonance imaging of the temporal bone. Neuroradiology 2010; 52: 785– 807 [DOI] [PubMed] [Google Scholar]
- 20. Rajan GP, Ambett R, Wun L, et al. Preliminary outcomes of cholesteatoma screening in children using non-echo-planar diffusion-weighted magnetic resonance imaging. Int J Pediatr Otorhinolaryngol 2010; 74: 297– 301 [DOI] [PubMed] [Google Scholar]
- 21. Schwartz KM, Lane JI, Bolster BD, et al. The utility of diffusion-weighted imaging for cholesteatoma evaluation. AJNR Am J Neuroradiol 2011; 32: 430– 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Annet L, Duprez T, Grandin C, et al. Apparent diffusion coefficient measurements within intracranial epidermoid cysts in six patients. Neuroradiology 2002; 44: 326– 28 [DOI] [PubMed] [Google Scholar]
- 23. Thiriat S, Riehm S, Kremer S, et al. Apparent diffusion coefficient values of middle ear cholesteatoma differ from abscess and cholesteatoma admixed infection. AJNR Am J Neuroradiol 2009; 30: 1123– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kösling S, Bootz F. CT and MR imaging after middle ear surgery. Eur J Radiol 2001; 40: 113– 18 [DOI] [PubMed] [Google Scholar]
- 25. De Foer B, Vercruysse JP, Bernaerts A, et al. Middle ear cholesteatoma: non-echo-planar diffusion-weighted MR imaging versus delayed gadolinium-enhanced T1-weighted MR imaging—value in detection. Radiology 2010; 255: 866– 72 [DOI] [PubMed] [Google Scholar]