What is more expensive, carotid endarterectomy or stenting? The authors used Medicare data from elderly patients to explore this issue and identify variables that increased costs. Data were obtained from government sources for 2001-2008 and a total of 12,485 stentings and 181,200 endarterectomies were studied. Not surprisingly, the payments for stenting were $3241 less than costs and those for endarterectomy were $1318 less than costs. Greater disparities between payment and costs were found for patients with unfavorable outcomes. Draw your own conclusions.
Abstract
BACKGROUND AND PURPOSE:
Hospitals struggle to provide care for elderly patients based on Medicare payments. Amid concerns of inadequate reimbursement, we sought to evaluate the hospitalization costs for recipients of CEA and CAS placement, identify variables associated with increased costs, and compare these costs with Medicare reimbursements.
MATERIALS AND METHODS:
All CEA and CAS procedures were extracted from the 2001–2008 NIS. Average CMS reimbursement rates for CEA and CAS were obtained from www.CMS.gov. Annual trends in hospital costs were analyzed by Sen slope analysis. Associations between LOS and hospital costs with respect to sex, age, discharge status, complication type, and comorbidity were analyzed by using the Wilcoxon rank sum test. Least-squares regression models were used to predict which variables had the greatest impact on LOS and hospital costs.
RESULTS:
The 2001–2008 NIS contained 181,200 CEA and 12,485 CAS procedures. Age and sex were not predictive of costs for either procedure. Among favorable outcomes, CAS was associated with significantly higher costs compared with CEA (P < .0001). Average Medicare payments were $1,318 less than costs for CEA and $3,241 less than costs for CAS among favorable outcomes. Greater payment-to-cost disparities were noted for both CEA and CAS in patients who had unfavorable outcomes.
CONCLUSIONS:
The 2008 Medicare hospitalization payments were substantially less than median hospital costs for both CAS and CEA. Efforts to decrease hospitalization costs and/or increase payments will be necessary to make these carotid revascularization procedures economically viable for hospitals in the long term.
Carotid revascularization therapies have been shown to reduce the incidence of stroke among patients with atherosclerotic carotid artery stenosis.1 Although CEA remains the most common surgical method for revascularization, CAS deployment has gained support over the past decade. Outcomes from the recent CREST revealed that patients older than 69 years of age had better outcomes with CEA, while patients younger than 69 years of age had better outcomes with stent placement.2 Although the results from CREST demonstrate clinical utility for both therapeutic modalities, particularly among asymptomatic presentations, scant large-scale data exist to identify potential disparities between costs and reimbursements among both favorable and unfavorable postoperative outcomes. As asymptomatic presentations represent most revascularization procedures and are more likely to have fewer confounding peri-procedural cost variables, we focused our cost analysis on this subset of individuals. In the current study, we evaluated the NIS data from 2001 to 2008 to ascertain costs associated with hospitalization for carotid revascularization in asymptomatic patients in order to identify variables predictive of hospital costs and to determine if Medicare reimbursements are sufficient to account for these costs.
Materials and Methods
Data Acquisition
ICD-9 procedure codes were used to independently identify cases of CEA (38.12, available from 2001–2008) and CAS (00.63, available from 2004–2008) from the 2001–2008 NIS hospital discharge data base (Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality, Rockville, Maryland).3 Age, sex, discharge status (including in-hospital mortality), LOS, total hospital charges, and hospital-specific mean cost-to-charge ratios were extracted from the NIS dataset for each procedure (CEA, CAS). Discharge status was grouped into 3 categories: “unfavorable discharges” requiring skilled care (STH, SNF, HHC), “favorable discharges” to home, and “in-hospital mortality.”
Asymptomatic cases (ICD-9: 433.10, 433,30) were included for further analysis, while symptomatic cases of carotid artery occlusion, as determined by the presence of transient ischemic attack (ICD-9: 435.9, 362.34) and/or carotid artery occlusion with cerebral infarction (ICD-9: 433.11), were excluded. Among procedures performed on asymptomatic patients, postoperative complications including stroke (ICD-9: 997.02) and cardiovascular complications (ICD-9: 997.1) were identified. CCS codes, available from HCUP, were used in concert with ICD-9 codes to identify pre-existing comorbid conditions within patient cohorts. These conditions included ARF (CCS: 157), CRF (CCS: 158), MI (CCS: 100), CHF (CCS: 108), COPD (CCS: 127), DM (CCS: 49), HTN (CCS: 98), CVD (ICD-9: 424.0–424.3), and HLD (CCS: 53).
Hospital costs were the primary end point examined in this analysis. Hospital costs were determined by multiplying the total hospital charges, documented in the NIS core file, by the weighted GAPICC ratio for each hospital on record in the NIS annual hospital file data.4 This conversion was performed to account for differences in operational expenses and billing practices. Inflation adjustment over the 8-year period was performed by using the consumer price index calculator,5 and total costs were adjusted to 2008 US dollar amounts unless otherwise specified. Medicare reimbursement schedules were obtained from the CMS Web site.6
Statistical Analysis and Multivariate Regression Models
All statistical analyses were performed by using software (JMP, version 9; SAS, version 9; SAS Institute, Cary, North Carolina). Significance was estimated by using a P value of less than .05. Data were presented as median scores with IQR due to non-normal data distributions, unless otherwise specified.7 Trends over time in hospital costs were detected by using the Sen slope method (Q score) as a test for both magnitude and significance.8 Significant relationships between costs and potential predictors were determined by using the Wilcoxon signed-rank test. Significant differences among 3 or more data groupings (discharge status, comorbidity) were determined by using ANOVA tests. Post hoc Tukey HSD tests were performed on significant ANOVA results to identify significant paired comparisons.
Predictors of hospital costs and lengths of stay for CEA and CAS were determined via multivariate standard least-squares regression analysis by using an identity-linkage function. Age, sex, discharge status, complications, and comorbidities were transformed to binary responses and treated as effect variables in these 3 models. Estimate coefficients found to be significant represented the predicted change in predictor variable (LOS or cost) for every unit change in effect variable. Predicted intercept data were presented as the unadjusted response variable for each model in the modified Forest plot. Adjustment from each estimate variable was shown as a deviation from this baseline value.
Results
Demographic, Outcome, and Cost Data
Among asymptomatic cases retrieved from the 2001–2008 NIS, 181,200 CEA procedures and 12,485 CAS procedures were recorded within 57,663,486 hospital admissions records, representing 0.31% and 0.02% of all recorded hospitalizations, respectively. The demographic characteristics and hospital outcomes, including cost, length of stay, and discharge status of patients undergoing these therapies, are shown in Table 1.
Table 1:
Demographic characteristics of carotid endarterectomy and carotid stenting recipients (NIS data 2001–2008)
| CEA (2001–2008) | CAS (2004–2008) | |
|---|---|---|
| Total Population | 181 200 | 12 485 |
| Gender | ||
| Male | 103 817 (57) | 7486 (60) |
| Female | 77 255 (43) | 4997 (40) |
| Age | ||
| <69 | 42 198 (23) | 3159 (25) |
| ≥69 | 138 970 (77) | 9326 (75) |
| Discharge Status | ||
| Home | 159 701 (88) | 11 097 (89) |
| Short-term facility | 528 (0.3) | 72 (0.6) |
| Skilled nursing facility | 8823 (4.8) | 704 (5.6) |
| Home health care | 10 926 (6.0) | 487 (3.9) |
| Death | 1020 (0.6) | 100 (0.8) |
| Complications | ||
| Stroke | 1799 (1.0) | 208 (1.7) |
| Cardiac complications | 3704 (2.0) | 287 (2.3) |
| Comorbidities | ||
| Acute renal failure | 643 (0.4) | 246 (2) |
| Chronic renal failure | 1564 (0.9) | 789 (6) |
| Myocardial infarction | 438 (0.2) | 178 (1) |
| Congestive heart failure | 2965 (1.6) | 1420 (11) |
| COPD | 4622 (2.6) | 2140 (17) |
| Diabetes | 15 131 (8.4) | 3249 (26) |
| Hypertension | 37 065 (20.4) | 8052 (64) |
| Valvular disease | 8273 (4.6) | 641 (5) |
| Hyperlipidemia | 30 501 (16.8) | 6566 (53) |
Changes Over Time in Hospital Costs and Reimbursements for CEA and CAS
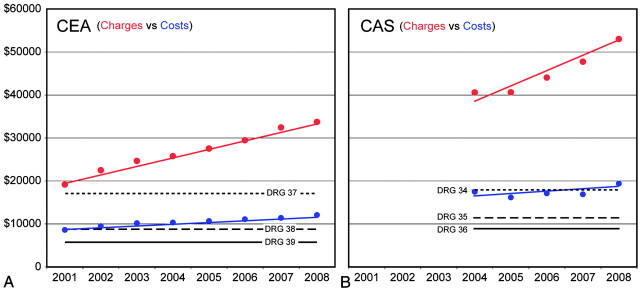
Yearly uncorrected mean hospital costs, charges, and 2008 Medicare average reimbursement rates for CEA and CAS are shown in Fig 1. As hospital charges vary considerably with operational overhead, hospital costs represent a more standardized means to account for these differences. The 2008 reimbursement schedule, representing the average Medicare reimbursement amounts for CEA and CAS, is shown in Table 2. In both CEA and CAS cases, Medicare reimbursement for uncomplicated cases was found to be significantly lower than hospital costs for similar outcomes (CEA, P < .0001; CAS, P < .0001). Trend analysis revealed that unadjusted hospital costs for both procedures have been significantly increasing over time (CEA: Q = 421, P < .001; CAS: Q = 424, P < .01).
Fig 1.
Annual hospital charges and costs for CEA and CAS. Annual (mean ± SD) unadjusted hospital charges (red circles) and costs (blue circles) are shown for CEA (left) and CAS (right). Sen slope trend-lines are shown for each annual data series (colored lines). 2008 stratified CMS average payments, based upon DRG codes for CEA and CAS, are overlaid in A and B, respectively (Table 2, solid line: no complication or comorbidity; dashed line: minor complication or comorbidity; dotted line: major complication or comorbidity).
Table 2:
CMS reimbursement schedule for CEA and CAS
| 2008 CMS Average Reimbursement | DRG Code | U.S. Dollars |
|---|---|---|
| CEA | ||
| Without comorbidities or complications | (DRG 039) | $ 5,909 |
| With comorbidities or complications | (DRG 038) | $ 7,955 |
| With major comorbidities or complications | (DRG 037) | $12,258 |
| CAS | ||
| Without comorbidities or complications | (DRG 036) | $ 9,196 |
| With comorbidities or complications | (DRG 035) | $10,290 |
| With major comorbidities or complications | (DRG 034) | $13,779 |
LOS Analysis
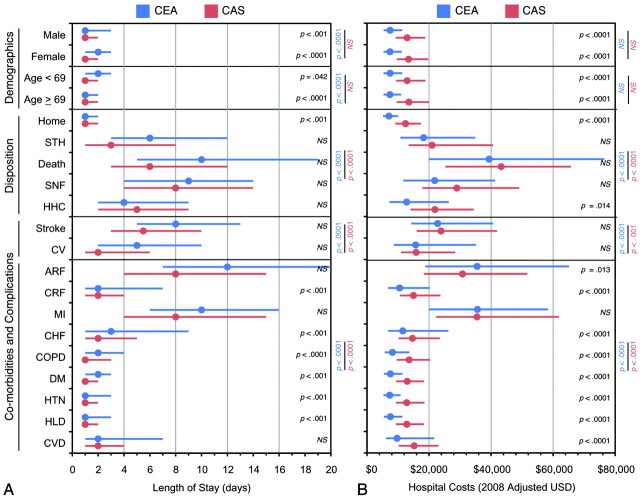
The relationship between patient and outcome variables with LOS, sorted by revascularization procedure, is shown in Fig 2 A. Significance for each intervariable (CEA vs CAS) and paired-variable response test (CAS variable 1 vs 2, CEA variable 1 vs 2) is shown separately. When CEA and CAS were examined separately, female sex (CEA only), age >69 years of age (CEA only), and unfavorable discharges (CEA and CAS) were associated with significantly increased LOS relative to males, age <69, and discharges to home, respectively. For both procedures, stroke was associated with significantly longer LOS relative to CV complications, and severe comorbidities (ARF, MI, CHF) significantly increased LOS by as much as 10–12 days relative to less severe comorbid conditions (DM, HTN, HLD). When CEA and CAS were directly compared, recipients of CAS had significantly shorter LOS among both genders, age strata, patients discharged to home, and among selected comorbidities (CRF, CHF, COPD, DM, HTN, HLD). Lengths of stay among unfavorable discharges and among procedures associated with postoperative complications were not significantly different between CEA and CAS.
Fig 2.
Demographic and outcome variables affecting length of stay and hospital costs for CEA and CAS. Median ± IQR data are shown for LOS (A) and 2008-adjusted hospital costs (B) among preselected demographic and treatment variables for CEA (blue) and CAS (red). Circles represent median values and lines represent interquartile ranges. Tests for significance are color coded as follows: intervariable test CEA vs CAS (black); intravariable tests for CEA (blue) and CAS (red).
Hospital Cost Analysis
The relationship between patient and outcome variables with hospital costs, sorted by revascularization procedure, is shown in Fig 2B. Significance for each intervariable (CEA vs CAS) and paired-variable response test (CAS variable 1 vs 2, CEA variable 1 vs 2) is shown. When CEA and CAS were examined separately, unfavorable discharges, relative to discharges to home, were associated with significantly higher hospitalization costs (CEA: F = 446, P < .0001; CAS: F = 447, P < .0001). Hospital costs did not significantly vary with sex or age stratum. In both procedures, stroke was associated with significantly higher costs relative to postoperative CV complications; and severe comorbidities (ARF, MI, CHF) significantly increased costs relative to less severe comorbid conditions (CRF, CHF, COPD, DM, HTN, HLD; CEA: F = 446, P < .0001; CAS: F = 447, P < .0001). When CEA and CAS were compared directly, recipients of CAS had significantly higher hospitalization costs among both genders, age strata, patients who discharged to home, patients discharged to home health care environments, and among selected comorbidities (COPD, DM, HTN, HLD, and valvular disease).
Multivariate Model Results
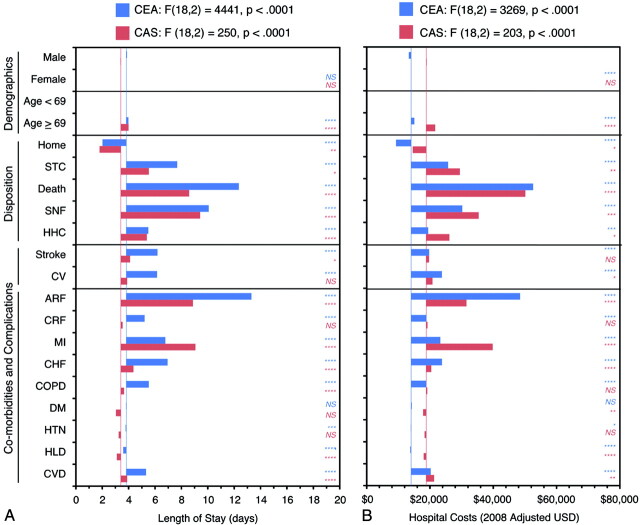
Multivariate regression analysis, shown in Fig 3, was performed to extricate the individual effects of highly interrelated variables (eg, demographics, discharge outcomes, clinical outcomes, and common comorbidities), and revealed numerous demographic and clinical outcomes to be predictive of length of stay and hospital costs among CEA and CAS procedures. Unadjusted values for LOS (CEA, 3.8 days; CAS, 3.4 days) and costs (CEA, $13,740; CAS, $18,837) are represented by the blue (CEA) and red (CAS) vertical lines. Sex was not predictive of significantly altered LOS, but was predictive of modestly lower hospital costs ($775) among CEA cases but not among CAS cases. Patients >69 years of age were predicted to have slightly longer hospitalization periods (CEA: 0.15 days, P < .0001; CAS: 0.59 days, P < .0001) and incur higher hospital costs (CEA: $990, P < .0001; CAS: $2,864, P < .0001). Discharge to home was the strongest negative predictor of LOS (CEA: −1.7 days, P < .0001; CAS: −1.8 days, P = .0089) and hospital cost (CEA: −$4,859, P < .0001; CAS: −$4,250, P < .0001). In all cases, unfavorable discharges were predictive of significantly longer LOS (CEA: P < .0001, all cases; CAS: P ≤ .0445, all cases) and higher costs (CEA: P < .0001, all cases; CAS: P ≤ .0261, all cases), particularly in hospital death and discharges to a skilled nursing facility. Postoperative complications following CEA were more likely to extend hospitalization time (stroke, 2.4 days; CV, 2.3 days) and increase hospital costs (stroke, $5,680; CV, $9,663), whereas similar complications were less predictive of increases following CAS. Severe comorbidities (ARF, MI, CHF) led to the greatest increases in length of stay and hospital costs for both procedures, yet subtle differences were observed. CEA recipients with acute renal failure were predicted to have more extended, costly hospitalization (LOS, 9.5 day increase; costs, $34,413 increase) compared with CAS recipients (LOS, 5.5 day increase; costs, $12,778 increase). In contrast, CAS recipients with documented myocardial infarction were predicted to have more extended, costly hospitalization (LOS, 5.7 day increase; costs, $21,059 increase) compared with CEA recipients (LOS, 2.9 day increase; costs, $9205 increase). In contrast, most minor comorbid conditions, with the exception of valvular heart disease, were predicted to only modestly affect LOS or costs.
Fig 3.
Predictors of length of stay and hospital costs for CEA and CAS by using multivariate regression analysis. Regression estimates for demographic and treatment variables for CEA (blue) and CAS (red) are shown for LOS (A) and 2008-adjusted hospital costs (B). The unadjusted response variable for each model, representing the baseline model value, is represented by the vertical colored lines (CEA, blue; CAS, red). Variable adjustments are shown by positive or negative bar-graph deflections from this baseline value. Regression results for each model are shown. Significance for each variable is coded as follows: ****P < .0001; ***P < .001; **P < .01; *P < .05; NS P ≥ .05).
Medicare Payment versus Hospital Cost Analysis
Data from the 2001–2008 NIS data base revealed that the 2008 adjusted cost of hospitalization with a favorable discharge outcome were $7,375 for endarterectomy and $12,668 for carotid stent placement. The median costs for uncomplicated CEA and CAS were $1,466 and $3,272 more than the average 2008 Medicare payments for CEA and CAS, respectively (Table 2). Complications and comorbidies added to the hospitalization costs (Figs 2 and 3). For example, patients with chronic renal insufficiency, a minor CC, incurred median hospitalization costs of $10,802 for CEA and $15,216 for CAS; and patients with myocardial infarction, a severe comorbid condition, incurred median hospitalization costs of $35,657 for CEA and $35,529 for CAS. The disparities between hospitalization costs and Medicare payments were substantially higher among these complicated discharges. Based upon our findings, median hospital costs exceeded average Medicare payments as follows: $2,847 for CEA and $4,926 for CAS in cases involving intermediate CC, and $23,399 for CEA and $21,750 for CAS in cases with severe comorbid condition complications.
Discussion
Our study reveals that, even among the cohorts for which costs would be most modest, for example, patients with the best clinical outcomes, Medicare reimbursement is substantially less than costs incurred by hospitals associated with CEA and CAS procedures. Furthermore, the hospitalization costs associated with CAS are approximately $5,000 higher than CEA for patients with favorable outcomes. Presumably, this relates, in large part, to costs of the endovascular devices used for CAS. However, the average 2008 Medicare payment for an uncomplicated CAS (DRG 36) was only $3,287 more than the payment for uncomplicated CEA (DRG 39), so hospitals may be at more risk for financial loss with CAS than with CEA.
Beginning in 2008, Medicare payments for CAS and CEA changed to account for patients with moderate and major comorbidities and complications (Table 2). Among uncomplicated procedures representing 90% of all cases examined, the 2008 modifications to the Medicare reimbursement schedule continue to inadequately cover even the most favorable outcomes. The remaining 10% of procedures involved complications and comorbidities that were anticipated to lengthen hospitalization and increase fees. In these cases, where hospital fees were 4 or more times higher than favorable outcomes, 2008 Medicare payment rates result in an even larger disparity between cost and reimbursement. Because the Medicare payment schedule is not designed to keep pace with the inflationary trends in hospitalization costs, the disparity between payments and costs would not be expected to improve over time.
Finally, our data show that in-hospital death, though uncommon, incurs the highest median hospital costs and displays the widest interquartile range. This observation suggests that hospitals vary considerably with respect to their management of the most severe complications. Because in-hospital death was not associated with the longer lengths of stay, we assume these additional fees are a result of additional procedural interventions. These data further demonstrate that the difference between hospital costs and Medicare payments is larger for major complications and argue for revision and/or restratification of the complication reimbursement schedule.
This study has several limitations due to utilization of an extremely large retrospective data base with a predefined data structure. First, coding errors are a well-established limitation of such data sources and have been shown to exist within the NIS data.9 Yet it has been suggested that such errors follow a random distribution and do not manifest in a systemic fashion, and thus do not impact the statistical results because they are diluted within the enormous size of the NIS dataset. Second, the predefined data limits of the NIS prevent us from ascertaining causal relationships and outcomes following hospitalization that may better explain observed effects in statistical regression models. Third, hospital charges within the NIS are not itemized in a manner that would permit isolation of the procedural cost versus hospitalization costs. However, in the context of this study, analysis of nonitemized charges may be more reflective of the true costs of hospitalization for each of the respective procedures. Fourth, although we provide the hospital charge data in Fig 1, caution is urged in using this metric, as hospital costs are a more reliable measurement of the normalized expenditure for each procedure.
Conclusions
Our findings indicate that typical costs for hospitalization for both carotid endarterectomy and carotid stent placement exceed payments under the revised CMS reimbursement system, regardless of outcome. Efforts to decrease hospitalization costs and/or increase payments will be necessary to make these carotid revascularization procedures economically viable in the long term.
ABBREVIATIONS:
- ARF
acute renal failure
- CAS
carotid artery stent
- CC
comorbid condition
- CCS
clinical classification software
- CEA
carotid endarterectomy
- CHF
congestive heart failure
- CMS
Centers for Medicare & Medicaid Services
- COPD
chronic obstructive pulmonary disease
- CREST
Carotid Revascularization Endarterectomy versus Stent placement Trial
- CRF
chronic renal failure
- CV
cardiovascular
- CVD
cardiac valve disease
- DM
diabetes mellitus
- DRG
diagnosis related group
- GAPICC
group average payer inpatient cost-to-charge
- HCUP
Healthcare Cost and Utilization Project
- HHC
home health care
- HLD
hyperlipidemia
- HSD
honestly significantly different
- HTN
hypertension
- ICD9
international classification of diseases, 9th edition
- IQR
interquartile range
- LOS
length of stay
- MI
myocardial infarction
- NIS
National Inpatient Sample
- SNF
skilled nursing facility
- STH
short-term hospitalization
Footnotes
Disclosures: David Kallmes—UNRELATED: Grants/Grants Pending: eV3, Micrus, MicroVention, NFocus, Sequent, Cook, ArthroCare, Stryker; Royalties: UVA patent foundation, Comments: Fusion patent; Payment for Development of Educational Presentations: CareFusion, eV3. Harry J. Cloft—UNRELATED: Grants/Grants Pending: Cordis.* (*Money paid to institution).
References
- 1. Levy EI, Mocco J, Samuelson RM, et al. Optimal treatment of carotid artery disease. J Am Coll Cardiol 2008; 51: 979– 85 [DOI] [PubMed] [Google Scholar]
- 2. Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363: 11– 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Healthcare Cost and Utilization Project (HCUP). HCUP Database website. 1998–2004. Agency for Healthcare Research and Quality, Rockville, Maryland. http://www.hcup-us.ahrq.gov/databases.jsp. Accessed Feb 20, 2011 [Google Scholar]
- 4. Healthcare Cost and Utilization Project (HCUP). Cost-to-charge ratio. 1998–2004. Agency for Healthcare Research and Quality, Rockville, Maryland. http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp. Accessed Feb 20, 2011 [Google Scholar]
- 5.Bureau of Labor Statistics. Consumer Price Index Inflation Calculator. Website calculator. [Accessed Feb 20, 2011]. http://www.bls.gov/data/inflation_calculator.htm.
- 6.Centers for Medicare & Medicaid Services. CMS website. [Accessed Feb 18, 2011]. http://www.cms.gov.
- 7. Qualls M, Pallin DJ, Schuur JD. Parametric versus nonparametric statistical tests: The length of stay example. Acad Emerg Med 2010; 17: 1113– 21 [DOI] [PubMed] [Google Scholar]
- 8. Salma T, Anttila P, Ruoho-Airola T, et al. Publications on air quality. No 31. Detecting trends of annual values of atmospheric pollutants by the Mann-Kendall test and Sen's slope estimates. Helsinki, Finland: Finnish Meteorological Institute; 2002 [Google Scholar]
- 9. Berthelsen CL. Evaluation of coding data quality of the HCUP National Inpatient Sample. Top Health Inf Manage 2000; 21: 10– 23 [PubMed] [Google Scholar]