SUMMARY:
Autosomal dominant polycystic kidney disease is a genetic disorder affecting 1 in 1000 people worldwide and is associated with an increased risk of intracranial aneurysms. It remains unclear whether there is sufficient net benefit to screening this patient population for IA, considering recent developments in imaging and treatment and our evolving understanding of the natural history of unruptured aneurysms. There is currently no standardized screening protocol for IA in patients with ADPCKD. Our review of the literature focused on the above issues and presents our appraisal of the estimated value of screening for IA in the setting of ADPCKD.
Autosomal dominant polycystic kidney disease is a genetic disorder affecting 1 in 1000 people worldwide and is associated with an increased risk of intracranial aneurysms.1 The average life expectancy of a patient with ADPCKD ranges from 53 to 70 years, depending on the subtype.2 It remains unclear whether there is sufficient net benefit in screening this patient population for IA, considering recent developments in imaging and treatment and evolving understanding of the natural history of unruptured aneurysms. There is currently no standardized screening protocol for IA in patients with ADPCKD.
The criteria supporting an ideal screening program can be organized by the following: 1) disease: sufficient prevalence and morbidity in the target population; 2) diagnosis: technique efficacy and safety; and 3) therapy: treatment efficacy, safety, cost-effectiveness, and improved outcome with early treatment.3,4
The following review of the literature focuses on these criteria and presents our appraisal of the estimated value of screening for IA in the setting of ADPCKD.
Disease
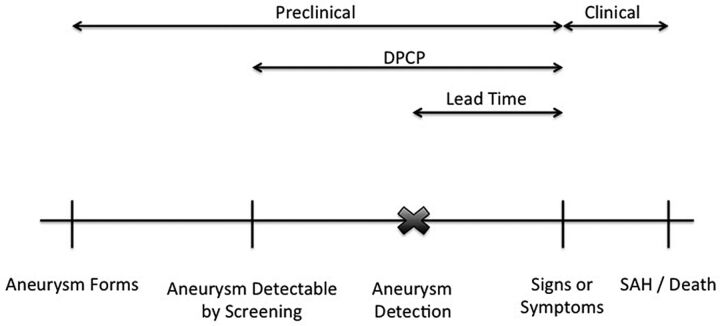
Screening effectiveness is dependent on the natural history of the disease (Fig 1). Specific to IA, the preclinical phase between aneurysm formation and symptom development/rupture can be variable and the clinical phase between symptom development and SAH/death can be short or nonexistent. The lead time (the time between aneurysm formation and the development of signs/symptoms) determines screening frequency and feasibility.
Fig 1.
Specific to IA, the preclinical phase between formation and symptom development/rupture can be quite variable, and the clinical phase between symptom development and SAH/death can be quite short or nonexistent. Lead time is dependent on screening frequency. DPCP indicates detectable preclinical phase. Adapted from Morrison65 by permission of Oxford University Press, USA.
Prevalence
ADPCKD has been associated with a widely variable reported prevalence of IA: 4%,5,6 5%,7 10%,8 11.7%,9 14%,10 22.5%,5 and 41.2%.11 Of the 18%–22% of patients with ADPCKD who also have a family history of IA, reported prevalence rates are more tightly grouped (ie, 22%,7 25.8%,9 and 27.3%5,10,12). In comparison, the general population carries an estimated prevalence of 0.4%–6%, depending on the study.8 Most retrospective autopsy series and reviews put the general population prevalence between 2% and 3%.13–16
In 1 large meta-analysis, ADPCKD was found to be the single greatest risk factor for IA development—greater than atherosclerosis and family history of IA.8 In fact, 10% of patients with undiagnosed ADPCKD will have IA rupture as their presenting symptom,13 and 6% of all patients with ADPCKD will die due to subarachnoid hemorrhage.17 IA rupture is considered the most severe complication of ADPCKD.18
The largest prospective screening study to date was recently published by Xu et al,19 with a sample size of 355 patients with ADPCKD screened by 3T time-of-flight MRA. Of these, 12.4% were found to have an IA, with the percentage increasing to 21.6% in patients with a family history of IA or hemorrhagic stroke versus 11% prevalence in those without these risk factors. The prevalence was found to be as high as 23.3% in the 60- to 69-year-old group. All aneurysms, except for 1 giant aneurysm (25 mm), were <10 mm, though 11% were >7 mm (ie, of the original group, 1.36% had an aneurysm of >7 mm). All aneurysms were located in the anterior circulation (49% in the ICA, 26% in the MCA, 25% in the anterior communicating artery, and 2% in the anterior cerebral artery). Multiple aneurysms were found in 18.2% of patients. No correlation was found with hypertension, renal function, liver cysts, or duration of disease.
Data from the International Study of Unruptured Intracranial Aneurysms (ISUIA),20 Stehbens,16 and Fox15 used to represent the general population data can be compared with those in larger series studies on patients with ADPCKD by Xu et al (n = 355),19 Schievink et al (n = 41),5 and Chauveau et al (n = 71) (Table 1).12
Table 1:
Aneurysm characteristics in patients with ADPCKD versus the general population
| Characteristics | General Population | ADPCKD |
|---|---|---|
| Male/female distribution | 71%–76% female | 46%–58% female |
| Aneurysm location | 80%–90% anterior circulation | 90%–100% anterior circulation |
| Multiple aneurysms | 35% | 18%–31% |
Morbidity
Intracranial aneurysms can cause local mass effect and thromboembolism; however, the most feared event is aneurysm rupture causing catastrophic SAH. Currently, the best prospective data regarding rupture risk come from the ISUIA trial.20 These data support a 5-year cumulative rupture rate of 0%–50%, depending on the location and size of the aneurysm (Table 2), with anterior circulation aneurysms of <7 mm having extremely low rates of rupture. Following the release of these data, the neurosurgical community exposed several weaknesses of the ISUIA study design. Specifically, ISUIA conducted a prospective but nonrandomized recruitment, predisposing it to selection bias in which small aneurysms deemed to be higher risk could have been excluded and prophylactically treated at the discretion of neurosurgeons on the basis of morphology or other risk factors (hypertension, smoking, alcohol abuse, family history). Patients with unruptured aneurysms were invited for inclusion, and their SAH-free survival was backdated to the time of their aneurysm diagnosis, whereas patients experiencing an SAH may not have been able to respond. Most controversial was the discrepancy of the ISUIA findings based on the fact that a large proportion of ruptured IAs present with sizes <7 mm. One published 10-year series revealed a mean ruptured aneurysm diameter of only 7.5 mm.21 Finally, the subanalyses and post hoc grouping of the aneurysm sizes and locations rather than prospective validation of an a priori hypothesis were criticized.
Table 2:
Five-year cumulative rupture rates from the ISUIA trial for patients without a history of SAH
| Artery | <7 mm | 7–12 mm | 13–24 mm | ≥25 mm |
|---|---|---|---|---|
| Cavernous carotid | 0% | 0% | 3% | 6.4% |
| ACA/MCA/ICA | 0% | 2.6% | 14.5% | 40% |
| PCA, PcomA, vertebrobasilar | 2.5% | 14.5% | 18.4% | 50% |
Note:—PCA indicates posterior cerebral artery; PcomA, posterior communicating artery; ACA, anterior cerebral artery.
Several explanations have attempted to reconcile the apparent size discrepancy for IA risk stratification. Wiebers et al22 suggested that aneurysms initially develop during a short time (hours to weeks), enlarging to a diameter that is constrained by the elasticity limits of the artery. If the aneurysm does not exceed the elasticity limit and remains unruptured, it undergoes compensatory stabilization via collagen formation. Once stabilized, however, larger aneurysms are at greater risk for further growth and rupture due to increased wall stress, according to the law of Laplace (exponentially increased wall stress with larger diameters). Matsubara et al23 supported this theory; in their 17-month study, aneurysm growth/bleb formation directly correlated with aneurysm size because only 2.4% (<5 mm) and 9.1% (5–9 mm) of smaller aneurysms progressed versus 50% (10–20 mm) of larger aneurysms. Further analysis revealed that the percentage of aneurysms exhibiting growth increased with time: 1-, 2-, and 3-year cumulative rupture risks were 2.5%, 8%, and 17.6%. Burns et al24 conducted a similar study with a longer follow-up of 47 months. The risk of aneurysm enlargement again correlated with aneurysm size: 6.9% (<8 mm), 25% (8–12 mm), and 83% (>13 mm).
Gibbs et al25 evaluated 21 patients with ADPCKD with documented asymptomatic IA detected on screening examinations. All aneurysms were small (<6.5 mm). On follow-up, 1 aneurysm grew (by 1 mm), and 1 patient developed a new MCA aneurysm (2 mm) during the screening period (mean, 81 months). The authors recommended screening only patients with ADPCKD and a family history of aneurysm rupture or SAH. Because nearly 10% of the patients in this small study exhibited aneurysm growth or new aneurysm development, the reasoning behind the authors' conclusions is unclear. Their analysis was further complicated by nonsurgical interventions, including aggressive antihypertensive therapy, lipid control, and tobacco cessation, which could potentially lower the risk of aneurysm growth and rupture.
A larger study by Schrier et al26 evaluated the efficacy of rescreening 76 patients with ADPCKD 10 years after an initial screening examination with negative findings by using CTA, MRA, or DSA (depending on patient-specific factors). They found that 2 patients developed aneurysms in the interval period, though 1 may have previously existed on retrospective analysis. During 10 years, 1 patient developed a 10-mm vertebral artery aneurysm, a 10-mm extracranial ICA aneurysm, and a 4-mm MCA aneurysm requiring surgical clipping.
Following IA rupture, the patient faces a mortality risk ranging from 10% to 67%, depending on the study, with another 10%–38% becoming permanently dependent and disabled.12,27 Patients with ADPCKD with IA rupture have a mortality rate similar to that of the general population (46%),13 though they present with SAH earlier in life, with a mean age of 35–45 years, with 64% of aneurysms rupturing before the patient reaches 50 years of age.5,13,28,29 In a single study, 10% of aneurysms in patients with ADPCKD ruptured before the patients reached 21 years of age,13 significantly earlier than the rate of the general population (mean age, 50–54 years).27,30
Diagnosis
Imaging Modalities
Nonimaging-based screening/risk-stratification methods have been evaluated with little success. Markers such as renal function do not correlate with the risk of having an IA; up to 50% of patients with ADPCKD with IAs have completely normal renal function.31 No correlation has been found with liver cysts or mitral valve abnormalities, and 29% of these patients were normotensive.13 A correlation has been found with the duration of hypertension, but not with hypertension itself or duration since the diagnosis of ADPCKD.21
Evidence is emerging that the associated vascular defects in ADPCKD may be due to mutations in the PKD1 and PKD2 genes (located on the short arm of chromosome 16).32,33 Abnormalities of these genes in mouse models correspond with increased rates of arterial dissection, arterial rupture, and intracranial vascular abnormalities.34 There is some evidence that the location of the genetic mutation may help prognosticate risk for IA in patients with ADPCKD (mutations in the 5′ region of PKD1 pose a higher risk than those at the 3′ end), though further investigation would be needed before this information could be used in a clinical setting.35
Traditionally, the criterion standard for IA evaluation has been conventional angiography or DSA, which yields a high spatial resolution of 0.1-mm2 pixels, optimum contrast due to direct intra-arterial bolus delivery with background subtraction, and high temporal resolution (2–6 frames/s) that can depict flow patterns within an aneurysm. Modern flat-panel 3D DSA acquisitions can generate an isotropic volume dataset of 0.2 mm3, which can be visualized as a reconstructed 3D model or 2D multiplanar sections. The quality of DSA is operator-dependent, varying with the degree of vessel superselection, injection rates and volumes, and number of projections, including any supplementary 3D DSA with postprocessed reconstructions. Regions of competitive flow such as at the vertebral-basilar junction and anterior communicating artery may be difficult to opacify due to a lack of contrast in the blood pool. Differences in geographic distortion and 2D planar imaging views can make subtle changes in aneurysm size difficult to assess on serial studies, unless 3D imaging is performed.
Recent advances in CT and MR imaging technology have made these modalities increasingly attractive options for IA screening. Multidetector CTA and 3T time-of-flight MRA can generate spatial resolutions of about 0.5-mm2 per pixel, whereas previous generation scanners provided a resolution of 1 mm2 per pixel. CTA relies on attenuation differences between iodinated contrast and surrounding tissues that can be suboptimal at the skull base in the presence of heavy mural calcification or metallic hardware. In fact, embolization coils and microsurgical clips used to treat IAs will cause metallic streak and beam-hardening artifacts on CTA, often yielding nondiagnostic scans. Furthermore, venous contamination seen with inadequately bolus-timed CTA can also limit assessment of the intracranial circulation.
Time-of-flight MRA relies predominantly on flow-related signal and, combined with suppression techniques, gives excellent contrast resolution with negligible interfering venous signal. Decreased temporal resolution with MRA predisposes it to motion artifacts, resulting in acquisition times that may require >5 minutes. In addition, turbulent flow can result in rapid dephasing and intraluminal signal loss, though these issues are less problematic on 3T MR imaging platforms. Contrast-enhanced TOF MRA is marred by venous signal, and the limited time window for dynamic contrast-enhanced arterial phase MRA results in relatively decreased spatial resolution. MR imaging–based techniques are sensitive to metallic susceptibility artifacts from either dental hardware or implanted devices and, at times, degrade images to nondiagnostic quality, as encountered with microsurgical aneurysm clips. Intraluminal stents are difficult to assess with noninvasive methods due to their intraluminal placement and cause moderate susceptibility artifacts on MRA. Although they may be better assessed with a postgadolinium protocol, confirmation with CTA or invasive DSA may be required for definitive diagnosis. Conversely, endovascular coils cause minimal MR imaging artifacts when ultrashort TEs are used with TOF MRA, permitting the assessment of residual filling in coiled aneurysms as an excellent screening technique for aneurysm recurrence.
Efficacy and Safety
Several studies have compared MRA and CTA with DSA with respect to diagnostic accuracy for IAs. According to a recent study, 3T TOF MRA demonstrated a screening sensitivity of 67% for aneurysms of <3 mm, 79% for those of 3–5 mm, and 95% for those of >5 mm.36 Because many patients have multiple aneurysms (∼30%),37 a per-patient screening sensitivity is a more accurate metric for assessing the effectiveness of a technique. In this study, the per-patient screening sensitivity and specificity were 96% and 92%, respectively. Multidetector CTA had a slightly lower per-patient screening sensitivity of 95% and a slightly higher screening specificity of 96%. Another recent study focused on CTA revealed a similar screening sensitivity of 95% for aneurysms of >7 mm.38 One study focused on small aneurysms (<5 mm) and found CTA sensitivity to be higher than that of DSA with equivalent specificity.39
Due to the relatively invasive nature of DSA and the small but definite risk of stroke as well as the cost, dedicated staff and time commitment, and patient discomfort involved in the procedure, DSA has become less attractive as a screening technique for IA. One study estimates the complication risk at 1.3%, with a 0.5% risk of permanent neurologic complications.40
Noncontrast TOF MRA has become the primary noninvasive screening technique, with the advantage of avoiding the use of potentially nephrotoxic or allergenic contrast media and avoiding the placement of an IV line. Adding a gadolinium-enhanced MRA to the protocol may add the risk of the rare but serious nephrogenic systemic sclerosis. Nephrogenic systemic sclerosis is noted to occur in 1%–7% of patients with a GFR <30 mL/min due to the use of certain gadolinium-based compounds, though it is exceedingly rare in patients with normal GFR.41 General MR imaging contraindications include pacemakers, other implanted hardware, and occasionally claustrophobia. All intracranial clips will cause prominent susceptibility artifacts, and some older clips are MR imaging incompatible due to the risk of clip movement. All endovascular coils, stents, and liquid embolic materials are MR imaging compatible.
Because 50% of patients with ADPCKD with aneurysms have normal renal function and many others have only slightly altered creatinine clearance, CTA using iodinated contrast media remains a viable option for many potential screening subjects. Risks of CTA include allergic reactions (0.18%–0.6%),41 contrast-induced nephrotoxicity or acute tubular necrosis, radiation, and intravenous extravasations. Contrast nephropathy is dependent on numerous factors including acute renal failure, dehydration, and elevated serum creatinine concentration or reduced GFR (<60 mL/min), though a serum creatinine level of <2 mg/dL is considered to be a sufficiently low risk for most patients with chronic renal failure.41 The radiation dose following a CTA of the head ranges from 1.6–1.9 mSv,42 which is roughly equivalent to the dose of an x-ray of the spine, 16 chest x-rays, or 7 months of background radiation.43 Comparatively, the radiation dose for a noncontrast head CT is 1.7–2.7 mSv.42
Cost
The cost of diagnostic imaging procedures varies among institutions. The 2012 Medicare technical reimbursement rate and the 2010 National 50th Percentile charge provide reliable figures for comparison (KnowledgeSource version 4.9.1; MedAssets, Alpharetta, Georgia). For CTA, the figures for Medicare and the National 50th Percentile are $740 and $2000 respectively. For MRA without contrast, the figures are $718 and $2050, whereas adding gadolinium to the study increases the reimbursement by $300. Thus, there is no significant difference in the up-front cost of CTA versus MRA. In our experience, a small number of patients will require a CTA to clarify a finding on an initial MRA, whereas the opposite almost never occurs. With more widespread use of 3T MRA, this should become less common. Less measurable cost worth considering is incurred by the use of sedation with postprocedure observation, which would be more common with MRA due to the longer procedure time, and issues with claustrophobia. CTA carries the risk of contrast reaction or soft-tissue extravasation, which could require a period of observation or admission in the rare severe cases.
Therapy
Following diagnostic screening and discovery of an IA, treatment versus conservative management must be contemplated. If treatment is deemed prudent, further study of the patient and aneurysm anatomy is required to determine whether microsurgical clipping or endovascular coil embolization is the preferred treatment. A multidisciplinary team experienced in both endovascular and microsurgical techniques along with neuro-critical care expertise, usually at a tertiary care center, is the ideal environment. Treatment decisions will depend on aneurysm size, location, anatomy/morphology (narrow/wide neck, saccular versus fusiform, incorporation of parent or branching vessels), patient age, comorbidities, and institutional/individual surgeon outcomes.
Efficacy and Safety
Microsurgical clipping and endovascular coil embolization have their inherent advantages and disadvantages, and a full comparison is beyond the scope of this review. While microsurgical clipping was previously the standard of care, coil embolization has matured rapidly in recent years and is a more frequent treatment alternative with technological advancements and the advent of adjunctive balloon and stent-assisted techniques.44 Numerous studies have shown a lower periprocedural morbidity and mortality associated with endovascular treatment and lower rates of discharge to long-term care facilities.44,45 Some authors have suggested that >90% of aneurysms can now be effectively treated by using endovascular techniques.46 However, the durability of coil embolization remains frequently debated due to the increased risk of residual aneurysm components or neck remnants, interval rupture or rebleeding (<1%), and the additional risk of retreatment. Despite these concerns, the largest and most recent retrospective analysis, by using the National Inpatient Sample data base, noted in-hospital mortality of 1.2% for clipping and 0.6% for coiling of unruptured aneurysms. In this study, the combined morbidity and mortality from coiling decreased from 6.2% to 4.3% versus clipping, which decreased from 16.9% to 13.2% during 2001–2008.44 These outcomes are per procedure, and the risk per patient would increase in cases of retreatment, the latter being negligible after clipping.
Renowden et al47 reported that 6% of 1631 patients required retreatment after coiling of 1834 mostly ruptured aneurysms. They found a lower morbidity in retreatment coiling procedures compared with the initial procedure: 3 cases of thromboembolism from 99 recoiling procedures. Overall, combined morbidity and mortality decreased from 14.8% to 7.6%. The important point is that due to modern techniques and increasing use of endovascular methods, treatment morbidity and mortality have significantly improved in comparison with the older risk-benefit analyses.
Nonsurgical interventions are a reasonable alternative to consider in treatment planning. Long-term hypertension has been correlated with an increased risk of IA rupture.48 Patients on anticoagulants have a doubled mortality rate after IA rupture.49 Other modifiable factors such as smoking, heavy alcohol consumption, oral contraceptive pills, atherosclerosis, ischemic heart disease in women, and hyperlipidemia have all been associated with an increased risk of aneurysmal SAH in the general population.50 These risk factors have not, however, been specifically evaluated in the ADPCKD population. While there is no direct evidence that modification of these risk factors would then decrease the risk of rupture, it would seem judicious to address these factors with all patients after the diagnosis of an IA in the hopes of mitigating their risk.
Risk-Benefit Analysis
Multiple authors have addressed the risks and benefits of screening for IAs and their treatment. Crawley et al51 performed a risk-benefit analysis in 1999 focused on screening patients without polycystic kidney disease with a strong family history of IA and concluded that screening causes net harm. This was the only major analysis with a negative result; explanations include relying on pre-ISUIA data, relatively low MRA sensitivity/specificity (90%), relatively high treatment morbidity and mortality rates (8%), a relatively low incidence (9.8%), and a relatively low 0.8% annual risk of rupture.
One of the most complex risk-benefit analyses to date was performed by Takao and Nojo.52 Data were obtained from the prospective arm of the ISUIA and from large meta-analyses. Fifty years of age was chosen for their model because it approximated the mean age in the ISUIA trial. The choice of a 50-year-old cohort in this study was significant because as discussed earlier, 64% of patients with ADPCKD present with IA rupture before this age. Endovascular treatment was found to be effective from a risk-benefit standpoint in anterior circulation aneurysms of >7 mm, cavernous carotid aneurysms of >13 mm, and posterior circulation aneurysms <24 mm. Surgical repair was effective in anterior circulation aneurysms of >13 mm and in posterior circulation aneurysms of 7–12 mm.
Butler et al53 conducted another comprehensive risk-benefit screening analysis. They determined that a single initial screening MRA in all 20-year-old patients with ADPCKD would increase the mean life expectancy without neurologic disability by 1 year. Hence, a 35-year-old patient would expect a mean 0.5-year increase in life expectancy; but declines in screening value were noted with increasing age. Of screened patients, those who have an aneurysm discovered and then treated will have an additional 10.8 years of life (on average) without neurologic deficit. Mitchell et al54 reported similar findings in their risk-benefit analysis. Patients with a >20-year life expectancy were found to benefit from treatment for all posterior circulation aneurysms and all anterior circulation aneurysms of >7 mm.
Studies specifically evaluating the treatment of IAs in patients with ADPCKD are limited. As described earlier, these patients may have an increased risk of spontaneous dissection due to arterial wall abnormalities secondary to defects in the PKD1 and PKD2 genes. One study by Chapman et al6 showed a higher risk of transient complications after angiography in patients with ADPCKD (25%) versus controls (10%), though the complication rates of both groups appeared markedly elevated. Two of 32 patients with ADPCKD experienced transient carotid artery vasospasm, and 1 had asymptomatic vertebral artery dissection without neurologic sequelae.
Cost-Effectiveness
While average initial hospital costs for endovascular coil embolization and surgical clipping are greater than Medicare reimbursement rates,55 this alone does not address the overall cost of a screening program. Multiple authors have performed elaborate cost-effectiveness analyses regarding aneurysm screening and treatment in this population.
Takao and Nojo52 defined cost-effectiveness as a cost <$100,000 per QALY, a commonly used value in the United States,56 and found both endovascular treatment and surgical clipping to be valuable from a cost perspective in the treatment of IAs of >7 mm. The costs per QALY ranged from as low as $600 for surgical clipping in anterior circulation aneurysms of >25 mm to $482,500 for surgical clipping of posterior circulation aneurysms of <7 mm. If one used prospective data from Xu et al,19 11% of the aneurysms discovered on initial screening in their study could be cost-effectively treated by using the analysis of Takao and Nojo. If one used retrospective data from Schievink et al,5 35%–78% of the aneurysms in patients with ADPCKD could be treated cost-effectively on the basis of this analysis.
Butler et al53 also addressed cost-effectiveness in their previously described study. As long as the aneurysm prevalence was above 1.1%, a general screening program in 20-year-old patients with ADPCKD was shown to be cost saving. Numerous studies have shown the general aneurysm prevalence in patients with ADPCKD to be at least 4%, and Xu et al19 demonstrated the prevalence in patients younger than 29 years of age to be 2.4%. No aneurysm size criteria were used in this study, and all aneurysms were hypothetically repaired surgically. Johnston et al57 determined via their cost-utility analysis that any intracranial aneurysm that is symptomatic or >10 mm or any patient with a prior history of SAH could be cost-effectively treated with a net gain in QALY.
While most aneurysms of <7 mm have a low risk of rupture and may not require treatment, their early detection would result in a more concentrated follow-up, which may detect aneurysm growth indicative of an unstable lesion; the additional cost of such an approach would have to be factored in.
A very recent and detailed analysis by Bor et al58 evaluated and compared numerous screening models. The study was specifically focused on patients (without ADPCKD) with a family history of 2 first-degree relatives with SAH and used an annual IA incidence of 0.3%–0.7% and an annual rupture risk of 1%–2%. While screening was cost-effective regardless of the model chosen, the largest health benefit was obtained by screening every other year from 20–80 years of age at a cost of $20,000/QALY, well within the acceptable range in the United States. Their recommended model was screening every 7 years from 20–80 years of age at a cost of $10,749/QALY, within the acceptable range in the United Kingdom and the Netherlands. The costs shown above compare favorably with those in other accepted screening programs and interventions such as digital mammography, colonoscopies, dialysis, and seatbelt use.59,60
Recommendations
Currently, no official standardized societal recommendations exist for IA screening in patients with ADPCKD, but various recommendations can be found in the literature. They include (only) screening patients with both ADPCKD and a family history of IA or SAH, a prior aneurysm rupture, high-risk occupations, undergoing major elective surgery, or having a “warning headache” or severe anxiety regarding the issue.13,61–63 Weibers et al22 provided recommendations for following unruptured IAs in any high-risk population (including ADPCKD): annual MRA/CTA for 2–5 years, then every 2–5 years thereafter if the aneurysms are stable. Butler et al53 suggested screening every 2–3 years in patients with ADPCKD with a family history of IA and every 5–20 years in those without. Torres et al64 advised rescreening every 5–10 years in patients with initial negative screening examination findings. In patients with ADPCKD with known aneurysms considered suitable for surveillance, they suggested biannual or annual imaging to confirm stability and then transitioning to less frequent intervals. Xu et al19 strongly recommended screening patients with ADPCKD with a family history of IA or SAH, though they did not provide detailed guidelines or specifically comment on patients without this history.
If one incorporates the most recent data regarding the incidence, screening, treatment risk, and cost-benefit analyses, screening patients with ADPCKD via CTA or MRA will improve life expectancy in a cost-effective and, at times, cost-saving manner. We recommend screening all patients with ADPCKD by noncontrast 3T TOF MRA at the time of initial diagnosis with follow-up scans at intervals of, at most, 10 years and as short as 2 years, depending on patient-specific risk factors, including the following: family history of IA or SAH, prior SAH, neurologic symptoms, hypertension, smoking, alcohol abuse, high-risk professions (such as pilots), or those undergoing major elective surgery. We would consider coil embolization of most posterior circulation aneurysms and anterior circulation aneurysms of >7 mm, though treatment of smaller IAs may be contemplated on the basis of additional patient and aneurysm risk factors (including irregular morphology). Patients with aneurysms unsuitable for coil embolization with adjunctive balloon or stent-assisted techniques may be offered surgical clipping on multidisciplinary consensus. Newly diagnosed IAs would undergo biannual TOF MRA imaging for the first 2 years and every 2–5 years thereafter if stable. Appropriate medical management to reduce modifiable risk factors for aneurysm growth/rupture (smoking/alcohol cessation, antihypertensive therapy, and, when possible, avoidance of blood thinners) is also recommended.
ABBREVIATIONS:
- ADPCKD
autosomal dominant polycystic kidney disease
- GFR
glomerular filtration rate
- IA
intracranial aneurysm
- QALY
quality-adjusted life years
- TOF
time-of-flight
REFERENCES
- 1. Dalgaard OZ. Bilateral polycystic disease of the kidneys: a follow-up of 284 patients and their families. Acta Med Scan Suppl 1957;328:1–255 [PubMed] [Google Scholar]
- 2. Wilson P. Polycystic kidney disease. N Engl J Med 2004;350:151–64 [DOI] [PubMed] [Google Scholar]
- 3. Obuchowski NA, Graham RJ, Baker ME, et al. Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol 2001;176:1357–62 [DOI] [PubMed] [Google Scholar]
- 4. Black WC, Welch HG. Screening for disease. AJR Am J Roentgenol 1997;168:3–11 [DOI] [PubMed] [Google Scholar]
- 5. Schievink WI, Torres VE, Piepgras DG, et al. Saccular intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1992;3:88–95 [DOI] [PubMed] [Google Scholar]
- 6. Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneuryms in autosomal dominant polycystic kidney disease. N Engl J Med 1992;327:916–20 [DOI] [PubMed] [Google Scholar]
- 7. Huston J, 3rd, Torres VE, Sulivan PP, et al. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1993;3:1871–77 [DOI] [PubMed] [Google Scholar]
- 8. Rinkel GJ, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29:251–56 [DOI] [PubMed] [Google Scholar]
- 9. Ruggieri PM, Poulos N, Masaryk TJ, et al. Occult intracranial aneurysms in polycystic kidney disease: Screening with MR angiography. Radiology 1994;191:33–39 [DOI] [PubMed] [Google Scholar]
- 10. Graf S, Schischma A, Eberhardt KE, et al. Intracranial aneurysms and dolichoectasia in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2002;17:819–23 [DOI] [PubMed] [Google Scholar]
- 11. Wakabayashi T, Fujita S, Ohbora Y, et al. Polycystic kidney disease and intracranial aneurysms. J Neurosurg 1983;58:488–91 [DOI] [PubMed] [Google Scholar]
- 12. Chauveau D, Pirson Y, Verellen-Dumoulin C, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int 1994;45:1140–46 [DOI] [PubMed] [Google Scholar]
- 13. de la Monte SM, Moore GW, Monk MA, et al. Risk factors for the development and rupture of intracranial berry aneurysms. Am J Med 1985;78:957–64 [DOI] [PubMed] [Google Scholar]
- 14. Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol 1990;34:361–65 [DOI] [PubMed] [Google Scholar]
- 15. Fox JL, ed. Intracranial Aneurysms. New York: Springer-Verlag; 1983:15–18 [Google Scholar]
- 16. Stehbens WE. Pathology of the Cerebral Blood Vessels. St. Louis: Mosby; 1972:351–470 [Google Scholar]
- 17. Fick GM, Johnson AM, Hammond WS, et al. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1995;5:2048–56 [DOI] [PubMed] [Google Scholar]
- 18. Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int 1994;46:951. [DOI] [PubMed] [Google Scholar]
- 19. Xu HW, Yu SQ, Mei CL, et al. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke 2011;42:204–06 [DOI] [PubMed] [Google Scholar]
- 20. Wiebers DO, Wisnant JP, Juston J, 3rd, et al. International Study of Unruptured Intracranial Aneurysms investigators: unruptured intracranial aneurysms—natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–10 [DOI] [PubMed] [Google Scholar]
- 21. Wiebers DO, Whisnant JP, Sundt TM, et al. The significance of unruptured intracranial saccular aneurysms. J Neurosurg 1987;66:23–29 [DOI] [PubMed] [Google Scholar]
- 22. Wiebers DO, Piepgras DG, Meyer FB, et al. Pathogenesis, natural history, and treatment of unruptured intracranial aneurysms. Mayo Clin Proc 2004;79:1572–83 [DOI] [PubMed] [Google Scholar]
- 23. Matsubara S, Hadeishi H, Suzuki A, et al. Incidence and risk factors for the growth of unruptured cerebral aneurysms: observation using serial computerized tomography angiography. J Neurosurg 2004;101:908–14 [DOI] [PubMed] [Google Scholar]
- 24. Burns JD, Huston J, 3rd, Layton KF, et al. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke 2009;40:406–11 [DOI] [PubMed] [Google Scholar]
- 25. Gibbs G, Huston J, Qian Q, et al. Follow-up of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney International 2004;65:1621–27 [DOI] [PubMed] [Google Scholar]
- 26. Schrier R, Belz M, Johnson A, et al. Repeat imaging for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease with initially negative studies: a prospective ten-year follow-up. J Am Soc Nephrol 2004;15:1023–28 [DOI] [PubMed] [Google Scholar]
- 27. Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997;28:660–64 [DOI] [PubMed] [Google Scholar]
- 28. Lozano AM, Leblanc R. Cerebral aneurysms and polycystic kidney disease: a critical review. Can J Neurol Sci 1992;19:222–27 [PubMed] [Google Scholar]
- 29. Chapman AB, Johnson AM, Gabow PA. Intracranial aneurysms in patients with autosomal dominant polycystic kidney disease: how to diagnose and who to screen. Am J Kidney Dis 1993;22:526–31 [DOI] [PubMed] [Google Scholar]
- 30. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2000;31:1843–50 [DOI] [PubMed] [Google Scholar]
- 31. Chauveau D, Pirson Y, Verellen-Dumoulin C, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int 1994;45:1140. [DOI] [PubMed] [Google Scholar]
- 32. Bichet D, Peters D, Patel AJ, et al. Cardiovascular polycystins: insights from autosomal dominant polycystic kidney disease and transgenic animal models. Trends Cardiovasc Med 2006;16:292–98 [DOI] [PubMed] [Google Scholar]
- 33. Kip SN, Hunter LW, Ren Q, et al. [Ca21]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circ Res 2005;96:873–80 [DOI] [PubMed] [Google Scholar]
- 34. Hassane S, Claij N, Lantinga-van Leeuwen IS, et al. Pathogenic sequence for dissecting aneurysm formation in a hypomorphic polycystic kidney disease 1 mouse model. Arterioscler Thromb Vasc Biol 2007;27:2177–83 [DOI] [PubMed] [Google Scholar]
- 35. Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 2003;361:2196. [DOI] [PubMed] [Google Scholar]
- 36. Hiratsuka Y, Miki H, Kiriyama I, et al. Diagnosis of unruptured intracranial aneurysms: 3T MR angiography vs. 64-channel multi-detector row CT angiography. Magn Reson Med Sci 2008;7:169–78 [DOI] [PubMed] [Google Scholar]
- 37. Chauveau D, Pirson Y, Verellen-Dumoulin C, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int 1994;45:1140. [DOI] [PubMed] [Google Scholar]
- 38. van Gelder JM. Computed tomographic angiography for detecting cerebral aneurysms: implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery 2003;53:597–605, discussion 605–06 [DOI] [PubMed] [Google Scholar]
- 39. Villablanca J, Jahan R, Hooshi P, et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. AJNR Am J Neuroradiol 2002;23:1187–98 [PMC free article] [PubMed] [Google Scholar]
- 40. Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003;227:522–28 [DOI] [PubMed] [Google Scholar]
- 41. ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media, Version 8. Reston, Virginia: American College of Radiology; 2012 [Google Scholar]
- 42. Mnyusiwalla A, Aviv R, Symons S. Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiology 2009;51:635–40 [DOI] [PubMed] [Google Scholar]
- 43. RadiologyInfo.org. Radiological Society of North America. What are x-rays and what do they do? 2012. http://www.radiologyinfo.org/en/pdf/sfty_xray.pdf. Accessed September 1, 2012
- 44. Brinjikji W, Rabinstein A, Nasr D, et al. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol 2011;32:1071–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alshekhlee A, Mehta S, Edgell RC, et al. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke 2010;41:1471–76 [DOI] [PubMed] [Google Scholar]
- 46. Pierot L, Spelle L, Vitry F. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008;39:2497–504 [DOI] [PubMed] [Google Scholar]
- 47. Renowden SA, Koumellis P, Benes V, et al. Retreatment of previously embolized cerebral aneurysms: the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol 2008;29:1401–04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Juvela S. Prehemorrhage risk factors for fatal intracranial aneurysm rupture. Stroke 2003;34:1852–57 [DOI] [PubMed] [Google Scholar]
- 49. Rinkel G, Prins N, Algra A. Outcome of aneurysmal subarachnoid hemorrhage in patients on anticoagulant treatment. Stroke 1997;28:6–9 [DOI] [PubMed] [Google Scholar]
- 50. Wardlaw J, White P. The detection and management of unruptured intracranial aneurysms. Brain 2000;123:205–21 [DOI] [PubMed] [Google Scholar]
- 51. Crawley F, Clifton A, Brown M. Should we screen for familial intracranial aneurysm? Stroke 1999;30:312–16 [DOI] [PubMed] [Google Scholar]
- 52. Takao H, Nojo T. Treatment of unruptured intracranial aneurysms: decision and cost effectiveness analysis. Radiology 2007;244:755–66 [DOI] [PubMed] [Google Scholar]
- 53. Butler W, Barker F, Crowell R. Patients with polycystic kidney disease would benefit from routine magnetic resonance angiographic screening for intracerebral aneurysms: a decision analysis. Neurosurgery 1996;38:506–15, discussion 515–16 [DOI] [PubMed] [Google Scholar]
- 54. Mitchell P, Gholkar A, Vindlacheruvu R, et al. Unruptured intracranial aneurysms: benign curiosity or ticking bomb? Lancet Neurol 2004;3:85–92 [DOI] [PubMed] [Google Scholar]
- 55. Brinjikji W, Kallmes D, Lanzino G, et al. Hospitalization costs for endovascular and surgical treatment of ruptured aneurysms in the United States are substantially higher than Medicare payments. AJNR Am J Neuroradiol 2012;33:1037–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Laupacis A, Feeny D, Detsky A, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473–81 [PMC free article] [PubMed] [Google Scholar]
- 57. Johnston SC, Gress DR, Kahn JG. Which unruptured cerebral aneurysms should be treated? A cost-utility analysis. Neurology 1999;52:1806–15 [DOI] [PubMed] [Google Scholar]
- 58. Bor S, Koffjberg H, Wermer M, et al. Optimal screening strategy for familial intracranial aneurysms: a cost-effectiveness analysis. Neurology 2010;74:1671–79 [DOI] [PubMed] [Google Scholar]
- 59. Tosteson A, Stout N, Fryback D, et al. Cost-effectiveness of digital mammography screening. Ann Intern Med 2008;148:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tengs TO, Adams ME, Pliskin JS, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal 1995;15:369–90 [DOI] [PubMed] [Google Scholar]
- 61. Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010;17:173–80 [DOI] [PubMed] [Google Scholar]
- 62. Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2002;13:269–76 [DOI] [PubMed] [Google Scholar]
- 63. Wakai S. Polycystic kidneys and aneurysms. J Neurosurg 1999;90:985–86 [DOI] [PubMed] [Google Scholar]
- 64. Torres V, Harris P, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007;369:1287–301 [DOI] [PubMed] [Google Scholar]
- 65. Morrison AS. The natural history of disease in relation to measures of disease frequency. In: Morrison AS. Screening in Chronic Disease. 2nd ed. New York: Oxford University Press; 1992:57 [Google Scholar]