Abstract
BACKGROUND AND PURPOSE:
Radiologic methods for the diagnosis of chemical radiculitis associated with anular tears in the lumbar spine have been rare. Provocative diskography is one of the methods for diagnosing diskogenic chemical radiculitis but is invasive. A reliable imaging method for replacing provocative diskography and diagnosing chemical radiculitis is required. Our aim was to investigate the value of 3D MR radiculography depicted by rendering imaging in the diagnosis of symptomatic chemical radiculopathy associated with anular tears.
MATERIALS AND METHODS:
The study population consisted of 17 patients (age range, 32–88 years) with unilateral radiculopathy. Symptomatic chemical radiculopathy was confirmed with provocative CT diskography and/or provocative selective nerve root block for agreement of sides and levels. Through adhering to the principles of selective excitation (Proset imaging), we acquired 3D coronal FFE sequences with selective water excitation. Morphologic changes in the ipsilateral symptomatic nerve root caused by chemical radiculopathy were compared with those in the contralateral nerve root on 3D MR lumbosacral radiculography.
RESULTS:
Pain reproduction at the contrast-leak level during diskography (n = 4) and selective nerve root injection (n = 13) showed concordant pain in all patients. All patients with symptomatic chemical radiculopathy showed nerve root swelling in both ipsilateral levels and sides on 3D MR radiculography. The most common nerve root affected by the chemical radiculopathy was the L5 nerve root (n = 13), while the most common segment exhibiting nerve root swelling was the exit nerve root (n = 16).
CONCLUSIONS:
All patients with radicular leg pain caused by chemical radiculopathy showed nerve root swelling on 3D MR radiculography. We believe that in cases without mechanical nerve root compression caused by disk herniation or stenosis in the lumbar spine, nerve root swelling on 3D MR radiculography in patients with radiculopathy associated with an anular tear may be relevant in the diagnosis of symptomatic chemical radiculopathy.
Commonly recognized causes of sciatica include disk herniation and spinal stenosis; however, some patients presenting with radiculopathy can show no evidence of nerve root compression on MR imaging. Patients with anular tears may experience low back pain or radiation of pain into the lower extremity. Recently, it has been observed that introduction of the nucleus pulposus into the epidural space, in the absence of mechanical compression, induces pronounced morphologic and functional changes in the nerve roots. Chemical radiculitis is an inflammatory condition of the nerve root, which may result from the rupture of the anulus fibrosus and dissemination of disk fluid along the nerve root sheath.1 The concept of radiculitis was first described by Lewin in 1943, when he discussed the condition of irritation of the lumbar and sacral nerve roots.2 Muramoto et al3 demonstrated that prostaglandin E2, a chemical mediator of inflammation, could provoke an ectopic eruption of impulses from the nerve roots in an in vitro canine model, indicating that it might play a role in irritation of the nerve roots. Also Peng et al4 reported that leakage of chemical mediators or inflammatory cytokines, which are produced in the painful disk, into the epidural space through anular tears could lead to injury to adjacent nerve roots and that the leakage might constitute the primary pathophysiologic mechanism of radiating leg pain without disk herniation.
Radiologic methods for the diagnosis of chemical radiculitis associated with anular tears in the lumbar spine have been rare. Diskography for the diagnosis of chemical radiculitis is controversial and poorly defined. Nevertheless, provocative diskography is one of the methods for diagnosing diskogenic chemical radiculitis.5 Slipman et al6 reported that inclusion criteria for the diagnosis of chemical radiculitis consisted of symptoms of extremity pain, MR imaging void of local nerve root pathology, an electromyographic study positive for an acute radiculopathy, and a positive fluoroscopically guided diagnostic selective nerve root block.
Byun et al7 showed that the perianular enhancement caused by chemical radiculitis on contrast lumbar MR imaging could cause radiculopathy. They suggested that perianular enhancement could be detected, in some cases, without any chemical radiculitis. However, morphologic evaluation of the nerve root caused by chemical radiculitis was not described.
The aim of this study was to investigate the value of 3D MR radiculography depicted by rendering imaging in the diagnosis of symptomatic chemical radiculopathy associated with anular tears.
Materials and Methods
The study population consisted of 17 patients (age range, 32–88 years; mean age, 56.6 years; 10 men, 7 women) with chemical radiculopathy. All patients with anular tears had unilateral pain localized to the leg or buttock. Patients with bilateral radiculopathy and disk herniation or spinal stenosis were excluded in our study. Also patients with tumor, synovial cyst, other intradural or extradural lesions, and peripheral neuropathy were excluded. Inclusion criteria in our study were the following: 1) unilateral radiculopathy without compressive lesions in the spinal canal and foraminal and extraforaminal zones of the lumbar spine on MR imaging, 2) an anular tear on MR images and/or CT diskography, 3) concordant pain during provocative CT diskography and/or provocative selective nerve root block for agreement of side and level, and 4) improvement of clinical symptoms after selective nerve root injection. Symptomatic nerve root was confirmed with provocative CT diskography (n = 4) and/or provocative selective nerve root block (n = 13) for agreement of side and level. Because diskography is more invasive compared with provocative selective nerve root block, selective nerve root block for confirmation and treatment in most of cases was performed. The presence of an anular tear was confirmed by CT diskography and/or MR imaging.
A 3-month follow-up 3D MR radiculography after selective nerve root block was performed in 3 patients. A control group (10 subjects) for nerve root evaluation on 3D MR radiculography was included. Criteria for control group subjects were no radiculopathy, no disk herniation, and no spinal stenosis.
MR imaging was performed by using a 1.5T scanner (Intera; Philips Healthcare, Best, the Netherlands) with a spine-array coil. With respect to spin-echo sequences, axial and sagittal T1- (TR/TE, 583/12 ms), turbo T2-weighted images (TR/TE, 3800/128 ms), and contrast-enhanced (gadopentetate dimeglumine, Magnevist; Schering, Berlin, Germany) axial T1-weighted images with fat suppression were obtained with the following parameters: 4-mm section thickness with a 0.4- to 0.7-mm overlapping section gap; 160 × 160 FOV; 4 NEX; and a 212 × 130 matrix. Contrast-enhanced T1-weighted images were obtained in 14 patients with symptomatic chemical radiculopathy. The 3D coronal FFE sequence with selective water excitation adhering to the principles of the selective excitation technique (Proset imaging) was acquired under the following acquisition parameters: 1-mm section thickness without an overlapping section gap; 250-mm FOV; 256 × 256 matrix; TR, 23.2 ms; TE, 13.8 ms; 8° flip angle; and 2 signal-intensity acquisitions. Forty coronal source FFE images for each subject were obtained. To obtain images of all lumbosacral nerve roots and DRG, we set the imaging plane to be parallel to the longitudinal axis of the lumbar spinal canal and centered on the level of the L3 vertebral body. One parallel regional saturation slab was added to suppress the signal intensity from the vessels. The resultant whole-imaging slab had an anteroposterior thickness of 4 cm, which fully covered the intervertebral foraminal region and extraforaminal zone from the anterior third of the vertebral body to the anterior margin of the spinous process. For image processing of direct volume rendering, an Aquarius 3D workstation equipped with commercially available automated analysis 3D rendering software (TeraRecon, San Mateo, California) was used.
Provocative diskography was performed in 4 patients. Diskography was performed in 1 or several intervertebral disk levels to confirm corresponding radiculopathy. Pain reproduction on injection of contrast medium during diskography was evaluated. CT was performed to identify the site of contrast leakage and the location of anular tears after diskography. Provocative selective nerve root block for confirmation of the radiculopathy was performed in 13 patients. All 14 patients demonstrated perianular enhancement caused by anular tears on contrast-enhanced axial T1-weighted images with fat suppression.
The spindle-shaped nerve root in the extraforaminal or foraminal zones was defined as DRG on Proset source images or 3D MR radiculography. In general, the nerve root that exited the spine at a particular level was referred to as the exiting nerve root. Another nerve root segment went across the disk and exited the spine at the next level below. It was called the traversing nerve root. On 3D MR radiculography, the nerve root in the extraforaminal zone beyond DRG was defined as the exit nerve. The proximal nerve segment between the thecal sac and DRG was defined as the transverse nerve root.
Swelling of the DRG and nerve roots was defined as positive when either size was bigger than the normal contralateral side. Morphologic changes in the ipsilateral symptomatic nerve root caused by chemical radiculopathy were compared with those in the contralateral nerve root on 3D MR lumbosacral radiculography. The relationship between morphologic change in the symptomatic nerve root on 3D MR lumbosacral radiculography and provocative diskography or selective nerve root block for confirmation of the radiculopathy was analyzed. We evaluated the relationship between morphologic changes in the symptomatic nerve root on 3D MR lumbosacral radiculography and perianular enhancement on contrast-enhanced axial T1-weighted images with fat suppression.
Axial Proset MPR images were selected for quantitative analysis of signal intensity and width in the lumbar nerve roots. The width and signal intensity of the symptomatic nerve root were compared with those in the contralateral asymptomatic nerve root on axial Proset MPR images. The width of the transverse nerve root was measured at the midpoint perpendicular to the longitudinal axis. The width of the exit nerve root was measured at just 1 cm caudal to the distal end of the DRG. The width of the DRG was measured vertically at the midpoint perpendicular to the longitudinal axis of the DRG. Three small regions of interest in each segment of ipsilateral and contralateral lumbar nerve roots were generated by an experienced radiologist by using the MR imaging console software. Placement of a region of interest generated a mean value for signal intensity of the pixels enclosed by the region of interest. The SIR of the nerve root to the paraspinal muscle was evaluated. 3D MR lumbosacral radiculography was interpreted by an experienced radiologist and spine physician.
Interobserver and intraobserver variability of the nerve root swelling on 3D MR radiculography was assessed by using κ statistics. A κ value of <0.40 indicated poor agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and ≥0.81, almost perfect agreement. A paired t test was used to compare differences of width and signal intensity between symptomatic nerve roots and asymptomatic contralateral nerve roots. Statistical calculations were done with the Statistical Package for the Social Sciences, Version 19.0 (SPSS, Chicago, Illinois). A P value < .005 was statistically significant.
All patients with symptomatic chemical radiculitis were treated with a selective nerve root block. Clinical symptom evaluation with the Oswestry Disability Index and the visual analog scale was performed after and before treatment.
Our study was approved by the institutional review board of our hospital.
Results
The L5-S1 disk was involved in 13 patients with chemical radiculopathy, while the L4–5 disk was involved in 3. One case of chemical radiculopathy was detected at the L2–3 level.
Pain reproduction at the contrast leak level during diskography showed concordant pain in all 4 cases. Pain reproduction during provocative selective nerve root injection showed concordant pain in all 13 patients.
The clinical symptoms and MR imaging findings are summarized in Table 1. All patients with symptomatic chemical radiculopathy showed nerve root swelling in the ipsilateral level and side on 3D MR radiculography. Swelling of only the exit nerve root was observed in 6 patients, while swelling of the entire segment of the nerve root was seen in 7 (Fig 1D). Swelling of the DRG and exit nerve root was found in 3 patients (Fig 2 D), and 1 patient had swelling of the transverse nerve root.
Table 1:
Clinical symptoms and MR imaging findings of the patients with chemical radiculitis
| No. | Age (yr)/Sex | Symptoms | NR Lesion | Segment of Swelling | Location of Perianular Enhancement | ODI, Before | VAS, After | Provocation |
|---|---|---|---|---|---|---|---|---|
| 1 | 51/M | Lt buttock, calf pain | Lt L5 | DRG, exit | For and extra | 18.5 | 0.0 | SNI |
| 2 | 53/M | Lt leg pain | Lt L5 | Entire | For and extra | 33.4 | 0.0 | SNI |
| 3 | 62/M | Lt leg pain | Lt L5 | Entire | – | 44.6 | 25, 3 | SNI |
| 4 | 49/F | Rt buttock, lat thigh pain | Rt L5 | DRG, exit | For and extra | 25.4 | 13.2 | SNI |
| 5 | 69/M | Lt leg pain, numbness | Lt L5 | Entire | For and extra | 22.3 | 16.1 | Diskography |
| 6 | 53/M | Lt leg pain | Lt L5 | Exit | For and extra | 42.5 | 21.1 | SNI |
| 7 | 62/M | Rt leg pain | Rt L5 | Exit | Extra | 62.7 | 33.2 | SNI |
| 8 | 35/M | Rt leg pain, toe weakness | Rt L5 | Exit | For and extra | 22.3 | 11.1 | SNI |
| 9 | 76/F | Lt buttock, leg pain | Lt L5 | Entire | For and extra | 54.7 | 22.2 | Diskography |
| 10 | 32/M | Rt leg pain | Rt L5 | Exit | Extra | 33.6 | 31.3 | SNI |
| 11 | 51/F | Lt buttock, leg pain | Lt L5 | Exit | – | 18.4 | 0.0 | SNI |
| 12 | 56/F | Lt buttock, leg pain | Lt L5 | Exit | Extra | 44.5 | 22.1 | SNI |
| 13 | 69/F | Rt buttock, leg pain | Rt L5 | Entire | For and extra | 54.6 | No | SNI |
| 14 | 44/M | Lt leg pain | Lt L4 | DRG, exit | For and extra | No | 80% | SNI |
| 15 | 88/M | Rt leg pain | Rt L4 | Entire | For and extra | 51.5 | 11.2 | SNI |
| 16 | 54/F | Rt anterior, lat leg pain | Rt L4 | Trans | – | No | 90% | Diskography |
| 17 | 59/F | Lt leg pain | Lt L2 | Entire | For and extra | 56.6 | 0.0 | Diskography |
Note:—NR indicates nerve root; ODI, Oswestry Disability Index; VAS, visual analogue scale; Rt, right; Lt, left; exit, exit nerve root; Trans, transverse nerve root; Entire, entire segments of nerve root; For, foraminal zone; extra, extraforaminal zone; lat, lateral; SNI, selective nerve root injection; No, no response of symptoms; Before, before treatment; After, after treatment; %, degree of symptom improvement; –, no contrast study.
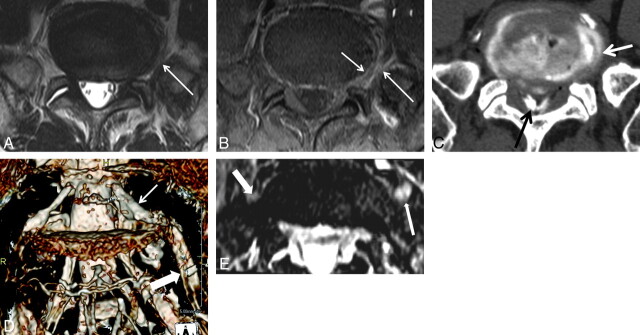
Fig 1.
A 69-year-old man with left leg pain. A, There is intermediate signal intensity (arrow) between the outer anulus and left L5 nerve root on the T2-weighted image. B, Contrast-enhanced T1-weighted image with fat suppression shows abnormal enhancement at the left perianular extraforaminal zone (large arrow) and outer anulus (small arrow) in L5-S1. Pain reproduction at this level during diskography shows concordant pain. C, CT diskography shows an anular tear and left posterolateral extraforaminal leak (arrow) of the contrast media from an anular tear. There is contrast media (black arrow) at the extradural spinal canal. D, Diffuse swelling of the entire left L5 nerve root (thick arrow) including DRG (thin arrow) is demonstrated on 3D MR radiculography. E, Axial Proset MPR image demonstrates that the left L5 exit nerve root (long arrow) along the ventral surface of the sacral ala is larger and higher in signal intensity compared with the right L5 exit nerve root (thick arrow).
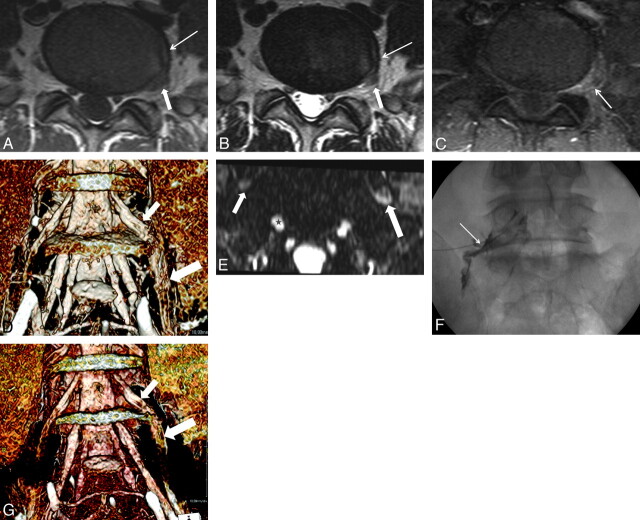
Fig 2.
A 51-year-old man with left buttock and calf pain. A and B, T1- (A) and T2-weighted (B) images show an anular tear (arrow) at the left lateral margin of the L5-S1 disk. There is intermediate signal intensity due to the fibrovascular and granulation tissue between the outer anulus and left L5 nerve root (short arrow). There is no apparent disk herniation. C, There is abnormal enhancement at the perianular extraforaminal zone (arrow) in L5-S1 on the contrast-enhanced T1-weighted image with fat suppression. D, Diffuse swelling of the left L5 dorsal root ganglion (arrow) and exit nerve root (thick arrow) is demonstrated on 3D MR radiculography. E, Axial Proset MPR image shows the left L5 exit nerve root (long arrow) along the ventral surface of the sacral ala to be larger and higher in signal intensity compared with the contralateral nerve root (short arrow). Star indicates the right S1 nerve root. F, Pain reproduction at this level (arrow) during a selective nerve root block (prone position) shows concordant pain. At 1 month after selective nerve root block at the left L5 nerve root, clinical symptoms are completely improved. G, However, a 3-month follow-up 3D MR radiculography reveals decreased swelling at the left L5 nerve root.
Our study found that there was an apparent correlation between nerve root swelling on 3D MR radiculography and clinical symptoms, including provocative selective nerve root block and diskography. In 14 patients, perianular enhancement caused by chemical radiculitis demonstrated a thick and linear enhancement along margins of the anular tear on contrast-enhanced axial T1-weighted images with fat suppression (Figs 1B and 2C). Perianular enhancement on MR imaging study corresponded to nerve root swelling on 3D MR radiculography for level and side. Comparison between quantitative measurements of the symptomatic nerve roots and those of the contralateral asymptomatic nerve roots on axial Proset MPR images are summarized in Table 2. In all cases with chemical radiculitis, the width of DRG and the nerve roots was larger than those of the contralateral asymptomatic nerve roots on axial Proset MPR images (paired t test, P < .005). The nerve/muscle SIR of the symptomatic nerve root was higher than that of the asymptomatic contralateral nerve root in segments of the DRG and exit nerve (Figs 1E and 2E) (paired t test, P < .005). However, there was no statistical difference of SIR in the transverse segment.
Table 2:
Comparison between quantitative measurements of the symptomatic nerve roots and those of the contralateral asymptomatic nerve roots on Proset images
| Side | Mean Value of Nerve Root Diameter (mm) |
Mean Value of SIRa |
||||
|---|---|---|---|---|---|---|
| Transverse | DRG | Exit | Transverse | DRG | Exit | |
| Ipsilateral | 4.2 ± 0.9 | 6.33 ± 0.12 | 7.2 ± 1.9 | 2.66 ± 1.48 | 2.51 ± 1.67 | 1.77 ± 0.75 |
| Contralateral | 3.3 ± 0.77 | 5.15 ± 0.98 | 4.72 ± 1.1 | 2.57 ± 1.41 | 2.14 ± 1.53 | 1.27 ± 0.54 |
Note:—Transverse indicates transverse nerve root; Exit, exit nerve root.
Width of the nerve roots with chemical radiculitis is larger than that of the contralateral asymptomatic nerve roots (paired t test, P < .005). SIR of the DRG and exit nerve roots with chemical radiculitis is higher than that of the contralateral asymptomatic nerve roots (paired t test, P < .005).
Although clinical symptoms were completely improved within 1 month, nerve root swelling caused by chemical radiculitis was improved to a decreased thickness at 3-month follow-up 3D MR radiculography in 3 patients (Fig 2F). In all control groups, a morphologic difference (thickness) of both sides of the DRG and nerve roots at the same levels was not detected.
Agreement with respect to the recognition of the nerve root swelling was excellent (κ values for intraobserver and interobserver = 1.00 and 0.87).
Discussion
Chemical radiculitis is an inflammatory condition of the nerve root, which may result from the rupture of the anulus fibrosus and dissemination of the disk fluid along the nerve root sheath. The nucleus pulposus has been demonstrated to induce axonal degeneration, myelin edema, and intravascular coagulation; increase endoneural fluid pressure; reduce intraneural blood flow; and reduce nerve root conduction velocity.8,9
Several studies have been performed to understand the role of inflammation in radicular pain. Leukocytes, macrophages, and lymphocytes have been found at the site of surgically created porcine disk protrusion in vivo.10 Saal et al11 found elevated levels of PLA2, the rate-limiting enzyme in the chemical cascade that liberates arachidonic acid, prostaglandins, and leukotrienes, in the disk material obtained from patients surgically treated for radiculopathy due to disk herniation. Further, Chen et al12 demonstrated that PLA2 promoted loss of myelin, breakdown of myelin sheaths, and vacuolar degeneration, ultimately creating hypersensitive regions allowing ectopic discharges. Peng et al13 investigated the histologic features of 19 specimens of lumbar intervertebral disks from 17 patients with diskogenic low-back pain during posterior lumbar interbody fusion. They suggested that the zone of granulation tissue with extensive innervations along the anular tears in the posterior part of the painful disk might be responsible for causing the pain of diskography and of diskogenic low-back pain. Also they reported that the adjacent tissues surrounding the anular tears were replaced by disorganized and vascularized granulation and scar tissue.
One of our previous studies revealed that the perianular enhancement adjacent to anular tears on MR imaging may be relevant in the diagnosis of symptomatic chemical radiculitis.7 In the current study, perianular enhancement on MR imaging corresponded with nerve root swelling on 3D MR radiculography for level and side. However, we suggest that perianular enhancement may be detected in asymptomatic patients without chemical radiculitis.
On 3D MR radiculography, all patients with radiculopathy caused by chemical radiculopathy showed ipsilateral nerve root swelling. We suggest that nerve root swelling due to chemical radiculopathy may be caused by inflammation and granulation of adjacent tissues surrounding anular tears. We propose 3D MR radiculography as a helpful method for diagnosing chemical radiculopathy.
Peng et al4 reported that the L5 and S1 nerve roots were the most common nerve root lesions caused by chemical radiculitis. In our results, the most common nerve root lesion was at the L5 nerve root. Furthermore, the most common segment for nerve root swelling was the exit nerve root (n = 16). We suggest a relationship between inflammatory perianular enhancement at the extraforaminal or foraminal zones and swelling of the exit nerve root.
Anular tears manifest on MR imaging as HIZs. The presence of HIZs within the posterior anulus seen on T2-weighted MR imaging has aroused great interest and even controversy among many investigators, with respect to whether these HIZs are closely associated with a concordant pain response on awake diskography.13,14 Although detection of symptomatic anular tears on MR imaging in patients with radiculopathy has been very important, nonspecific HIZs on T2-weighted images seem to be an unreliable marker of painful anular tears. Therefore, provocative diskography has become the criterion standard in the definitive diagnosis of diskogenic pain associated with anular tears; however, it is invasive.
Slipman et al6 mentioned that a biochemical etiology in chemical radiculitis–associated anular tear is likely to play a significant role in radiculopathy and radicular pain. Patients with motor abnormalities, normal findings on MR imaging, positive findings on electromyography, and a positive diagnostic selective nerve root block were included. The study provided confirmatory clinical evidence and supported the existence of a nonstructural, biochemical mechanism by which radiculopathy can occur. In general, provocative diskography or diagnostic selective nerve root block or both are confirmatory for a chemical radiculopathy–associated anular tear. To our knowledge, there are no noninvasive imaging techniques for diagnosing chemical radiculitis.
There are several articles regarding the changes in spinal nerve roots by using 3D MR imaging. Kim et al15 reported that DRG swelling and running course abnormality of the L5 exiting root on coronal source images of MR myelography were useful findings in diagnosing L5 root compression at the L5-S1 foramen or extraforamen. Furthermore, Kikkawa et al16 studied gadolinium-enhanced 3D MR imaging fast low-angle shot in the evaluation of symptomatic lumbosacral nerve roots. They reported enhancement of the symptomatic nerve roots in patients with radiculopathy. However, morphologic changes in the nerve root in chemical radiculitis associated with anular tears by using 3D MR imaging had not yet been studied. Zhang et al17 investigated the effectiveness of 3D high-spatial-resolution diffusion-weighted MR neurography based on steady-state free precession in the diagnosis of sciatica. They mentioned that the presence of nerve root compression or increased T2 signal-intensity changes can be observed in all patients with sciatica.
In patients with cervical radiculopathy, the signal intensity of the cervical spinal nerves on high-resolution MR neurography was evaluated by Erdem et al.18 They reported that a markedly increased signal intensity in the distal portion of the affected spinal nerves was found. Nerve/muscle SIR measurements of the affected spinal nerves showed a significantly increased intensity compared with the noninvolved spinal nerves. In our study, nerve/muscle signal-intensity ratio on Proset MPR images showed significantly increased values compared with the contralateral nerve roots. We suggest that enlargement and increased signal intensity of the nerve root associated with chemical radiculitis indicate edema of the nerve root caused by inflammation and irritation associated with an anular tear.
The Proset is a selective-excitation technique used to suppress either water or fat by exploiting the difference between water and fat resonance frequencies. With Proset, the signal intensity of fat has been completely suppressed and the details of the nerve root and DRG were delineated.19 In our study, the nerve root and DRG are well-depicted on 3D MR rendering images by using source images based on Proset.
Contrary to patients with chemical radiculopathy, the control group showed no definite swelling or abnormal thickness of nerve roots on 3D MR rendering imaging based on Proset. In cases with unilateral radiculopathy, spine MR imaging without compressive or stenotic lesions, and positive provocative diskography and/or diagnostic selective nerve block, abnormal nerve root swelling on 3D MR radiculography may suggest chemical radiculopathy.
Conclusions
All patients with radiculopathy caused by chemical radiculitis showed nerve root swelling on 3D MR radiculography. We believe that in cases without mechanical nerve root compression caused by disk herniation or stenosis in the lumbar spine, nerve root swelling on 3D MR radiculography in patients with radiculopathy may be relevant in the diagnosis of symptomatic chemical radiculitis.
ABBREVIATIONS:
- DRG
dorsal root ganglia
- FFE
fast-field echo
- HIZ
high-intensity zone
- MPR
multiplanar reformations
- PLA2
phospholipase A2
- SIR
signal-intensity ratio of the nerve root to the paraspinal muscle
Footnotes
This work was supported by the Yeungnam University research grants in 2009.
References
- 1. Marshall LL, Trethewie ER, Curtain CC. Chemical radiculitis: a clinical, physiological and immunological study. Clin Orthop Rel Res 1977: 61–67 [PubMed] [Google Scholar]
- 2. Lewin P. Backache and Sciatic Neuritis. Philadelphia: Lea & Febiger; 1943. [Google Scholar]
- 3. Muramoto T, Atsuta Y, Iwahara T, et al. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Int Orthop 1997; 21: 172– 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peng B, Wu W, Li Z, et al. Chemical radiculitis. Pain 2007; 127: 11– 16 [DOI] [PubMed] [Google Scholar]
- 5. Kloth D. Chemical radiculopathy. Pain physician 2003; 6: 139, author reply 139–140 [PubMed] [Google Scholar]
- 6. Slipman CW, Isaac Z, Lenrow DA, et al. Clinical evidence of chemical radiculopathy. Pain Physician 2002; 5: 260– 65 [PubMed] [Google Scholar]
- 7. Byun WM, Ahn SH, Ahn MW. Significance of perianular enhancement associated with anular tears on magnetic resonance imagings in diagnosis of radiculopathy. Spine (Phila Pa 1976) 2008; 33: 2440– 43 [DOI] [PubMed] [Google Scholar]
- 8. Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine 1993; 18: 1425– 32 [PubMed] [Google Scholar]
- 9. Otani K, Arai I, Mao GP, et al. Nucleus pulposus-induced nerve root injury: relationship between blood flow and motor nerve conduction velocity. Neurosurgery 1999; 45: 614– 19, discussion 619–20 [DOI] [PubMed] [Google Scholar]
- 10. Habtemariam A, Virri J, Gronblad M, et al. Inflammatory cells in full-thickness anulus injury in pigs: an experimental disc herniation animal model. Spine 1998; 23: 524– 29 [DOI] [PubMed] [Google Scholar]
- 11. Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine 1990; 15: 674– 78 [DOI] [PubMed] [Google Scholar]
- 12. Chen C, Cavanaugh JM, Ozaktay AC, et al. Effects of phospholipase A2 on lumbar nerve root structure and function. Spine 1997; 22: 1057– 64 [DOI] [PubMed] [Google Scholar]
- 13. Peng B, Hou S, Wu W, et al. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur Spine J 2006; 15: 583– 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim CH, Jee WH, Son BC, et al. Discogenic lumbar pain: association with MR imaging and CT discography. Eur J Radiol 2005; 54: 431– 37 [DOI] [PubMed] [Google Scholar]
- 15. Kim SB, Jang JS, Lee SH. Morphologic changes of L5 root at coronal source images of MR myelography in cases of foraminal or extraforaminal compression. J Korean Neurosurg Soc 2009; 46: 11– 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kikkawa I, Sugimoto H, Saita K, et al. The role of Gd-enhanced three-dimensional MRI fast low-angle shot (FLASH) in the evaluation of symptomatic lumbosacral nerve roots. J Orthop Sci 2001; 6: 101– 09 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Song L, Meng Q, et al. Morphological analysis in patients with sciatica: a magnetic resonance imaging study using three-dimensional high-resolution diffusion-weighted magnetic resonance neurography techniques. Spine 2009; 34: E245– 50 [DOI] [PubMed] [Google Scholar]
- 18. Erdem CZ, Erdem LO, Cagavi F, et al. High resolution MR neurography in patients with cervical radiculopathy [in Turkish]. Tani Girisim Radyol 2004; 10: 14– 19 [PubMed] [Google Scholar]
- 19. Shen J, Wang HY, Chen JY, et al. Morphologic analysis of normal human lumbar dorsal root ganglion by 3D MR imaging. AJNR Am J Neuroradiol 2006; 27: 2098– 103 [PMC free article] [PubMed] [Google Scholar]