SUMMARY:
Intracranial atherosclerotic disease may constitute the most common cause of ischemic stroke worldwide; yet, in the developed world, imaging research has largely focused on extracranial atherosclerosis. Many studies in populations of Asian, African, and Hispanic descent demonstrate the preponderance of intracranial stenosis compared with carotid stenosis. This review examines the clinical presentations of MCA atherosclerosis and stenosis and the use of noninvasive MR imaging in the assessment of intracranial vasculature. MRA is a well-validated technique that offers great advantage over traditional angiography. Advances in high-resolution MR imaging of MCA stenosis have the potential to yield excellent visualization of plaque. Future developments in high-resolution MR imaging to depict intracranial atherosclerosis are explored in this review; these advances will guide endovascular therapy and the comparison of novel interventions.
Stroke is a common cause of morbidity and mortality, with cerebrovascular disease constituting the second most frequent cause of death worldwide.1 While the burden of cerebrovascular disease in the developing world is receiving greater attention, its importance is underestimated.2 Intracranial atherosclerotic disease is reaching greater clinical prominence as potentially the greatest contributor to the burden of cerebrovascular disease worldwide.3 Previously, atherosclerotic disease within intracranial vessels was not well-appreciated clinically, perhaps due to the inability to visualize the MCA noninvasively or the greater emphasis on the more easily imaged carotid arteries. In an early study of MCA syndromes, Lhermitte et al4 alleged that “atherosclerotic thrombosis of MCA is probably a rare event and the primary cause . . . lies in the proximal ICA . . . for an almost inaccessible artery appears to be of little significance.” Thus, early investigations established a precedent of emphasizing extracranial atherosclerosis at the expense of investigation of other manifestations of cerebrovascular disease.
In examining only extracranial disease, investigators may be detecting only advanced atherosclerosis. Some argue that MCA disease may precede extracranial atherosclerosis; this sequence is suggested by the lack of substantial carotid stenosis in a group of patients with intracranial atherosclerosis, while nearly all those with extracranial stenosis also had concomitant intracranial stenosis.5 Therefore, detecting intracranial atherosclerosis may enable clinicians to intervene earlier.
There is much controversy about the degree of MCA stenosis responsible for stroke, with some groups arguing that MCA stenosis is not important in the white population on the basis of a small TCD sonography study.6 Yet a host of postmortem and imaging studies implicate MCA atherosclerosis as a likely cause of stroke.7–9 Further investigation is necessary to evaluate the prevalence and importance of MCA disease as an etiology of ischemic injury.
Clinical Syndrome
Stroke is a multifaceted clinical entity comprising several different syndromes; each has differing etiologies and varied pathophysiology. For instance, stenosis of the extracranial carotid arteries is either incidental or unlikely to be implicated in lacunar strokes, whereas atherosclerosis of the smaller cerebral arteries is the predominant identifiable cause of lacunar stroke.10,11 As the largest intracranial artery, the MCA is the most frequently involved in stroke and transient ischemic attacks. MCA stenosis appears to occur with lacunar and striatocapsular infarcts, perhaps due to the occlusion of small perforating arteries by atheroemboli.12,13 When one considers intracranial atherosclerotic disease, MCA disease can be defined as the presence of an atherosclerotic lesion within the MCA in the absence of cardiogenic embolism.14
Intracranial Atherosclerosis
The common risk factors implicated in most atherosclerotic disease processes (eg, hypertension, hyperlipidemia, diabetes, and smoking) are most likely at play in atherosclerosis of the intracranial arteries such as the MCA.15 Atherosclerosis was implicated in 27.5% of cases of MCA stenosis in an early clinicopathologic correlation study but is most likely much more frequent.4 There may exist some key differences in the location of atherosclerotic MCA stenosis versus other causes of MCA stenosis; atherosclerosis appears in the more proximal portions of the artery.16 In terms of the nature of atherosclerotic lesions within the MCA, a postmortem study revealed an increased risk of infarct in MCA plaques with a large lipid area, intraplaque hemorrhage, neovascularization, and thrombus.17
Presentation
Atherosclerotic disease of the MCA is responsible generally for lacunar and borderzone infarcts as well as partial anterior circulation deficits, whereas cortical and territorial infarcts tend to be associated with cardioembolism or internal carotid disease.16,18 The presentation of MCA-related ischemic symptoms appears to be less severe than that of ICA-related events, an observation supported by the finding of lower National Institutes of Health Stroke Scale scores in MCA disease compared with ICA disease.18 This relative increase in the severity of ICA disease over MCA disease may be due to the larger size of ICA lesions, which are capable of completely occluding the MCA lumen rapidly in the setting of acute thromboembolism, which is thought to be the final pathway of ischemic symptoms from carotid atherosclerosis.19
Epidemiology
The incidence of intracranial atherosclerotic disease in the general population is poorly understood; knowledge about the occurrence of this pathology is inferred from a few postmortem studies of patients with stroke. Early studies investigating intracranial atherosclerosis purported that this disease process was an infrequent cause of stroke and potentially discouraged much-needed investigation.4 Recent studies challenge these notions claiming the rarity of intracranial atherosclerosis. A substantial study of >300 stroke fatalities in Paris showed that intracranial atherosclerotic plaque occurred in 59% of patients and 37.2% of all patients had intracranial plaque that was stenotic.8 Specifically, the MCA was diseased in 28.9% of fatal stroke postmortem examinations; of these, roughly half of the plaque was 30%–74% stenotic and one-quarter of the plaque was severely stenotic (>75%) or completely occlusive.8 Another postmortem study of patients with MCA territory stroke detected MCA stenosis on the ipsilateral side in 19% and on the contralateral side in 7% of cases.7 Thus, MCA disease appears frequently in fatal stroke in certain cohorts and may cause substantial stenosis in many patients.
Clinical population studies also bear out the substantial impact of MCA disease in certain populations. A high incidence of intracranial stenosis is reported in African, Asian, and Hispanic populations, but not in whites.9 In a study of 850 Korean patients with ischemic syndromes, 12.6% were found to have isolated MCA disease16; an even larger study found that MCA stenosis was the most common type of atherosclerotic lesion in approximately one-third of patients with stroke.20 MCA stenosis was identified in 36% of Korean patients with small striatocapsular infarcts.13 Estimates of the responsibility of intracranial stenosis in causing stroke are 33%–50% in China.9,21,22 Asymptomatic patients with vascular risk factors also frequently have significant degrees of intracranial stenosis. TCD sonography identified MCA stenosis in 12.6% of at-risk individuals in a study conducted in Hong Kong.23 Therefore, while estimates vary widely, MCA disease appears quite common in several large cohorts.
Risk, Management, and Outcomes
As suggested by the results of a large postmortem study, stroke history appears to be associated with intracranial stenotic plaque, not simply the presence of nonstenotic atherosclerotic plaque.8 Stroke risk with severe intracranial artery stenosis appears related to the degree of stenosis with a hazard ratio of 2.03 (P = .0025) of symptom recurrence for severe (≥70%) stenosis.24A prospective study of MCA disease found a 12.5% annual risk of stroke in symptomatic patients and a 2.8% annual risk in asymptomatic individuals with significant MCA stenosis or occlusion25; similarly in symptomatic patients, the Warfarin-Aspirin Symptomatic Intracranial Disease trial found an 18.6% recurrence during approximately 2 years of follow-up.26 These recurrences occurred more frequently in the same territory as the initial symptoms (73%); more concerning, almost half of these strokes were disabling.26 Because the progression of MCA stenosis appears to be quite common, suggested by 61% serial angiography progression of stenosis during approximately 2 years, routine monitoring of stenosis with time may be even more important than in carotid disease.27 Knowing the prognosis of particular types and degrees of stenotic MCA disease is important in determining target populations for angioplasty, stent placement, or medical management.
Currently, management of MCA stenosis is generally decided on symptom status: Asymptomatic patients typically receive platelet inhibitors such as aspirin or clopidogrel and symptomatic patients are prescribed anticoagulation.25,28,29 Much uncertainty regarding the ideal medical management of MCA stenosis exists and varies by patient characteristics; some argue that asymptomatic MCA atherosclerosis carries little risk,30 whereas previously symptomatic patients with intracranial atherosclerosis have a particularly high recurrence risk if they fail antithrombotic therapy.31 Regardless of symptom status, risk-factor modification to address vascular risks is of paramount importance in preventing all-cause morbidity and mortality, especially because MCA stenosis in patients with diabetes is an independent predictor of vascular mortality.32 Large studies examining treatment effects in individuals with MCA stenosis are needed to guide appropriate medical management.
Severe stenosis with recent symptoms carries much greater risk than asymptomatic stenosis and may necessitate endovascular therapy, including stent placement as reviewed at greater length by Turan et al.33 Some groups recommend urgent endovascular therapy for patients with impending stroke who have failed best medical therapy.34 Angioplasty has been the most common endovascular therapy for intracranial stenosis and may have some ability to reduce the risk of stroke in selected patient populations, with an annual stroke rate of 3.36%.35–37 Studies of endovascular therapy show that stent placement can be performed with high success and low adverse event rates within the MCA and that stent placement reduces residual postoperative stenosis compared with angioplasty.38–40 The clinical importance of recanalization by stent placement or angioplasty in reducing stroke or risk of death remains to be decided; current evidence is summarized by Gröschel et al.41 Another option for restoring blood flow in MCA disease is superficial temporal artery−MCA bypass, which appears to have some success in medically refractory symptomatic patients and improves perfusion.42,43 To resolve these pressing questions, larger long-term prospective clinical trials of therapy for MCA stenosis are needed, particularly in asymptomatic patients, to ascertain the ability of medical therapy to prevent the development of ischemic symptoms.
Imaging Findings
Most early imaging studies of the MCA have focused on assessment of lumen status and have been largely reliant on invasive DSA or CTA. More recently, the degree of stenosis has been determined on the basis of flow velocity calculated from TCD sonography; this method offers fair assessment of MCA stenosis and may have a place in clinical practice in identifying patients requiring detailed imaging methods such as high-resolution MR imaging.44–46 Limitations of these methods include the small risk associated with nonionizing radiation in traditional and CT angiography and limited repeatability and low sensitivity in the case of sonography.47 DSA should play little role in modern imaging because it is invasive, exposes patients to a substantial amount of radiation, and provides no information about the cerebral parenchyma or the vessel wall. Noninvasive imaging of the MCA with MR imaging may offer benefits, including excellent spatial resolution of the vessel wall, avoiding radiation use, incorporation with stroke imaging protocols, and excellent repeatability. With the use of increasingly advanced imaging, it may be found that previously idiopathic stroke cases were associated with intracranial plaque not seen by conventional angiographic imaging methods.
MRA
The detection of stenosis within intracranial vessels such as the MCA appears best accomplished via noninvasive screening methods such as MRA and CTA. The criterion standard, DSA, should be regarded as too invasive to screen for stenosis following ischemic events despite its greater level of spatial resolution. TOF MRA was first compared with DSA in the 1990s, showing up to 100% detection of intracranial artery occlusion.48,49 Since then, this technique has rapidly emerged as a common means of imaging the intracranial arteries noninvasively without using gadolinium; TOF MRA takes advantage of the contrast between nonsaturated spins in the blood entering the imaging plane and the stationary adjacent tissue, which remains saturated.
The evidence regarding the accuracy of MRA in detecting disease within the MCA varies, with diverse criteria for diagnosis, vessels imaged, and comparators. Early studies report the sensitivity and specificity of MRA in detecting vessel occlusion as 88%–100% and 95%–97%, whereas studies looking at the ability to detect nonocclusive stenosis report lower figures50,51; in 1 particular study, “substantial” stenosis detection could be achieved with 86% sensitivity and specificity.51 Another study showed 100% sensitivity for the detection of moderate-to-complete stenosis.52 MRA offers good equivalency with DSA for detection of >50% stenosis with reported sensitivity, specificity, and accuracy of 92%, 91%, and 91%, respectively, but falls short of the 100% and 99% sensitivity and specificity of CTA.53
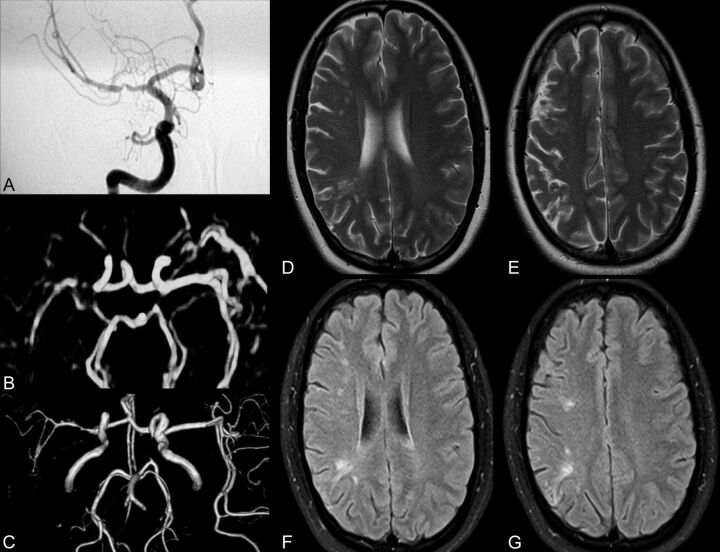
A later study reported much poorer performance of MRA in detecting intracranial stenosis, with a sensitivity and specificity of 70% and 99% versus 98% and 98% for CTA.54 An earlier comparison of CTA and MRA suggested that interpretation of MRA is more reliable than that of CTA.55 Higher field strength scanners may carry additional benefits in improving signal intensity–to-noise ratio and background suppression; other sequences, such as novel sensitivity encoding TOF MRA protocols, also have substantially abbreviated acquisition times.56,57 In a trial of 3D TOF MRA of intracranial steno-occlusive disease in 139 patients on a 3T scanner, 2 observers demonstrated 78%–85% sensitivity and 95% specificity in detecting >50% stenosis and 100% and 99% sensitivity and specificity for the detection of complete occlusion with excellent interobserver agreement.58 Figure 1 depicts the correspondence of contrast-enhanced MRA, TOF MRA, and DSA in a patient with recent ischemic lesions seen on brain imaging.
Fig 1.
Comprehensive imaging of a patient with recent stroke depicting left MCA stenosis. A−G, DSA (A) confirms contrast-enhanced MRA (B) and volume-reduced TOF MRA (C) findings of severe stenosis within the right MCA in a recently symptomatic patient with small ischemic lesions seen on T2 (D and E) and FLAIR (F and G). MRA (B and C) overestimates the degree of stenosis in this particular case.
The differences among studies might reflect the inclusion of lesser degrees of stenosis, as seen in the study by Bash et al54 or limitations of the poor statistical power in small-scale studies. Although CTA performs better overall compared with MRA for the purpose of determining degree of stenosis and is not subject to the flow void artifacts and overestimation frequently encountered in MRA studies, CTA also carries the burden of radiation exposure, use of iodinated contrast, postprocessing steps, and technical limitations related to calcification of the arterial wall.53,54 MRA is not without key limitations: Regions of extremely narrowed lumen can appear completely occluded due to dephasing of blood concomitant to the increased velocity of spins through the vessel due to narrowing.59 This flow void phenomenon, nevertheless, indicates a region of extremely narrowed lumen and may not affect clinical practice.60 In addition, review of source images rather than maximum intensity projection images alone aids in reducing this source of artifacts.52 Technical advancements in imaging protocols and the use of high-field-strength scanners all improve the ability of MRA to detect stenosis.57
One key distinct clinical benefit of MR imaging−based studies is that MRA may be used with other acquisitions to offer a comprehensive imaging protocol to ascertain MCA status in addition to the presence of ischemic lesions in the setting of suspected MCA stenosis. Ideally, MRA will be an effective screening technique for patients with suspected MCA syndromes to detect stenosis within the intracranial vessels and to indicate the need for high-resolution MR imaging to accurately image the stenotic region. Using the information gained from MRA could predict risk; in particular, concurrent intracranial and extracranial stenosis on MRA elevates the risk of cerebrovascular events and death.61 Imaging the MCA may have importance in risk assessment other than simply addressing MCA disease—preoperative imaging of patients undergoing carotid endarterectomy also suggests the ability of MCA signal-intensity abnormalities to predict the risk of postoperative ischemic events with excellent sensitivity.62 MRA may also track changes in the vessel lumen with time; serial MRA has already been used by some groups to study the progression of stenosis in lieu of DSA or TCD.63 Progression of stenosis within the intracranial vessels on repeat MRA has been associated with vascular risk factors such as diabetes and smoking.63
MRA could serve as a means of monitoring patients at increased risk of MCA disease and could be used to track regression with therapy. In a study of cilostazol and aspirin versus aspirin alone, MRA was used to monitor changes in MCA stenosis.64 MRA demonstrated that the degree of stenosis progressed less in symptomatic patients receiving cilostazol and aspirin but did not differ with treatment in asymptomatic patients.64 On the other hand, a randomized double-blind placebo-controlled study of simvastatin use demonstrated no significant change in stenosis on MRA during 2 years.65
Another novel application of MRA is the evaluation of intracranial in-stent stenosis with quantitative MRA by using TOF MRA and phase-contrast MR imaging to measure blood flow. In a study of 14 patients with intracranial stents, quantitative MRA results matched DSA findings in the case of a restenosed MCA stent66; these authors used quantitative MRA to demonstrate the hemodynamic effects of stent placement.67 Thus, MRA of the MCA already shows promise in monitoring the response to pharmacologic and surgical intervention, but rigorous investigation of these new applications of MRA is needed.
High-Resolution MR Imaging
Conventional angiographic methods including MRA and CTA provide detailed information about the lumen status of the intracranial vessels, including the MCA. These methods, however, fall short in characterizing the presence of nonocclusive atherosclerotic disease; this requires visualization of plaque and the ability to derive information about plaque composition. There are a variety of reasons why detection and depiction of nonstenotic atherosclerotic disease is of paramount importance, as has been advocated in the case of carotid atherosclerosis.68 Even large plaques may not cause discernable stenosis because of arterial wall remodeling and expansion, which thereby obfuscates findings by angiographic and luminology methods.69 Clinically, atherosclerosis, yielding a low degree of stenosis, can still confer an enhanced risk of symptoms in extracranial atherosclerosis; this assumption may be accurate in intracranial disease.3 Neovascularization and lipid area were associated with greater risk than stenosis in a postmortem study.17 In a small study of patients with MCA disease, high-resolution MR was able to image a small plaque that did not yield stenosis on MRA; high-resolution MR imaging may provide detailed information about smaller degrees of stenosis than other imaging methods.70 Detection of these small lesions may carry clinical import because rupture of a nonocclusive plaque potentially causes ischemic symptoms via a thromboembolic pathway.
Quantitative Assessment of Plaque Burden
The simplest use of high-resolution MR imaging of the MCA is the calculation of the degree of MCA stenosis, which may be stated as such:
where the reference lumen area is the area of the nonoccluded lumen, preferably at a proximal segment.71,72
There are limitations to such a calculation because the use of a reference site is dependent on the tortuosity of the MCA and factors related to section selection such as the particular region of the MCA imaged and the imaging parameters, section spacing, and section thickness. One potential means of ascertaining the normal lumen measurement for a specific site of stenosis is to image the equivalent segment of the contralateral MCA as implied by others73; this method is equally subject to artifact and, furthermore, presumes unilateral disease.
Other calculations that may be performed independent of these limitations include the wall area at the point of maximal stenosis. Increased wall thickness seen in patients with recent acute ischemic stroke had an average maximal thickness of 2.34 mm compared with 0.51 mm in control subjects (P < .001).74 From the standpoint of distinguishing symptomatic and asymptomatic individuals, the degree of stenosis is a poor differentiating factor (P = .327), but wall area was substantially greater in symptomatic individuals in 1 small study (12.90 versus 8.20, P < .001).71 Nevertheless, all of these quantitative assessments of plaque within the MCA are dependent on section thickness and, thereby, are subject to overestimation attributable to partial volume effects.73
Plaque Characterization
In terms of overall morphology, atherosclerotic lesions are expected to be eccentric, with a reduction in lumen area due to focal arterial wall thickening. High-field high-resolution MR imaging has been performed with success in other intracranial vascular beds, including the cervical and superficial cranial arteries.75 High-resolution MCA imaging could theoretically indicate the presence of vulnerable plaque components such as a lipid-rich necrotic core or intraplaque hemorrhage. Current literature posits that the properties of plaque within the intracranial arteries are the same as those of the carotid arteries.17 On the basis of the large body of carotid and coronary atherosclerosis literature, it is hypothesized that multiple contrast weightings may provide an insight into plaque components as summarized from multispectral histology-validated carotid imaging in Table 1. Normal multispectral imaging of the MCA is shown in Fig 2. Unfortunately, histologic valida-tion of the imaging appearance of atherosclerotic plaque within the MCA is hampered by the need for postmortem examination of patients previously imaged. It is generally assumed that the signal intensity relative to the graymatter or the pterygoid muscle on each sequence in the MCA matches that of the plaque component relative to the sternocleidomas-toid for carotid imaging.76
Table 1:
| Plaque Characteristic | TOF MRA | T1-Weighted | PD-Weighted | T2-Weighted |
|---|---|---|---|---|
| Fibrous cap | Isointense/hypointense | Isointense | Isointense/hyperintense | Hyperintense |
| Lipid-rich necrotic core | Isointense | Isointense/hyperintense | Hyperintense | Hypointense |
| Hemorrhage | Hyperintense | Hyperintense to hypointense (with age of hemorrhage) | Hypointense to hyperintense | Hypointense to hyperintense |
| Calcification | Hypointense | Hypointense | Hypointense | Hypointense |
Note:—PD indicates proton attenuation.
Fig 2.
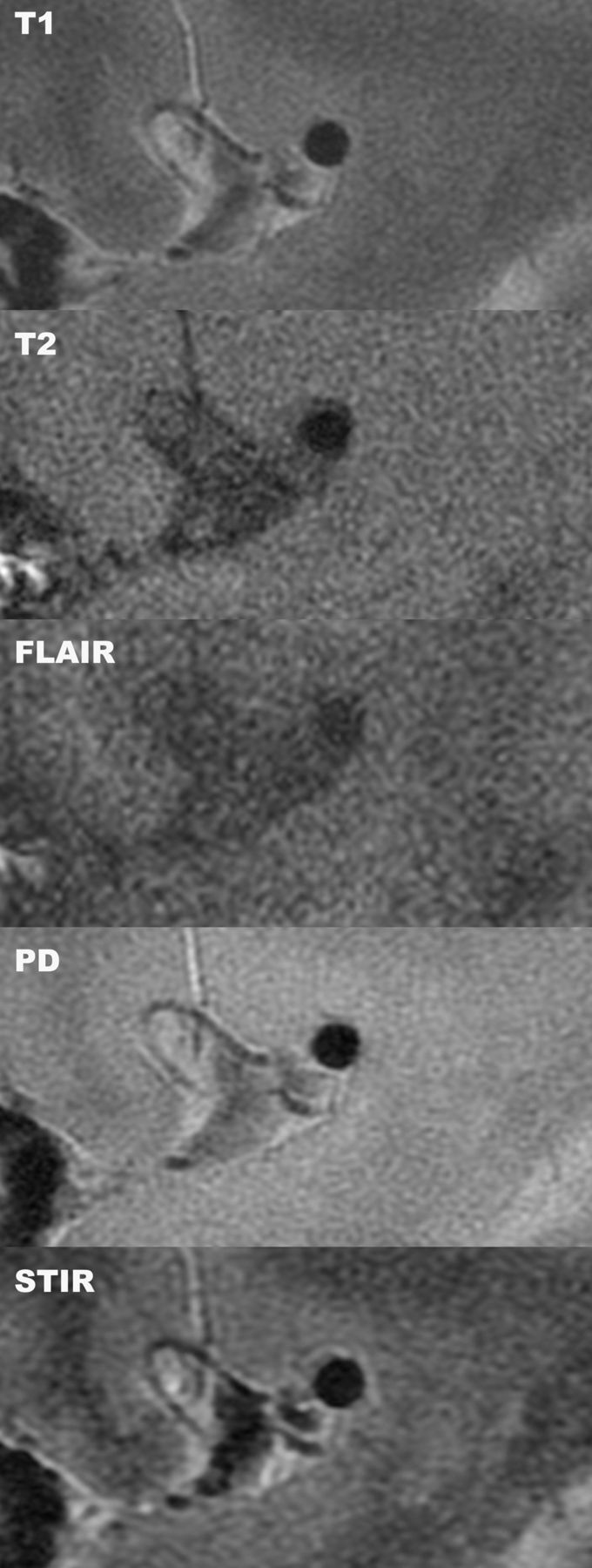
Multicontrast high-resolution MR imaging of a normal MCA. Sagittal high-resolution MR imaging of the MCA in a healthy volunteer demonstrates the ability of multiple sequences to depict the arterial wall and lumen in detail.
Most prior reports of high-resolution MR imaging of the MCA (summarized in Table 2) have focused on T2-weighted imaging findings within the arterial wall by using 3T scanners.71,77 Figure 3A, –B exemplifies the focused use of high-resolution T2-weighted sagittal imaging to resolve the plaque morphology following localization of a stenotic lesion on MRA. T2-weighted high-resolution MR imaging yielded moderate-to-good inter- and intraobserver reproducibility for detection of wall abnormalities and wall thickening.77
Table 2:
Studies of the MCA using high-resolution MR imaging
| Study | Population | Field Strength | Findings |
|---|---|---|---|
| Swartz et al (2009)79 | 37 Patients with focal neurologic deficits | 3T | Enhancing eccentric plaque in patients with intracranial atherosclerotic disease |
| Xu et al (2010)71 | 26 Symptomatic and 28 asymptomatic patients with MCA stenosis | 3T | Symptomatic patients had larger wall area and expansive remodeling |
| Li et al (2009)77 | 48 Patients with suspected MCA disease | 3T | High-resolution MR imaging can visualize plaque not seen with MRA |
| Niizuma et al.(2008)70 | 3 Patients with perforator infarcts | 3T | High-resolution T2-weighted images detected a thin plaque that did not demonstrate stenosis on MRA |
| Klein et al (2006)73 | 6 Patients with documented high-grade MCA stenosis | 1.5T | Focal arterial wall thickening is seen on high-resolution MR imaging of patients with previously identified MCA stenosis |
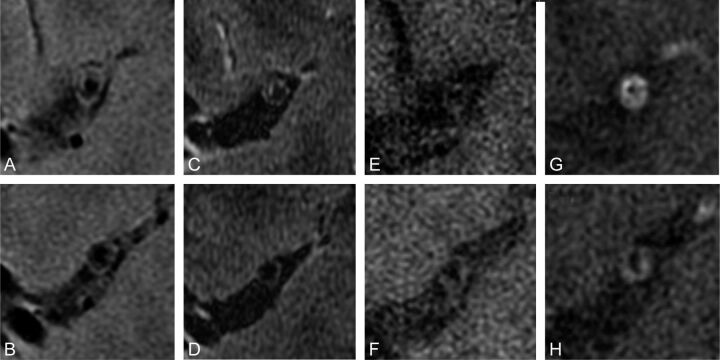
Fig 3.
Multispectral high-resolution MR imaging of a symptomatic patient with MCA disease. T2-weighted (A and B), spin-echo inversion recovery (C and D), T1-weighted (E and F), and postgadolinium T1-weighted (G and H) images depict a large eccentric contrast-enhancing plaque within the lumen of a recently symptomatic patient on the ipsilateral side of symptom presentation.
Detection of plaque characteristics within the MCA has the potential to indicate whether plaque is at risk of rupture (eg, the plaque has a thinned or ruptured fibrous cap) or is stable (eg, it has a thick fibrous cap), assuming MCA atherosclerosis pathophysiology parallels that in the carotid artery.78 A protective fibrous cap in MCA atherosclerosis appears as a bright band adjacent to the lumen on T2-weighted images.70,71
Focal enhancement with contrast administration may potentially identify hemorrhagic plaque composition within the MCA, but in 1 small study, only approximately 15% of patients had enhancement as exemplified in Fig 3G, -H.79 Enhancement could potentially be used to indicate the risk of plaque causing symptoms; however, an earlier study of just 6 patients with known MCA stenosis found that both asymptomatic and remotely symptomatic patients demonstrated gadolinium enhancement of plaque.73 A bright arterial wall on unenhanced FLAIR T1 may be seen in acute hemorrhage or dissection of the MCA.79 In 1 case study, bright T1 hyperintense signal intensity (relative to the adjacent pterygoid muscle) within an MCA plaque was seen in a recently symptomatic patient, implicating intraplaque hemorrhage as the likely cause of this patient's symptoms.76 Because there are no validated imaging appearances for plaque components in the MCA, the actual ability of high-resolution imaging to discern particular components remains tentative.
Recommended Imaging Protocol
Imaging of the MCA for the clinical indication of ischemic symptom evaluation should be integrated with routine stroke protocols, incorporating whole-brain axial FLAIR, diffusion-weighted imaging, and apparent diffusion coefficient sequences in addition to traditional T1- and T2-weighted sequences. Diffusion-weighted sequences are essential because it is well-established that these investigations detect early cerebral ischemia not seen on other sequences and can determine the age of infarcts. TOF MRA of the brain should be performed to yield an overview of the degree of intracranial stenotic lesions. In addition, high-resolution multispectral MR imaging should be performed to specifically image the MCA in clinically suspected cases; the combination of MRA and MR imaging has been used with success in diagnosing intracranial arterial dissections.80 Because of the need for excellent spatial resolution to image these small vessels, the examination of intracranial vasculature must be performed on high-field-strength clinical scanners (ie, 3T and perhaps, with future investigations, 7T, as has already been shown in ex vivo vascular imaging) to take advantage of improved signal intensity–to-noise ratio.81,82 Our group and others strongly emphasize the inadequacy of 1.5T scanners for this protocol.79
The investigation of the MCA should consist of parasagittal sequences oriented perpendicular to the direction of the MCA; these sequences may be directed specifically to image the site of highest degree of stenosis seen on previously acquired MRA images or to nonspecifically sample the vessel of interest (ie, the symptomatic side of the MCA). Because atherosclerosis is a systemic disease and the literature reports contralateral stenoses frequently, the asymptomatic side of the MCA should also be imaged, though the clinical usefulness of this strategy to detect asymptomatic lesions is still unproven.83 In this protocol, we recommend using T1, T2, short tau inversion recovery, FLAIR T1, and fast spin-echo proton-attenuation−weighted sequences for the purpose of providing multispectral characterization of the arterial wall plaque in a manner similar to that validated in carotid MR imaging.84,85 Figure 2 depicts the appearance of these high-resolution sagittal MCA sequences in a healthy volunteer. Contrast-enhanced T1 sequences may be useful in offering better contrast between the fibrous cap and lipid-rich necrotic core atheroma and may also correlate with vulnerable plaque and the risk of symptoms as suggested in the carotid imaging literature. However, the benefit yielded by the use of contrast enhancement in the MCA is uncertain at present because there is no compelling evidence of contrast aiding in the detection of atherosclerotic disease within the MCA.
Future Developments
Wider adoption of high-resolution MR imaging of the MCA for the evaluation of patients with ischemic symptoms has the potential of altering presently held assumptions about the extent of intracranial atherosclerosis as a causative factor in stroke. The application of alternate MR imaging techniques—such as nanoparticle contrast agents targeted to plaque components and the use of dynamic contrast-enhanced imaging to detect neovascularization—could yield even more detailed depiction of the plaque constituents. All of these proposed means of segmenting MCA plaque into its components must be validated.86,87 One means is to examine the postmortem histology of patients previously imaged, but a more feasible, though not ideal, alternative is to use quantitative imaging methods to match the plaque component values from MCA imaging with those histologically validated values in carotid artery imaging.
Unfortunately, the progress gained in the research setting may not reach the clinical arena easily. Several practical limitations hamper clinical use of high-resolution MR imaging of the MCA: The requirement of a 3T scanner and technical expertise restricts these studies to tertiary academic medical centers, and the section selection of regions of the MCA for sagittal acquisition necessitates the involvement of well-trained technicians and active input from neuroradiologists. Additional studies of the reproducibility of high-resolution MR imaging of the MCA are needed to ensure that intracranial atherosclerosis can be followed with serial imaging with time: Evidence of excellent reproducibility would support the use of high-resolution MR imaging in this setting to track disease progression and response to therapy. Moreover, although the mere detection of MCA disease in symptomatic patients does not connote causality, prospective studies examining MCA status and the risk of subsequent events could reveal important associations between high-resolution MR imaging findings and clinical risk.
Aside from these technical considerations, broader studies of diverse populations are needed to test the commonly held assumptions alleging greater prevalence in non-Western populations. The Barcelona-Asymptomatic Intracranial Atherosclerosis Study will address the clinical importance of atherosclerotic disease in the intracranial vessels in a large population by using a combination of TCD and MRA imaging and will provide much needed information on the long-term clinical risk of asymptomatic intracranial stenosis.88
Summary
MCA atherosclerosis is increasingly recognized as an important contributor to the risk of stroke worldwide. MRA can offer evaluation of MCA stenosis with good accuracy compared with DSA but still only provides information about vessel patency alone. High-resolution MR imaging offers additional insight into atherosclerotic disease within the MCA by visualizing the arterial wall and plaque constituents. Additional work to correlate imaging findings with risk of future outcomes is necessary to offer clinical significance.
ABBREVIATIONS:
- TOF
time-of-flight
- TCD
transcranial Doppler
References
- 1. World Health Organization. Causes of Death. Geneva, Switzerland: World Health Organization; 2004. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part2.pdf. Accessed September 15, 2011. [Google Scholar]
- 2. Sridharan SE, Unnikrishnan JP, Sukumaran S, et al. Incidence, types, risk factors, and outcome of stroke in a developing country: the Trivandrum Stroke Registry. Stroke 2009; 40: 1212– 18 [DOI] [PubMed] [Google Scholar]
- 3. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke 2011; 42 (1 suppl): S20– 23 [DOI] [PubMed] [Google Scholar]
- 4. Lhermitte F, Gautier JC, Derouesne C, et al. Ischemic accidents in the middle cerebral artery territory: a study of the causes in 122 cases. Arch Neurol 1968; 19: 248– 56 [DOI] [PubMed] [Google Scholar]
- 5. Suwanwela NC, Chutinetr A. Risk factors for atherosclerosis of cervicocerebral arteries: intracranial versus extracranial. Neuroepidemiology 2003; 22: 37– 40 [DOI] [PubMed] [Google Scholar]
- 6. Wardlaw JM, Doubal FN, Eadie E, et al. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc Dis 2011; 31: 12– 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein IF, Labreuche J, Lavallee PC, et al. Is moderate atherosclerotic stenosis in the middle cerebral artery a cause of or a coincidental finding in ischemic stroke? Cerebrovasc Dis 2010; 29: 140– 45 [DOI] [PubMed] [Google Scholar]
- 8. Mazighi M, Labreuche J, Gongora-Rivera F, et al. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008; 39: 1142– 47 [DOI] [PubMed] [Google Scholar]
- 9. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158– 59 [DOI] [PubMed] [Google Scholar]
- 10. Mead GE, Lewis SC, Wardlaw JM, et al. Severe ipsilateral carotid stenosis and middle cerebral artery disease in lacunar ischaemic stroke: innocent bystanders? J Neurol 2002; 249: 266– 71 [DOI] [PubMed] [Google Scholar]
- 11. Rajapakse A, Rajapakse S, Sharma JC. Is investigating for carotid artery disease warranted in non-cortical lacunar infarction? Stroke 2011; 42: 217– 20 [DOI] [PubMed] [Google Scholar]
- 12. Lyrer PA, Engelter S, Radu EW, et al. Cerebral infarcts related to isolated middle cerebral artery stenosis. Stroke 1997; 28: 1022– 27 [DOI] [PubMed] [Google Scholar]
- 13. Bang OY, Heo JH, Kim JY, et al. Middle cerebral artery stenosis is a major clinical determinant in striatocapsular small, deep infarction. Arch Neurol 2002; 59: 259– 63 [DOI] [PubMed] [Google Scholar]
- 14. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial—TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35– 41 [DOI] [PubMed] [Google Scholar]
- 15. Gongora-Rivera F, Labreuche J, Jaramillo A, et al. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke 2007; 38: 1203– 10 [DOI] [PubMed] [Google Scholar]
- 16. Lee PH, Oh SH, Bang OY, et al. Isolated middle cerebral artery disease: clinical and neuroradiological features depending on the pathogenesis. J Neurol Neurosurg Psychiatry 2004; 75: 727– 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen XY, Wong KS, Lam WW, et al. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis 2008; 25: 74– 80 [DOI] [PubMed] [Google Scholar]
- 18. Lee PH, Oh SH, Bang OY, et al. Infarct patterns in atherosclerotic middle cerebral artery versus internal carotid artery disease. Neurology 2004; 62: 1291– 96 [DOI] [PubMed] [Google Scholar]
- 19. Bonati LH, Wetzel SG, Gandjour J, et al. Diffusion-weighted imaging in stroke attributable to internal carotid artery dissection: the significance of vessel patency. Stroke 2008; 39: 483– 85 [DOI] [PubMed] [Google Scholar]
- 20. Kim YD, Choi HY, Cho HJ, et al. Increasing frequency and burden of cerebral artery atherosclerosis in Korean stroke patients. Yonsei Med J 2010; 51: 318– 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu HM, Tu YK, Yip PK, et al. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke 1996; 27: 650– 53 [DOI] [PubMed] [Google Scholar]
- 22. Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology 1998; 50: 812– 13 [DOI] [PubMed] [Google Scholar]
- 23. Wong KS, Ng PW, Tang A, et al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology 2007; 68: 2035– 38 [DOI] [PubMed] [Google Scholar]
- 24. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555– 63 [DOI] [PubMed] [Google Scholar]
- 25. Kern R, Steinke W, Daffertshofer M, et al. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology 2005; 65: 859– 64 [DOI] [PubMed] [Google Scholar]
- 26. Famakin BM, Chimowitz MI, Lynn MJ, et al. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke 2009; 40: 1999– 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akins PT, Pilgram TK, Cross DT, 3rd, et al. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke 1998; 29: 433– 38 [DOI] [PubMed] [Google Scholar]
- 28. Block F, Hoang PA. Oral anticoagulation in symptomatic intracranial stenoses [in German]. Nervenarzt 2003; 74: 523– 26. Epub 2003 May 10 [DOI] [PubMed] [Google Scholar]
- 29. Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology 1995; 45: 1488– 93 [DOI] [PubMed] [Google Scholar]
- 30. Kremer C, Schaettin T, Georgiadis D, et al. Prognosis of asymptomatic stenosis of the middle cerebral artery. J Neurol Neurosurg Psychiatry 2004; 75: 1300– 03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology 2000; 55: 490– 97 [DOI] [PubMed] [Google Scholar]
- 32. Thomas GN, Chen XY, Lin JW, et al. Middle cerebral artery stenosis increased the risk of vascular disease mortality among type 2 diabetic patients. Cerebrovasc Dis 2008; 25: 261– 67 [DOI] [PubMed] [Google Scholar]
- 33. Turan TN, Derdeyn CP, Fiorella D, et al. Treatment of atherosclerotic intracranial arterial stenosis. Stroke 2009; 40: 2257– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta R, Schumacher HC, Mangla S, et al. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology 2003; 61: 1729– 35 [DOI] [PubMed] [Google Scholar]
- 35. Marks MP, Marcellus M, Norbash AM, et al. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke 1999; 30: 1065– 69 [DOI] [PubMed] [Google Scholar]
- 36. Marks MP, Marcellus ML, Do HM, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol 2005; 26: 525– 30 [PMC free article] [PubMed] [Google Scholar]
- 37. Yoon W, Seo JJ, Cho KH, et al. Symptomatic middle cerebral artery stenosis treated with intracranial angioplasty: experience in 32 patients. Radiology 2005; 237: 620– 26 [DOI] [PubMed] [Google Scholar]
- 38. Leung TW, Yu SC, Lam WW, et al. Would self-expanding stent occlude middle cerebral artery perforators? Stroke 2009; 40: 1910– 12 [DOI] [PubMed] [Google Scholar]
- 39. Siddiq F, Vazquez G, Memon MZ, et al. Comparison of primary angioplasty with stent placement for treating symptomatic intracranial atherosclerotic diseases: a multicenter study. Stroke 2008; 39: 2505– 10 [DOI] [PubMed] [Google Scholar]
- 40. Suh DC, Kim JK, Choi JW, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol 2008; 29: 781– 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gröschel K, Schnaudigel S, Pilgram SM, et al. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke 2009; 40: e340– 47 [DOI] [PubMed] [Google Scholar]
- 42. Tsai ST, Yen PS, Wang YJ, et al. Superficial temporal artery-middle cerebral artery bypass for ischemic atherosclerotic middle cerebral artery disease. J Clin Neurosci 2009; 16: 1013– 17 [DOI] [PubMed] [Google Scholar]
- 43. O JH, Jang KS, Yoo Ie R, et al. Assessment of cerebrovascular reserve before and after STA-MCA bypass surgery by SPECT and SPM analysis. Korean J Radiol 2007; 8: 458– 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao S, Lam WW, Chan YL, et al. Optimal values of flow velocity on transcranial Doppler in grading middle cerebral artery stenosis in comparison with magnetic resonance angiography. J Neuroimaging 2002; 12: 213– 18 [PubMed] [Google Scholar]
- 45. Navarro JC, Lao AY, Sharma VK, et al. The accuracy of transcranial Doppler in the diagnosis of middle cerebral artery stenosis. Cerebrovasc Dis 2007; 23: 325– 30 [DOI] [PubMed] [Google Scholar]
- 46. Kaps M, Stolz E, Allendoerfer J. Prognostic value of transcranial sonography in acute stroke patients. Eur Neurol 2008; 59 (suppl 1): 9– 16 [DOI] [PubMed] [Google Scholar]
- 47. Lien LM, Chen WH, Chen JR, et al. Comparison of transcranial color-coded sonography and magnetic resonance angiography in acute ischemic stroke. J Neuroimaging 2001; 11: 363– 68 [DOI] [PubMed] [Google Scholar]
- 48. Heiserman JE, Drayer BP, Keller PJ, et al. Intracranial vascular stenosis and occlusion: evaluation with three-dimensional time-of-flight MR angiography. Radiology 1992; 185: 667– 73 [DOI] [PubMed] [Google Scholar]
- 49. Sawada M, Yano H, Shinoda J, et al. Symptomatic middle cerebral artery stenosis and occlusion: comparison of three-dimensional time-of-flight magnetic resonance angiography with conventional angiography. Neurol Med Chir (Tokyo) 1994; 34: 682– 85 [DOI] [PubMed] [Google Scholar]
- 50. Korogi Y, Takahashi M, Mabuchi N, et al. Intracranial vascular stenosis and occlusion: diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology 1994; 193: 187– 93 [DOI] [PubMed] [Google Scholar]
- 51. Stock KW, Radue EW, Jacob AL, et al. Intracranial arteries: prospective blinded comparative study of MR angiography and DSA in 50 patients. Radiology 1995; 195: 451– 56 [DOI] [PubMed] [Google Scholar]
- 52. Korogi Y, Takahashi M, Nakagawa T, et al. Intracranial vascular stenosis and occlusion: MR angiographic findings. AJNR Am J Neuroradiol 1997; 18: 135– 43 [PMC free article] [PubMed] [Google Scholar]
- 53. Hirai T, Korogi Y, Ono K, et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. AJNR Am J Neuroradiol 2002; 23: 93– 101 [PMC free article] [PubMed] [Google Scholar]
- 54. Bash S, Villablanca JP, Jahan R, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005; 26: 1012– 21 [PMC free article] [PubMed] [Google Scholar]
- 55. Wong KS, Lam WW, Liang E, et al. Variability of magnetic resonance angiography and computed tomography angiography in grading middle cerebral artery stenosis. Stroke 1996; 27: 1084– 87 [DOI] [PubMed] [Google Scholar]
- 56. Willinek WA, Born M, Simon B, et al. Time-of-flight MR angiography: comparison of 3.0-T imaging and 1.5-T imaging–initial experience. Radiology 2003; 229: 913– 20 [DOI] [PubMed] [Google Scholar]
- 57. Willinek WA, Gieseke J, von Falkenhausen M, et al. Sensitivity encoding (SENSE) for high spatial resolution time-of-flight MR angiography of the intracranial arteries at 3.0 T. Rofo 2004; 176: 21– 26 [DOI] [PubMed] [Google Scholar]
- 58. Choi CG, Lee DH, Lee JH, et al. Detection of intracranial atherosclerotic steno-occlusive disease with 3D time-of-flight magnetic resonance angiography with sensitivity encoding at 3T. AJNR Am J Neuroradiol 2007; 28: 439– 46 [PMC free article] [PubMed] [Google Scholar]
- 59. Furst G, Hofer M, Sitzer M, et al. Factors influencing flow-induced signal loss in MR angiography: an in vitro study. J Comput Assist Tomogr 1995; 19: 692– 99 [DOI] [PubMed] [Google Scholar]
- 60. Nederkoorn PJ, van der Graaf Y, Eikelboom BC, et al. Time-of-flight MR angiography of carotid artery stenosis: does a flow void represent severe stenosis? AJNR Am J Neuroradiol 2002; 23: 1779– 84 [PMC free article] [PubMed] [Google Scholar]
- 61. Man BL, Fu YP, Chan YY, et al. Use of magnetic resonance angiography to predict long-term outcomes of ischemic stroke patients with concurrent stenoses in Hong Kong. Cerebrovasc Dis 2009; 28: 112– 18 [DOI] [PubMed] [Google Scholar]
- 62. Suzuki T, Ogasawara K, Hirooka R, et al. Preoperative single-slab 3D time-of-flight magnetic resonance angiography predicts development of new cerebral ischemic events after carotid endarterectomy: clinical article. J Neurosurg 2009; 111: 141– 46 [DOI] [PubMed] [Google Scholar]
- 63. Miyazawa N, Akiyama I, Yamagata Z. Analysis of incidence and risk factors for progression in patients with intracranial steno-occlusive lesions by serial magnetic resonance angiography. Clin Neurol Neurosurg 2007; 109: 680– 85 [DOI] [PubMed] [Google Scholar]
- 64. Kwon SU, Cho YJ, Koo JS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005; 36: 782– 86. Epub 2005 Mar 3 [DOI] [PubMed] [Google Scholar]
- 65. Mok VC, Lam WW, Chen XY, et al. Statins for asymptomatic middle cerebral artery stenosis: the Regression of Cerebral Artery Stenosis study. Cerebrovasc Dis 2009; 28: 18– 25 [DOI] [PubMed] [Google Scholar]
- 66. Prabhakaran S, Wells KR, Jhaveri MD, et al. Hemodynamic changes following Wingspan stent placement: a quantitative magnetic resonance angiography study. J Neuroimaging 2011; 21: e109– 13. Epub 2009 Jan 22 [DOI] [PubMed] [Google Scholar]
- 67. Prabhakaran S, Warrior L, Wells KR, et al. The utility of quantitative magnetic resonance angiography in the assessment of intracranial in-stent stenosis. Stroke 2009; 40: 991– 93. Epub 2009 Jan 22 [DOI] [PubMed] [Google Scholar]
- 68. Wasserman BA, Wityk RJ, Trout HH, 3rd, et al. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 2005; 36: 2504– 13 [DOI] [PubMed] [Google Scholar]
- 69. Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987; 316: 1371– 75 [DOI] [PubMed] [Google Scholar]
- 70. Niizuma K, Shimizu H, Takada S, et al. Middle cerebral artery plaque imaging using 3-Tesla high-resolution MRI. J Clin Neurosci 2008; 15: 1137– 41 [DOI] [PubMed] [Google Scholar]
- 71. Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010; 212: 507– 11 [DOI] [PubMed] [Google Scholar]
- 72. Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643– 46 [PMC free article] [PubMed] [Google Scholar]
- 73. Klein IF, Lavallee PC, Touboul PJ, et al. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology 2006; 25 67: 327–29 [DOI] [PubMed] [Google Scholar]
- 74. Park JK, Kim SH, Kim BS, et al. Imaging of intracranial plaques with black-blood double inversion recovery MR imaging and CT. J Neuroimaging 2011; 21: e64– 68 [DOI] [PubMed] [Google Scholar]
- 75. Markl M, Uhl M, Wieben O, et al. High-resolution 3T MRI for the assessment of cervical and superficial cranial arteries in giant cell arteritis. J Magn Reson Imaging 2006; 24: 423– 27 [DOI] [PubMed] [Google Scholar]
- 76. Turan TN, Bonilha L, Morgan PS, et al. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging 2011; 21: e159– 61 [DOI] [PubMed] [Google Scholar]
- 77. Li ML, Xu WH, Song L, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis 2009; 204: 447– 52 [DOI] [PubMed] [Google Scholar]
- 78. Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006; 240: 464– 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-Tesla contrast-enhanced MRI. Neurology 2009; 72: 627– 34 [DOI] [PubMed] [Google Scholar]
- 80. Hirai T, Korogi Y, Murata Y, et al. Intracranial artery dissections: serial evaluation with MR imaging, MR angiography, and source images of MR angiography. Radiat Med 2003; 21: 86– 93 [PubMed] [Google Scholar]
- 81. Buhk JH, Ries T, Finck-Wedel AK, et al. Possibilities and limitations in imaging the intracranial arteries in the context of a contrast-enhanced whole-body magnetic resonance angiographic screening protocol at 1.5 versus 3 Tesla. J Comput Assist Tomog 2011; 35: 4– 8 [DOI] [PubMed] [Google Scholar]
- 82. Jahnke C, Dietrich T, Paetsch I, et al. Experimental evaluation of the detectability of submillimeter atherosclerotic lesions in ex vivo human iliac arteries with ultrahigh-field (7.0 T) magnetic resonance imaging. Int J Cardiovasc Imaging 2007; 23: 519– 27 [DOI] [PubMed] [Google Scholar]
- 83. Kim YD, Choi HY, Jung YH, et al. Mirror pattern of cerebral artery atherosclerosis in patients with ischaemic stroke. Eur J Neurol 2009; 16: 1159– 64 [DOI] [PubMed] [Google Scholar]
- 84. Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001; 104: 2051– 56 [DOI] [PubMed] [Google Scholar]
- 85. Watanabe Y, Nagayama M. MR plaque imaging of the carotid artery. Neuroradiology 2010; 52: 253– 74 [DOI] [PubMed] [Google Scholar]
- 86. McAteer MA, Akhtar AM, von Zur Muhlen C, et al. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis 2010; 209: 18– 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kerwin WS, O'Brien KD, Ferguson MS, et al. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 2006; 241: 459– 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lopez-Cancio E, Dorado L, Millan M, et al. The population-based Barcelona-Asymptomatic Intracranial Atherosclerosis Study (ASIA): rationale and design. BMC Neurol 2011; 11: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Honda M, Kawahara I, Kitagawa N, et al. Asymptomatic carotid artery plaques: use of magnetic resonance imaging to characterize vulnerable plaques in 6 cases. Surg Neurol 2007; 67: 35– 39 [DOI] [PubMed] [Google Scholar]
- 90. Saam T, Cai JM, Cai YQ, et al. Carotid plaque composition differs between ethno-racial groups: an MRI pilot study comparing mainland Chinese and American Caucasian patients. Arterioscler Thromb Vasc Biol 2005; 25: 611– 16 [DOI] [PubMed] [Google Scholar]
- 91. Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002; 15: 62– 67 [DOI] [PubMed] [Google Scholar]