Abstract
The dimorphic fungus Sporothrix globosa is the predominant etiologic agent causing sporotrichosis in China, particularly in the northeast. It has been demonstrated that the incubation temperature and growth phase can influence in vitro antifungal susceptibility profiles of S. schenckii sensu stricto and S. brasiliensis (sibling species of S. globosa). Few studies have reported on the antifungal susceptibility of S. globosa, especially using large numbers of isolates. In this study, we assessed the susceptibility of 80 isolates of S. globosa originating from Jilin Province, northeastern China, to six antifungal agents (itraconazole, terbinafine, voriconazole, posaconazole, fluconazole, and amphotericin B), at varying incubation temperatures and in different fungal growth phases. The isolates were most sensitive to terbinafine (geometric mean [GM] of the minimum inhibitory concentration [MIC]: 0.0356 μg/ml for the mycelial phase at 30 °C, 0.0332 μg/ml for the mycelial phase at 35 °C, and 0.031 μg/ml for the yeast phase, respectively), followed by posaconazole (GM of the MIC: 4.2501 μg/ml for the mycelial phase at 30 °C, 1.4142 μg/ml for the mycelial phase at 35 °C, and 0.7195 μg/ml for the yeast phase, respectively) and itraconazole (GM of the MIC: 6.8448 μg/ml for the mycelial phase at 30 °C, 3.1383 μg/ml for the mycelial phase at 35 °C, and 1.0263 μg/ml for the yeast phase, respectively). The isolates were relatively resistant to fluconazole (GM of the MIC: 76.7716 μg/ml for the mycelial phase at 30 °C, 66.2570 μg/ml for the mycelial phase at 35 °C, and 24.4625 μg/ml for the yeast phase, respectively) and voriconazole (GM of the MIC: 26.2183 μg/ml for the mycelial phase at 30 °C, 13.6895 μg/ml for the mycelial phase at 35 °C, and 1.3899 μg/ml for the yeast phase, respectively). For all the tested azole drugs, the MICs at 30 °C were significantly higher than those at 35 °C (P < 0.001); for all agents except terbinafine, the MICs of S. globosa in the yeast phase were significantly lower than those of the strains in the mycelial phase (P < 0.001). These results show that the sensitivities of S. globosa to antifungal compounds are dependent on incubation temperature and growth phase. To the best of our knowledge, this is the largest study of antifungal susceptibility of S. globosa isolates reported to date. To establish epidemiological cutoff values for S. globosa, further antifungal susceptibility testing studies by independent laboratories located in different regions and using uniform conditions are required.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00316-y) contains supplementary material, which is available to authorized users.
Keywords: Growth phase, Sporothrix globosa, Sporothrix schenckii, Antifungal susceptibility, Incubation temperature
Introduction
Sporotrichosis is a chronic subcutaneous mycosis affecting humans and animals caused by species of the dimorphic fungi of the genus Sporothrix, such as S. brasiliensis, S. schenckii sensu stricto, and S. globosa [1, 2]. S. globosa is distributed worldwide, with isolates reported from Latin America, Spain, Japan, Madagascar, Iran, India, and China [3–5]. In Jilin Province, northeastern China, where the largest number of sporotrichosis cases are reported in the world, almost all the patients are from underdeveloped rural areas [6]. S. globosa is the only etiologic agent of sporotrichosis reported in this area [7]. The predominant types of infection are fixed and lymphocutaneous sporotrichosis; these conditions are not life-threatening, but require long-term, regular chemotherapy. Therefore, the length and cost of treatment are important factors affecting patient compliance, and consequently influence the effect of treatment. Clinical resistance to antifungals in sporotrichosis patients has also been reported recently [8]. Research findings from antifungal susceptibility studies are important in guiding clinical therapy.
An incubation temperature of 35 °C is recommended for in vitro antifungal susceptibility testing using the broth microdilution method for filamentous fungi (nondermatophyte molds) of the Clinical and Laboratory Standards Institute (CLSI; reference method M38); 30 °C can also be used for certain fungi [9]. When compared with S. schenckii and S. brasiliensis, the poor thermotolerance of S. globosa limits growth at 35 °C [7, 10]. Therefore, an incubation time of > 72 h is needed for S. globosa, compared with 48–72 h for the S. schenckii complex, to guarantee the quality of the growth control. As a result, the susceptibility testing parameters for S. schenckii and S. brasiliensis are not consistent with those for S. globosa. There are also significant differences of antifungal susceptibility between the mycelial and yeast growth phases of S. schenckii and S. brasiliensis [11, 12]. However, there has been no assessment of the influence of incubation temperature and growth phase on the susceptibility of S. globosa using a large number of isolates.
This study investigated the in vitro antifungal susceptibility of 80 isolates of S. globosa originating from Jilin Province, northeastern China, to six antifungal agents at different incubation temperatures and by growth phase.
Materials and methods
Fungal strains and culture conditions
Eighty isolates of S. globosa from human sporotrichosis patients were obtained from different regions of Jilin Province, northeastern China (Fig. 1). All the isolates were identified as S. globosa by morphology and molecular identification (amplification and sequencing of the calmodulin gene, CAL; see supplementary material). The origins of isolates and GenBank accession numbers are shown in Table 1.
Fig. 1.
Maps showing the origin of Sporothrix globosa isolates in Jilin Province, northeastern China
Table 1.
The 80 isolates of Sporothrix globosa used in this study
| Origin | Isolate ID | Gender (M/F) | Age (year) | Clinical form | GenBank accession code |
|---|---|---|---|---|---|
| Baicheng City (n = 5) | FHJU11051803 | M | 0.25 | F | KY350125 |
| FHJU11053102 | F | 57 | D | KY350126 | |
| FHJU11110301 | F | 43 | F | KY349940 | |
| FHJU11122805 | M | 34 | F | KY349945 | |
| FHJU12061704 | F | 44 | F | KY349939 | |
| Baishan City (n = 3) | FHJU09042601 | F | 54 | F | KY349965 |
| FHJU10122702 | F | 24 | F | KY350091 | |
| FHJU13031801 | F | 51 | F | KY349978 | |
| Changchun city (n = 15) | FHJU11061001 | M | 9 | L | KY350041 |
| FHJU12013003 | F | 68 | L | KY350077 | |
| FHJU12020401 | F | 42 | F | KY350082 | |
| FHJU12021201 | F | 51 | L | KY350084 | |
| FHJU12021506 | F | 54 | L | KY350093 | |
| FHJU12031903 | M | 33 | F | KY350042 | |
| FHJU12032401 | F | 60 | L | KY350062 | |
| FHJU12050403 | F | 9 | L | KY350039 | |
| FHJU12052202 | F | 79 | F | KY350063 | |
| FHJU12052302 | F | 62 | L | KY350079 | |
| FHJU12061503 | F | 47 | F | KY350067 | |
| FHJU12061601 | F | 37 | F | KY350083 | |
| FHJU12062301 | F | 6 | F | KY350045 | |
| FHJU12082002 | F | 64 | F | KY350070 | |
| FHJU13032302 | M | 17 | F | KY350001 | |
| Jilin City (n = 10) | FHJU11030803 | F | 75 | L | KY350129 |
| FHJU11052001 | M | 4 | F | KY349993 | |
| FHJU11102001 | M | 4 | F | KY349972 | |
| FHJU12021602 | F | 44 | L | KY349987 | |
| FHJU12030604 | F | 5 | F | KY349988 | |
| FHJU12031206 | M | 60 | F | KY349983 | |
| FHJU12032601 | M | 6 | F | KY349992 | |
| FHJU12040304 | M | 12 | F | KY349990 | |
| FHJU12062602 | F | 54 | L | KY349984 | |
| FHJU12091101 | F | 59 | L | KY349973 | |
| Liaoyuan City (n = 7) | FHJU11021301 | F | 46 | F | KY349979 |
| FHJU11021806 | M | 48 | D | KY349976 | |
| FHJU11061302 | F | 70 | L | KY350006 | |
| FHJU11070404 | M | 59 | L | KY350005 | |
| FHJU11120503 | M | 1.5 | F | KY349994 | |
| FHJU12061702 | F | 28 | L | KY350004 | |
| FHJU13041102 | F | 42 | F | KY349975 | |
| Siping City (n = 15) | FHJU11011004 | M | 3 | F | KY349996 |
| FHJU11011202 | F | 47 | L | KY350034 | |
| FHJU11021107 | F | 6 | L | KY350012 | |
| FHJU11022201 | M | 48 | L | KY349998 | |
| FHJU11022802 | F | 67 | L | KY350018 | |
| FHJU12020201 | F | 55 | F | KY350023 | |
| FHJU12022803 | M | 2 | F | KY350037 | |
| FHJU12033001 | M | 82 | L | KY350009 | |
| FHJU12040302 | F | 70 | F | KY349995 | |
| FHJU12041004 | F | 44 | F | KY350130 | |
| FHJU12050903 | M | 55 | L | KY350022 | |
| FHJU12051605 | F | 24 | F | KY350010 | |
| FHJU12062901 | F | 4 | F | KY350000 | |
| FHJU12080901 | F | 54 | F | KY349997 | |
| FHJU13032301 | M | 14 | L | KY350019 | |
| Songyuan City (n = 15) | FHJU11062006 | F | 50 | F | KY350028 |
| FHJU11081502 | M | 25 | F | KY349970 | |
| FHJU11090602 | F | 58 | F | KY349957 | |
| FHJU11112801 | M | 55 | F | KY349969 | |
| FHJU11120502 | F | 75 | F | KY350132 | |
| FHJU12021402 | F | 62 | L | KY350029 | |
| FHJU12022102 | F | 54 | L | KY349971 | |
| FHJU12030901 | M | 43 | F | KY350035 | |
| FHJU12032602 | F | 58 | F | KY350031 | |
| FHJU12032804 | F | 5 | F | KY349935 | |
| FHJU12041902 | M | 55 | F | KY350033 | |
| FHJU12051002 | F | 64 | F | KY349966 | |
| FHJU12082201 | F | 55 | L | KY349937 | |
| FHJU13031803 | F | 46 | F | KY349962 | |
| FHJU13040501 | M | 10 | F | KY349961 | |
| Tonghua City (n = 5) | FHJU11111102 | M | 44 | L | KY350050 |
| FHJU11122402 | F | 50 | F | KY350027 | |
| FHJU12053001 | F | 49 | F | KY350025 | |
| FHJU13022601 | F | 58 | L | KY350003 | |
| FHJU13051103 | F | 7 | F | KY350002 | |
| Yanbian Korean Autonomous Prefecture (n = 5) | FHJU11102401 | F | 3 | F | KY350047 |
| FHJU11121301 | F | 77 | L | KY350049 | |
| FHJU11122602 | M | 7 | L | KY350118 | |
| FHJU12031204 | F | 68 | L | KY350119 | |
| FHJU12040702 | M | 65 | F | KY350115 |
F, female; M, male; FHJU, First Hospital of Jilin University
Clinical form: F, fixed cutaneous; L, lymphocutaneous; D, disseminated cutaneous
Strains of Candida parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) were used as quality control strains, and were provided by Dr. Yu Zhang (Second Hospital of Jilin University, Changchun, China). S. globosa ATCC MYA-4912 was used as the reference strain. Mycelial-phase strains were obtained by growth on potato-dextrose-agar (PDA; BD, Franklin Lakes, NJ, USA) at 30 °C for 10–14 days. To convert strains into the yeast phase, mycelial cultures were subcultured on brain heart infusion (BHI; BD)-agar at 35 °C for 7 days. Three successive passages were performed to obtain the yeast phase.
Antifungal drugs
Six antifungal compounds were used: itraconazole (ITZ; Tokyo Chemical Industry Co., Ltd. Tokyo, Japan), terbinafine (TRB; Tokyo Chemical Industry Co., Ltd.), voriconazole (VCZ; Tokyo Chemical Industry Co., Ltd.), posaconazole (PCZ; Toronto Research Chemicals, Toronto, ON, Canada), and amphotericin B (AMB; Sigma-Aldrich, St. Louis, MO, USA), which were dissolved in dimethyl sulfoxide (Bio Basic Inc., Toronto, ON, Canada) at 1.6 mg/ml as a stock solution, and fluconazole (FCZ; Tokyo Chemical Industry Co., Ltd.), which was dissolved in water at 2.56 mg/ml. The final concentrations used in susceptibility tests were 0.06–32 μg/ml for AMB and VCZ, 0.03–16 μg/ml for ITZ and PCZ, 0.0078–4 μg/ml for TRB, and 128–0.25 μg/ml for FCZ.
Mycelial and yeast inoculum preparation
Antifungal susceptibility tests were conducted according to CLSI protocols M27 and M38 for yeast and mycelial phases respectively with some modifications [9, 13]. Subculture of S. globosa mycelial phase was incubated on PDA at 30 °C for 7 days, and then 1 ml of sterile 0.85% saline solution was added. The conidia were resuspended by scraping the culture surface with a sterile tip. The suspension was transferred to a 2-ml sterile tube and allowed to settle for 5 min. The upper homogeneous suspension was transferred to a sterile tube and mixed by vortexing for 15 s. The density of the suspension was adjusted to an optical density (OD) of 0.09–0.13 based on the absorbance at 530 nm. This suspension was diluted 1:50 in RPMI 1640 culture medium (Gibco, Life Technologies, NY, USA) with 3-(N-morpholino) propanesulfonic acid (Genview, Beijing, China) buffer (pH 7.0). The concentration of this suspension was approximately 0.4 × 104 to 5 × 104 colony-forming units (CFU)/ml.
Subculture of S. globosa yeast phase was followed by three passages of growth on BHI-agar at 35 °C for 7 days. A suspension was produced as described above for the mycelial phase and adjusted to 1 × 106 to 5 × 106 CFU/ml with sterile 0.85% saline by counting the yeast cells using a hemocytometer. This suspension was then diluted 1:50 in sterile 0.85% saline and subsequently 1:20 in RPMI 1640 culture medium, resulting in a concentration of 1 × 103 to 5 × 103 CFU/ml. The concentration of the inoculum was checked by determination of colony counts on PDA.
Antifungal susceptibility test
Inocula (yeast or mycelial) were used to fill each well of 96-well round-bottom microplates (Greiner Bio-One, Frickenhausen, Germany) with 0.1 ml of suspension. The cell suspension was then diluted with 0.1 ml of RPMI 1640 culture medium containing serial dilutions of antifungal compounds to obtain the final concentration. Cell suspensions without antifungal compounds and RPMI 1640 culture medium were used as growth controls and blank controls, respectively. The microplates were incubated at 35 °C (yeast phase) for 72 h, or at 30 °C and 35 °C (mycelial phase) for 48–72 h. The results were detected by visual observation. For the mycelial phase, the minimum inhibitory concentration (MIC) of FCZ was defined as the minimum concentration that inhibited 50% of growth, the MIC of TRB was defined as the minimum concentration that inhibited 80% of growth, and the MICs of all other drugs were defined as the minimum concentration that inhibited 100% of growth compared with the growth controls. For the yeast phase, the MIC of AMB was defined as the minimum concentration that inhibited 100% of growth, the MIC of TRB was defined as the minimum concentration that inhibited 80% of growth, and the MICs of triazoles were defined as the minimum concentration that inhibited 50% of growth compared with the growth controls.
To determine minimal fungicidal concentrations (MFCs), 10-μl aliquots from wells showing 100% growth inhibition were spread on Sabouraud dextrose agar (BD) plates in duplicate and incubated at 30 °C for 7 days. The MFC was defined as the lowest concentration of antifungal that killed approximately 99% to 99.5% of the fungi (i.e., such that < 3 colonies grew) [14, 15]. All tests were conducted in duplicate [11].
Statistical analysis
The Mann–Whitney U test was used for comparisons between results from the yeast and mycelial phases and between results for the antifungal agents (SPSS software v18.0, IBM, New York, NY, USA). A P value < 0.05 was considered statistically significant. For each antifungal tested, values of MIC50 and MIC90 (i.e., the lowest antifungal concentrations able to inhibit 50% and 90% of the fungal growth of the isolates, respectively), as well as MFC50 and MFC90 (i.e., the lowest concentrations able to kill 50% and 90%, respectively, of the fungal isolates), were calculated.
Results
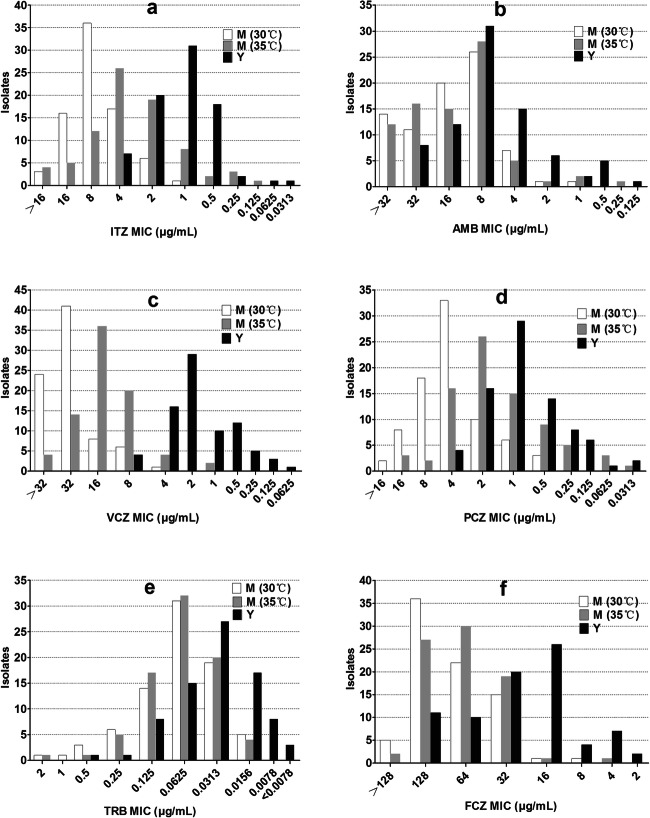
The antifungal susceptibility profiles of 80 isolates of S. globosa in the mycelial and yeast phases are presented in Table 2. The MICs for the quality control strains were consistent with CLSI guidelines, and the results for the reference strain S. globosa ATCC MYA-4912 are shown in the supplementary material. First, the effects of six antifungal drugs were compared. Regardless of the growth phase or incubation temperature, TRB showed the lowest MICs, significantly lower than for all the other tested antifungals (all P values < 0.001). Meanwhile, the geometric means (GMs) of the MICs of TRB were 0.0356 μg/ml for the mycelial phase at 30 °C, 0.0332 μg/ml for the mycelial phase at 35 °C, and 0.031 μg/ml for the yeast phase, respectively. FCZ was the antifungal with the highest MICs and the GMs were 76.7716 μg/ml for the mycelial phase at 30 °C, 66.2570 μg/ml for the mycelial phase at 35 °C, and 24.4625 μg/ml for the yeast phase, respectively. The MICs of PCZ obtained with the mycelial phase at 30 °C and 35 °C and the yeast phase were significantly lower than those of all the other antifungal drugs except TRB (all P values < 0.05). In both the mycelial (30 °C and 35 °C) and yeast phases, > 60% of the S. globosa isolates tested had MICs within the range of three serial dilutions of the antifungal concentration (Fig. 2). The ranges of the MICs for AMB and PCZ for the mycelial phase at 35 °C were wider than those for the other agents evaluated, ranging from 0.25–> 32 to 0.0313–16 μg/ml, respectively.
Table 2.
MICs of six antifungal agents against the mycelial and yeast growth phases at different incubation temperatures of 80 isolates of S. globosa
| Growth phase/temp (°C) | Parameter | MIC (μg/ml) | |||||
|---|---|---|---|---|---|---|---|
| ITZ | AMB | VCZ | PCZ | TRB | FCZ | ||
| M/30 | Range | 0.25– > 16A,a,b | 1– > 32A,a,b | 4– > 32A,a,b | 0.5– > 16A,a | 0.078–1 | 8– > 218A,a,b |
| GM | 6.8448 | 13.2232 | 26.2183 | 4.2501 | 0.0356 | 76.7716 | |
| MIC50 | 8 | 16 | 32 | 4 | 0.0313 | 128 | |
| MIC90 | 16 | 32 | > 32 | 16 | 0.125 | > 128 | |
| M/35 | Range | 0.125– > 16B,a,b | 0.25– > 32a,b | 1–32B,a,b | 0.0313–16B,a | 0.0078–1 | 4– > 218B,a,b |
| GM | 3.1383 | 12.6626 | 13.6895 | 1.4142 | 0.0332 | 66.2570 | |
| MIC50 | 4 | 16 | 16 | 2 | 0.0313 | 64 | |
| MIC90 | 16 | 32 | 32 | 4 | 0.0625 | > 218 | |
| Y/35 | Range | 0.0313–4C,a,b | 0.125–32C,a,b | 0.0625–8C,a,b | 0.0313–4C,a | < 0.0078–3 | 2–128C,a,b |
| GM | 1.0263 | 6.1156 | 1.3899 | 0.7195 | 0.031 | 24.4625 | |
| MIC50 | 1 | 8 | 2 | 1 | 0.0313 | 16 | |
| MIC90 | 2 | 16 | 4 | 2 | 0.125 | 64 | |
MIC, minimum inhibitory concentration; GM, geometric mean; MIC50, MIC at which 50% of the isolates were inhibited; MIC90, MIC at which 90% of the isolates were inhibited; ITZ, itraconazole; AMB, amphotericin B; VCZ, voriconazole; PCZ, posaconazole; TRB, terbinafine; FCZ, fluconazole
A indicates a significant difference (P < 0.001) compared with the MICs in the yeast phase (Mann–Whitney U test)
B indicates a significant difference (P < 0.001) compared with the MICs in the mycelial phase at 30 °C (Mann–Whitney U test)
C indicates a significant difference (P < 0.001) compared with the MICs in the mycelial phase at 35 °C (Mann–Whitney U test)
a indicates a significant difference (P < 0.05) in the MICs compared with TRB in the same conditions (Mann–Whitney U test)
b indicates a significant difference (P < 0.05) in the MICs compared with PCZ in the same conditions (Mann–Whitney U test)
Fig. 2.
Distribution of itraconazole (a), amphotericin B (b), voriconazole (c), posaconazole (d), terbinafine (e), and fluconazole (f) minimum inhibitory concentrations, tested at 30 or 35 °C against the mycelial (M) and yeast (Y) phases of S. globosa isolates (n = 80) from Jilin Province, northeastern China
The MICs of S. globosa in the yeast phase were significantly lower than the MICs for the mycelial phase at both temperatures for AMB, VCZ, ITZ, PCZ, and FCZ (P < 0.001); the only exception was for TRB. The MICs obtained for the mycelial phase at 30 °C were significantly higher than those obtained for the same phase at 35 °C for VCZ, ITZ, PCZ, and FCZ (P < 0.001), but not AMB and TRB. The susceptibility to VCZ was the most significantly different between the two phases; the GMs of the MICs for the mycelial phase at 30 °C and 35 °C were nearly 26 and 10 times higher, respectively, than that for the yeast phase. The GM of the MIC for AMB at 30 °C (13.2232 μg/ml) was higher than that at 35 °C (12.6626 μg/ml), although this difference was not significant (P = 0.902).
As the MICs for most isolates in the mycelial phase at 30 °C were near or above the maximal drug concentrations, the MFCs for the mycelial phase at 30 °C were not compared. The MFC values for TRB were significantly lower than those of the other compounds (P < 0.001), and the GMs of the MFC measured for the mycelial phase at 35 °C and the yeast phase were 0.1272 μg/ml and 0.0989 μg/ml, respectively. The MFC90 values for the antifungals tested against both phases were higher than the maximum concentrations of the antifungal agents in the susceptibility testing, except for TRB and PCZ. For the azoles used against the yeast phase, the MFCs were at least eightfold higher than the MICs. Meanwhile, the MFCs for AMB and TRB were fourfold higher than the MICs (Table 3).
Table 3.
MFCs of six antifungal agents against the mycelial and yeast growth phases of 80 isolates of S. globosa
| Growth phase/temp (°C) | Parameter | MFC (μg/ml) | |||||
|---|---|---|---|---|---|---|---|
| ITZ | AMB | VCZ | PCZ | TRB | FCZ | ||
| M/35 | Range | 2– > 16A,a,b | 1– > 32A,a,b | > 32A,a,b | 0.25–> 16A,a | 0.0156–4 | 64–> 128a,b |
| GM | > 16 | > 32 | > 32 | 10.3747 | 0.1271 | > 128 | |
| MFC50 | > 16 | 32 | > 32 | 16 | 0.125 | > 128 | |
| MFC90 | > 16 | > 32 | > 32 | > 16 | 0.25 | > 128 | |
| Y/35 | Range | 0.5– > 16a,b | 0.5–> 32a,b | 1– > 32a,b | 0.25–> 16a | 0.0078–2 | 32–> 128a,b |
| GM | 13.5714 | 22.82432 | 19.0273 | 8.4268 | 0.0989 | > 128 | |
| MFC50 | 16 | 32 | 32 | 8 | 0.25 | > 128 | |
| MFC90 | > 16 | > 32 | > 32 | 16 | 2 | > 128 | |
MFC, minimum fungicidal concentration; GM, geometric mean; MFC50, MFC at which 50% of the isolates were killed; MFC90, MFC at which 90% of the isolates were killed; ITZ, itraconazole; AMB, amphotericin B; VCZ, voriconazole; PCZ, posaconazole; TRB, terbinafine; FCZ, fluconazole
A indicates a significant difference (P < 0.005) in the MFCs compared with the yeast phase (Mann–Whitney U test)
a indicates a significant difference (P < 0.05) in the MFCs compared with TRB in the same conditions (Mann–Whitney U test)
b indicates a significant difference (P < 0.05) in the MFCs compared with PCZ in the same conditions (Mann–Whitney U test)
Discussion
The conditions used for in vitro susceptibility testing, such as incubation temperature and growth phase, were closely related to the sensitivity to antifungal agents of S. schenckii sensu stricto and S. brasiliensis [11, 16]. However, there are few reports for S. globosa, especially studies assessing large numbers of isolates. Virulence profiles indicate that S. globosa is less pathogenic than S. brasiliensis and S. schenckii, which might explain why fewer sporotrichosis patients are identified as infected with S. globosa compared with the other species [10]. The thermotolerance of S. globosa is also lower than that of the other two species; at a culture temperature of 35 °C, S. globosa grows slowly or is growth limited, whereas S. schenckii and S. brasiliensis grow rapidly in the same conditions [10, 17]. Therefore, the conditions used for in vitro susceptibility testing of the S. schenckii complex (CLSI recommend 35 °C for 48–72 h) are probably not suitable for S. globosa. However, there is no previous report on the sensitivity to antifungals of S. globosa at different temperatures.
Our in vitro susceptibility profiles demonstrated that as the incubation temperature of the microplates increased, the MICs of the antifungals tested against S. globosa were significantly decreased, except for AMB and TRB. Trilles et al. [12] found that the incubation temperature exerted a significant influence on the MICs of drugs toward S. schenckii sensu lato. However, their study showed the changing trends of the MICs were independent of the classification of the antifungal agents in their study, e.g., the GMs of the MICs for ITZ were 4.08 μg/ml at 30 °C and 1.47 μg/ml at 35 °C, while the GMs of the MICs for VCZ were 9.81 and > 16 μg/ml, respectively. Such results are mostly related to the species of the isolates tested. For S. globosa, the higher incubation temperature would be accompanied by prolonged incubation time (> 72 h), which can be attributed to the lower thermotolerance of this strain. Our study showed, in contrast to triazoles, that the GM of the MICs for AMB exhibited no difference between incubation at 35 °C and 30 °C (13.2232 μg/ml at 30 °C and 12.6626 μg/ml at 35 °C). Similar results were also found for Paracoccidioides brasiliensis [18], another dimorphic fungus. This difference between drug types is associated with the mechanisms by which they inhibit fungal growth. The triazoles competitively inhibit the activity of intracellular cytochrome P450 [19] and lanosterol 14 alpha-demethylase in fungi [20], while AMB inhibits the synthesis of the fungal membrane by binding to ergosterol [21]. The process of AMB binding with ergosterol could be more effective at higher temperatures [22].
In our study, the MICs of the antifungals for S. globosa in the yeast phase were significantly lower than those for the fungus in the mycelial phase. These results were consistent with previous reports [4, 23]. Mahmoudi et al. assayed the antifungal susceptibilities of four S. globosa isolates, collected from Iran, to five antifungal agents and the results showed that the MICs of ITZ, VCZ, and FCZ were lower for the fungus in the yeast phase than in the mycelial phase [4]. Two isolates from Argentina also showed differences between phases with respect to AMB, FCZ, and ITZ [23]. It is possible that the structure of the cell wall changes when S. globosa transitions to the yeast phase, making its cell wall more permeable than that in the mycelial phase.
Since S. schenckii was reclassified as a complex [10, 17], several in vitro antifungal susceptibilities have been reassessed to verify the differences among different strains. Marimon tested 92 isolates belonging to five species of the S. schenckii complex in vitro using 12 antifungal agents, and the results showed significant differences in their responses [24]; Stopiglia tested the susceptibility of 85 isolates identified as S. schenckii, S. brasiliensis, and S. globosa to six antifungal agents and found no difference among different strains [25]. However, because of a lack of sufficient isolates, it is difficult to assess whether the antifungal susceptibility is different among different species of the S. schenckii complex. Recently, epidemiologic cutoff values (ECVs) for antifungal agents against S. schenckii sensu stricto and S. brasiliensis have been proposed, based on data obtained from 17 independent laboratories for a large number of isolates [26]. The experimental conditions for establishing the MICs were an inoculum of conidial suspension and an incubation temperature of 35 °C. Meanwhile, the MICs of five antifungal agents against S. globosa were also tested. The most frequently obtained MICs against S. schenckii sensu stricto, S. brasiliensis, and S. globosa were 1, 1, and 2 μg/ml, respectively, for AMB; 0.5, 1, and 0.5 μg/ml, respectively, for ITZ; 1, 1, and 0.5 μg/ml, respectively, for PCZ; 16, 8, and 8 μg/ml, respectively, for VCZ; and 0.5, 0.06, and 0.06 μg/ml, respectively, for TRB. The antifungal susceptibilities of these fungi showed differences, and S. globosa was the most sensitive to the antifungal agents. The GMs of the MICs of S. globosa were 1.35 μg/ml for AMB, 0.61 μg/ml for ITZ, 0.75 μg/ml for PCZ, 6.21 μg/ml for VCZ, and 0.57 μg/ml for TRB [26]. The isolates tested in the present study were sensitive to TRB and resistant to VCZ. However, the GM of the MIC of most of the isolates tested with AMB in a previous study [24] was significantly lower than that observed in the present study (12.6626 μg/ml), which might be related to the origin of the isolates. We found no significant association between the clinical characteristics of sporotrichosis (e.g., the clinical form, patient gender, patient age) and the antifungal susceptibility of S. globosa. Hence, the clinical manifestation of the disease is most likely related to the immune status of the patient, rather than the susceptibility of the infecting strain.
Our in vitro susceptibility profiles showed that TRB was the most active agent against the 80 isolates of S. globosa tested regardless of incubation temperature or growth phase, which was consistent with previous reports [27, 28]. Our previous survey showed that the average clinical treatment with TRB was for 2.21 months with a dose of 250 mg/day for cutaneous or lymphocutaneous sporotrichosis, compared with 1.86 months (200 mg/day) for treatment with ITZ [29]. A similar observation was reported in Brazil, where the cure rates for TRB (250 mg/day, 11.5 weeks) and ITZ (100 mg/day, 11.8 weeks) were 92.7% and 92%, respectively [30]. Although TRB showed the potential to treatment systemic sporotrichosis when evaluated in an experimental model [31], the clinical application of TRB is less effective, which might be related to distribution of the pathogen in the host [21]. Furthermore, the optimal dose and duration of treatment by TRB is a subject of debate among clinical practitioners [32]. TRB is still not recommended as a first-line treatment for cutaneous sporotrichosis. It is also not recommended for disseminated sporotrichosis [31]. This emphasizes that discordance may exist between in vitro and in vivo conditions, which indicates caution is needed when interpreting in vitro antifungal susceptibility results.
In our study, PCZ showed higher activity against S. globosa than ITZ; the latter is the first-line therapy in treatment of sporotrichosis [33]. Nonetheless, PCZ is not the first choice for treatment of normal cutaneous or lymphocutaneous sporotrichosis because of its price and limited availability. PCZ can be administered in difficult treatment of disseminated sporotrichosis or after failure of treatment with ITZ [34].
To the best of our knowledge, this is the largest study of in vitro antifungal susceptibility of S. globosa isolates reported to date. We have found that the sensitivities of S. globosa to antifungal compounds are dependent on the incubation temperature and growth phase. To establish ECVs, it is necessary to conduct studies in uniform conditions and obtain more data from independent laboratories located in different regions.
Electronic supplementary material
(DOC 37 kb)
Acknowledgments
We thank Catherine Dandie, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding information
This study received funding from the National Natural Science Foundation of China [grant numbers 81573060, 81703136, and 81803147] and the Science & Technology Project Foundation of Jilin Province [grant number 20170204060SF].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Beer ZW, Duong TA, Wingfield MJ. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Stud Mycol. 2016;83:165–191. doi: 10.1016/j.simyco.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9(12):e4190. doi: 10.1371/journal.pntd.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53(1):3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoudi S, Zaini F, Kordbacheh P, Safara M, Heidari M. Sporothrix schenckii complex in Iran: molecular identification and antifungal susceptibility. Med Mycol. 2016;54(6):593–599. doi: 10.1093/mmy/myw006. [DOI] [PubMed] [Google Scholar]
- 5.Rasamoelina T, Maubon D, Raharolahy O, Razanakoto H, Rakotozandrindrainy N, Rakotomalala FA, Bailly S, Sendrasoa F, Ranaivo I, Andrianarison M, Rakotonirina B, Andriantsimahavandy A, Rabenja FR, Andrianarivelo MR, Ramarozatovo LS, Cornet M. Sporotrichosis in the Highlands of Madagascar, 2013-2017(1) Emerg Infect Dis. 2019;25(10):1893–1902. doi: 10.3201/eid2510.190700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao L, Song Y, Zhou JF, Cui Y, Li SS. Epidemiological and clinical comparisons of paediatric and adult sporotrichosis in Jilin Province, China. Mycoses. 2020;63(3):308–313. doi: 10.1111/myc.13045. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, Cui Y, Zhen Y, Yao L, Shi Y, Song Y, Chen R, Li S. Genetic variation of Sporothrix globosa isolates from diverse geographic and clinical origins in China. Emerg Microbes Infec. 2017;6(10):e13–e88. doi: 10.1038/emi.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischman GO, Rodrigues AM, Fernandes GF, et al. Atypical clinical presentation of Sporotrichosis caused by Sporothrix globosa resistant to Itraconazole. Am J Trop Med Hyg. 2016;94(6):1218–1222. doi: 10.4269/ajtmh.15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CLSI Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 3rd ed. CLSI standard M38. Wayne, PA: Clinical and Laboratory Standards Institute; 2017
- 10.Almeida-Paes R, de Oliveira LC, Oliveira MME, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed Res Int. 2015;2015:212308–212310. doi: 10.1155/2015/212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchotene KO, Brandolt TM, Klafke GB, Poester VR, Xavier MO. In vitro susceptibility of Sporothrix brasiliensis: comparison of yeast and mycelial phases. Med Mycol. 2017;55(8):869–876. doi: 10.1093/mmy/myw143. [DOI] [PubMed] [Google Scholar]
- 12.Trilles L, Fernandez-Torres B, Dos SLM, et al. In vitro antifungal susceptibilities of Sporothrix schenckii in two growth phases. Antimicrob Agents Chemother. 2005;49(9):3952–3954. doi: 10.1128/AAC.49.9.3952-3954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. CLSI standard M27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017
- 14.Espinel-Ingroff A. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J Clin Microbiol. 2001;39(3):954–958. doi: 10.1128/JCM.39.3.954-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J Clin Microbiol. 2002;40(10):3776–3781. doi: 10.1128/jcm.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Huang H, Feng P, Zhang J, Zhong Y, Xue R, Xie Z, Li M, Xi L. In vitro activity of itraconazole in combination with terbinafine against clinical strains of itraconazole-insensitive Sporothrix schenckii. Eur J Dermatol. 2011;21(4):573–576. doi: 10.1684/ejd.2011.1400. [DOI] [PubMed] [Google Scholar]
- 17.Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45(10):3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz RC, Werneck SM, Oliveira CS, et al. Influence of different media, incubation times, and temperatures for determining the MICs of seven antifungal agents against Paracoccidioides brasiliensis by microdilution. J Clin Microbiol. 2013;51(2):436–443. doi: 10.1128/JCM.02231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelkonen O, Maenpaa J, Taavitsainen P, Rautio A, Raunio H. Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica. 1998;28(12):1203–1253. doi: 10.1080/004982598238886. [DOI] [PubMed] [Google Scholar]
- 20.Brilhante RS, Paiva MA, Sampaio CM, et al. Azole resistance in Candida spp. isolated from Catu Lake, Ceara, Brazil: an efflux-pump-mediated mechanism. Braz J Microbiol. 2016;47(1):33–38. doi: 10.1016/j.bjm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86–96. doi: 10.1016/j.bcp.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Pasricha S, Payne M, Canovas D, Pase L, Ngaosuwankul N, Beard S, Oshlack A, Smyth GK, Chaiyaroj SC, Boyce KJ, Andrianopoulos A. Cell-type-specific transcriptional profiles of the dimorphic pathogen Penicillium marneffei reflect distinct reproductive, morphological, and environmental demands. G3 (Bethesda) 2013;3(11):1997–2014. doi: 10.1534/g3.113.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordoba S, Isla G, Szusz W, et al. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses. 2018;61(7):441–448. doi: 10.1111/myc.12760. [DOI] [PubMed] [Google Scholar]
- 24.Marimon R, Serena C, Gene J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Ch. 2008;52(2):732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottonelli Stopiglia CD, Magagnin CM, Castrillón MR et al (2013) Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med Mycol:1–9. 10.3109/13693786.2013.818726 [DOI] [PubMed]
- 26.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, Govender NP, Guarro J, Johnson EM, Kidd SE, Pereira SA, Rodrigues AM, Rozental S, Szeszs MW, Ballesté Alaniz R, Bonifaz A, Bonfietti LX, Borba-Santos LP, Capilla J, Colombo AL, Dolande M, Isla MG, Melhem MSC, Mesa-Arango AC, Oliveira MME, Panizo MM, Pires de Camargo Z, Zancope-Oliveira RM, Meis JF, Turnidge J (2017) Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Ch 61(10). 10.1128/AAC.01057-17 [DOI] [PMC free article] [PubMed]
- 27.Galhardo MC, De Oliveira RM, Valle AC, et al. Molecular epidemiology and antifungal susceptibility patterns of Sporothrix schenckii isolates from a cat-transmitted epidemic of sporotrichosis in Rio de Janeiro, Brazil. Med Mycol. 2008;46(2):141–151. doi: 10.1080/13693780701742399. [DOI] [PubMed] [Google Scholar]
- 28.Silveira CP, Torres-Rodriguez JM, Alvarado-Ramirez E, et al. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J Med Microbiol. 2009;58(Pt 12):1607–1610. doi: 10.1099/jmm.0.007609-0. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Li SS, Zhong SX, Liu YY, Yao L, Huo SS. Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J Eur Acad Dermatol Venereol. 2013;27(3):313–318. doi: 10.1111/j.1468-3083.2011.04389.x. [DOI] [PubMed] [Google Scholar]
- 30.Francesconi G, Francesconi DVA, Passos SL, et al. Comparative study of 250 mg/day terbinafine and 100 mg/day itraconazole for the treatment of cutaneous sporotrichosis. Mycopathologia. 2011;171(5):349–354. doi: 10.1007/s11046-010-9380-8. [DOI] [PubMed] [Google Scholar]
- 31.Meinerz AR, Xavier MO, Cleff MB, et al. Efficacy of terbinafine and itraconazole on a experimental model of systemic sporotrichosis. Braz J Microbiol. 2008;39(4):734–737. doi: 10.1590/S1517-838220080004000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan VK. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:272376–272313. doi: 10.1155/2014/272376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kauffman CA, Bustamante B, Chapman SW, Pappas PG. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(10):1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 34.Paixao AG, Galhardo M, Almeida-Paes R, et al. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res Ther. 2015;12:16. doi: 10.1186/s12981-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 37 kb)