Abstract
BACKGROUND AND PURPOSE:
A recent study identified a preprocedural P2Y12 reaction units value of <60 or >240 as a strong independent predictor of perioperative thromboembolic and hemorrhagic complications after treatment of cerebral aneurysms with the Pipeline Embolization Device. This study aimed to determine whether a last-recorded P2Y12 reaction units value of <60 or >240 predicts thromboembolic and hemorrhagic complications up to 6 months after treatment of cerebral aneurysms with the Pipeline Embolization Device in the same patient cohort.
MATERIALS AND METHODS:
We recorded patient and aneurysm characteristics, P2Y12 receptor antagonist administered, P2Y12 reaction units value with VerifyNow, procedural variables, and thromboembolic and hemorrhagic complications up to 6 months after Pipeline Embolization Device procedures at our institution during an 8-month period. Complications causing a permanent disabling neurologic deficit or death were considered major. Multivariate regression analysis was performed to identify independent predictors of thromboembolic and hemorrhagic complications.
RESULTS:
Forty-four patients underwent 48 Pipeline Embolization Device procedures at our institution during the study period. There were 11 thromboembolic and hemorrhagic complications up to 6 months after treatment in our cohort (22.9%), 5 of which were major (10.4%). A last-recorded P2Y12 reaction units value of <60 or >240 was the only independent predictor of all (P = .002) and major (P = .03) thromboembolic and hemorrhagic complications in our cohort. Most patients (71%) required, on average, 2 adjustments to the dose or type of P2Y12 receptor antagonist to remain within the 60–240 target P2Y12 reaction units range.
CONCLUSIONS:
In our cohort, a last-recorded P2Y12 reaction units value of <60 or >240 was the only independent predictor of all and major thromboembolic and hemorrhagic complications up to 6 months after Pipeline Embolization Device procedures.
Endovascular treatment of cerebral aneurysms with the Pipeline Embolization Device (PED; Covidien/ev3, Irvine, California) requires its deployment within the lumen of the parent artery to allow the vessel to endothelialize along the PED and exclude the aneurysm from the circulation. This process carries the risk of thromboembolic complications because platelets could become activated, adhere to the PED, form thrombus, and cause either in situ PED thrombosis or distal thromboembolization. Hence, PED procedures are usually performed under dual antiplatelet therapy (DAT) with aspirin and a P2Y12 receptor antagonist such as clopidogrel, prasugrel, or ticagrelor. However, DAT carries the risk of hemorrhagic complications, with parenchymal intracerebral hemorrhage (ICH) being the most potentially devastating.
Case series of 12–191 patients with cerebral aneurysms treated with the PED have reported a wide range of thromboembolic and hemorrhagic complications, with the risk of cerebral infarction ranging from 0% to 14% and the risk of ICH ranging from 0% to 11%.1–16 Among other factors, the variability in thromboembolic and hemorrhagic complications after PED procedures could be due to differences in patient responses to the P2Y12 receptor antagonists administered while the PED endothelializes.
The P2Y12 receptor plays a central role in platelet activation and aggregation. Clopidogrel and prasugrel cause irreversible inhibition of the P2Y12 receptor, while ticagrelor causes reversible inhibition of this receptor. VerifyNow (Accumetrics, San Diego, California) is a point-of-care platelet function test that measures the degree of P2Y12 receptor inhibition after stimulation with adenoside diphosphate, a P2Y12 receptor agonist. This assay has been found to correlate strongly with light transmittance aggregometry, the criterion standard for quantification of platelet reactivity, in patients treated with clopidogrel, prasugrel, or ticagrelor.17–20 VerifyNow results are reported in P2Y12 reaction units (PRUs), with a lower PRU value corresponding to a higher degree of P2Y12 receptor inhibition and, hence, a decreased likelihood of platelet activation and aggregation; and a higher PRU value corresponding to a lower degree of P2Y12 receptor inhibition and, hence, an increased likelihood of platelet activation and aggregation. A recent study of 44 patients who underwent 48 PED procedures for treatment of cerebral aneurysms at our institution found that a preprocedural PRU value of <60 or >240 measured with VerifyNow was the strongest independent predictor of perioperative thromboembolic and hemorrhagic complications occurring up to postoperative day 30.16
The aim of this study was to determine whether a last-recorded PRU value of <60 or >240 (up to the occurrence of a complication, if any) predicts thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in the same cohort of patients with cerebral aneurysms treated with the PED at our institution.
Materials and Methods
Our study was approved by the institutional review board of our hospital and conducted in compliance with the Health Insurance Portability and Accountability Act. We conducted a retrospective analysis to examine the predictors of thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients who underwent treatment of cerebral aneurysms with the PED at our institution from November 17, 2011 to July 23, 2012.
Medical Record Review
We recorded baseline patient characteristics, dose and type of P2Y12 receptor antagonist administered, P2Y12 receptor inhibition with the VerifyNow test (in PRUs) up to the occurrence of a thromboembolic or hemorrhagic complication (if any), aspirin dose, aneurysm characteristics, number of PEDs deployed, technical difficulties, procedure time, postprocedural corticosteroid regimen, and the incidence and severity of thromboembolic and hemorrhagic complications occurring up to 6 months after the PED procedure. Procedures were considered technically difficult if there was PED herniation into the aneurysm, incomplete PED opening requiring balloon angioplasty for adequate wall apposition, PED migration during deployment requiring a second PED to cover the aneurysm neck, or concurrent treatment of another cerebral aneurysm. Thromboembolic and hemorrhagic complications were reviewed by a panel of 3 neurointerventionalists and were designated “major” if they caused a permanent disabling neurologic deficit or death.
DAT Protocol
P2Y12 receptor inhibition was assessed with the VerifyNow test in all patients before the procedure and 10 and 30 days after any changes to the dose or type of P2Y12 receptor antagonist administered, after changes to medications that may affect clopidogrel or prasugrel metabolism, or at any time if the patient was symptomatic with abnormal bruising/bleeding or focal neurologic deficits.
The target P2Y12 receptor inhibition initially was 80–200 PRUs and was subsequently expanded to 60–240 PRUs after July 27, 2012. For most elective PED procedures (83%), DAT was started 10 days before the procedure with 325 mg aspirin daily and 75 mg clopidogrel daily. The clopidogrel response was assessed the day before the procedure. Clopidogrel hyporesponders received a 60-mg prasugrel loading dose the day before the procedure followed by 10 mg prasugrel daily, and the initial prasugrel response was assessed on the day of the procedure. Clopidogrel hyper-responders were placed on every other day, every third day, every Monday and Friday, every fourth day, or every fifth day dosing regimens as needed to reach the target PRU range. Prasugrel hyporesponders received a 180-mg loading dose of ticagrelor followed by 90 mg twice a day with the initial ticagrelor response assessed before the procedure. Prasugrel hyper-responders in follow-up testing initially had the daily prasugrel dose reduced to 5 mg and, if needed, were subsequently placed on every other day or every third day dosing regimens to reach the target PRU range. Although the aforementioned adjustments to the dose or type of the P2Y12 receptor antagonist administered were made according to the preprocedural PRU value, elective PED procedures were not rescheduled to a later date to reach the target PRU range before PED deployment.
For urgent/emergent PED procedures, a 60-mg loading dose of prasugrel was administered followed by 10 mg prasugrel daily, with the initial prasugrel response assessed before the procedure and follow-up testing performed as described above.
PED Procedure
The PED procedure was performed with the patient under general anesthesia by a team of 2 neurointerventionalists by using transfemoral access in a dedicated biplanar neuroangiographic unit (Axiom Artis; Siemens, Erlangen, Germany). Heparinization was used throughout the procedure to achieve an activated clotting time 2–2.5 times baseline. A triaxial system was used with a 6F long sheath (Shuttle; Cook, Bloomington, Indiana), a distal access catheter (ReFlex; Covidien/ev3 or Neuron; Penumbra, Alameda, California), and a Marksman microcatheter (Covidien/ev3). A 0.016-inch (Headliner; Terumo, Tokyo, Japan) or 0.014-inch (Traxcess; MicroVention, Aliso Viejo, California or Avigo; Covidien/ev3) microwire was used to advance the Marksman microcatheter across the aneurysm neck. The PED device was deployed across the aneurysm neck under fluoroscopic guidance. A postdeployment DynaCT (Siemens) angiography performed to ensure adequate PED vessel wall apposition and aneurysm neck coverage. Final biplanar angiography was performed to document patency of the intracranial vasculature. Hemostasis was achieved with an Angio-Seal device (St. Jude Medical, Minnetonka, Minnesota). Heparinization was not continued postprocedure.
Statistical Analysis
Statistical analysis was performed using MedCalc 11.1 software package (MedCalc Software, Mariakerke, Belgium). First, we performed univariate analysis with the χ2 or Fisher exact test for each variable to identify the predictors of all and major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort. Then, we performed multivariate regression analysis to identify the independent predictors of all and major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort. A P value ≤ .05 was considered statistically significant.
Results
From November 17, 2011, to July 23, 2012, forty-four patients underwent 48 PED procedures to treat 54 cerebral aneurysms at our institution. Thirty-six patients were women (81.8%), and 8, men (18.2%). Mean age was 59.2 years (median, 63 years; range, 31–81 years). Seven patients had a remote history of subarachnoid hemorrhage (15.9%), and 10, a family history of cerebral aneurysms (22.7%). Ten aneurysms were symptomatic (18.5%), 11 had recurred after coiling (20.4%), and 27 were incidental (50%). Mean maximum aneurysm size was 8.4 mm (median, 5.8 mm; range, 1.9–27.6 mm). Mean aneurysm neck size was 4.8 mm (median, 4 mm; range, 1.1–17 mm). Mean procedure time was 67.7 minutes (median, 50.5 minutes; range, 28–220 minutes). Seventeen procedures were technically difficult (35.4%), with a mean procedure time of 108.7 minutes (median, 91 minutes; range, 56–220 minutes). Mean number of PEDs deployed was 1.3 (range, 1–5).
Mean last-recorded PRU value up to the occurrence of a thromboembolic or hemorrhagic complication (if any) was 130.2 (median, 132.5; range, 0–292). Mean time interval from initiation of DAT to the last P2Y12 receptor inhibition test up to the occurrence of a thromboembolic or hemorrhagic complication (if any) was 103.3 days (median, 83 days; range, 7–233 days).
Thromboembolic and Hemorrhagic Complications Occurring Up to 6 Months after Treatment of Cerebral Aneurysms with the PED and Associated Last-Recorded PRU Values
Table 1 summarizes the incidence of thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort according to the last-recorded PRU value, using a PRU value of >240 as a proposed cutoff for P2Y12 receptor underinhibition21 and a PRU value of <60 as a proposed cutoff for P2Y12 receptor overinhibition.
Table 1:
Thromboembolic and hemorrhagic complications occurring up to 6 months after treatment of cerebral aneurysms with the PED according to last-recorded PRU value
| All Complications |
P Valueb | Thromboembolic Complications |
P Valueb | Hemorrhagic Complications |
P Valueb | ||||
|---|---|---|---|---|---|---|---|---|---|
| All (%) | Majora (%) | All (%) | Majora (%) | All (%) | Majora (%) | ||||
| All procedures (n = 48) | 11 (22.9) | 5 (10.4) | 6 (12.5) | 1 (2.1) | 5 (10.4) | 4 (8.3) | |||
| PRU <60 (n = 9) | 5 (55.6) | 3 (33.3) | 1 (11.1) | 0 | 4 (44.4) | 3 (33.3) | |||
| PRU 60–240 (n = 37) | 4 (10.8) | 1 (2.7) | 3 (8.1) | 0 | 1 (2.7) | 1 (2.7) | |||
| PRU >240 (n = 2) | 2 (100) | 1 (50) | <.001/.011 | 2 (100) | 1 (50) | .014/.042 | 0 | .004/.036 | |
Resulting in death or permanent disabling neurologic deficits.
For difference in all/major complications.
There were 6 thromboembolic (12.5%) and 5 hemorrhagic (10.4%) complications occurring up to 6 months after treatment in our cohort. Of these, 5 were major (10.4%): Three resulted in a permanent disabling neurologic deficit (6.2%, one thromboembolic, 2 hemorrhagic), and 2 ICHs resulted in death (4.2%).
The 4 perioperative thromboembolic complications in our cohort (one of which was major) have been described previously.16 There were 2 additional thromboembolic complications occurring after the perioperative period in our cohort: 1 patient with an asymptomatic delayed PED thrombosis after treatment of a blister ICA aneurysm identified at the time of the 6-month follow-up angiogram (PRU 193), and 1 patient with a delayed perforator infarction causing nondisabling deficits in short-term memory and executive planning after treatment of an anterior cerebral artery aneurysm (PRU 205, Fig 1). The patient with the major perioperative thromboembolic complication had a markedly elevated PRU value (PRU 292) at the time of the complication.
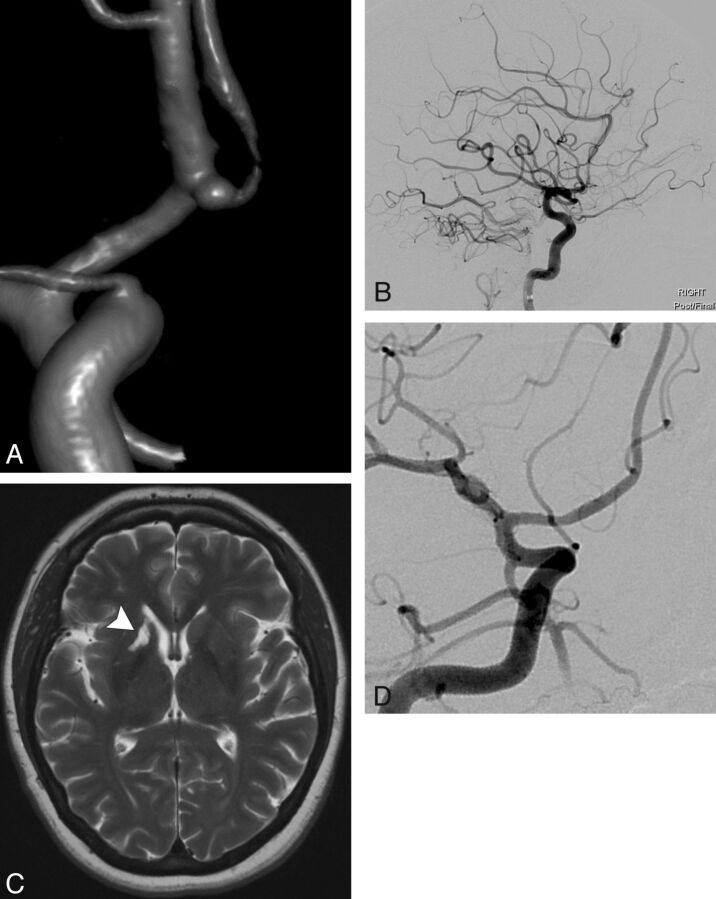
Fig 1.
A 47-year-old patient with a family history of ruptured cerebral aneurysms presented with a small right anterior cerebral artery aneurysm identified at screening. The patient elected to undergo endovascular treatment with the PED. A, 3D image demonstrates a 2-mm right A1/A2 segment anterior cerebral artery aneurysm with the anterior communicating artery arising from the aneurysm sac. B, Final lateral right ICA angiogram after uncomplicated deployment of a single 2.5 × 10 mm PED across the aneurysm neck demonstrates patent cerebral vasculature. The patient demonstrated conversion to a clopidogrel hyper-response (PRU 52) 30 days after initiation of DAT and underwent 2 clopidogrel dose adjustments to remain within the target P2Y12 receptor inhibition range of 60–240 PRU. A final dosing regimen of 75 mg clopidogrel every third day was instituted 94 days after initiation of DAT. At the time of the 6-month follow-up angiogram, the patient's spouse stated that for the past 3–4 months the patient had experienced memory deficits, cognitive changes, and weight gain. MR imaging examination of the brain was performed immediately, and P2Y12 receptor inhibition testing showed a PRU of 205. C, T2WI demonstrates an old infarction in the right caudate head, anterior limb of the right internal capsule, and right putamen (arrowhead), consistent with a perforator infarction (likely in the territory of the right recurrent artery of Heubner), which was not present in pretreatment imaging. D, Six-month follow-up catheter angiogram demonstrates complete exclusion of the right anterior cerebral artery aneurysm from the circulation with a widely patent PED but no flow across the anterior communicating artery. The patient underwent neurologic and neuropsychological consultation and had nondisabling deficits in short-term memory, attention, problem solving, and self-regulation with near-complete resolution after outpatient rehabilitation.
The 4 perioperative hemorrhagic complications in our cohort (3 of which were major) were all ICHs ipsilateral to the PED and have been described previously.16 An additional ICH contralateral to the PED occurred on postoperative day 50 in a patient with postmortem examination–proven amyloid angiopathy at the site of the ICH (PRU 58, Fig 2). Of note, 80% of hemorrhagic complications, including 3 of the 4 major complications (75%), occurred in patients who had decreased PRU values shortly before (PRU 0) or at the time of the ICH (PRU 2, 10, and 58).
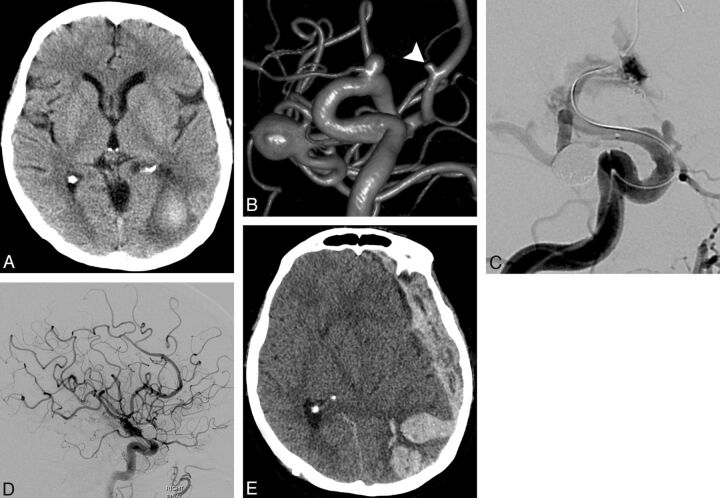
Fig 2.
A 63-year-old patient with a long-standing history of cigarette smoking presented with a 3-week history of visual disturbance. A, NCCT demonstrates a subacute ICH in the left occipital lobe. The patient underwent further evaluation with contrast-enhanced brain MR imaging, CTA, and conventional angiography, none of which demonstrated an etiology for the ICH. B, 3D image of a right ICA angiogram demonstrates 3 incidental cerebral aneurysms, with a 2-mm right A2 segment anterior cerebral artery aneurysm (arrowhead). There were 2 additional incidental left-sided cerebral aneurysms, not in the vicinity of the ICH (not shown). Three months after presentation, a repeat contrast-enhanced brain MR image showed expected evolution of the left occipital ICH without new foci of hemorrhage or abnormal enhancement. The patient expressed a strong desire to undergo endovascular treatment of all the incidental cerebral aneurysms, despite the fact that amyloid angiopathy was the leading differential diagnosis for the ICH. After careful discussion with the patient and family regarding the risk of potentially-devastating rehemorrhage, DAT with aspirin and clopidogrel was instituted, and the right MCA bifurcation aneurysm was treated uneventfully with stent-assisted coil embolization. C, Sixteen days later, balloon-assisted coil embolization of the 2-mm right anterior cerebral artery aneurysm was complicated by aneurysm rupture. Hemostasis was achieved with immediate balloon inflation, and the aneurysm was ultimately treated with several coils and a 2.5 × 12 mm PED across its neck. D, Final lateral right ICA angiogram demonstrates patent cerebral vasculature. The patient recovered from the subarachnoid hemorrhage and was discharged to a rehabilitation facility on postoperative day 25. The patient demonstrated conversion to a clopidogrel hyper-response (PRU 34) 26 days after initiation of DAT and underwent 3 clopidogrel dose adjustments in an attempt to reach the target P2Y12 receptor inhibition range of 60–240 PRU. A dosing regimen of 75-mg clopidogrel every fourth day was instituted 65 days after initiation of DAT, but follow-up P2Y12 receptor inhibition testing had not been completed. E, On postoperative day 50, the patient was found unresponsive at home. NCCT demonstrates a recurrent left occipital ICH with extensive subdural extension causing a severe rightward midline shift. PRU at the time of the ICH was 58. The patient died that evening. Postmortem examination revealed amyloid angiopathy affecting the left occipital lobe.
Overall, 4 of the 5 (80%) major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort occurred in patients who had markedly elevated (292, thromboembolic complication) or decreased (0, 10, 58, hemorrhagic complications) PRU values shortly before or at the time of the complication.
Predictors of Thromboembolic and Hemorrhagic Complications Occurring Up to 6 Months after Treatment of Cerebral Aneurysms with the PED
The On-line Table summarizes the predictors of thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort. In univariate analysis, a last-recorded PRU value of <60 or >240 (P < .001) and a technically difficult procedure (P = .036) were predictors of all thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort, while a last-recorded PRU value of <60 or >240 (P = .01) was the only predictor of major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort.
In multivariate regression analysis, a last-recorded PRU value of <60 or >240 was the only independent predictor of all (P = .002) and major (P = .03) thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort.
Variability in Clopidogrel Response and Clinical Management of DAT
Table 2 summarizes the final P2Y12 receptor antagonist dosing regimen administered and associated major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in our cohort.
Table 2:
Final P2Y12 receptor antagonist dosing regimen and associated major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the PED
| No. of Patients (%) | Major Complications (%) | Outside Target P2Y12 Receptor-Inhibition Range (PRU 60–240)a (%) | |
|---|---|---|---|
| All patients | 44 (100) | 5 (11.4) | 4 (80) |
| Clopidogrel | 31 (71) | 3 (9.7) | 3 (100) |
| 150 mg daily | 3 (9.7) | 1 Infarction, 1 ICH (66.7) | 2 (100) |
| 75 mg daily | 15 (48.3) | 0 | |
| 75 mg QOD | 2 (6.5) | 0 | |
| 75 mg Q3D | 3 (9.7) | 0 | |
| 75 mg QMF | 5 (16.1) | 0 | |
| 75 mg Q4D | 2 (6.5) | 1 ICH (50)b | 1 (100) |
| 75 mg Q5D | 1 (3.2) | 0 | |
| Prasugrel | 12 (27) | 2 (16.7) | 1 (50) |
| 10 mg daily | 6 (50) | 1 ICH (16.7) | 1 (100) |
| 5 mg daily | 5 (41.7) | 1 ICH (20) | 0 |
| 5 mg QOD | 1 (8.3) | 0 | |
| Ticagrelor | 1 (2) | 0 | |
| 90 mg BID | 1 (100) |
Note:—QOD indicates every other day; Q3D, every third day; QMF, every Monday and Friday; Q4D, every fourth day; Q5D, every fifth day; BID, twice a day.
Shortly before or at the time of a major complication.
Recurrent ICH with subdural extension contralateral to the PED on postoperative day 50 in a patient with postmortem examination–proven amyloid angiopathy at the site of ICH (PRU 58 at the time of ICH, Fig 2).
Forty-two patients underwent elective PED procedures (95.5%), and 2 patients were started on aspirin/prasugrel DAT for urgent/emergent PED procedures (4.5%) in our cohort. With the initial target P2Y12 receptor inhibition range of 80–200 PRUs as the reference, in preprocedural testing 11 patients were considered clopidogrel hyporesponders (26.2%; mean PRU, 272.8; median, 262; range, 207–399), 9 patients were considered clopidogrel hyper-responders (21.4%; mean PRU, 48.1; median, 54; range, 9–73), and 22 patients were within the target PRU range (52.4%; mean PRU, 141.9; median, 155.5; range, 83–197).
Among the 22 patients who were initially within the target PRU range, 17 became clopidogrel hyper-responders in follow-up testing (77.3%; mean PRU, 23.4; median, 10; range, 1–78). Among these, 7 were symptomatic (41.2%; five with abnormal bruising/bleeding and 2 with ICH). Mean time from initiation of DAT to conversion to a clopidogrel hyper-response was 41.6 days (median, 30 days; range, 16–200 days). No patients became clopidogrel hyporesponders in follow-up P2Y12 receptor inhibition testing.
During the study period, 31 patients required at least 1 adjustment to the dose or type of P2Y12 receptor antagonist administered to remain within the expanded target P2Y12 receptor inhibition range of 60–240 PRUs (70.5%), with a mean of 2.1 adjustments per patient (median, 2; range, 1–5) and a mean time from initiation of DAT to last adjustment of 49.9 days (median, 43 days; range, 8–149 days). Four patients temporarily exhibited a PRU of >240 after a dose adjustment (12.9%; 3 prasugrel, 1 clopidogrel) without experiencing thromboembolic complications.
Discussion
The overall incidence of thromboembolic (12.5%) and hemorrhagic (10.4%) complications up to 6 months after PED procedures in our cohort is within the range of previously published studies (0%–14% and 0%–11%, respectively).1–15 The overall risk of major complications causing a permanent disabling neurologic deficit or death in our cohort was 10.4%, which is higher than the risk of serious complications associated with endosaccular aneurysm treatment with coils (approximately 3%–4%). However, the target patient population for PED procedures is usually those with large and wide-neck aneurysms, which are either not treatable by coil embolization or are at an increased risk of recurrence, requiring multiple treatments. Nevertheless, the higher risk of major thromboembolic and hemorrhagic complications associated with flow diversion must be strongly considered when offering this treatment technique to patients with potentially coilable or clipable cerebral aneurysms.
The issue of P2Y12 receptor underinhibition in patients undergoing coronary interventions has been well-studied in the cardiology literature, and a PRU cutoff of >240 has been proposed to indicate significant residual platelet reactivity.21 In our cohort, 26% of patients were considered clopidogrel hyporesponders in preprocedural testing using a PRU cutoff of >200 (using a PRU cutoff of >240, 21% of patients would have been considered clopidogrel hyporesponders). The proportion of clopidogrel hyporesponders in our cohort was lower than that reported by Akbari et al22 (32.9%) and Lee et al23 (42.9%) in patients undergoing various types of neurointerventional procedures also tested with VerifyNow using a <40% “P2Y12% inhibition” cutoff. Although 4 patients in our cohort experienced nondisabling thromboembolic complications with last-recorded PRU values of <240 (including a delayed asymptomatic PED thrombosis and a delayed perforator infarction), the patient with the major disabling thromboembolic complication had a last-recorded PRU value of >240 (PRU 292).
The important issue of P2Y12 receptor overinhibition in patients undergoing PED procedures has not been well examined in the literature. In our cohort, 21% of patients were considered clopidogrel hyper-responders in preprocedural testing using a PRU cutoff of <80 (using a PRU cutoff of <60, 14% of patients would have been considered clopidogrel hyper-responders). Our findings are similar to those reported by Goh et al24 in a cohort of 47 patients undergoing various types of neurointerventional procedures also tested with VerifyNow (15% using a >72% “P2Y12% inhibition” cutoff). Most important, using a PRU cutoff of <80, we evidenced a conversion to a clopidogrel hyper-response in follow-up testing in 77% of patients who had initially been within the target PRU range in preprocedural testing (using a PRU cutoff of <60, sixty percent of patients would have exhibited a conversion). Overall, P2Y12 receptor overinhibition was evidenced in 62% (PRU cutoff of <80) to 50% (PRU cutoff of <60) of patients who received clopidogrel in our cohort.
While the etiology of perioperative ICHs ipsilateral to the PED is likely multifactorial, P2Y12 receptor overinhibition in the perioperative period may play an important role. In our cohort, 3 of the 4 patients (75%) with perioperative ICHs were found to have P2Y12 receptor overinhibition with markedly decreased PRU values (0, 2, 10) at the time of or just before the ICH. Similarly, Goh et al24 also found that P2Y12 receptor overinhibition in the perioperative period placed patients undergoing various types of neurointerventional procedures at significantly increased risk of experiencing major perioperative hemorrhagic complications (43%). A study of 133 coil embolization procedures to treat unruptured cerebral aneurysms reported a 30% rate of periprocedural thromboembolic diffusion-weighted imaging–positive lesions within 72 hours of the intervention.25 Furthermore, a pathologic study of 3 patients who had fatal ICHs after PED procedures demonstrated basophilic material occluding the blood vessels around the ICH,26 which may represent the hydrophilic coating of the catheters or wires used in these procedures. Therefore, it is plausible that some of the perioperative ICHs ipsilateral to the PED could be explained by hemorrhagic transformation of subclinical infarctions caused by embolization of different types of embolic materials (air bubbles, atherosclerotic plaque, thrombus, or hydrophilic coating from the catheters or wires) in the setting of P2Y12 receptor overinhibition. Moreover, the occurrence of a fatal amyloid ICH contralateral to the PED outside the perioperative period in 1 patient with a decreased PRU value (PRU 58) in our cohort underscores the importance of avoiding P2Y12 receptor overinhibition for the entire duration of P2Y12 receptor antagonist administration to prevent major hemorrhagic complications that may not be directly related to the PED procedure.
In our cohort, a last-recorded PRU value of <60 or >240 was the only independent predictor of all and major thromboembolic and hemorrhagic complications up to 6 months after treatment of cerebral aneurysms with the PED. Although maintaining patients within the target PRU range of 60–240 has proved to be challenging in our experience—with 71% of patients requiring, on average, 2 adjustments to the dose or type of P2Y12 receptor antagonist administered—the possibility of averting major thromboembolic and hemorrhagic complications justifies the requisite time and effort. Future prospective studies are needed to determine whether active P2Y12 receptor antagonist management to maintain patients within a target P2Y12 receptor inhibition range (proposed PRU 60–240) in preprocedural and follow-up testing, including rescheduling patients who are outside this range in preprocedural testing to make the P2Y12 receptor antagonist adjustments needed to reach the target PRU range before PED deployment, could lower the risk of thromboembolic and hemorrhagic complications after PED procedures.
Following the findings of our study, we have changed our DAT protocol for PED procedures (Table 3). Due to the risk of potentially-devastating rehemorrhage, we do not institute DAT - and hence do not perform PED procedures - in patients with a history of unexplained intracranial hemorrhage or suspected amyloid angiopathy. For elective PED procedures, we currently initiate DAT 17 days before the procedure and perform 2 preprocedural P2Y12 receptor inhibition tests: the first after ten 75-mg clopidogrel doses to adjust the dosing schedule according to the PRU value (if needed), and the second the day before the procedure to ensure that the patient is within the 60–240 target PRU range before PED deployment. Elective PED deployment is not undertaken until the patient is within the 60–240 target PRU range in preprocedural testing performed no earlier than the day before the procedure. Due to the increased risk of major hemorrhagic complications with prasugrel encountered by our group and reported by Akbari et al,22 and the occurrence of a major ipsilateral ICH on postoperative day 20 in a patient receiving a 5-mg daily prasugrel dose in our cohort (PRU 185), we no longer administer prasugrel to patients considered clopidogrel hyporesponders or who must undergo urgent/emergent PED procedures. Patients who must undergo urgent/emergent PED procedures are started on ticagrelor before the procedure (180 mg × 1, then 90 mg twice a day) without P2Y12 receptor inhibition testing and are transitioned to clopidogrel on postoperative day 30 following the protocol described in Table 3. We do not perform P2Y12 receptor inhibition testing in patients who receive ticagrelor because the reversible nature of the P2Y12 receptor inhibition caused by this medication may preclude dose adjustments and the only patient whom we have tested with VerifyNow while on ticagrelor therapy consistently exhibited markedly decreased PRU values (30, 6, 8).
Table 3:
Current DAT protocol for PED procedures
| Initiation of DAT | 17 Days before procedure |
| Target P2Y12 receptor inhibition | PRU 60–240 |
| Target aspirin inhibition | ≥50% |
| Initial aspirin dose | 81 mg daily |
| Initial clopidogrel dose | 75 mg daily |
| Preprocedural aspirin inhibition testinga | After ten 81-mg aspirin doses or the day before procedure |
| Preprocedural P2Y12 receptor-inhibition testing | After ten 75-mg clopidogrel doses and the day before procedure |
| Hyporesponse to aspirin (< 50% inhibition) | Aspirin, 325 mg daily |
| Clopidogrel dosing schedules | 0) 150 mg dailyb; 1) 75 mg daily; 2) 75 mg QOD; 3) 75 mg Q3D; 4) 75 mg QMF; 5) 75 mg Q5D; 6) 75 mg Q7D; 7) 75 mg PRN to reach PRU ≥60 |
| Hyporesponse to clopidogrel (PRU > 240) | Go back 1 step in clopidogrel dosing schedule |
| Hyper-response to clopidogrel (PRU < 60) | PRU 40–59: advance 1 step in clopidogrel dosing schedule |
| PRU 10–39: advance 2 steps in clopidogrel dosing schedule | |
| PRU < 10: advance 3 steps in clopidogrel dosing schedule | |
| Reschedule procedure | PRU < 60 or >240 on the day before procedure |
| Postprocedural P2Y12 receptor-inhibition testing | 7–10 and 30–40 Days after any clopidogrel dose adjustment, after changes to medications that may affect clopidogrel metabolism, or at any time if symptomatic with abnormal bruising/bleeding or focal neurologic deficits |
Note:—QOD indicates every other day; Q3D, every third day; QMF, every Monday and Friday; Q5D, every fifth day; Q7D, once a week; PRN, dosing schedule as needed.
Aspirin inhibition testing performed with a standard collagen platelet aggregation assay.
Hyporesponders to 150-mg daily clopidogrel dose (PRU > 240) are started on ticagrelor (180 mg × 1, then 90 mg twice a day) the day before the procedure without further P2Y12 receptor-inhibition testing.
The limitations of our study are the modest sample size and retrospective nature.
Conclusions
In our cohort, a last-recorded PRU value of <60 or >240 was the only independent predictor of all and major thromboembolic and hemorrhagic complications occurring up to 6 months after treatment of cerebral aneurysms with the PED, and most patients required 2 adjustments to the dose or type of P2Y12 receptor antagonist administered to remain within the 60–240 target PRU range.
Supplementary Material
Acknowledgments
We acknowledge Sandee K. Verootis, Radiology Department, Abbott Northwestern Hospital, for her contribution in the data collection for this study.
ABBREVIATIONS:
- DAT
dual antiplatelet therapy
- ICH
parenchymal intracerebral hemorrhage
- PED
Pipeline Embolization Device
- PRU
P2Y12 reaction units
Footnotes
Disclosures: Josser E. Delgado Almandoz—RELATED: Other: Coviden/ev3, Comments: travel expenses for a Pipeline training course, UNRELATED: Consultancy: Covidien/ev3. Yasha Kadkhodayan—UNRELATED: Other: Penumbra,* Comments: site principal investigator for industry-sponsored trial. David E. Tubman—RELATED: Consulting Fee or Honorarium: MicroVention, Covidien, Lake Region Medical, UNRELATED: Consultancy: MicroVention, Covidien, Lake Region Medical, Payment for Lectures (including service on Speakers Bureaus): Covidien, Royalties: Cook, Payment for Development of Educational Presentations: Covidien. *Money paid to the institution.
Paper previously presented in part at: Ninth Annual Meeting of the Society of NeuroInterventional Surgery, July 23–26, 2012, San Diego, California; and International Stroke Conference 2013, February 6–8, 2013, Honolulu, Hawaii.
REFERENCES
- 1. Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: the Buenos Aires experience. Neurosurgery 2009;64:632–42 [DOI] [PubMed] [Google Scholar]
- 2. Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the Pipeline embolization device. AJNR Am J Neuroradiol 2010;31:1139–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lubicz B, Collignon L, Raphaeli G, et al. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: preliminary experience in 20 patients with 27 aneurysms. World Neurosurg 2011;76:114–19 [DOI] [PubMed] [Google Scholar]
- 5. Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012;54:369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McAuliffe W, Wycoco V, Rice H, et al. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the Pipeline embolization device. AJNR Am J Neuroradiol 2012;33:164–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deutschmann HA, Wehrschuetz M, Augustin M, et al. Long-term follow-up after treatment of intracranial aneurysms with the Pipeline embolization device: results from a single center. AJNR Am J Neuroradiol 2012;33:481–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz JP, Chow M, O'Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 2012;33:603–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colby GP, Lin LM, Gomez JF, et al. Immediate procedural outcomes in 35 consecutive Pipeline embolization cases: a single-center, single-user experience. J Neurointerv Surg 2013;5:237–46 [DOI] [PubMed] [Google Scholar]
- 10. Chitale R, Gonzalez LF, Randazzo C, et al. Single center experience with Pipeline stent: feasibility, technique and complications. Neurosurgery 2012;71:679–91 [DOI] [PubMed] [Google Scholar]
- 11. Phillips TJ, Wenderoth JD, Phatouros CC, et al. Safety of the Pipeline embolization device in treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol 2012;33:1225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the Pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012;33:1436–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Kelly CJ, Spears J, Chow M, et al. Canadian experience with the Pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2013;34:381–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kan P, Siddiqui AH, Veznedaroglu E, et al. Early postmarket results after treatment of intracranial aneurysms with the Pipeline embolization device: a U.S. multicenter experience. Neurosurgery 2012;71:1080–87 [DOI] [PubMed] [Google Scholar]
- 15. Yu SC, Kwok CK, Cheng PW, et al. Intracranial aneurysms: midterm outcome of Pipeline embolization device: a prospective study in 143 patients with 178 aneurysms. Radiology 2012;265:893–901 [DOI] [PubMed] [Google Scholar]
- 16. Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J Neurointerv Surg 2013; 5 Suppl 3:iii3–10 [DOI] [PubMed] [Google Scholar]
- 17. von Beckerath N, Pogatsa-Murray G, Wieczorek A, et al. Correlation of a new point-of-care test with conventional optical aggregometry for the assessment of clopidogrel responsiveness. Thromb Haemost 2006;95:910–11 [DOI] [PubMed] [Google Scholar]
- 18. Jakubowski JA, Payne CD, Li YG, et al. The use of the VerifyNow P2Y12 point-of-care device to monitor platelet function across a range of P2Y12 inhibition levels following prasugrel and clopidogrel administration. Thromb Haemost 2008;99:409–15 [DOI] [PubMed] [Google Scholar]
- 19. Varenhorst C, James S, Erlinge D, et al. Assessment of P2Y(12) inhibition with the point-of-care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am Heart J 2009;157:562.e1–9 [DOI] [PubMed] [Google Scholar]
- 20. Jeong YH, Bliden KP, Antonino MJ, et al. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J 2012;164:35–42 [DOI] [PubMed] [Google Scholar]
- 21. Bonello L, Tantry US, Marcucci R, et al. Working group on high on-treatment platelet reactivity: consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919–33 [DOI] [PubMed] [Google Scholar]
- 22. Akbari SH, Reynolds MR, Kadkhodayan Y, et al. Hemorrhagic complications after prasugrel (Effient) therapy for vascular neurointerventional procedures. J Neurointerv Surg 2013;5:337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee DH, Arat A, Morsi H, et al. Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. AJNR Am J Neuroradiol 2008;29:1389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goh C, Churilov L, Mitchell P, et al. Clopidogrel hyper-response and bleeding risk in neurointerventional procedures. AJNR Am J Neuroradiol 2013;34:721–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altay T, Kang HI, Woo HH, et al. Thromboembolic events associated with endovascular treatment of cerebral aneurysms. J Neurointerv Surg 2011;3:147–50 [DOI] [PubMed] [Google Scholar]
- 26. Deshmukh V, Hu YC, McDougall CG, et al. Histopathological assessment of delayed ipsilateral parenchymal hemorrhages after the treatment of paraclinoid aneurysms with the Pipeline embolization device [abstract]. Neurosurgery 2012;71:E551–52 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.