Abstract
Strain K001 was isolated from a cyanobacterial culture derived from Abrolhos, a reef bank microbial mat (South Atlantic Ocean—Brazil). Cells of K001 are Gram stain–negative, catalase and oxidase-positive, non-motile, rod-shaped, and with or without appendages. Phylogenetic analysis based on 16S rRNA gene sequences showed that strain K001 belongs to the genus Muricauda. The highest strain K001 16S rRNA gene identity, ANI, and dDDH, respectively, are with M. aquimarina (98.90%, 79.23, 21.60%), M. ruestringensis (98.20%, 80.82, 23.40%), and M. lutimaris (97.86%, 79.23, 22.70%). The strain grows at 15–37 °C and between 0.5 and 10% NaCl. The major fatty acids of strain K001 are iso-C15:0, iso-C15:1 G, iso-C17:0 3-OH, and summed feature 3 (C16:1 ω6c and/or C16:1 ω7c). The polar lipids are represented by phosphatidylethanolamine, three unidentified aminolipids, and three unidentified polar lipids. The major respiratory quinone is MK-6. The G+C content of the DNA of strain K001 is 41.62 mol%. Based on polyphasic analysis of strain K001, it was identified as a novel representative of the genus Muricauda and was named Muricauda brasiliensis sp. nov. The type strain is K001 (=CBMAI 2315T = CBAS 752T).
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-020-00400-3.
Keywords: Flavobacteriaceae, Muricauda brasiliensis, Cyanobacterial culture, Abrolhos reef bank
In order to investigate heterotrophic bacteria associated with cyanobacterial cultures from Abrolhos reef bank (South Atlantic Ocean—17° 57′ S, 38° 42′ W), several strains were isolated. The strain K001 was selected for further analysis in part due to its potential for pigment production. Based on 16S rRNA gene sequence analyses, strain K001 belongs to the genus Muricauda, which was proposed in 2001 by Bruns et al. [1]. The genus Muricauda belongs to family Flavobacteriaceae, order Flavobacteriales, class Flavobacteria, and phylum Bacteroidetes. The name of the group refers to an appendage contiguous to the outer membrane present in some cells of species belonging to the genus [1]. As of January 2020, the genus is comprised of 19 species: M. ruestringensis, M. flavescens, M. aquimarina, M. lutimaris, M. lutaonensis, M. olearia, M. beolgyonensis, M. antarctica, M. taeanensis, M. zhangzhouensis, M. pacifica, M. lutea, M. marina, M. indica, M. iocasae, M. nanhaiensis, M. amoyensis, M. hymeniacidonis, and M. alvinocaridis [1–18]. All members of the genus are rod-shaped Gram-negative bacteria isolated from a variety of saline environments such as intertidal, deep-sea, and mangrove sediments; surface seawater; sand; and oil-polluted seawater [1–18]. In this study, we described the phenotypic, genetic, and phylogenetic characteristics of strain K001 that is proposed as a novel species in the genus Muricauda.
Isolation and ecology
Strain K001 was isolated during the effort to obtain pure cultures of heterotrophic bacteria from non-axenic marine cyanobacterial cultures, available at Culture Collection of Microorganisms (CCMR) at the Universidade Federal do Rio de Janeiro—Rio de Janeiro—Brazil. The chosen cyanobacterial cultures are from mats from Abrolhos reef bank, a national marine park in the South Atlantic Ocean. The cyanobacterial culture CCMR0080 was the source from which the strain K001 was isolated. To isolate the K001 strain, 0.1 mL of culture medium containing a clump of filamentous cyanobacteria was spread on plates with marine agar 2216 (MA; Difco) and incubated for 48 h at 28 °C. Isolated colonies were streaked on fresh MA plates to obtain pure cultures. The pure culture of strain K001 was stored at − 80 °C with 15% (v/v) glycerol in liquid broth 2216 (Difco).
16S ribosomal RNA phylogeny
Genomic DNA of K001 was extracted with GenElute bacterial genomic DNA kit (Sigma-Aldrich), according to the manufacturer’s instructions. Genomic DNA was amplified by PCR using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1494R (5′-TACGGCTACCTTGTTACGAC-3′), adapted from Lane, 2001 [19]. The nearly full-length 16S rRNA gene sequence (1490 nt), generated at Macrogen (South Korea), is available at GenBank/EMBL/DDBJ under accession number MN996941.
The EzTaxon-e server (www.ezbiocloud.net) was used to calculate the similarity between K001 16S rRNA gene sequence and other strains [20]. Sequences of K001 and associated strains were aligned using the CLUSTAL_W program implemented in the software package MEGA (v7.0) [21, 22]. Phylogenetic trees were constructed using the same program. Neighbor-joining (NJ) [23, 24] and maximum-likelihood (ML) [25] trees were reconstructed based on the Kimura 2-parameter model [25], with reliability confirmed by bootstrap analysis based on 1000 resamplings.
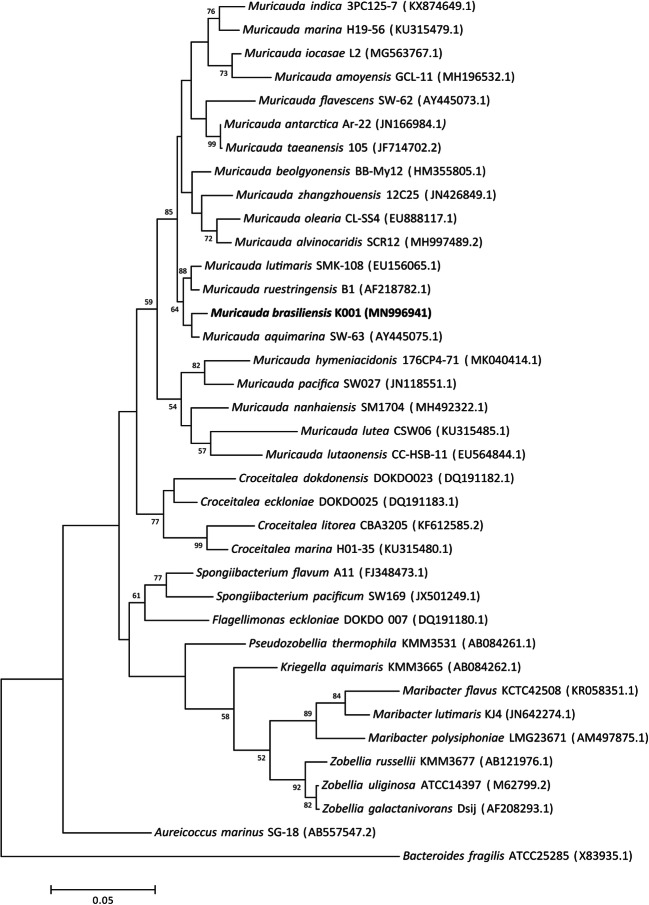
According to the nearly complete 16S rRNA gene sequence, the species most closely related to K001 were M. aquimarina (98.90% 16S rRNA gene sequence similarity), M. ruestringensis (98.20%), and M. lutimaris (97.86%). The range of similarity between the strain K001 and those other 16 known species of the genus Muricauda was 97.86–92.72%. In the phylogenetic trees, neighbor-joining, and maximum-likelihood, the strain K001 always forms a subclade with M. aquimarina, M. ruestringensis, and M. lutimaris and a branch together with M. aquimarina (Figs. 1 and S1; available in the online version of this manuscript). The supertree generated with 31 phylogenetic marker proteins show a similar topology of the 16S rRNA gene sequence phylogenetic trees, with the strain K001 falling within a subclade with M. aquimarina, M. ruestringensis, and M. lutimaris (Fig. S2, available in the online version of this manuscript).
Fig. 1.
Maximum-likelihood (ML) phylogenetic tree based on 16S rRNA gene sequences, showing the position strain K001. Bootstrap values above 50% based on 1000 resamplings are shown at branch nodes. Bar, 0.05 substitutions per nucleotide position. Bacteroides fragilis ATCC25285 was used as an outgroup
Genome features
The draft genome sequence of strain K001 was described in a previous work [26]. Briefly, a DNA library was prepared using a Nextera XT kit (Illumina), and sequencing was performed using an Illumina NextSeq500 platform with paired-end reads 150 base reads at the Genome Research Core, University of Illinois at Chicago. Raw sequence data were quality trimmed, and de novo assembled with software package CLC Genomics Workbench (Qiagen, Hilden, Germany). Prokka—version 1.12—was used to annotate the genome using the UniProt database [26]. As proposed by Chun et al., the overall genome related index (OGRI) was determined between strain K001 and each species of the genus Muricauda with whole-genome sequences available in public databases [27, 28]. The average nucleotide identity (ANI) was calculated with ANI calculator tool available online in the EZBioCloud platform (https://www.ezbiocloud.net/tools/ani) [29]. Digital DNA–DNA hybridization (dDDH) values were calculated using the Genome-to-Genome Distance Calculator from Leibniz Institute—DSMZ (http://ggdc.dsmz. de/ggdc.php) [30]. The level of contamination in the assembled genome was evaluated with CheckM [31], and the calculation of the sequencing depth of coverage was performed with the Burrows-Wheeler Aligner (BWA) software package [32]. A phylogenomic tree was reconstructed with AMPHORA2. The sequences of 31 phylogenetic markers were concatenated and then aligned with MAFFT [33, 34]. A tree was constructed using Fasttree [35]. Additionally, two online platforms were used to confirm the results of strain K001 identity, TYGS (Type Strain Genome Server, from DSMZ) and TrueBac ID (from EZBioCloud) [36, 37] (report in supplementary material, available in the online version of this manuscript).
The draft genome of strain K001 is 3.79 Mb, distributed in 55 contigs [26]. The GC content is 41.62 mol%, matching with the expected range to the genus Muricauda (39.9–50.70 mol%) [1–18]. The average coverage was approximately 300x, and the N50 and L50 values for the assembly were 232,816 bp and 5 contigs, respectively [26]. The estimated level of contamination was 0.65%. The major ANI value was calculated between strain K001 and M. ruestringensis (80.82), followed by M. lutimaris (79.23) and M. aquimarina (79.23). M. ruestringensis was also the strain with the highest value of dDDH (23.40%), followed by M. lutimaris (22.70%) and M. aquimarina (21.60%). Both values indicate K001 is a distinct species, with ANI < 95% and dDDH < 70% [27]. Both automated pipelines, TYGS and TrueBac ID, showed similar results demonstrating that the strain K001 is a new species within the genus Muricauda. These results corroborate the manually conducted OGRI analyses.
Physiology and chemotaxonomy
The strain DSM 13258T Muricauda ruestringensis was acquired from the DSMZ Leibiniz Institute collection of microorganisms for comparative phenotypic analysis. Both strains, K001 and M. ruestringensis, were cultured in DIFCO 2216 broth or agar plate for 48 h at 30 °C, except when otherwise indicated.
Cell morphology and cell appendage presence, typical feature Muricauda genus, were observed by light microscopy (data not shown) or scanning electron microscopy (SEM) in liquid and solid media (Fig. 2). Gram staining was performed using the Hucker staining method, and the cells were visualized with a Leica DM750 microscope [38]. For SEM, cells were washed three times with phosphate-buffered saline (PBS) and then fixed on Karnovsky fixative solution (2% (v/v) paraformaldehyde, 2% (v/v) glutaraldehyde, 3% (w/v) sucrose, and 0.005 M CaCl2 in 0.05 M cacodylate buffer), followed by a second fixation with 1% (w/v) Osmium tetroxide and adsorption onto poly-l-lysine-coated coverslips. The samples were then dehydrated with acetone (50/70/90/100%, v/v) and drying at critical point with liquid CO2, and covered with a thin layer of gold (approximately 10 nm) [39]. Images were generated with a scanning electron microscope JEOL JSM-7001F (JEOL Ltd., Tokyo, Japan).
Fig. 2.

Scanning electron micrograph showing appendages found on some cells of K001 (magnification × 6000). Bar, 1 μm
Optimum growth temperature was assessed by growth for 1 week on agar plates at 4, 10, 15, 20, 28, 30, 33, 37, and 42 °C. Optimum pH (from 4.0–9.0 in 1.0-unit intervals) was tested in broth medium with pH adjusting with HCl and NaOH. Tolerance to different concentrations of NaCl was determined at 30 °C in broth medium adapted from Bruns et al. [1] ((0.25% (w/v) Bacto Peptone, 0.15% (w/v) yeast extract in artificial seawater—0.64 g KCl, 4.53 g MgCl2.6H2O, 5.94 g Mg2SO4.7H2O, and 1.3 g CaCl2 in 1 L of distilled water) with correspondent concentration of NaCl (0–5%, w/v, in 0.5-unit intervals, 6–9%, w/v, in 1.0-unit intervals)). The capability to grow with NaCl as sole salt source was tested in broth medium with 2.25% (w/v) NaCl, 0.25% (w/v) Bacto Peptone, and 0.15% (w/v) yeast extract dissolved in distilled water. Hydrolysis of Tween 20, 60, and 80 was tested in agar plate with the addition of 1% (v/v) of the respective Tween type [38]. Hydrolysis of starch was performed in agar plate with the addition of 0.50% (w/v) of starch and revealed with iodine vapor after 48 h [40]. Catalase activity was evaluated by bubble formation in 3% (v/v) hydrogen peroxide solution. Flexirubin-type pigments were determined by color changes in cell biomass after the addition of 20% (w/v) KOH solution [41]. Carotenoid pigments were determined by acetonitrile extraction 100% (v/v) followed by chromatographic analysis. Carotenoids were separated and analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC). Shimadzu LC-10A instrument, equipped with an RPI8 column and photodiode array detector, was used for separation, and carotenoids were identified based on retention time and spectrogram profile (300–600 nm). The ability to grow under anaerobic condition was tested in the agar medium supplemented with cysteine hydrochloride (0.05% w/v), sodium sulfide (0.05% w/v), and potassium nitrate (0.10% w/v) [16]. Anaerobic glass jars were prepared under a nitrogen atmosphere and sealed with rubber stoppers and aluminum seals. Antibiotic susceptibility patterns were determined by applying antibiotic discs over cells spread in a plate with Drigalski spreader. The plates were incubated at 33 °C for 48 h. Susceptibility was indicated by halo formation around the disc. The evaluated antibiotics and concentration were (μg per disc) ciprofloxacin (5 μg), vancomycin (30 μg), clindamycin (2 μg), oxacillin (1 μg), sulphonamides (300 μg), chloramphenicol (30 μg), amikacin (30 μg), norfloxacin (10 μg), polymyxin B (300 U), ofloxacin (5 μg), erythromycin (15 μg), ceftazidime (30 μg), penicillin G (10 U), ceftriaxone (30 μg), tetracycline (30 μg), cephalexin (30 μg), cefazolin (30 μg), doxycycline (30 μg), gentamycin (10 μg), ampicillin (10 μg), and piperacillin + tazobactam (110 μg).
Oxidase activity, additional enzyme activities, and carbon assimilation were tested with API ZYM and API 20 NE Biomerieux kits according to manufacturer’s instructions, except for artificial seawater addition for cell inoculum preparation. Quinones and polar lipids from strain K001 were recovered with a two-step extraction protocol. In the first step, a mixture of hexane-methanol (1:2, v/v) was used to retrieve quinones in the hexane phase. The polar lipids were recovered from the 0.30% (w/v) methanol-aqueous NaCl mixture, resultant from the first stage. The quinones were analyzed using a RP-HPLC instrument equipped with an RPI8 column and UV-light monitoring system. Polar lipids were analyzed by two-dimensional silica gel thin layer chromatography and revealed using typical spray reagents for specific functional groups [38, 42–44]. The analyses of the quinones and polar lipids, as well the API ZYM and API 20 NE metabolic tests, were performed on strain K001 by the Identification Services of DSMZ Leibiniz Institute.
Fatty acid analyses followed the standard procedure of Sherlock Microbial Identification System (Sherlock, version 6.2). Briefly, cells (approx. 40 mg) of K001 and DSM 13258 were harvested after 48 h/30 °C growth on MA and transferred to Teflon-capped glass tubes, following saponification of the fatty acids in 1 mL methanol/sodium hydroxide (150 mL deionized water, 150 mL methanol, 45 g sodium hydroxide) at 100 °C for 30 min. Two milliliters of 6 mol L−1 methane in HCl were added to each tube and the mixtures were incubated at 80 °C for 10 min. The separation of the fatty acid methyl esters from the acidic to the organic phase was carried out by adding 1.25 mL hexane:tertbutyl ether (1:1). After shaking for 10 min, the lower phase was discarded and the upper phase containing the fatty acid methyl esters was recovered, washed with 0.25 mol L−1 NaOH, and stored at 20 °C until analysis. Fatty acid methyl esters were analyzed by gas chromatography (Agilent 7890A) using the RTSBA6 method/library [45, 46].
Comparative analysis of protein profile by MALDI-TOF mass spectrometry was performed to further demonstrate the distinctive phenotype of K001 and the reference DSM 13258 strains [47, 48]. Analyses were conducted using extracted proteins [49]. Samples were spotted in technical sextuplicates on 96-well target steel plate and covered by 1 μL of 10 mg/mL α-cyano-4-hydroxycinnamic acid (HCCA) matrix (50% (v/v) acetonitrile, 0.30% (v/v) trifluoroacetic acid) and let it dry at room temperature. Sample spectra were obtained in an Autoflex Speed II MALDI-TOF/TOF (Bruker Daltonics) in positive linear mode, 2000–20,000 m/z range, acquiring 2000 successful shots per spot using FlexControl 3.0 software. Obtained spectra were processed and analyzed in MALDI Biotyper 3.0 software [47, 50]. Main spectra profile (MSP) was created using the technical sextuplicates, compared to Biotyper database and between K001 and DSM 13258. All experiments were performed in biological quadruplicates. A dendrogram was constructed using MSP information from each replicate on MALDI Biotyper 3.0 software.
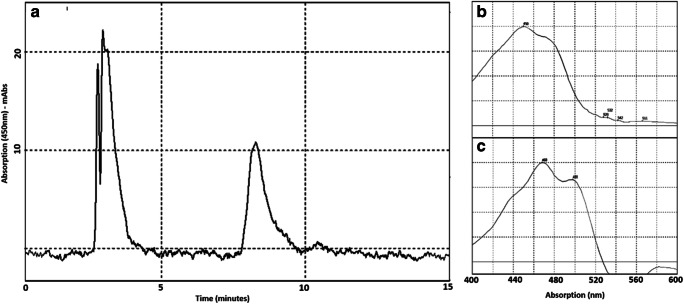
The K001 cells are rod-shaped Gram-negative and some of them present appendage, like the other species in the genus Muricauda. Different from M. ruestringensis, not all strain K001 appendages show a vesicle in the top of structure. However, as K001 was isolated from a mat-forming cyanobacterial culture, the role of the appendage probably is the same proposed to M. ruestringensis, to connect cells to each other or to substrate. The small flocks can be observed in liquid media culture of strain K001 in 24 h under agitation, instead of only older culture as related to M. ruestringensis. The main phenotypic characteristics are summarized in Table 1. Despite the phenotypic similarity with the related strains M. ruestringensis DSM 13258T and M. aquimarina SW-63T, strain K001 has shown the absence of Lipase (C14) in API ZYM test and the inability to the assimilation of capric acid, in API 20 NE test. Growth under anaerobic condition, motility, and flexirubin-type pigment production was not observed. The presence of carotenoids pigments was confirmed (Fig. 3). The carotenoid pigment zeaxanthin was present, as described in other species of the genus [51, 52]. The complete physiological and biochemical characteristics are described in the species description.
Table 1.
General characteristics from strain K001 and reference strains. Strains: 1, K001T; 2, M. ruestringensis DSM 13258T (data from Bruns et al. 2001, and this study); 3, M. aquimarina SW-63T (data from Yoon et al. 2005); 4, M. lutimaris SMK-108T (data from Yoon et al. 2008). Kits API ZYM and API 20 NE data are from Yang et al. 2013; +, positive; −, negative; W, weak positive; ND, no data
| Characteristic | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Colony color | Yellow | Yellow | Yellow | Yellow |
| Cell size (μm) | ||||
| Length | 1.1–2.4 | 1.1–2.7 | 2.5–6.0 | 0.8–5.0 |
| Width | 0.3–0.5 | 0.3–0.6 | 0.2–0.5 | 0.2–0.4 |
| Growth conditions | ||||
| Optimal temperature (°C) | 28–33 | 20–30 | 30–37 | 30 |
| NaCl (%, w/v) | 0.5–9 | 0.5–9 | 2* | 1–10 |
| pH range | 6–8 | 6–8 | 7* | 5–8 |
| Facultatively anaerobic growth | – | + | – | + |
| Catalase activity | + | + | + | + |
| Hydrolysis | ||||
| Starch | – | – | – | – |
| Tween 20 | + | + | + | + |
| Tween 60 | + | + | + | – |
| Tween 80 | + | + | + | – |
| API ZYM† | ||||
| Esterase lipase (C8) | + | + | + | w |
| Lipase (C14) | – | + | + | + |
| Valine arylamidase | + | + | + | + |
| Cystine arylamidase | + | + | + | + |
| Trypsin | + | + | + | + |
| a-Chymotrypsin | + | + | + | + |
| Acid phosphatase | + | + | + | + |
| Naphthol-AS-BI-phosphohydrolase | + | + | + | + |
| a-Galactosidase | + | + | + | w |
| b-Galactosidase | + | + | – | w |
| a-Mannosidase | + | + | + | + |
| API 20NE† | ||||
| Urease | – | – | – | – |
| Gelatin hydrolysis | – | – | – | + |
| Assimilation of capric acid | – | + | + | + |
| Assimilation of phenylacetic acid | – | – | – | + |
| Catalase | + | + | + | w |
| DNA G+C content (mol%) | 41.62 | 41 | 44.10–44.20 | 41.10 |
*Optimal condition
†Data of reference strains from Yang et al. 2013
Fig. 3.
Chromatogram profile of (A) strain K001, showing peaks of carotenoid pigments. HPLC analysis conditions: RP-column C18 Vydac (218TP54), mobile phase 100% (v/v) MeOH, flow 1.0 ml/min. Spectrogram profile (range 400–600 nm) of main chromatogram peaks (B and C)
The fatty acid profile of K001 (Table 2) is similar to that of the reference strains, except for the abundance of the major fatty acids iso-C15:0 (35.21%), iso-C15:1 G (14.48%), iso-C17:0 3-OH (22.55%), and summed feature 3 (8.93%). Profiles of the minor fatty acids revealed profiles typical of the genus, though some unique features of strain K001 were identified, e.g., C14:0 (0.85%), C18:0 (2.73%), iso-C17:0 (0.67%), C18:1ω9c (0.98%) and absence of iso-C14:0, anteiso-C15: 1 A, C15: 0 3-OH, summed feature 8. The MK6 quinone was detected, as expected for the genus Muricauda. Analysis of polar lipids demonstrated the presence of phosphatidylethanolamine, three unidentified aminolipids, and three unidentified polar lipids, similar to profiles from other members of the genus but different from M. ruestringensis DSM 13258T for the presence of the aminolipids (Fig. S3, available in the online version of this manuscript).
Table 2.
Cellular fatty acid composition (%) of strain K001 and related Muricauda species. Strains: 1, K001T; 2, M. ruestringensis DSM 13258T; 3, M. aquimarina SW-63T; 4, M. lutimaris SMK-108T. K001 and DSM 13258T data are from this study; other strain data are from Yang et al. 2013. The table presents data of fatty acids accounting for > 0.5%, the values italicized represent the most abundant fatty acids in profile
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Straight-chain | ||||
| C14:0 | 0.85 | - | - | - |
| C16:0 | 4.20 | 1.73 | 1.40 | 1.80 |
| C18:0 | 2.73 | - | 1.40 | 1.40 |
| C18:0 10-methyl | - | - | - | - |
| Branched | ||||
| iso-C13:0 | - | - | - | 0.60 |
| iso-C14:0 | - | - | 0.50 | - |
| iso-C15:0 | 35.21 | 46.10 | 24.60 | 27.20 |
| iso-C15:1 G | 14.48 | 17.48 | 28.30 | 36.40 |
| iso-C16:0 | 0.77 | - | 0.70 | - |
| iso-C17:0 | 0.67 | 0.61 | - | - |
| iso-C18:1 H | - | - | - | 0.70 |
| anteiso-C15:0 | 0.79 | 1.42 | 3.10 | 2.40 |
| anteiso-C15:1 A | - | - | 0.70 | 0.80 |
| Unsaturated | ||||
| C12:1 at 11–12 | - | - | - | 2.20 |
| C13:1 at 12–13 | - | - | - | 0.50 |
| C15:1ω6c | - | - | 0.80 | 1.00 |
| C17:1ω6c | - | - | 0.60 | 0.70 |
| C17:1ω8c | - | - | - | - |
| C18:1ω5c | - | - | 0.60 | - |
| C18:1ω9c | 0.98 | - | 0.80 | 1.20 |
| Hydroxy | ||||
| C15:0 2-OH | - | - | - | - |
| C15:0 3-OH | - | - | 1.40 | 1.30 |
| iso-C15:0 3-OH | 4.24 | 3.65 | 5.20 | 3.30 |
| C16:0 3-OH | 0.82 | 0.51 | - | - |
| iso-C16:0 3-OH | 1.16 | 0.74 | 3.60 | 4.60 |
| C17:0 2-OH | - | - | 0.80 | - |
| C17:0 3-OH | - | - | - | - |
| iso-C17:0 3-OH | 22.55 | 18.68 | 15.20 | 5.20 |
| Summed features* | ||||
| 3 | 8.93 | 3.19 | 2.60 | 1.20 |
| 4 | - | 0.60 | - | - |
| 8 | - | - | 1.90 | 2.90 |
| 9 | 1.61 | 1.93 | 2.20 | 1.60 |
*Summed feature occurs when having an imperfect peak separation. Summed feature 3 represents C16:1 ω6c and/or C16:1 ω7c; summed feature 4 represents iso-C17:1 I and/or anteiso-C17:1 B; summed feature 8 represents C18: 1 ω7c and/or C18: 1 ω6c; summed feature 9 represents iso-C17: 1 ω9c and/or C16: 010-methyl
The MALDI-TOF protein profile spectra obtained from direct colony or protein extraction of strains K001 and reference strain M. ruestringensis DSM 13258T were used to create main spectra profiles (MSP) for each strain and condition. Direct colony spotting spectra were less reproducible and were not used for further analysis. The protein extraction MSP analyses in MALDI Biotyper 3.0 software (Bruker Daltonics) showed an identification score lower than 2.0 for both strains, indicating that these species are not represented in MALDI Biotyper database. Moreover, when comparing strain K001 to M. ruestringensis DSM 13258T, scores were lower than 2.0 for all six biological replicates, indicating that these organisms belong to different species. Dendrogram analysis of generated MSP shows that K001 and M. ruestringensis DSM 13258T are grouped in different clades, even when an external group is added (Fig. S4, available in the online version of this manuscript).
Based in OGRI analyses from whole-genome sequence data, phylogenetic information provided by the 16S rRNA gene sequence and by phenotypic characterization, the strain K001 is considered as a novel species in the genus Muricauda, for which the name Muricauda brasiliensis sp. nov. is proposed.
Description of Muricauda brasiliensis sp. nov.
Muricauda brasiliensis (bra.si.li.en′sis N.L. masc./fem. adj. brasiliensis refer of or pertaining to the country from where the type strain was isolated, Brazil).
Cells are Gram stain–negative, rod-shaped, non-flagellated, non-motile, approximately 0.3–0.5 wide and 1.1–2.4 μm long, and strictly aerobic. Appendages are present in many cells after 48-h incubation in DIFCO 2216, a trait common with other species in the genus Muricauda. Under agitation, cells form aggregates on broth culture after 24 h. In DIFCO 2216 agar plate, colonies are yellow, translucent, circular, convex, with regular edges, and present 1–2-mm diameter after 48 h. The growth occurs between 15 and 37 °C, but not at 4 °C and 42 °C. The NaCl range supported is 0.5–9%; growth also occurs at NaCl as sole salt at 2.25% in broth medium but not in absence of this salt. Growth pH range is from 6 to 8. The cells are catalase and oxidase positive. The ability to hydrolyze Tween 20, 60, and 80, and starch is not present. Carotenoids-type pigments are present, but flexirubin-type are not. The cells are positive for esculin hydrolysis, β-galactosidase activity, assimilation of glucose, arabinose, mannose, N-acetylglucosamin, maltose, and malate; and negative to nitrate reduction, indole formation, glucose fermentation, arginindihydrolase and urease activities, gelatin hydrolysis, and assimilation of mannitol, gluconate, caprate, adipate, citrate, and phenylacetate. The cells are positive to alkaline phosphatase, esterase (C4), esterase lipase (C8), leucin-arylamidase, valin-arylamidase, cystin-arylamidase, trypsin, chymotrypsin, acid phosphatase, naphtol-AS-BI-phosphatase, a-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, and α-manosidase; and negative to lipase (C14), β-glucoronidase and α-fucosidase. K001 is susceptible to ciprofloxacin, clindamycin, chloramphenicol, norfloxacin, ofloxacin, erythromycin, ceftazidime, ceftriaxone, tetracycline, cephalexin, doxycycline, ampicillin, and piperacillin + tazobactam; but not to amikacin, cefazolin, gentamycin, penicillin g, polymyxin b, sulphonamides, oxacillin, and vancomycin. The major fatty acids are iso-C15:0 (35.21%), iso-C17:0 3-OH (22.55%), and iso-C15:1 G (14.48%). MK-6 is the predominant respiratory quinone. The main polar lipids include phosphatidylethanolamine, three unidentified aminolipids, and three unidentified polar lipids.
Protologue
The type strain is K001 (=CBMAI 2315T=CBAS 752T), isolated from a cyanobacterial culture. The genomic G+C content (mol) is 41.62%. The GenBank/EMBL/DDBJ accession numbers of K001T for the 16S rRNA gene sequence is MN996941, and the draft genome sequence is QBTW00000000.
Electronic supplementary material
(PDF 500 kb)
(TSV 228 bytes)
(PDF 56.1 kb)
Acknowledgments
We thank the Laboratory of Environmental Sanitation, Department of Civil and Environmental Engineering, University of Brasilia (Brazil), for the infrastructure to maintain the cultures and to perform some analyses. We thank the DNA Services Facility in Research Resources Center, University of Illinois at Chicago, Chicago (USA) for the sequencing service. We are grateful to the Microscopy and Microanalysis Laboratory of the University of Brasilia (Brazil), for their collaboration in carrying out the scanning electron microscopy analysis. We thank the Laboratory of Toxinology (Department of Physiological Sciences, Biological Sciences Institute, University of Brasilia, Brazil), the Laboratories of Graduate Program in Genomics Science and Biotechnology (Catholic University of Brasilia, Brasilia, Brazil), Laboratory of Mass Spectrometry (EMBRAPA Genetic Resources and Biotechnology, Brasilia, Brazil), and the Laboratories of the Department of Microbiology (Federal University of Viçosa, Viçosa, Brazil) for the infrastructure to perform part of the experiments of this work. We thank Dr. Manuela da Silva for the K001 microbial collections deposition efforts.
Abbreviations
- MA
marine agar DIFCO 2216
- MB
marine broth DIFCO 2216
- MALDI-TOF
matrix-assisted laser desorption/ionization
- MS
mass spectrogram
- dDDH
digital DNA–DNA hybridization
- ANI
average nucleotide identity
- TYGS
Type Strain Genome Server
- MIDI
Sherlock Microbial Identification System
Funding
This research was supported by a grant from BioTecMar (no. 408339/2013-6) of CNPq (Ministry of Science, Technology, Innovations and Communications—Brazil), the Foundation for Research Support of the Federal District (FAP-DF), and Coordination for the Improvement of Higher Education Personnel (CAPES).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Accession numbers: The GenBank/EMBL/DDBJ accession numbers of K001T strain for the 16S rRNA gene sequence is MN996941, and the draft genome sequence is QBTW00000000.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bruns A, Rohde M, Berthe-Corti L. Muricauda ruestringensis gen. nov., sp. nov., a facultatively anaerobic, appendaged bacterium from German North Sea intertidal sediment. Int J Syst Evol Microbiol. 2001;51:1997–2006. doi: 10.1099/00207713-51-6-1997. [DOI] [PubMed] [Google Scholar]
- 2.Wu YH, Yu PS, Zhou YD, Xu L, Wang CS, Wu M, Oren A, Xu XW. Muricauda antarctica sp. nov., a marine member of the Flavobacteriaceae isolated from Antarctic seawater. Int J Syst Evol Microbiol. 2013;63:3451–3456. doi: 10.1099/ijs.0.048355-0. [DOI] [PubMed] [Google Scholar]
- 3.Su Y, Yang X, Wang Y, Liu Y, Ren Q, Zhang XH. Muricauda marina sp. Nov., isolated from marine snow of yellow sea. Int J Syst Evol Microbiol. 2017;67:2446–2451. doi: 10.1099/ijsem.0.001992. [DOI] [PubMed] [Google Scholar]
- 4.Dang Y, Sun Y, Sun L, Yuan X, Li Y, Qin Q et al (2019) Muricauda nanhaiensis sp. nov., isolated from seawater of the South China Sea. Int J Syst Evol Microbiol 69:2089–2094. 10.1099/ijsem.0.003437 [DOI] [PubMed]
- 5.Hwang CY, Kim MH, Bae GD, Zhang GI, Kim YH, Cho BC. Muricauda olearia sp. nov., isolated from crude-oil-contaminated seawater, and emended description of the genus Muricauda. Int J Syst Evol Microbiol. 2009;59:1856–1861. doi: 10.1099/ijs.0.007708-0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Gao X, Qiao Y, Wang Y, Zhang XH. Muricauda pacifica sp. Nov., isolated from seawater of the South Pacific Gyre. Int J Syst Evol Microbiol. 2015;65:4087–4092. doi: 10.1099/ijsem.0.000542. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Jin HM, Jeon CO. Muricauda taeanensis sp. nov., isolated from a marine tidal flat. Int J Syst Evol Microbiol. 2013;63:2672–2677. doi: 10.1099/ijs.0.047647-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, Li Y, Guo Q, Lai Q, Wei J, Zheng T, Tian Y. Muricauda zhangzhouensis sp. nov., isolated from mangrove sediment. Int J Syst Evol Microbiol. 2013;63:2320–2325. doi: 10.1099/ijs.0.040881-0. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Lai Q, Yan P, Shao Z. Roseovarius amoyensis sp. nov. and Muricauda amoyensis sp. nov., isolated from the Xiamen coast. Int J Syst Evol Microbiol. 2019;69:3093–3101. doi: 10.1099/ijsem.0.003595. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Yu M, Zhou S, Fu T, Sun W, Wang L et al (2020) Muricauda alvinocaridis sp. nov., isolated from shrimp gill from the Okinawa Trough. Int J Syst Evol Microbiol 70:1666–1671. 10.1099/ijsem.0.003953 [DOI] [PubMed]
- 11.Lee SY, Park S, Oh TK, Yoon JH. Muricauda beolgyonensis sp. nov., isolated from a tidal flat. Int J Syst Evol Microbiol. 2012;62:1134–1139. doi: 10.1099/ijs.0.032581-0. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JH, Lee MH, Oh TK, Park YH. Muricauda flavescens sp. nov. and Muricauda aquimarina sp. nov., isolated from a salt lake near Hwajinpo Beach of the East Sea in Korea, and emended description of the genus Muricauda. Int J Syst Evol Microbiol. 2005;55:1015–1019. doi: 10.1099/ijs.0.03051-0. [DOI] [PubMed] [Google Scholar]
- 13.Park JS. Muricauda hymeniacidonis sp. Nov., isolated from sponge of Hymeniacidon sinapium. Int J Syst Evol Microbiol. 2019;69:3800–3805. doi: 10.1099/ijsem.0.003683. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Liu X, Lai Q, Du Y, Sun F, Shao Z. Muricauda indica sp. nov., isolated from deep sea water. Int J Syst Evol Microbiol. 2018;68:881–885. doi: 10.1099/IJSEM.0.002602. [DOI] [PubMed] [Google Scholar]
- 15.Liu SQ, Sun QL, Sun YY, Yu C, Sun L. Muricauda iocasae sp. Nov., isolated from deep sea sediment of the South China Sea. Int J Syst Evol Microbiol. 2018;68:2538–2544. doi: 10.1099/ijsem.0.002870. [DOI] [PubMed] [Google Scholar]
- 16.Arun AB, Chen WM, Lai WA, Chao JH, Rekha PD, Shen FT, Singh S, Young CC. Muricauda lutaonensis sp. nov., a moderate thermophile isolated from a coastal hot spring. Int J Syst Evol Microbiol. 2009;59:2738–2742. doi: 10.1099/ijs.0.007930-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Yang X, Liu J, Wu Y, Zhang XH. Muricauda lutea sp. nov., isolated from seawater. Int J Syst Evol Microbiol. 2017;67:1064–1069. doi: 10.1099/ijsem.0.001792. [DOI] [PubMed] [Google Scholar]
- 18.Yoon JH, Kang SJ, Jung YT, Oh TK. Muricauda lutimaris sp. nov., isolated from a tidal flat of the Yellow Sea. Int J Syst Evol Microbiol. 2008;58:1603–1607. doi: 10.1099/ijs.0.65659-0. [DOI] [PubMed] [Google Scholar]
- 19.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/JB.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution (N Y) 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 26.Vizzotto CS, Lopes FAC, Green SJ, Steindorff AS, Walter JM, Thompson FL, Krüger RH. Draft genome sequence of Muricauda sp. strain K001 isolated from a marine cyanobacterial culture. Genome Announc. 2018;6:1–2. doi: 10.1128/genomeA.00451-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu XW, de Meyer S, Trujillo ME. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 28.Chun J, Rainey FA. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int J Syst Evol Microbiol. 2014;64:316–324. doi: 10.1099/ijs.0.054171-0. [DOI] [PubMed] [Google Scholar]
- 29.Yoon SH, Ha S, Min LJ, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek. Int J Gen Mol Microbiol. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 30.Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed]
- 31.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Eisen JA. A simple, fast, and accurate method of phylogenomic inference. Genome Biol. 2008;9:R151. doi: 10.1186/gb-2008-9-10-r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price MN, Dehal PS, Arkin AP. Fasttree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ha S-M, Kim CK, Roh J, Byun J-H, Yang S-J, Choi S-B, Chun J, Yong D. Application of the whole genome-based bacterial identification system, TrueBac ID, using clinical isolates that were not identified with three matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems. Ann Lab Med. 2019;39:530–536. doi: 10.3343/alm.2019.39.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 2019;10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy, Beveridge, Breznak, Marzluf, Schmidt, Snyder, editors. Methods for general and molecular microbiology, Third Edition. 3a edition. American Society of Microbiology; 2007
- 39.Souza W (ed) (2007). Técnicas de microscopia eletrônica aplicadas às ciências biológicas. 3a edição. Sociedade Brasileira de Microscopia, Rio de Janeiro
- 40.Raney F, Oren A (2011) Methods in microbiology. In: Recent titles in the series, vol 38. Academic Press, London
- 41.Bernardet JF, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148. doi: 10.1099/00207713-46-1-128. [DOI] [Google Scholar]
- 42.Tindall BJ. Lipid composition of Halobacterium lacusprofundi. FEMS Microbiol Lett. 1990;66:199–202. doi: 10.1016/0378-1097(90)90282-U. [DOI] [Google Scholar]
- 43.Tindall BJ. A comparative study of the lipid composition of Halobacterium saccharovorum from various sources. Syst Appl Microbiol. 1990;13:128–130. doi: 10.1016/S0723-2020(11)80158-X. [DOI] [Google Scholar]
- 44.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 45.Sasser M (1990) Fatty acid profiling by gas chromatography. Tech. Note 101 - MIDI, Newark
- 46.(2015) Fatty acid profiling by gas chromatography - for the Sherlock MIS. Microbial ID, Inc., Newak
- 47.Ramasamy D, Mishra AK, Lagier JC, Padhmanabhan R, Rossi M, Sentausa E, Raoult D, Fournier PE. A polyphasic strategy incorporating genomic data for the taxonomic description of novel bacterial species. Int J Syst Evol Microbiol. 2014;64:384–391. doi: 10.1099/ijs.0.057091-0. [DOI] [PubMed] [Google Scholar]
- 48.Vandamme P, Peeters C. Time to revisit polyphasic taxonomy. Antonie van Leeuwenhoek. Int J Gen Mol Microbiol. 2014;106:57–65. doi: 10.1007/s10482-014-0148-x. [DOI] [PubMed] [Google Scholar]
- 49.Agustini BC, Silva LP, Bloch C, Bonfim TMB, Da Silva GA. Evaluation of MALDI-TOF mass spectrometry for identification of environmental yeasts and development of supplementary database. Appl Microbiol Biotechnol. 2014;98:5645–5654. doi: 10.1007/s00253-014-5686-7. [DOI] [PubMed] [Google Scholar]
- 50.Schumann P, Maier T. MALDI-TOF mass spectrometry applied to classification and identification of bacteria. vol. 41. 1st ed. Elsevier Ltd.; 2014. 10.1016/bs.mim.2014.06.002
- 51.Prabhu S, Rekha PD, Arun AB (2014) Zeaxanthin biosynthesis by members of the genus Muricauda. Polish J Microbiol 63:115–119. 10.33073/pjm-2014-017 [PubMed]
- 52.Prabhu S, Pd R, Young CC, Hameed A, Lin SY, Ab A. Zeaxanthin production by novel marine isolates from coastal sand of India and its antioxidant properties. Appl Biochem Biotechnol. 2013;171:817–831. doi: 10.1007/s12010-013-0397-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 500 kb)
(TSV 228 bytes)
(PDF 56.1 kb)