Abstract
BACKGROUND AND PURPOSE:
Parotid gland BCA is a rare benign tumor. Only a few studies describing the imaging features of BCA have been published. This study investigated CT and sonography characteristics of BCA of the parotid gland.
MATERIALS AND METHODS:
Demographics of patients with BCA were evaluated, and lesion characteristics of CT (n = 22) and sonography (n = 20) were reviewed. These cases were grouped into 3 types: type 1 tumors, located at the superficial region of superficial lobe of the parotid gland; type 2 tumors, located at the deeper region of superficial lobe; and type 3 tumors, located in the deep lobe. Imaging findings were correlated with pathology.
RESULTS:
Sixteen patients (73%) were female and 6 (27%) were male. The mean age was 51.5 years (SD 10.2; range 32–73). The size of the tumors was less than 30 mm. The sizes of type 1, type 2, and type 3 tumors were 11.4 ± 3.29 mm, 19.3 ± 5.44 mm, and 26 ± 3.6 mm, respectively. The CT attenuation increase was 64.5 ± 19 HU on contrast CT. The type 1 tumors were solid (11/11), showed homogeneous or slightly heterogeneous enhancement on CT, and were homogeneously or slightly heterogeneously hypoechoic on sonography. Cystic changes tended to occur in type 2 (7/8) or type 3 (2/3) tumors, which showed obvious heterogeneous attenuation on CT and anechoic on sonography.
CONCLUSIONS:
The BCA tends to be small and shows early intense enhancement. The solid tumor is common in the superficial region of the parotid gland, and cystic lesions occur mostly in the deeper parts of the superficial lobe or in the deep lobe.
Salivary gland neoplasms account for <3% of all tumors. Most are benign, and the parotid gland is the most common site.1 BCA is a rare benign epithelial tumor, accounting for approximately 1%–3% of all parotid gland benign tumors, far less common than parotid pleomorphic adenoma and Warthin tumor.2,3 BCA was first described as a distinct clinical and pathologic entity by Kleinsasser and Klein4 in 1967 and was recognized as a histologically distinct entity by the WHO in 1991. In 2005, BCA was classified by the WHO as 1 of 9 subcategories of salivary gland epithelial tumors. BCA is composed of basaloid cells, sharply delineated from the stroma by basement membrane, and a mesenchymal component or chondromyxoid stroma should be absent.5
Because of BCA's low prevalence, previous reports on CT findings of parotid gland BCA contain only sporadic cases6,7 or small case series.3,8,9 The largest series, with 14 cases, was reported by Chawla et al.5 In this study, we report 22 cases with BCA in the parotid gland and describe the CT and sonography features of this tumor. The tumors were grouped into 3 types based on location and size distribution, and CT enhancement pattern and sonography echogenicity were analyzed. Imaging findings were correlated with pathology.
Materials and Methods
A retrospective search through the PACS and histopathology records, from October 2006 to January 2011, in a single tertiary hospital dedicated to tumor diagnosis and treatment revealed 22 consecutive patients with BCA of the parotid gland who underwent superficial or total parotidectomy. All patients underwent preoperative CT and 20 had a preoperative sonography. None of the patients underwent biopsy before CT scanning and sonography. CT was performed by using a 16-section scanner (Sensation; Siemens, Erlangen, Germany). The images were acquired with 4-mm contiguous section thickness and a FOV of 230 × 230 mm, and a matrix of 512 × 512. Scan coverage included the external auditory canal to the thoracic inlet. For contrast-enhanced images, a bolus intravenous dose of 80 mL of nonionic iodinated contrast agent (320 mg I /mL) (Optiray; Tyco Healthcare, Montreal, Canada) was given to all patients at the rate of 3 mL per second. The scan was initiated 40 seconds after the onset of contrast injection. Delayed scan was not performed. Sonography was performed by using the sonography system LOGIC-7 (GE Healthcare, Milwaukee, Wisconsin) or the Sonoline Elegra (Siemens). The frequencies of the probes ranged from 8–12 MHz.
All images were reviewed in consensus by 2 radiologists. Tumors were assessed with regard to the location, size, border, enhancement attenuation, and enhancement pattern on the CT scan. Tumors in superficial and deep lobes of the parotid gland were distinguished by the location of the retromandibular vein, then, tumors of the superficial lobe were further divided into 2 types based on the following criteria.1 Type 1 tumors were close to the superficial border of the superficial lobe, and these tumors were distanced from the retromandibular vein. Type 2 tumors were adjacent to the retromandibular vein and distanced from the superficial border of the superficial lobe. Tumors at the deep lobe were termed type 3. The tumor size was measured in maximal dimension on the transverse plane. A tumor was considered to have a well-defined border if it was well demarcated from the rest of the parotid gland throughout. For assessment of the unenhanced and contrast-enhanced attenuation of tumors, a circular ROI was drawn on the soft tissue components of the tumor, avoiding cystic and necrotic areas that were defined as having a CT attenuation of 20 HU or less.5,8 The enhancement patterns were divided into homogeneous enhancement, slight heterogeneous enhancement, and obvious heterogeneous enhancement. Slight heterogeneous enhancement involves an enhanced tumor and irregular nonenhancing regions that have the same attenuation, being higher than 20 HU and therefore considered solid, on both the plain CT scan and contrast CT scan. Obvious heterogeneous enhancement involves an enhanced tumor and low attenuation regions, with a CT attenuation of 20 HU or less on plain CT scan, considered to be cystic or necrotic regions; these low attenuation regions demonstrate no enhancement on contrast CT.8 On sonography, the tumor was evaluated for its border and echogenicity. Compared with the surrounding parenchyma, echogenicity was discriminated as either hypoechoic or anechoic. No hyperechoic tumor was noticed in this study.
Results
This series included 16 women (72.8%) and 6 men (27.2%), aged between 32 and 73 years (mean 51.5 years, SD 10.2 years). In 4 patients, the tumor was found during an incidental healthy examination. Seventeen patients presented with a palpable mass without tenderness, while 1 patient had slight pain.
The CT and sonography characteristics of the tumors are summarized in the On-line Table. All 22 cases were single lesions. Fifteen tumors were on the left parotid gland, while the remaining tumors were located on the right side. The tumors were located in the superficial lobe in 19 patients (type 1 = 11; type 2 = 8) and in the deep lobe (type 3) in 3 cases. On CT, 21 cases showed well-defined borders (Fig 1), while 1 case (case 14, a type 2 tumor) showed heterogeneous enhancement with ill-defined borders (Fig 2). On sonography, all 20 cases showed a round or oval nodule, and 19 cases displayed well-defined borders. The CT attenuation of the soft-tissue portion of the tumors on unenhanced CT was 38.6 ± 9.5 HU (mean ± SD). The soft-tissue components showed early intense enhancement after contrast injection; the postcontrast CT attenuation was 103 ± 19.4 HU (mean ± SD) and the CT attenuation increase was 64.5 ± 19 HU (mean ± SD).
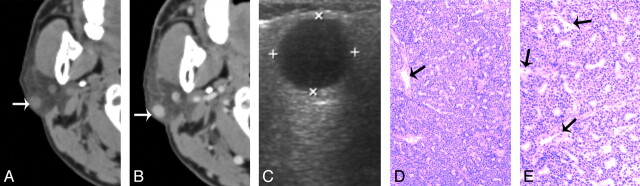
Fig 1.
A 55-year-old man with type 1 BCA in the right parotid gland A, and B, CT shows a round, well-defined, homogeneously enhancing lesion in the superficial region of the right parotid superficial lobe. The lesion is adjacent to the outer border of the parotid gland (white arrow). C, The tumor shows as well-defined and homogeneously hypoechoic on sonography. D, and E. Photomicrographs of tumor specimen (hematoxylin-eosin stain; ×50 and ×100) show capillaries (black arrows) and uniform tumor cell without hemorrhage, collagen deposition, or cystic change.
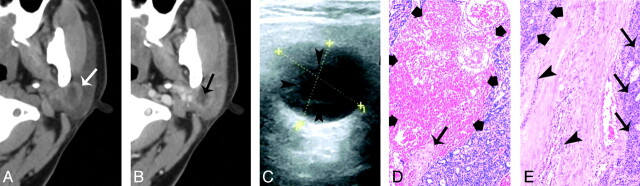
Fig 2.
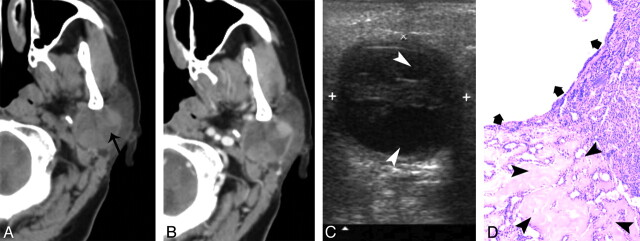
A 40-year-old woman with type 2 BCA in the left parotid gland. A and B, CT shows a round, ill-defined lesion (white arrow) with a cystic component (black arrow) in the deeper region. C, The lesion shows as heterogeneously hypoechoic, with an anechoic region (arrowheads) on sonography. The superficial border of tumor is irregular and ill defined. D, Photomicrograph (hematoxylin-eosin stain; ×50) demonstrates tumor cystic region with hemorrhage (thick arrows) and collagen deposition (thin arrow). E, Specimen histology (hematoxylin-eosin stain; ×50) shows the tumor tissue (thick arrows), the infiltration of tumor cells into the capsule (arrowhead), and tumor cells in surrounding tissue (thin arrows).
In type 1 tumors, well-defined round nodules located on the superficial region of the parotid superficial lobe were demonstrated on CT scan and sonography. The lesion sizes were 11.4 ± 3.29 mm (range 7–16 mm). All of the tumors were homogeneous on the plain CT scans; according to the enhancement pattern, the tumors could be further divided into 2 categories. In the first category (n = 5), the tumors showed homogeneous enhancement on the contrast CT scan. Of these 5 cases, 4 had sonography and displayed as homogeneously hypoechoic. The histology specimens of these 5 tumors showed small capillaries and tumorous tissue structure without hemorrhage, collagen deposition, or cystic change (Fig 1). In the second category (n = 6), the tumors showed slight heterogeneous enhancement on contrast CT scan. All 6 cases had sonography. Four cases showed as slightly heterogeneously hypoechoic, while the remaining 2 cases displayed as homogeneously hypoechoic. With tumors that had slight heterogeneous enhancement on contrast CT scan, specimen histology showed irregular collagen components within the tumor, which could be correlated to the low-attenuation region (Fig 3).
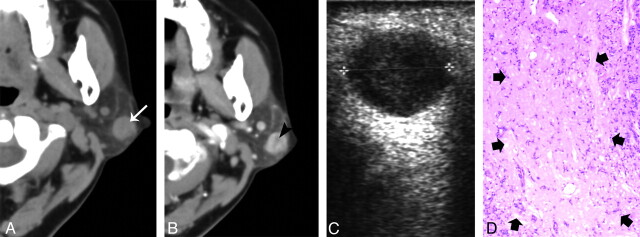
Fig 3.
A 54-year-old woman with type 1 BCA in the left parotid gland. A, Plain CT shows a round nodule with homogeneous attenuation located at the superficial region of the superficial lobe (white arrow). B, The tumor shows slight heterogeneous enhancement with a small, low attenuation component (black arrowheads) on contrast CT. C, Sonography shows well-defined heterogeneously hypoechoic lesion. D, Correlated to the low attenuation component on the postcontrast CT, a photomicrograph of a tumor section (hematoxylin-eosin stain; ×50) shows a collagen component (thick arrows).
Type 2 tumor sizes were 19.3 ± 5.44 mm (range 9–24 mm). Seven tumors had both plain and contrast-enhanced CT, and all demonstrated obvious heterogeneous enhancement with cystic regions (CT attenuation ≤20 Hu); the cystic regions became more recognizable on contrast CT. On sonography, these 7 cases showed as heterogeneously hypoechoic with an intratumoral anechoic region. With these cases, specimen histology showed cystic components and hemorrhage within the tumor (Figs 2 and 4). The remaining case (case 17) without contrast CT and sonography showed homogeneously attenuation on a plain CT scan. The specimen histology confirmed uniform tumorous tissue structure within the tumor.
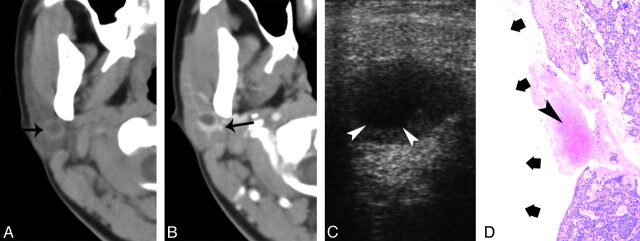
Fig 4.
A 32-year-old woman with type 2 BCA in the right parotid gland. A, Plain CT shows a round lesion (9 mm) with a cystic area (black arrow) located at the deeper region of the superficial lobe. B, Contrast-enhanced CT shows obvious enhancement of the peripheral tumor rim, whereas the cystic component does not enhance. The tumor is adjacent to the retromandibular vein (black arrow). C, Sonography shows a well-defined, hypoechoic nodule with an anechoic core (white arrowheads). D, Photomicrographs (hematoxylin-eosin stain; ×50) show a hemorrhage (arrowhead) and cystic region (thick arrows) within the tumor.
Type 3 tumor lesion sizes were 26 ± 3.6 mm (range 22–29 mm). The CT values were same as type 2 tumors. On CT, 2 tumors showed heterogeneous attenuation, with an obvious low-attenuation region that displayed as anechoic on sonography. One case (case 20) displayed homogenous enhancement on contrast CT and as homogenously hypoechoic on sonography. In cases 21 and 22, specimen histology confirmed the cystic changes within the tumor (Fig 5), whereas case 20 had uniform tumorous tissue structure.
Fig 5.
A 73-year-old man with type 3 BCA in the left parotid gland. A, Plain CT shows a round lesion with cystic area (black arrow). B, The lesion displays obvious heterogeneous enhancement on contrast CT scan. C, Sonography shows a well-defined hypoechoic tumor with an anechoic region (white arrowheads). D, Photomicrographs of tumor specimen (hematoxylin-eosin stain; ×50) show collagen deposition (arrow head) and tumor cystic component (thick arrows).
In type 2 and type 3 tumors, all cases showed well-defined borders on CT and sonography, except for case 14, a type 2 tumor. In this case, a partial margin of the tumor displayed as irregular and ill defined on the CT scan and sonography. A histology review revealed hemorrhage and collagen within the tumor. The capsule was irregular and accompanied by infiltration of tumor cells. Tumor cells were found in the surrounding tissue (Fig 2).
Discussion
The typical clinical feature of BCA is a slowly growing, asymptomatic, freely movable parotid nodule or mass. In the current study, 16 patients (72.8%) were female. A similar sex trend has been observed in several previous studies.2,3,5,9 The mean age of patients in this study was 51.5 years (range 32–73 years), and 50% of the patients were in the range between 50 and 60 years old. This was different from a few previous studies,5,8 where it was reported that BCA was often found in women over 60 years of age. In addition, in this study, BCA occurred with higher frequency in the left parotid gland (15/22, 68%) than the right parotid gland (7/22, 32%).
Parotid gland BCA has been assumed to be a rather small tumor, and its imaging features include the presence of contrast enhancement in a well-defined lesion.5,6,8,10 These features are also recognized in our study. The soft-tissue component of BCA masses showed early intense enhancement after intravenous contrast injection. The CT attenuation increase was 64.5 ± 19 HU. In one previous study,8 BCA showed significantly higher CT attenuation than pleomorphic adenoma on contrast-enhanced images. The marked enhancement of BCA was considered as related to its vascular architecture.9 The solid component of BCA usually has numerous endothelium-lined vascular channels, with prominent small capillaries and venules.11 Such vascularity might explain the intense enhancement of BCA.
We noticed that the tumors in the deeper region of the superficial lobe or in the deep lobe were much larger than the ones in the superficial region of the superficial lobe. The tumor size of parotid gland BCA varied, with type 3 > type 2 > type 1. It is likely that lesions in type 1 can be found earlier, due to being adjacent to the surface of the parotid gland, whereas tumors type 2 and type 3 tumors were less likely to be found by the patients themselves. The relationship between parotid gland BCA size and its location has not been reported previously.
In parotid gland BCA type 1, the tumors could show homogeneous enhancement or slight heterogeneous enhancement. The former demonstration often showed a round, solid, intense homogeneous enhancement nodule, with a well-defined border in the superficial region of the superficial lobe. BCA in the superficial region of the superficial lobe could also show slight heterogeneous enhancement on contrast CT, and these tumors demonstrated collagen components on the microscopic specimens. Chawla et al8 also reported BCA cases with inhomogeneous enhancement and described the presence of nonenhancing stellate areas on CT scan. It was reported that collagen components would most likely be responsible for the small, low attenuation region on the CT imaging.8 However, the sonography feature of BCA with collagen components was not uniform and displayed as slightly heterogeneously hypoechoic (4/6) or homogeneously hypoechoic (2/6). It is highly likely that it was easier to detect collagen regions on contrast CT than on sonography because collagen regions do not enhance. While CT covers the whole tumor, sonography may miss some sections containing collagen regions.
In this study, many type 2 and type 3 parotid gland BCA tumors showed obvious heterogeneous enhancement with cystic components. In previous studies,3,5,6,8 the maximal tumor dimension of all BCAs with cystic changes were smaller than 30 mm. Our result was in good agreement with those previous studies (On-line Table). Cystic change can even be found in a tumor that is 9 mm in maximal dimension on the transverse plane (On-line Table, case 12; Fig 4). We suggest that BCAs tend to be small tumors, even with cystic regions. In addition, our results showed cystic change occurred mostly in tumors at the deeper parts of the superficial lobe or in the deep lobe. On the contrary, the solid BCAs were common in the superficial parts of the superficial lobe. The results were not reported in previous studies. With nodules of less than 30 mm, together with cystic components in the deeper parts of the superficial or deep lobes, BCA should be considered as a possibility.
In addition, case 14 showed heterogeneous enhancement and ill-defined borders. The ill-defined margin was attributed to the infiltration of tumor cells into the capsules and surrounding tissue, which might indicate malignant transformation. According to previous studies, the malignant transformation rate of parotid BCA is about 4%.5,10
In this series, the sonography features of BCA were mostly consistent with CT features, except for 2 cases. However, for a parotid lesion, it is important to confirm the adjacent relationship between tumor and facial nerve (the facial nerve course can be inferred from the retromandibular vein) before operating. Sonography is often the initial imaging examination in patients with parotid gland mass because of its accessibility, low cost, and the capability of guided biopsy. But CT imaging is usually more intuitive for surgeons than sonography. Furthermore, CT is better for assessing the full tumor extent than sonography, especially when the tumor is located in or involves the deep lobe of the parotid gland.
The main differential diagnoses for BCA based on imaging features include pleomorphic adenoma and Warthin tumor. Pleomorphic adenoma is the most common benign neoplasm in the parotid gland, and tends to show a well-defined, lobulated homogeneous mass. These tend to be larger than BCA.6 The cystic change and hemorrhage are often seen in larger tumors (>3 cm).1 Some pleomorphic adenomas show ill-defined borders due to malignant transformation. Pleomorphic adenoma shows little or no enhancement in the immediate postcontrast scan but strong enhancement in the delayed scan.7,12,13 As opposed to pleomorphic adenoma, as shown in this study, BCA shows intense enhancement in the early phase, and this feature can be used to differentiate BCA from pleomorphic adenoma.5,7 Warthin tumor is the second most common benign tumor of the parotid gland. Warthin tumor is more common in elderly men (>50 years old) than in women, and its development is closely related to smoking habits. Multiplicity or bilateral involvement is seen in 10%–15% of Warthin tumor patients.1,5 Warthin tumor is most commonly located in the caudal portion of the parotid gland,14 while BCA can be found in any quadrant of the parotid gland.
Conclusions
This study suggests that the typical BCA tends to be small in size, especially in the superficial region of the superficial lobe of the gland. BCA shows intense enhancement in the early postcontrast phase. Solid lesions are common in the superficial region of the parotid gland, while cystic lesions occur mostly in the deeper parts of the superficial lobe or in the deep lobe. These features may help in differential diagnosis of BCA.
ABBREVIATIONS:
- BCA
basal cell adenoma
- WHO
World Health Organization
Footnotes
This study is partially supported by a direct grant for research of The Chinese University of Hong Kong (2041501).
References
- 1. Lee YY, Wong KT, King AD, et al. Imaging of salivary gland tumours. Eur J Radiol 2008; 66: 419– 36 [DOI] [PubMed] [Google Scholar]
- 2. Nagao K, Matsuzaki O, Saiga H, et al. Histopathologic studies of basal cell adenoma of the parotid gland. Cancer 1982; 50: 736– 45 [DOI] [PubMed] [Google Scholar]
- 3. Kawata R, Yoshimura K, Lee K, et al. Basal cell adenoma of the parotid gland: a clinicopathological study of nine cases—basal cell adenoma versus pleomorphic adenoma and Warthin's tumor. Eur Arch Otorhinolaryngol 2010; 267: 779– 83 [DOI] [PubMed] [Google Scholar]
- 4. Kleinsasser O, Klein HJ. Basal cell adenoma of the salivary glands [in German]. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 1967; 189: 302– 16 [PubMed] [Google Scholar]
- 5. Chawla AJ, Tan TY, Tan GJ. Basal cell adenoma of the parotid gland: CT scan features. Eur J Radiol 2006; 58: 260– 65 [DOI] [PubMed] [Google Scholar]
- 6. Jang M, Park D, Lee SR, et al. Basal cell adenoma in the parotid gland: CT and MRI findings. AJNR Am J Neuroradiol 2004; 25: 631– 35 [PMC free article] [PubMed] [Google Scholar]
- 7. Yerli H, Teksam M, Aydin E, et al. Basal cell adenoma of the parotid gland: dynamic CT and MRI findings. Br J Radiol 2005; 78: 642– 45 [DOI] [PubMed] [Google Scholar]
- 8. Chiu NC, Wu HM, Chou YH, et al. Basal cell adenoma versus pleomorphic adenoma of the parotid gland: CT findings. AJR Am J Roentgenol 2007; 189: 254– 61 [DOI] [PubMed] [Google Scholar]
- 9. Lee DK, Chung KW, Baek CH, et al. Basal cell adenoma of the parotid gland: characteristics of 2-Phase helical computed tomography and magnetic resonance imaging. J Comput Assist Tomogr 2005; 29: 884– 88 [DOI] [PubMed] [Google Scholar]
- 10. Nagao T, Sugano I, Ishida Y, et al. Carcinoma in basal cell adenoma of the parotid gland. Pathol Res Pract 1997; 193: 171– 78 [DOI] [PubMed] [Google Scholar]
- 11. Triest WE, Fried MP, Stanievich JF. Membranous basal cell adenoma of the hypopharynx. Arch Otolaryngol 1983; 109: 774– 77 [DOI] [PubMed] [Google Scholar]
- 12. Lev NH, Khanduja K, Morris PP, et al. Parotid pleomorphic adenomas: delayed CT enhancement. AJNR Am J Neuroradiol 1998; 19: 1835– 39 [PMC free article] [PubMed] [Google Scholar]
- 13. Choi DS, Na DG, Byun HS, et al. Salivary gland tumors: evaluation with two-phase helical CT. Radiology 2000; 214: 231– 36 [DOI] [PubMed] [Google Scholar]
- 14. Ikeda M, Motoori K, Hanazawa T, et al. Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 2004; 25: 1256– 62 [PMC free article] [PubMed] [Google Scholar]