SUMMARY:
Dural sealants are an adjunct to obtain watertight closure after intradural procedures. This study aims to characterize the appearance on MR imaging of 3 commonly employed dural sealants: fibrin glue, PEGH, and BSAG. To this end, patients who underwent spinal intradural procedures that included the use of dural sealant during closure were identified retrospectively. Post-operative data on 15 patients, including complications such as pseudomeningocele formation and infection, were gathered. The appearance of dural sealants on follow-up MR imaging scans within 3 days of surgery was analyzed. Fifteen patients were identified (5 with fibrin glue, 5 with PEGH, and 5 with BSAG applied during closure) with appropriately timed post-operative MR imaging scans. All 3 substances were identifiable based on anatomic location and imaging characteristics on post-operative MR imaging in standard T1, T1 PGFS, and T2 FSE. Definite differentiation between CSF and fibrin glue or PEGH was not possible with the T1 or T1 PGFS, or with the T2 FSE. Differences in intensity between CSF and BSAG were also not significant on either T1 sequence, but they were statistically significant on the T2 FSE. All patients had an uneventful post-operative course, and no patients developed post-operative pseudomeningocele at 30 days. This study concludes that water-based dural sealants such as fibrin glue and PEGH are difficult to differentiate from CSF on standard T1, T1 PGFS and T2 FSE, while BSAG is easily recognized on the T2 FSE. Recognition of water-based sealants therefore requires communication between the neurosurgeon and the neuroradiologist to avoid post-operative misidentification.
Watertight closure of the dura is a primary concern in the management of intradural spine tumors. Although Harvey Cushing asserted that “an accurate approximation of the dura in its two layers is desirable,”1 in the modern era, a single-layered dural closure with suture is the standard of care. To further reduce the chance of leakage, numerous adjunctive dural sealants have emerged in the last several years. By being applied to the suture line following primary repair, these substances are designed to bond with the tissue and fill small gaps in the closure, thus preventing leakage of CSF through the repair site.2,3
Three such substances are commonly used in our practice. Fibrin glue (TISSEEL; Baxter BioSurgery, Deerfield, Illinois) has been used as a dural sealant in both cranial and spinal cases. It relies on a solution of fibrinogen, aprotinin, and clotting factors. When mixed with a thrombin counterpart, the fibrin cross-links, causing coagulation and binding to surrounding tissues. The glue is subsequently degraded by natural fibrinolysis during several weeks. Hydrogel (DuraSeal; Covidien, Dublin, Ireland) is a compound based on polyethylene glycol and trilysine, a small amino acid. When trilysine is mixed with polyethylene glycol, the substances cross-link almost instantly, forming a flexible layer that adheres to surrounding tissues. It also has been shown effective in nonautologous dural repairs.4 The PEGH is subsequently broken down by hydrolysis during 4–8 weeks, and the byproducts are renally cleared. The BSAG polymer (BioGlue; CryoLife, Atlanta, Georgia) is primarily used in cardiovascular applications as a hemostatic and structural reinforcing agent along suture lines. It has been used in neurosurgical procedures to minimize the risk of cerebrospinal leak after dural closure and in sellar reconstruction following transsphenoidal surgery.5–7 BSAG is composed of a highly cross-linked protein polymer and takes several months to degrade, much longer than the other 2 water-based compounds.8
Both fibrin glue and PEGH have a high water content and thus have characteristics similar to CSF or reactive inflammation on MR imaging.9 Furthermore, they are applied over the dural repair, exactly where a postoperative CSF collection or infection may be expected to form. In patients treated with fibrin glue or PEGH, MR images obtained within the first few weeks of surgery to assess postoperative pseudomeningocele are difficult to interpret. The misinterpretation of dural sealant as a pseudomeningocele may therefore result in unnecessary lengthening of the hospital stay, invasive CSF diversion procedures, and reoperation. BSAG, on the other hand, is not water-based and should thus be distinguishable from CSF with postoperative MR imaging.
This study aims to characterize the appearance of fibrin glue, PEGH, and BSAG on MR imaging in the postoperative setting. One study has examined the radiographic appearance of PEGH in a canine model9; however, to our knowledge, there is no existing comparison of MR imaging appearance in patients in the immediate postoperative timeframe. The analysis will seek to describe the signal characteristics of these 3 sealants under MR imaging and to establish criteria for distinguishing appropriately placed dural sealants from pseudomeningocele.
Materials and Methods
Patient Population
We analyzed data collected from 15 patients undergoing spinal surgery for removal of intradural lesions at our medical center. All patients had fibrin glue, PEGH, or BSAG applied to the dural repair during closure. Patients who had sealant applied after repair of incidental dural tears were excluded from the study because the closure of unintentional dural rents is often imperfect and CSF leakage through the repair cannot be definitely excluded. Patients who underwent nonroutine MR imaging for new neurologic deficits or other unexpected symptoms were excluded as well.
The study was conducted under institutional review board approval (reference number 10-00271).
Data Collection
Data for these patients were collected retrospectively via chart review. Hospital and clinic records were reviewed to extract data pertaining to patient age, dates of surgery, intraoperative technique, and dates of routine follow-up imaging. MR images were included in the analysis if they occurred within 3 days of surgery and were ordered as routine studies. Postoperative courses were reviewed. To ensure that measurements of signal intensity in the extradural region on postoperative MRI were due to sealant and not to CSF, we excluded patients if clinical evidence of spinal fluid leak or pseudomeningocele was documented as a complication. Pathology reports were reviewed for any intraoperative specimens.
The final reports of the included MR images were reviewed, and all information involving imaging techniques and sequences performed was noted. A 3D volumetric analysis was conducted in a blinded fashion by the lead author (P.E.T.) under the oversight of a board-certified neuroradiologist (P.V.M.). Manual segmentation was performed with region-of-interest analysis to measure the volume (in cubic centimeters) of sealant by using a PACS workstation. Signal characteristics within the ROIs were quantified. CSF signal intensity was then measured in 5 randomly selected areas for each series. Region-of-interest intensity was then normalized as follows: mean region-of-interest intensity/mean CSF intensity. Determination of volumes was made without consideration of clinical outcome.
Statistical Analysis
Normalized sealant intensities were analyzed as continuous variables. Comparison of normalized sealant intensities between groups and MR images was conducted by using 2-way ANOVA. Post hoc analysis of subsets was conducted by using a Bonferroni correction.
Results
Fifteen patients were identified who fit the inclusion criteria, 5 each who received fibrin glue, PEGH, and BSAG. The patient demographics and clinical characteristics as well as pathologic diagnoses are summarized in the Table.
Patient demographics, location/type of sealant, and pathologic diagnoses
| Age (yr) | Sex | Level | Sealant | Pathologic Diagnosis |
|---|---|---|---|---|
| 51 | M | T11–L1 | PEGH | Schwannoma |
| 32 | M | T4–7 | Fibrin glue | Schwannoma |
| 37 | F | S1 | PEGH | Ependymoma |
| 67 | F | C4–6 | BSAG | Meningioma |
| 66 | F | L2–3 | BSAG | Schwannoma |
| 41 | M | L1 | Fibrin glue | Ependymoma |
| 21 | M | T12–L1 | PEGH | Ependymoma |
| 34 | F | L4–5 | PEGH | Ependymoma |
| 20 | M | T4–8 | Fibrin glue | Meningioma |
| 73 | F | L1–3 | BSAG | Ependymoma |
| 62 | F | T12–L1 | BSAG | Schwannoma |
| 38 | M | L3 | BSAG | Ependymoma |
| 67 | M | C12 | Fibrin glue | Schwannoma |
| 40 | F | T8–12 | PEGH | Ependymoma |
| 55 | M | L23 | Fibrin glue | Schwannoma |
Imaging techniques were largely similar throughout the patient population. MR imaging was performed by using a 1.5T magnet. The protocol typically included the following sequences: 1) T1-weighted spin-echo unenhanced, 2) T1-weighted spin-echo PGFS, and 3) T2-weighted FSE. Additional sequences were evaluated if available.
A total of 15 imaging studies were reviewed. Representative sections of the T1, T1 PGFS, and T2 FSE scans are depicted in Figs 1–3. On the initial postoperative scan, the mean signal intensity for voxels corresponding to fibrin glue standardized to intensity of CSF was 1.15 on T1, 0.98 on T1 PGFS, and 0.94 on T2 FSE. The mean signal intensity for voxels corresponding to PEGH standardized to CSF was 1.36 on T1, 1.44 on T1 PGFS, and 1.68 on T2 FSE. The mean standardized signal intensity for voxels corresponding to BSAG was 2.12 on T1, 1.70 on T1 PGFS, and 0.13 on T2 FSE. While there was no significant difference in intensity characteristics between fibrin glue and PEGH (P < .74), both of these substances demonstrated significantly different characteristics from BSAG (P < .01). Additionally, PEGH demonstrated a weak statistical trend toward hyperintensity on T2 FSE compared with CSF (P < .17). These signal characteristics are summarized in Fig 4.
Fig 1.
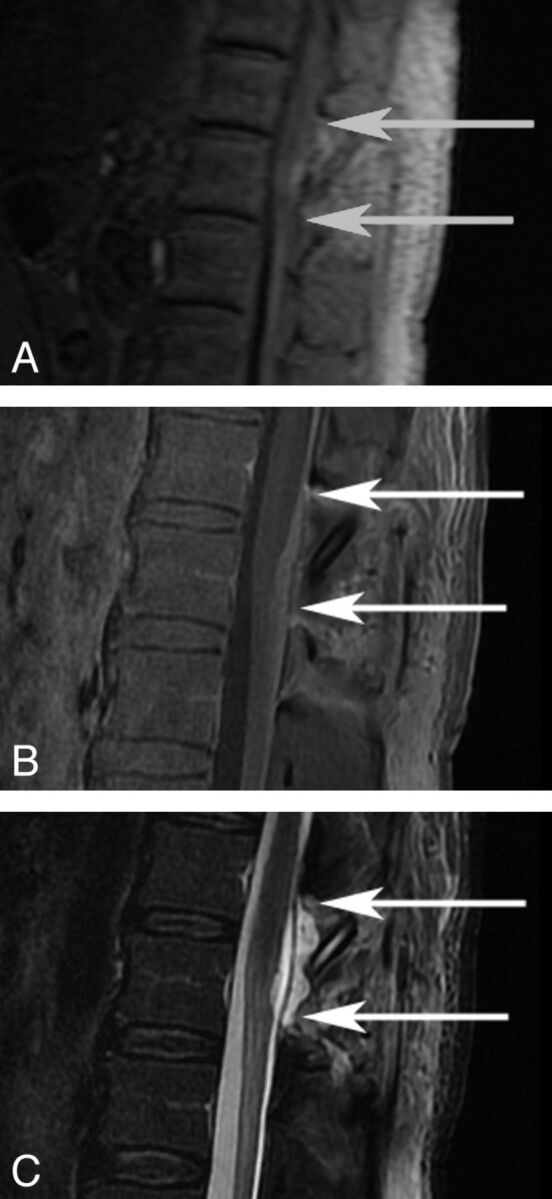
MR images of the lumbar spine after fibrin glue application. T1 (A), T1 PGFS (B), and T2 FSE (C) weighted sequences are depicted. Arrows depict the rostral and caudal limits of the region of application.
Fig 2.
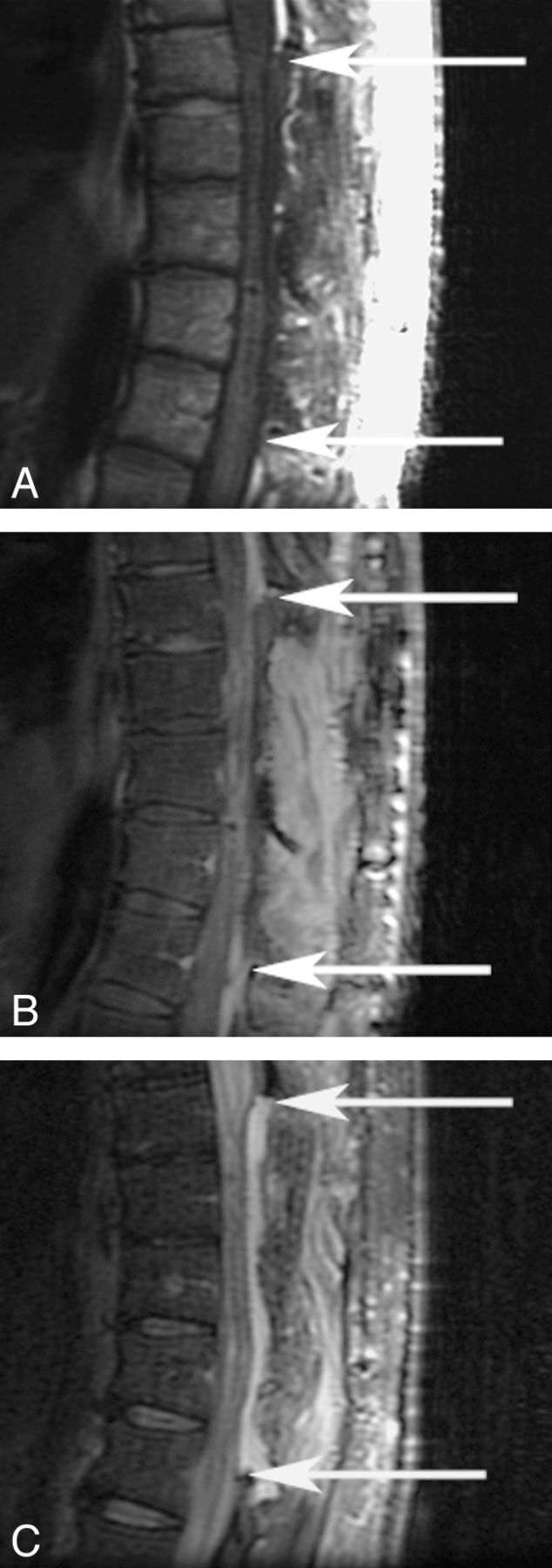
MR images of the thoracic spine after PEGH application. T1 (A), T1 PGFS (B), and T2 FSE (C) weighted sequences are depicted. Arrows depict the rostral and caudal limits of the region of application.
Fig 3.
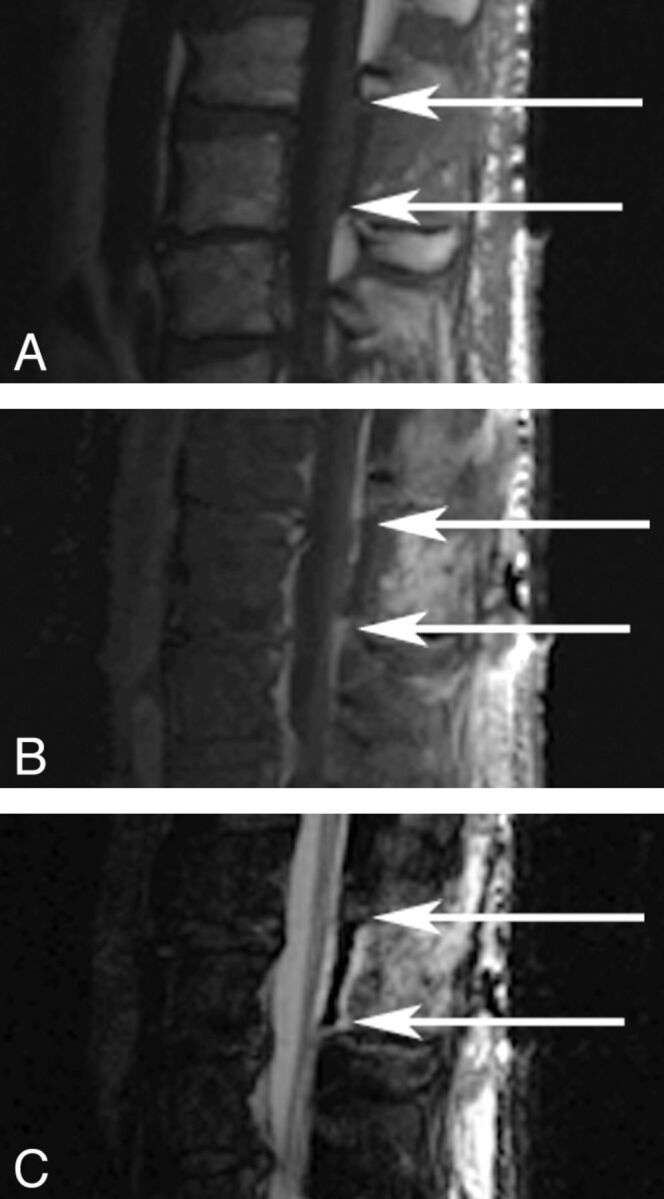
MR images of the lumbar spine after BSAG application. T1 (A), T1 PGFS (B), and T2 FSE (C) weighted sequences are depicted. Arrows depict the rostral and caudal limits of the region of application.
Fig 4.
The signal intensity of dural sealant standardized to the intensity of CSF.
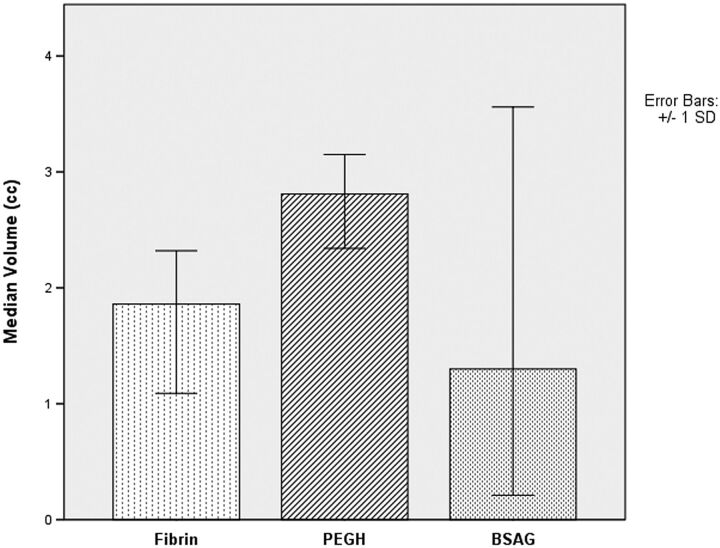
The median volume of fibrin glue applied, as calculated by region-of-interest measurement on the initial postoperative scan over T1, T1 PGFS, and T2 FSE sequences, was 1.86 cm3. Similarly, the median volume of PEGH was 2.81 cm3, while BSAG had a median volume of 1.30 cm3 (Fig 5). No significant difference was found among these 3 groups, indicating that the dural sealants were each used in relatively equal quantities.
Fig 5.
The volume of dural sealant applied as measured by postoperative MR imaging.
Discussion
Data suggest that the usage of a dural sealant may reduce the rates of CSF leakage, spinal headache, and pseudomeningocele formation after an intradural procedure.10–13 All 3 of the sealants in this study use basic biologic chemistry to accomplish their task. As such, they have similar properties to natural tissue. Fibrin glue and PEGH have a high water content and are readily absorbed by the body with time. The third, BSAG, has much lower water content and may take years to fully absorb. Because of these characteristics, however, both fibrin glue and PEGH are difficult to distinguish from CSF on MR imaging. This analysis has demonstrated that with conventional T1- and T2-weighted techniques, neither fibrin glue nor PEGH may be reliably differentiated from each other or from CSF, though a statistical trend toward T2-weighted hyperintensity compared with CSF is likely. Given that they are applied directly to the dura, they paradoxically mimic pseudomeningocele. In the clinical setting of a postoperative spine patient with vague symptoms and an MR image consistent with epidural pseudomeningocele, it is possible that misinterpretation of these sealants can lead to additional (and unnecessary) work-up and treatment.
The radiologists and neuroradiologists who read the scans of postoperative spine patients must therefore be given the necessary information to identify these sealants when used. By communicating their usage to the relevant radiologist, the surgeon can ensure that final radiology reports include the dural sealant within the differential of an epidural fluid collection.
Conclusions
Fibrin glue, PEGH, and BSAG are commonly used adjuncts in establishing a watertight closure following an intradural procedure. The imaging characteristics of fibrin glue and PEGH make them functionally indistinguishable from CSF on standard T1, T1 PGFS, and T2 FSE MR imaging sequences. BSAG is identifiable as low signal intensity compared with CSF on the T2 FSE sequence. Specific communication with the radiologist or neuroradiologist regarding the location and quantity of sealant used can increase the chance of correctly identifying the etiology of epidural collections.
ABBREVIATIONS:
- BSAG
bovine serum albumin and gluteraldehyde polymer
- FSE
fast spin- echo
- PEGH
polyethylene glycol hydrogel
- PGFS
postgadolinium fat saturation
Footnotes
Disclosures: Praveen Mummaneni—UNRELATED: Consultancy: Medtronic, DePuy Spine, Comments: I am no longer a consultant at this time, but I was in the past, Expert Testimony: private attorney offices,* Royalties: DePuy Spine, Quality Medical Publishers; Travel/Accommodations/Meeting Expenses Not Related to Activities Listed: DePuy Spine. Chrisopther Ames—UNRELATED: Consultancy: DePuy, Medtronic, Stryker, Payment for Lectures (including service on Speakers Bureaus): Trans1,* Patents (planned, pending, or issued): Fish & Richardson PC, Royalties: Aesculap, LAWx, Stock/Stock Options: Trans1, Doctors Research Group. *Money paid to the institution.
References
- 1. Cushing H. Surgery of the head. In: Keen W.W. ed. Surgery, Its Principles and Practice. Vol 3. Philadelphia: W.B. Saunders Co; 1908: 17–276 [Google Scholar]
- 2. Preul MC, Bichard WD, Spetzler RF. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery 2003; 53: 1189–98, discussion 1198–99 [DOI] [PubMed] [Google Scholar]
- 3. Boogaarts JD, Grotenhuis JA, Bartels RH, et al. Use of a novel absorbable hydrogel for augmentation of dural repair: results of a preliminary clinical study. Neurosurgery 2005; 57(1 suppl): 146–51, discussion 146–51 [DOI] [PubMed] [Google Scholar]
- 4. Weinstein JS, Liu KC, Delashaw JB, Jr, et al. The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. J Neurosurg 2010; 112: 428–33 [DOI] [PubMed] [Google Scholar]
- 5. Kumar A, Maartens NF, Kaye AH. Evaluation of the use of BioGlue in neurosurgical procedures. J Clin Neurosci 2003; 10: 661–64 [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Maartens NF, Kaye AH. Reconstruction of the sellar floor using Bioglue following transsphenoidal procedures. J Clin Neurosci 2003; 10: 92–95 [DOI] [PubMed] [Google Scholar]
- 7. Stylli SS, Kumar A, Gonzales M, et al. The biocompatibility of BioGlue with the cerebral cortex: a pilot study. J Clin Neurosci 2004; 11: 631–35 [DOI] [PubMed] [Google Scholar]
- 8. Yuen T, Kaye AH. Persistence of Bioglue in spinal dural repair. J Clin Neurosci 2005; 12: 100–01 [DOI] [PubMed] [Google Scholar]
- 9. Kacher DF, Frerichs K, Pettit J, et al. DuraSeal magnetic resonance and computed tomography imaging: evaluation in a canine craniotomy model. Neurosurgery 2006; 58(1 suppl): ONS140–47, discussion ONS140-47 [DOI] [PubMed] [Google Scholar]
- 10. Nakamura H, Matsuyama Y, Yoshihara H, et al. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: a randomized controlled trial. Spine (Phila Pa 1976) 2005; 30: E347–51 [DOI] [PubMed] [Google Scholar]
- 11. Than KD, Baird CJ, Olivi A.. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery 2008; 63(1 suppl 1): ONS182–86, discussion ONS186–87 [DOI] [PubMed] [Google Scholar]
- 12. Kassam A, Horowitz M, Carrau R, et al. Use of Tisseel fibrin sealant in neurosurgical procedures: incidence of cerebrospinal fluid leaks and cost-benefit analysis in a retrospective study. Neurosurgery 2003; 52: 1102–05, discussion 1105 [PubMed] [Google Scholar]
- 13. Preul MC, Campbell PK, Garlick DS, et al. Application of a new hydrogel dural sealant that reduces epidural adhesion formation: evaluation in a large animal laminectomy model. J Neurosurg Spine 2010; 12: 381–90 [DOI] [PubMed] [Google Scholar]