Abstract
We herein present a Brazilian guideline for the management of feline sporotrichosis, a mycosis caused by Sporothrix brasiliensis. This guideline is an effort of a national technical group organized by the Working Group on Sporothrix and Sporotrichosis of the International Society for Human and Animal Mycology (ISHAM). This publication intends to provide information on clinical-epidemiological aspects of this zoonosis, as well as a literature revision. Moreover, it gives some practical information on diagnosis and treatment of feline sporotrichosis. It also contains information that can be helpful for the prevention and control of S. brasiliensis transmission.
Keywords: Cat, Sporotrichosis, Epidemiology, Diagnosis, Treatment, S. brasiliensis, Guideline
Introduction
Cat-transmitted sporotrichosis caused by Sporothrix brasiliensis is an endemic neglected subcutaneous mycosis in Brazil. This zoonosis has been reported in the south and southeast regions for almost 21 years [1]. The geographic expansion of zoonotic sporotrichosis to the northeast region of the country occurred in the last 5 years [2]. Recently, feline and zoonotic cases were reported in other South American countries [3–5].
Sporothrix brasiliensis was only identified in 2007 [6] and included in the pathogenic clade of the Sporothrix genus [7]. Prior to the description of the new pathogenic cryptic species within the Sporothrix genus [6], sporotrichosis was attributed to a single etiological agent, Sporothrix schenckii, known as a low virulent fungal pathogen [1].
The first human case of sporotrichosis was described in 1898, by Benjamin Schenck in the USA and the causative agent was further identified as S. schenckii [8]. In 1907, Adolph Lutz and Affonso Splendore reported sporotrichosis in brown rats (Ratus novergicus) they collected in São Paulo City, Brazil. These authors also described, for the first time, the yeast parasitic phase of the fungus. Interestingly, they proposed that the rats could acquire sporotrichosis by ingestion as they succeed to experimentally infect rats using this route of infection [9].
The first evidence of cat-transmitted sporotrichosis was documented in the USA, in 1952 [10]. In 1955, in São Paulo State, southeast region of Brazil, the first human case of sporotrichosis associated with a sick cat was reported [11]. Several decades later, new cases of sporotrichosis in cats were reported in São Paulo and, among them, cases of human sporotrichosis due to cat-to-human transmission [12, 13]. Interestingly, in 1906, De Beurmann and Gougerot [14] isolated S. schenckii from the fur of rats, cats, and rabbits living in stables with infected horses. In conclusion, in the beginning of the twentieth century, naturally occurring sporotrichosis was described in animals and the possibility that healthy animals could carry the fungus was supported by some authors [9, 14, 15]. These historical observations have an important epidemiological significance and, somehow, can be considered as a trigger for the cat outbreak we are facing nowadays. In other words, the animal to human cycle of Sporothrix spp. was already reported but had no epidemiological significance until the cat-transmitted outbreak of sporotrichosis started in 1998, in Rio de Janeiro State, located in the southeast region of Brazil [16–20].
From 1907 to 2007, a century has passed until the discovery that sporotrichosis could be caused by at least three pathogenic species, S. schenckii, S. brasiliensis, or Sporothrix globosa [1]. Moreover, while S. schenckii is found worldwide, S. globosa and S. brasiliensis are geographically restricted and predominant in Asia and South America [18–21]. It is important to highlight here that, in Brazil, S. brasiliensis is now known as the major etiological agent (> 90% of the cases) of human and feline sporotrichosis [22–25]. This emerging pathogen is associated to a systemic disease in cats, is highly virulent, shows azole resistance, and is also related to severe clinical cases in humans including hospitalizations and deaths [26–38].
Due to the critical clinical-epidemiological panel of sporotrichosis associated with S. brasiliensis infection, the cat-to-human transmission and the high susceptibility of cats to this pathogen, a Brazilian guideline for the management of feline sporotrichosis caused by S. brasiliensis was proposed by the ISHAM Working Group on Sporothrix and Sporotrichosis. The aim of this guideline is to provide practical information that can help professionals in the management of this mycosis in cats, including clinical-epidemiological data and recommendations about diagnostic tools and treatment protocols. Furthermore, information that can be helpful to prevent and control S. brasiliensis transmission is also discussed.
Epidemiology
Feline sporotrichosis caused by S. brasiliensis
Currently, S. brasiliensis, S. schenckii, and Sporothrix humicola are the known causative agents of feline sporotrichosis [19, 20, 25, 39–42]. Cases of feline sporotrichosis were reported in the USA, Mexico, Argentina, Paraguay, Malaysia, Spain, Germany, Australia, Japan, Thailand, the UK, and Brazil [3–5, 17, 18, 41, 42]. In this last country, an impressive and exponential number of feline cases have been documented in several states [2, 17, 24, 29, 43, 44]. As already commented, in Brazil, the main etiological agent of feline sporotrichosis is S. brasiliensis [23–25, 31, 45, 46]. In contrast, in some Asian countries, S. schenckii has been described as the causal agent of sporotrichosis in cats [39–41, 47].
Sporotrichosis is acquired by the traumatic inoculation of the fungus into the skin via contact with contaminated plants, soil or decomposing organic matter, or less frequently, by inhalation of conidia. Cat-to-human and cat-to-cat transmission generally occurs through bites or scratches of sick animals [16]. Two pathogenic species generally follow an environmental route of transmission (sapronosis), S. schenckii and S. globosa [19]. The sapronotic route of infection affects specific occupational populations, mainly agricultural workers and gardeners [1, 19]. In contrast, S. brasiliensis is associated to zoonotic and cat-to-cat transmission [17, 19] and the infection occurs mainly through scratches, bites, or contact with the exudate of cutaneous lesions [43]. Some authors hypothesized that the cats’ infection may occur through inhalation route, to explain the high frequency of respiratory signs and nasal mucosal lesions, as well as the isolation of the fungus from the nasal cavity [43], lungs [48], and bronchoalveolar lavage washings [49]. Cats also play a key role as a source of infection due to the high fungal burden of yeast cells observed in the skin lesions [50] and the presence of the fungus in the claws and the oral cavity [43, 46]. The factors that lead to a high susceptibility of cats to S. brasiliensis infection, which frequently develop a severe and/or systemic disease, are still unknown.
Apparently, healthy cats have a minor role in S. brasiliensis transmission [46]. The hypothesis that they can propagate the fungus associated with their fur, is unlikely, but cannot be discharged [14]. It was reported a low frequency of fungal cells in the oral cavity and claws of healthy cats [46, 51, 52]. Dogs are also not directly involved in the transmission of Sporothrix spp. in view of the scarcity of fungal organisms in their cutaneous lesions [53] which is also observed in human lesions. Very few reports documented the dog-to-human transmission [14, 54].
A higher occurrence of the disease is reported in young adult male and unneutered cats. The presence of at least two cats per domicile was reported in the majority of the cases [44]. The mobility of cats in open areas surrounding their domiciles, their involvement in fights (especially for non-neutered male cats), and their habit of scratching tree trunks among other behavioral factors may facilitate the dispersal of Sporothrix spp. into the environment [55].
The importance of natural sources to acquire S. brasiliensis infection either for felines or other hosts is still not elucidated. A single study showed that S. brasiliensis was isolated in the feces collected from the small intestine of two-necropsied cats and also in feces samples collected from a pile of sand [24]. Feces from diseased cats may contaminate the soil contributing to spread S. brasiliensis by creating new environmental reservoirs as a source of contamination for animals and/or humans [24]. In Argentina, S. brasiliensis was also isolated from soil (cave of nine-banded armadillo) [4].
In addition, by a genome analysis of S. brasiliensis, it was hypothesized that this emerging species is evolutionarily more adapted to the parasitism of mammals compared to S. schenckii [56].
Spatial distribution of feline sporotrichosis caused by S. brasiliensis
Nowadays, Brazil is the country with the highest number of cases of feline sporotrichosis already reported all over the world. As mentioned before, feline sporotrichosis was first described in Brazil in the 1950s and its occurrence remained sporadic until the 1990s [12, 13, 57–61]. Two hundred and forty-four cases in dogs (from 1998 to 2014) and 5.113 cases in cats (from 1998 to 2017) were diagnosed and registered by a single institution of Rio de Janeiro State, Instituto Nacional de Infectologia Evandro Chagas, Fiocruz [2, 17, 62]. It is necessary to be aware that these numbers indicate the severity and endemicity of sporotrichosis but do not reflect the real incidence of this disease either in Rio de Janeiro State or in Brazil. After two decades, since the beginning of the outbreak of sporotrichosis due to S. brasiliensis infection in Rio de Janeiro State [17], the notification of the disease is still not compulsory in Brazil with few local exceptions. In conclusion, sporotrichosis is underestimated, is sub-notified, and remains as a neglected disease.
Feline sporotrichosis is now reported in all states of the southeastern region of Brazil: Rio de Janeiro [23, 25, 31, 46], São Paulo [24], Espírito Santo [63], Minas Gerais [64], Rio Grande do Sul [65], Paraná [66], and Santa Catarina [67]. Rio Grande do Sul State (RS) has the second largest series of feline sporotrichosis recorded in the literature [29, 45, 65] and the sporotrichosis outbreak was also registered in the late 1990s [68, 69]. Feline and human cases were reported in the municipalities of Rio Grande and Pelotas, RS [29, 45, 70–72]. Since 2014, cases of feline and human sporotrichosis were diagnosed in Paraná, mainly in Curitiba City (the state capital) and, according to data from the Zoonosis Surveillance Unit, between January 2016 to August 2019, 793 cases of feline sporotrichosis were reported (Farias M, personal communication).
As commented previously, feline sporotrichosis was recorded since the 1960s in São Paulo State [12, 13, 24]. The number of feline cases registered by the Centers for Zoonoses Control (CCZ) of São Paulo and Guarulhos cities (SP) had been increased since 2011 (Table 1).
Table 1.
Registered feline cases of sporotrichosis in some municipalities of São Paulo state, Brazil, for the period of 2011 to 2019
| City | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 # | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Guarulhos | 2 | 5 | 9 | 24 | 171 | 490 | 923 | 929 | 1229a | 3782 |
| São Paulo | 71 | 47 | 47 | 86 | 85 | 122 | 172 | 432 | 292 | 1354 |
| Diadema | 4 | 4 | 10 | 84 | 69 | 10 | 28 | 209 | ||
| Itaquaquecetuba | 14 | 45 | 1 | 1 | 61 | |||||
| Peruíbe | 4 | 24 | 32 | 60 | ||||||
| São José do Rio Preto | 2 | 34 | 36 | |||||||
| Caraguatatuba | 2 | 6 | 13 | 3 | 24 | |||||
| Arujá | 3 | 12 | 1 | 16 | ||||||
| Barueri | 2 | 8 | 10 | |||||||
| Campinas | 1 | 1 | 2 |
Source of the data: H. Montenegro, personal communication (Center of Zoonotic Control (CCZ) of São Paulo City) and W. Mansho, personal communication (Center of Zoonotic Control (CCZ) of Guarulhos City)
#Partial data from January 1 to July 31, 2019
aData from January 1 to December 31, 2019
In the last 5 years, an expansion of sporotrichosis occurred to several other regions of Brazil with cases reported in the following states: Mato Grosso [73], Mato Grosso do Sul, Distrito Federal [74], Rio Grande do Norte [75], Paraíba [76], Pernambuco [77, 78], Alagoas [79], Acre, and Pará [2]. Studies on the identification of the causative agent of feline sporotrichosis in the northeast region have not yet been documented. However, we believe that S. brasiliensis is responsible for the outbreaks in all Brazilian regions as it was previously reported as the predominant Sporothrix species, in Brazil [19].
In 2015, a local outbreak was registered in the city of Camaçari situated only 41 km distant from the most populated area of Bahia State, Salvador City (the capital of Bahia). Interestingly, between 2015 and 2018, no cases were reported in the city of Salvador, even though a public health surveillance, an alert for possible cases, was out in 2015. The first autochthonous cases were detected in Salvador City by March 2018, soon after the compulsory notification of animal and human sporotrichosis took place. Between March and December 2018, 289 suspected cases were reported in animals (92% felines). The casuistry and a rapid dispersion of cases among several neighborhoods of Salvador City suggests two hypotheses: sporotrichosis was under-reported before the compulsory notification (2018) and/or this zoonosis was introduced and spread rapidly in the municipality. The example of Salvador City illustrates how critical and important is the notification of sporotrichosis. The notification allows the public health system to monitor the disease, to investigate and diagnose cases in a timely manner, to register epidemiological data, and to propose the necessary interventions for the management and control of this disease. Sporotrichosis is also being alerted by health secretariats of other capitals and metropolitan areas of the northeast region of Brazil (Table 2). In conclusion, compulsory notification must be introduced in all Brazilian states by the Health Ministry; otherwise, this disease will continuously spread without any epidemiological control.
Table 2.
Feline sporotrichosis data available in the northeast region of Brazil, in the period of 2014 to 2019
| City (state) | Human confirmed cases | Animal confirmed cases | Period (month and/or year) |
|---|---|---|---|
| Rio Largo (AL)a | 1 | 1 | 2014 (Marques-Melo et al. 2014) |
| Camaçari (BA)a | 70 | – | 2015 to 2017I |
| 171 | – | January to December/2018I | |
| Salvador (BA)a | 56 (29) | 289 (141) | February to December/2018 (SMS, 2019) |
| João Pessoa (PB)a | 329 | – | January/2018 to May/2019II |
| – | 232 (222) | June/2018 to February/2019 (Costa MCL, 2019) | |
| No city (PE)b | 115 (59) | March/2014 to February/2016 (Silva et al. 2018) | |
| 322 | 2016 to 2018III | ||
| No city (RN)b | 131 | 195 (117) | 2015 to 2019IV |
aBrazilian states of Alagoas (AL), Bahia (BA), Paraíba (PB), Pernambuco (PE), and Rio Grande do Norte (RN)
bThe data is only available for the state without any municipality information
Source [in Portuguese]: Media, Technical Notes and Epidemiological Bulletins published by the Health Secretariat of the respective State or Municipality. Data were accessed on July 2, 2020 and are available at the following websites:
II. https://portalcorreio.com.br/joao-pessoa-registrou-329-casos-de-esporotricose-em-humanos/
Costa, M. C. L. Distribuição espacial da esporotricose felina no município de João Pessoa, estado da Paraíba, Brasil. 2019. Monografia (Graduação) - UFPB/CCA
Marques-Melo EH, Lessa DFS, Nunes ACBT, Chaves KP, Porto WJN, Notomi MK, Garrido LHA (2014) Felino doméstico como agente transmissor de esporotricose para humano: relato de primeiro caso no estado de Alagoas [article in Portuguese]. Rev. Baiana Saúde Pública 38:490–498
Silva GM, Howes JCF, Leal CAS, Mesquita EP, Pedrosa PM, Oliveira AAF, Silva LBG, Mota RA (2018) Surto de esporotricose felina na região metropolitana do Recife. Pesq Vet Bras 38:1767–1771
SMS. Secretaria Municipal da Saúde de Salvador. Diretoria de Vigilância da Saúde. Situação Epidemiológica da Esporotricose em Salvador – BA, 2018. Boletim Epidemiológico n° 06, 2019. Disponível em: http://www.emevz.ufba.br/esporotricose-em-salvador-voce-tambem-pode-notificar
Outside Brazil, to date, only Argentina has reported the occurrence of S. brasiliensis infection in humans and cats [4, 5] which is alarming because this suggests the transboundary expansion of sporotrichosis caused by S. brasiliensis to other Latin America countries [2]. Importantly, health authorities of Brazilian neighboring countries which have a dense transit of people and animals in a daily basis, such as Argentina, Paraguay, Bolivia, and Uruguay, should be aware to identify cases rapidly as well as, to implement policies for prevention and control of sporotrichosis.
Clinical aspects
Feline sporotrichosis is a subcutaneous mycosis that can be clinically presented as a single lesion or as multiple cutaneous lesions and, commonly, cats can develop a disseminated systemic disease. The most frequent clinical form is characterized by multiple cutaneous lesions with mucosal involvement, especially the nasal mucosa. However, cutaneous lesions may be absent in a few cases. Conjunctival, oral, and genital mucosa may also be affected. In addition, lymph node enlargement is frequently observed, while lymphangitis is less frequent [43, 80].
The incubation period after a Sporothrix spp. infection to the first signs is variable. The average incubation period is 14 days, but this incubation period may extend for months, similar to which is observed in humans [80].
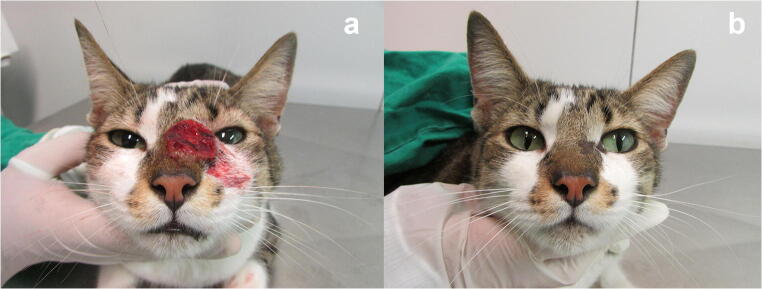
The skin lesions appear as nodules and ulcers in different anatomical sites. They are usually located on the head, especially in the nasal region [43] (Fig. 1). Tumor-like lesions are also observed (Fig. 2). The nodules may ulcerate and drain serosanguinolent and/or purulent exudates. Some lesions form crusts. Zones of necrosis exposing muscle and bone and myiasis can also occur [80, 81]. Cats usually exhibit a good general health condition even though they present multiple skin lesions and mucosal involvement [43]. Generally, hematologic and serum biochemical abnormalities are consistent with several infectious diseases. In the case of sporotrichosis, these abnormalities are more frequent in cats presenting multiple skin lesions [43].
Fig. 1.
Cat with sporotrichosis caused by Sporothrix brasiliensis showing multiple ulcerated skin lesions on the head
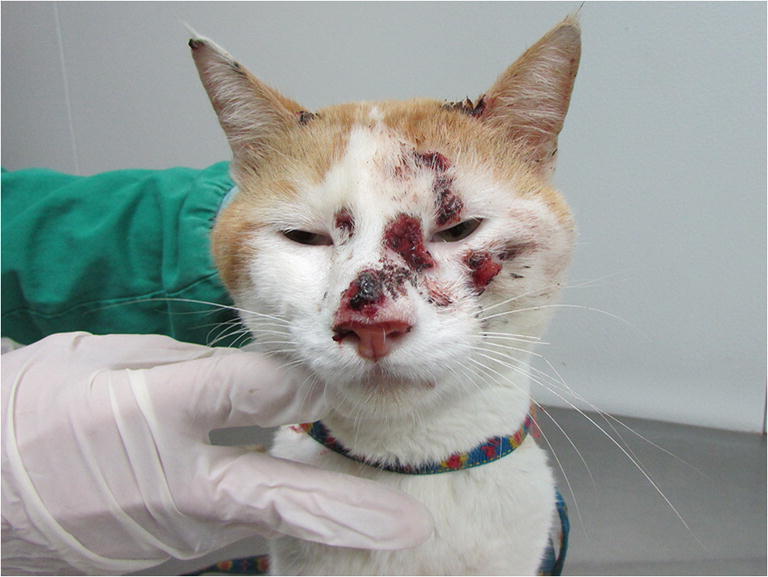
Fig. 2.

Cat with sporotrichosis caused by Sporothrix brasiliensis showing a tumor-like lesion on the nasal region
Extracutaneous signs, particularly respiratory signs (sneezing, dyspnea, and nasal discharge) are frequent [80]. The occurrence of respiratory signs may precede the appearance of cutaneous lesions or may be observed in cats without any skin lesion [43]. In general, the lesions located in the nasal region may reoccur after the clinical “cure” or are refractory to antifungal treatment [82–84]. Nasal mucosal lesions and respiratory signs were associated with treatment failure and death [31, 85]. Sporothrix brasiliensis infection is frequently associated with the nasal mucosal lesions and of upper respiratory signs in cats [25, 31, 84].
Disseminated sporotrichosis should be considered if the cat presents a history of lethargy, depression, anorexia, and fever [81]. Sporothrix spp. was observed by histopathology and/or could be isolated by culture from several organs of infected cats such as the lungs, heart, spleen, kidneys, lymph nodes, brain, adrenal glands, and liver, in specimens recovered after postmortem examination [48, 86–88]. Hematogenous dissemination may occur. This hypothesis is supported by the fact that Sporothrix spp. was isolated from peripheral blood of cats with either widespread cutaneous lesions or localized skin lesions [88].
The correlation between the severity of sporotrichosis and co-infection with feline immunodeficiency virus (FIV) or feline leukemia virus (FeLV) (progressive infection) remains controversial. However, severe cases of feline sporotrichosis are frequently described in animals apparently immunocompetent. Several reports support that dissemination of Sporothrix spp. is unrelated to FIV and/or FeLV co-infections [43, 85, 88]. Furthermore, Sporothrix-retrovirus co-infection in cats suggest an immunosuppression by their immunological parameters but, so far, no association has been observed between retrovirus co-infection, the duration of sporotrichosis treatment, and to the outcome of the disease [85, 88–91]. Other comorbidities or immunosuppressive conditions cannot be ruled out in cats with severe sporotrichosis. In addition, sporotrichosis is mainly reported in geographical areas of low socioeconomic status and poor health assistance, and the lack of vaccination and prophylactic deworming status in the population of cats should also be considered as a pre-condition for several comorbidities [91].
The differential diagnosis of feline sporotrichosis must include neoplasias (mainly squamous cell carcinoma), eosinophilic dermatosis, bacterial pyoderma, protothecosis, mycobacteriosis, cryptococcosis, histoplasmosis, phaeohyphomycosis, and American tegumentary leishmaniasis, among others. The veterinarians should be alert to the possibility of co-infection, in other words, to the parallel existence of sporotrichosis and other skin infections [80].
Diagnosis
The diagnosis of feline sporotrichosis depends on laboratorial tests, because clinical signs are nonspecific. The reference standard method is the mycological test that is based on the Sporothrix spp. isolation in culture media, its identification by morphological parameters and the culture conversion to the yeast phase. The disadvantages are (i) the necessity of a biosafety laboratory level 2 to manage Sporothrix spp. and other pathogenic fungi, following the current legislation; (ii) culture contamination is not uncommon, due to swab or biopsy samples contamination; and (iii) Sporothrix spp. is a slow-growing fungus and the reference standard method requires the conversion to the yeast phase by subculture at 37 °C. This represents a mean time to a diagnostic result around 30 days. Alternatively, cytopathological and histopathological examinations are very useful tools for the routine and preliminary diagnosis of this disease, especially in cats. Histopathology also requires a biopsy. Cytopathology is based on a cutaneous lesion imprint. For all these methods that will be described in detail, a negative result does not discharge the disease.
Immunohistochemistry and polymerase chain reaction (PCR) are other options for the diagnosis, but they are used principally in research and have not been implemented in the clinical routine, yet.
Recently, a serological test has been made available in private laboratories for the preliminary diagnosis of feline sporotrichosis. This ELISA test detects IgG antibodies against a purified antigen of Sporothrix spp. and was recently validated for all clinical forms of feline sporotrichosis.
Importantly, previous treatment with topical or systemic antifungal drugs can reduce the sensitivity of laboratorial exams for the diagnosis of Sporothrix infection.
Mycological test
Nowadays, definitive sporotrichosis diagnosis is based on the isolation and identification of Sporothrix spp. in culture by morphological parameters. Samples for fungal culture can be obtained by skin lesion exudate or mucosal secretions collected with a sterile swab, as well as lesion biopsies, lymph node aspirates, and organ fragments collected during necropsy [92].
Ideally, the clinical samples should be seeded as soon as possible in the appropriate culture media for fungal isolation. Alternatively, swabs containing the specimen may be stored in Stuart Transport Medium for a better preservation from the clinic to the laboratory. Skin biopsies should be placed in sterile saline for transportation to the laboratory. If immediate culturing is impossible, specimens should be stored at 4 °C for no longer than 8–10 h.
Sporothrix spp. is dimorphic fungus showing a mycelial phase and a yeast parasitic phase. Isolation is easily obtained after spreading of the biological samples on Sabouraud agar with chloramphenicol and on media with cycloheximide, such as Mycosel agar. After 5 to 7 days of incubation at 25 °C, filamentous hyaline colonies start to grow, and they may develop a dark color, usually in the centers of the colonies (Fig. 3). To identify an isolate as Sporothrix spp., one must demonstrate that it undergoes dimorphism by subculturing the fungus on enriched media such as Brain Heart Infusion agar (BHI agar) at 35 to 37 °C for 5 to 7 days. After Sporothrix spp. conversion to the yeast phase, colonies acquire a creamy aspect and a yellow to tan color [16]. Cultures should be observed for up to 30 days because of the possibility of late fungal growth.
Fig. 3.

Macromorphology of the colonies of S. brasiliensis (strain IPEC872), cultivated in PDA medium for 21 days at 30 °C
The mycelial phase is characterized by thin, hyaline, septate, and branched hyphae containing conidiophores whose apex forms a small vesicle with sympodially arranged denticles. Each denticle produces one conidium, each measuring approximately 2 to 4 μm and conidia are arranged in flower-like structures. Conidia become detached from the conidiophores, sometimes being arranged side by side in a row bilaterally to the hyphae. The yeast phase is pleomorphic, showing spindle-shaped and/or oval cells measuring 2.5 to 5 μm in diameter and resembling a “cigar” [93]. Round and oval yeast-like cells are commonly observed for S. brasiliensis [36]. The microculture can be necessary in some specific cases, either for confirmation by micromorphological identification or in the case of atypical strains. The PCR should also be considered to identify Sporothrix spp., after the fungus isolation in culture.
Direct microscopic examination
The cytopathological examination is a low-cost technique, simple to perform and the result is provided quickly. In addition, it does not require sophisticated technical training or complex laboratory structure, but accuracy and experience in identifying Sporothrix spp. are required [94–96]. The imprint quality on a glass-slide is critical for a good microscopic identification. Importantly, a negative cytopathological examination result does not ruled out a Sporothrix spp. infection and is recommended to be combined with other diagnostic tools.
In cats with sporotrichosis, the cytopathological examination from exudate of skin lesions usually reveals numerous yeast-like cells that may be oval, rounded, or cigar-shaped, with a diameter of 3 to 5 μm and a length of 5–9 μm. These structures may be located inside macrophages, neutrophils, and multinucleated or free giant cells, being surrounded by a transparent halo (Fig. 4) and may be similar to those observed in Histoplasma or Cryptococcus genus [94].
Fig. 4.
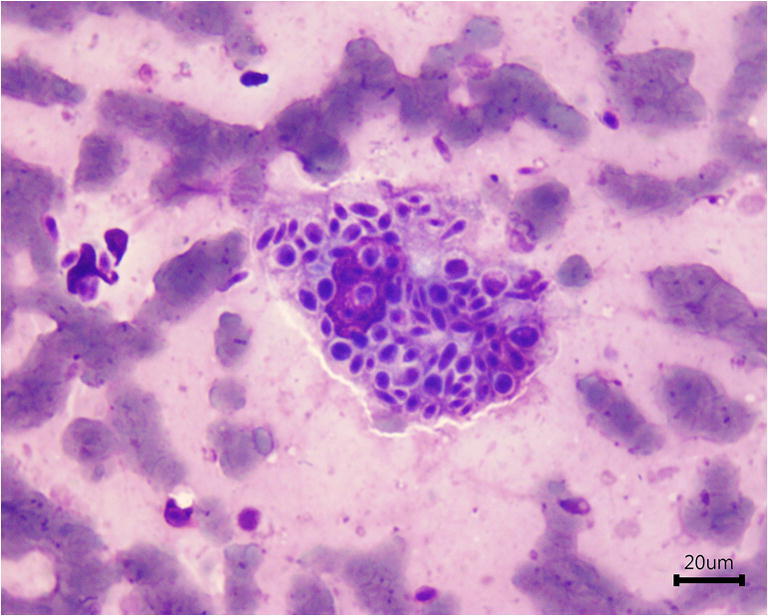
Impression smear of a skin lesion from a cat with sporotrichosis showing numerous cigar-shaped or oval yeast-like cells within macrophages and extracellular medium. The staining method used was the Quick Panoptic stain
The staining methods routinely used in cytopathological examination for the diagnosis of sporotrichosis are Romanowsky type (Quick Panoptic method) and/or Gram and Giemsa [96]. The stained slide should be analyzed by light microscopy using at least 40× objective lens and ideally 100× objective lens, for the accurate observation and identification of yeast-like structures suggestive of Sporothrix spp. [94].
This method has been widely used in the diagnosis of feline sporotrichosis due to the high fungal burden in the skin lesions of these animals, which facilitates the observation of fungal structures [95]. In general, the sensitivity of this method varies between 52.6 and 87% [94–97]. In cats under systemic antifungal treatment, the fungal burden tends to decrease leading to reduced sensitivity of the cytopathological exam [50]. A cohort study with 196 felines was performed to compare the effectiveness of the mycological test and the cytopathology test. The authors concluded that the mycological test was more sensitive (95.2%) in relation to the cytopathology (52.6%) [97]. In another study, 806 cases of feline sporotrichosis were included. Cytopathological examination of the skin lesions was positive in 636 cats, and the sensitivity of this method in relation to fungal culture was 78.9% [94].
Cytopathology is recommended for preliminary diagnosis in epizootic situations, as the positive result allows the initiation of the antifungal treatment before isolation of Sporothrix spp., especially when there are difficulties in performing fungal culture [94–96]. A recent study reveals that the cytopathological examination can be used to quantify the sequential fungal burden of skin lesions during feline treatment [50]. It is noteworthy that in order to have therapeutic monitoring using cytopathological examination, it should be collected a sequential exudate from the same skin lesion for quantitative comparison of the fungal burden. A cell block cytology was recently described as an efficient tool to diagnose sporotrichosis in cats [98].
Histopathological examination
The histopathological examination can be used for the preliminary diagnosis of feline sporotrichosis. This diagnostic tool may also give some information about the inflammatory response and other alterations related to the skin lesion [80].
Tissue samples are collected by biopsy or necropsy. The skin biopsy should be obtained from borders of active lesions with a punch (3–4 mm), after local antisepsis with 70% alcohol and anesthesia with 2% lidocaine hydrochloride. In cats, the sedation is strongly recommended. The samples are fixed in 10% buffered formalin, embedded in blocks of paraffin wax, microtome sectioned to 5 μm thickness, and stained [99].
The hematoxylin-eosin (H&E) is the routine staining recommended to observe inflammatory cells and pathological alterations associated with Sporothrix spp. infection. However, H&E staining does not allow a good visualization of yeast-like cells of Sporothrix spp., the parasitic phase, which can be confused with other pathogenic fungi and protozoa. In addition, the microscopic alterations observed are not disease-specific [92]. For these reasons, specific histochemical staining such as Grocott Methenamine Silver (GMS) (Fig. 5) and periodic acid-Schiff (PAS) should be used to identify the structure of Sporothrix spp. [43, 87].
Fig. 5.

Histological section from a skin lesion from cat with sporotrichosis stained by Grocott Methenamine Silver (GMS). Numerous dark-brown Sporothrix spp. yeast-like cells are observed
In feline sporotrichosis, the occurrence of well-formed granulomas is uncommon and is associated with a low fungal burden. In most cases, malformed granulomas with poorly activated histiocytes, which are often full of yeasts, are described. Neutrophils, lymphocytes, plasmocytes, mast cells, and eosinophils can also be observed [31, 100]. After the initiation of antifungal treatment, feline sporotrichosis lesions tend to have dermal fibrosis associated with pyogranulomatous infiltrate [31].
The yeast structures of Sporothrix spp. are oval, rounded, or cigar-shaped (4.0 and 6.0 μm) and may present narrow-base buds, most often single. Less frequently, hyphae and pseudohyphae can be observed [31]. The asteroid body is a typical structure observed in tissue specimens of sporotrichosis’ patients but this finding is uncommon in animals [100].
Development of new diagnostic tools
Serologic techniques have been shown to be useful as a diagnostic tool and for monitoring the treatment response or relapses of human sporotrichosis [101, 102]. Previously, ELISA tests based on a purified Sporothrix spp. antigen (SsCBF) and exoantigens had shown high sensitivity when evaluated with sera of cats with confirmed sporotrichosis from endemic areas [103]. To collect blood from cats, it is necessary to properly contain the animal, thus avoiding the risk of scratches or bites.
Based on a preliminary promisor result, the human ELISA test (SsCBF-ELISA) was adapted and validated with 62 sera of infected cats (confirmed either by cytopathological examination and/or fungal culture) and is now available in private laboratories, thus expanding the diagnostic panel. When cytopathological and serological tests are positive, the diagnosis of sporotrichosis presents a specificity of 89.3% and a positive predictive value of 92.5%, [104]).
The serology detection and quantification of IgG antibodies by the SsCBF-ELISA test was also studied as a therapeutic monitoring tool [105]. A serological scar may remain for months but the IgG levels drop accordingly to the therapeutic response. However, it is important to notice that continuous exposure to the infectious agent in a contaminated environment may keep high antibody levels and a positive serology (seroconversion). In any case, a diagnostic test result should always be associated with the clinical findings.
Molecular tests are important diagnostic tools for identifying Sporothrix. There is a growing demand for new methods of molecular diagnosis to identify pathogenic species in tissue samples. Few polymerase chain reaction (PCR) assays have been described for the detection of Sporothrix in these samples; however, they are not yet routinely used in the diagnosis of human and animal sporotrichosis [106, 107].
A PCR assay using chitin synthase 1 (CHS 1) was successfully performed for rapid and specific detection of Sporothrix DNA and gender-level identification from skin lesion fragment obtained by biopsy of a cat with sporotrichosis [106].
The detection and identification of Sporothrix at the species level has been described using sequences of calmodulin genes specific for PCR target species, which were used in different tissue samples and feces of experimentally infected mice [108]. In addition, the “rolling circle amplification” (RCA) technique has been used successfully in the detection and identification of Sporothrix spp. [109].
Treatment of feline sporotrichosis
Cats under systemic antifungal therapy, due to the consequent reduction in the fungal burden, do not appear to play a key role in the Sporothrix spp. transmission cycle. Therefore, the early treatment of feline sporotrichosis should be performed [50], based on a precise diagnosis. The cat’s general medical condition, the occurrence of respiratory signs as well as the number, extension, and location of the lesions are factors that may influence the prognosis. As feline sporotrichosis is hard to treat, it requires a long period of daily care and cats do not always respond well to treatment. A successful treatment depends on the owner’s cooperation and persistence [84].
The abandonment of feline sporotrichosis treatment is frequent (≈ 30–40%) and mainly occurs at the time that the cat’s owner observes the healing of the skin lesions. Therefore, the irregularity of the treatment may lead to the recurrence of the disease, imposing difficulties to the cure process [43, 110] and, in consequence, contributing to the transmission of Sporothrix spp.
In general, lesions on the nasal region of cats are difficult to treat. The severity and extension of the lesions (severe pyogranulomatous inflammatory infiltrates, high fungal loads, and extension of lesions to mucosa, cartilage, and bone) may hinder their healing [84].
There are a limited number of oral antifungal agents for the treatment of sporotrichosis in cats. The itraconazole demonstrated good in vitro activity against strains of S. brasiliensis isolated from cats [111–113]. However, the correlation between the in vitro antifungal susceptibility assay and the in vivo therapeutic response must be made with caution.
Itraconazole and potassium iodide are the most commonly used drugs to treat feline sporotrichosis [84]. Itraconazole remains the drug of choice for the treatment and its effectiveness as a monotherapy has already been reported [31, 43, 50, 69, 84, 85, 90, 113, 114]. In contrast, an increasing number of itraconazole-insensitive strains over time was documented [112, 116]. Generic and compounded itraconazole are not bioequivalent to the reference drug. The use of compounded itraconazole is not recommended, but the generic is a satisfactory alternative to the reference drug [117, 118].
It is important to note that treatment of feline sporotrichosis remains a challenge in so many cases. Reports of therapeutic failure and recrudescence are common, even when established therapeutic protocols are used [90]. Cats with multiple lesions and high fungal burdens tend to have persistent lesions and a higher risk of treatment failure when itraconazole monotherapy is used [31, 50], also a prolonged wound healing period [31].
Potassium iodide capsules is an option for the treatment of cats infected with S. brasiliensis. In addition, the potassium iodide cost greatly favors its use because it is less expensive than itraconazole [118]. Itraconazole combined with potassium iodide is an effective treatment in naive cats (Fig. 6), with a faster onset of action and a moderate percentage of adverse effects [90]. It also represents an important option for the treatment of refractory cases to itraconazole, especially for those cats presenting nasal mucosal lesions and/or respiratory signs [30]. Although the potassium iodide mechanism of action is still unclear, this drug is very effective to improved healing and promptly control the fungal burden [50].
Fig. 6.
a Cat with sporotrichosis caused by S. brasiliensis presenting an ulcerated lesion on the nasal region before antifungal treatment. b The clinical cure was achieved after 14 weeks of itraconazole (100 mg/24 h) and potassium iodide (2.5 mg/kg/24 h) therapy
Amphotericin B by intravenous administration is limited in cats because of serious adverse effects. The lipid formulations of amphotericin B have less nephrotoxicity than the conventional drug and their use is indicated for the treatment of disseminated forms sporotrichosis. However, the cost of these formulations may be prohibitive [120]. The use of intravenous liposomal amphotericin B combined with itraconazole was successfully described in a case of feline sporotrichosis refractory to itraconazole alone [84]. On the other hand, intralesional or subcutaneous amphotericin B, associated with oral itraconazole, is a good alternative in cases refractory to itraconazole monotherapy [82–84].
The potential of terbinafine for the treatment of sporotrichosis has been confirmed in human patients [121, 122], but its effectiveness in the treatment of animal sporotrichosis is unknown, so far. Recently, the successful use of terbinafine in the treatment of two dogs with sporotrichosis caused by S. brasiliensis was reported [123].
Fluconazole was used to treat a cat presenting cutaneous lesions and respiratory signs. This drug was reintroduced shortly after the discharge due to the recurrence of respiratory signs and the clinical cure was further achieved [114]. However, its effectiveness and safety in the treatment of feline sporotrichosis needs to be clarified.
Thermotherapy [124], surgical therapy [125, 126], and cryosurgery [127] are other options for the treatment of feline sporotrichosis. Their use should be carefully evaluated according to the case.
Recommended treatment regimens
According to the clinical evaluation of the animal and considering the severity of the case, i.e., the clinical condition, the number and/or location of skin/mucosal lesions, and the presence of extracutaneous signs, especially respiratory signs, it is recommended a itraconazole monotherapy or the association of itraconazole with potassium iodide (capsule). These drugs can be prescribed according to the doses described (Table 3). Also, in Fig. 7, an algorithm for the treatment of cats with sporotrichosis was demonstrated.
Table 3.
Recommended doses of itraconazole monotherapy or combined with potassium iodide (capsule) for feline sporotrichosis
*Previously recommended doses
Fig. 7.
Algorithm for the treatment of feline sporotrichosis
Itraconazole administered orally with food facilitates the absorption of this drug. This drug should not be administered with antacids (H2 receptor antagonists or proton pump blockers) because alkalinity decreases its absorption [119].
Itraconazole and potassium iodide capsules can be opened, and the dose (or a fraction) can be mixed in the wet food; however, beads (itraconazole) should remain intact [36, 120]. The administration of the opened capsules together with the food decreases the chances of contact with the diseased animal and the risk of zoonotic transmission.
Recommendations if drug toxicity is detected
Temporary drug suspension is necessary due to clinical and/or laboratory adverse reactions (nausea, anorexia, vomiting, increased serum ALT), until the appetite returns and/or serum hepatic enzymes return to reference levels (usually 1–2 weeks). Thus, clinical and biochemical monitoring is strongly recommended [120].
Cats with an elevation in transaminases levels may also receive a hepatoprotective therapy with oral silymarin (30 mg/kg, once a day) or S-adenosylmethionine (SAMe) (20 mg/kg, once a day) [128, 129].
Criterion of cure
The criterion for cure for feline sporotrichosis is still clinical, with the disappearance of all the signs. It is important to maintain the treatment for 1 month after the clinical cure. In cats presenting lesions (cutaneous and/or mucosal) on the nasal region and/or respiratory signs, the treatment should be maintained for 2 months after clinical cure to minimize the risk of recurrence. Clinical cure is observed regardless the initial clinical findings or co-infection with FIV and/or FeLV [31, 43, 50, 85, 89–91]. Recurrence after clinical cure may occur, which demonstrates the possibility of reactivation of the lesions in spite of the end of the treatment [84].
Euthanasia
Euthanasia may be indicated in situations of severe clinical condition or unsatisfactory therapeutic response (cases of clinical stagnation or worsening of the clinical condition). The indication of euthanasia should always be made by a veterinarian, after careful clinical evaluation of the animal. The carcasses must be incinerated to avoid environmental contamination.
General recommendations
The cat should remain isolated from other animals throughout the treatment, which may take few weeks to several months (median time = 4 months) [31, 43, 84, 85, 90]. The cooperation of the owners is important in attempting a successful therapy response.
The neutering/spaying of the cats is an important preventive and control policy. This surgical procedure encumbers the instinct for hunting, mating, and roaming the neighborhood and therefore may reduce the chance of transmission of Sporothrix spp. Importantly, cats clinically cured should be neutered/spaying.
Antifungal topical treatment is not recommended. The use of topical medications, like fly repellent ointment, must be made with caution in sick animals because of the risk of zoonotic transmission.
In cases of concurrent bacterial infection it is necessary to introduce a systemic antibiotic therapy. Glucocorticoids or any immunosuppressive drugs are contraindicated because the disease can worsen or recur.
As in humans, administration of any azole, terbinafine, and potassium iodide should be avoided during pregnancy [130, 131]. However, the use must be considered in pregnant cats because of the risk of zoonotic transmission and/or progression of the clinical signs.
Public health considerations and prevention
The reduction of public health risks due to zoonotic outbreaks, mainly in high density populated urban areas, requires prevention and control strategies. The frequency of zoonosis increased in the last decades. They may be related to poverty, poor sanitation, and anthropogenic changes in the environment [24].
In Brazil, feline sporotrichosis requires urgent preventive policies to avoid transmission of the main causative agent, S. brasiliensis [16]. The high risk of cat-to-human transmission along with the fact that the therapeutic regimen can be long and expensive may discourage the compliance of owners with the therapeutic protocols and the recommended care practices. In consequence, the prevention and control of this disease may be seriously affected [50]. Sporotrichosis can be cured if treated accordingly and, together, health professionals and animal owners are important players to contribute for the epidemiological control.
During the handling of sick cats, it is important to follow some specific biosafety procedures to reduce risks, such as the use of personal protective equipment (PPE). The use of disposable latex gloves and long sleeve disposable apron is compulsory for veterinarians and their assistants. After the removal of the gloves, hands, wrists, and forearms should be washed with soap. In cases of occurrence of continuous sneezing and/or multiple skin lesions, it is recommended to use facial mask N95 or PFF2 and safety goggles [132]. In addition, care must be taken to avoid injuries and contact with exudate from lesions when manipulating cats with sporotrichosis. In case of injury caused by scratches or bites of cats with sporotrichosis, it is recommended to wash them immediately with soap and to look for specialized medical assistance. Veterinarians should also alert caretakers and owners of cats with sporotrichosis to the possibility of zoonotic transmission [80].
Appropriate physical restraint or sedation of noncooperative patients must be done to allow a physical examination and the collection of biologic samples for laboratory tests.
Cages or transport containers must be decontaminated with sodium hypochlorite (1%), diluted 1:3 in water, for at least 10 min. After clinical examination, tables used should be disinfected with sodium hypochlorite solution (1%), followed by alcohol 70%, for at least 10 min, using disposable paper towels. Additionally, floors and walls must be cleaned and disinfected daily with sodium hypochlorite solution (1%) [132].
For public health purposes and to control epidemic cat-transmitted sporotrichosis, an effective and viable therapeutic regimen applied to cats under field conditions is necessary. Recent studies have shown that some therapeutic protocols may reduce the fungal burden in cats with sporotrichosis, even when the infection is caused by S. brasiliensis [31, 50]. The early treatment of feline sporotrichosis contributes for the disease control. Rapid preliminary diagnostic tests with good sensibility to confirm a clinical suspicious, like a cytopathological examination, can be helpful for this purpose [50].
Public awareness programs on sporotrichosis prophylaxis are required for (i) education for responsible ownership, which includes proper health care for the animals and neutering, and (ii) confinement of cats inside the home and limitation of the number of cats per household.
Animals with sporotrichosis that were euthanized and/or necropsied or evolved to death should not be buried, due to the possibility of soil contamination by Sporothrix spp. The carcasses must be packed in a milky white bag with a biological risk symbol and kept under refrigeration until the incineration [132]. General public health policies such as basic sanitation, regular garbage collection, and cleaning of uninhabited lots are also required [96].
The public health surveillance must be able to receive reports of suspicious cases of sporotrichosis for an early diagnosis and to act in a timely manner to contain this zoonosis.
The first cases of sporotrichosis in Guarulhos City (SP) were reported in 2011. Since then, the Technical Division of the Zoonosis Control Center of Guarulhos City (CCZ/Guarulhos) carried out the following several actions to control the disease: (i) identification and treatment of animal cases, (ii) guidance and care for animal management by the owners and, (iii) implantation of compulsory notification (W. Mansho, personal communication).
In the city of Salvador (BA) with a recent history of a zoonotic outbreak of sporotrichosis associated with cats, some actions for the disease control were implemented:
After receiving a notification, a technical team from the Center for Zoonoses Control (CZC), with the presence of a veterinarian, conducted an epidemiological and clinical investigation.
The laboratory diagnosis was a partnership with the Laboratory of Mycological Research of the Federal University of Bahia, where confirmatory cytopathological tests and fungal culture were carried out.
After the case was confirmed for sporotrichosis, a CZC team consisting of agents to combat endemic diseases (ACE) conducted home visits to identify new cases; instruct residents on sporotrichosis and responsible ownership; and publicize the CZC contacts.
Residents were informed about the benign evolution of sporotrichosis in both animals and humans once the correct treatment was provided, free of charge, encouraging suspect cases to be reported immediately to the public health service.
The preliminary analysis of the benefits due to these interventions indicated a reduction in the casuistry, especially when there is a joint action of the public health surveillance team with the family health team of the primary health care unit. These results are an encouragement for sporotrichosis to be included in the national list of compulsory notification, as well as to institutionalize the drug supply for the free treatment of humans and animals of low-income populations. Laboratory support is necessary for the confirmation of human and animal case. The Central Public Health Laboratory should be prepared to assist the municipalities that request it. In addition, interinstitutional partnerships should be encouraged involving the academia, research institutions following the One Health approach model advocated by the WHO, FAO, and OIE (2019) [133].
Technology and innovation are important for the development of new solutions which may improve this complex network and may help for the timely appropriate management of infectious diseases.
One Health
Whereas S. brasiliensis can be transmitted from animals to humans, surveillance should include the environment, in addition to people and animals, as advocated in the One Health approach [18, 134]. One Health is a collaborative, multisectoral, and multidisciplinary approach that recognizes the connection between people, animals, plants, and their shared environment. This is a challenge for human, animal, and environmental health experts to work together to achieve the best health outcomes for all [133]. From the One Health perspective, an integrated approach model for sporotrichosis prevention is presented (Fig. 8).
Fig. 8.
The One Health approach for prevention and control of sporotrichosis
The prevention and control policies to constraints the expansion of sporotrichosis cannot be effectively implemented and enforced by only one sector. A joint effort in this direction is necessary and urgent.
Notification of sporotrichosis
Up to now, sporotrichosis is a notifiable disease only in Brazil, and its incidence is based on previous studies in the literature.
In Brazil, compulsory notification is only made in the states of Rio de Janeiro and Pernambuco, in addition to the municipalities of Guarulhos (state of São Paulo), Conselheiro Lafaiete and Belo Horizonte (state of Minas Gerais), Salvador and Camaçari (state of Bahia), Natal (state of Rio Grande do Norte), and João Pessoa (state of Paraíba). Even in regions where the compulsory notification is not performed, veterinarians should notify the local Department of Health when animal sporotrichosis cases occur.
Rio de Janeiro was the first state where human and animal sporotrichosis have become a notifiable disease (2013 and 2014, respectively). Since then, all suspected human and animal sporotrichosis cases should be recorded in the Notification of Injury Information System according to the guidelines and routines established by the State Health Department of Rio de Janeiro.
In the city of Rio de Janeiro, a form for notification of feline and canine sporotrichosis cases was created in 2018, with the purpose of facilitating its filling by professionals, which is available on the website of the city of Rio de Janeiro. Notifications may be made by email: http://formsus.datasus.gov.br/site/formulario.php?id_aplicacao=34080. In case of impossibility to complete and send the form, it is important to communicate, even by telephone, the data that will make the investigation of the possible case feasible, such as the number of affected animals, address, and other basic data. When notification is compulsory, non-compliance is considered a serious federal sanitary infraction of the Code of Ethics of the exercise of veterinary medicine, subjecting the professional to penalties such as suspension of professional practice.
In order to facilitate notifications to animal health agencies, a mobile application (NOTVET) has recently been created. It can be used by any citizen who has knowledge of cases of animal diseases [135]. However, the creation of specific forms for sporotrichosis and mobile applications does not dispense campaigns to sensitize veterinarians about the importance of notification of sporotrichosis.
Making sporotrichosis a notifiable disease, it could increase awareness and potentially decrease the rate of zoonotic sporotrichosis. Thus, the notification should be mandatory in other states in Brazil, since this information could be used to investigate cases that could prevent the spread of infection and further cases, to assess the effects of public health interventions, as well as, to facilitate the early identification of outbreaks.
Vaccine candidates and perspectives
A specific and protective humoral response against Sporothrix spp. has already been observed [136, 137]. In recent years, potential antigenic targets for vaccine have been studied. It is known that the cell wall of this fungus is an important source of antigens for the development of vaccines. Recently, a study using monoclonal antibody against gp70 (mAbP6E7) in the treatment of sporotrichosis has shown promising results [138]. A vaccine based on peptides of a GP70 antigen showed protection against S. globosa [139] and S. brasiliensis [140]. Another study demonstrated the protective capacity of a vaccine based on recombinant enolase (rSsEno) and Montanide™ Pet Gel A (PGA) as an adjuvant against S. schenckii [141]. However, despite all efforts, there is no immunoprophylaxis or immunotherapy yet available against Sporothrix spp. infection either in cats or in humans.
Acknowledgments
The authors are grateful to Luisa Helena Monteiro de Miranda, Anna Barreto Fernandes Figueiredo, Manoel Marques Evangelista de Oliveira, Jéssica Sepulveda Boechat, Isabela Maria da Silva Antonio, and Maria Lopes Corrêa (Laboratório de Pesquisa Clínica em Dermatozoonoses em Animais Domésticos, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brazil); Andréa Salvador de Almeida (Centro de Controle de Zoonozes, Salvador, BA, Brazil); Romeika Karla dos Reis Lima; and Kamylla Moura Gadêlha (Canis & Catus Especialidades, Clínica Veterinária, Natal, RN, Brazil) for the technical support. SAP was supported in part by Jovem Cientista do Nosso Estado 2019 (JCNE)–FAPERJ (Grant No. E-26/202.737/2019) and has a productivity fellowship from “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).”
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lopes-Bezerra LM, Mora-Montes HM, Zhang Y, Nino-Vega G, Rodrigues AM, de Camargo ZP, de Hoog S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol. 2018;56:126–143. doi: 10.1093/mmy/myx103. [DOI] [PubMed] [Google Scholar]
- 2.Gremião IDF, Oliveira MME, Miranda LHM, Freitas DFS, Pereira SA. Geographic expansion of sporotrichosis, Brazil. Emerg Infect Dis. 2020;26:621–624. doi: 10.3201/eid2603.190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte JMG, Acosta VRW, Viera PMLF, Caballero AA, Matiauda GAG, Oddone VBR, Brunelli JGP. Esporotricosis trasmitida por gato doméstico. Reporte de un caso familiar. Rev Nac. 2017;9:67–76. [Google Scholar]
- 4.Córdoba S, Isla G, Szusz W, Vivot W, Hevia A, Davel G, Canteros CE. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Myc. 2018;61:441–448. doi: 10.1111/myc.12760. [DOI] [PubMed] [Google Scholar]
- 5.Etchecopaz AN, Lanza N, Toscanini MA, Devoto TB, Pola SJ, Daneri GL, Iovannitti CA, Cuestas ML. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J Mycol Med. 2019;30:100908. doi: 10.1016/j.mycmed.2019.100908. [DOI] [PubMed] [Google Scholar]
- 6.Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Beer ZW, Duong TA, Wingfield MJ. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Study Mycol. 2016;83:165. doi: 10.1016/j.simyco.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenck B. On refractory subcutaneous abscesses caused by a fungus possibly related to sporotrichia. John Hopkins Hosp. 1898;9:286–290. [Google Scholar]
- 9.Lutz A, Splendore A. Sobre uma micose observada em homens e ratos. Rev Med São Paulo. 1907;21:433–450. [Google Scholar]
- 10.Singer JI, Muncie JE. Sporotrichosis; etiologic considerations and report of additional cases from New York. New York State J Med. 1952;52:2147–2153. [PubMed] [Google Scholar]
- 11.Almeida F, Sampaio SAP, Lacaz CS, Fernandes JC. Statistical data on sporotrichosis; analysis of 344 cases [article in portuguese] An Bras Derm Sifilogr. 1955;30:9–12. [PubMed] [Google Scholar]
- 12.Larsson CE, Gonçalves MA, Araújo VC, Dagli MLZ, Correa B, Neto CF. Feline sporotrichosis: clinical and zoonotic aspects. Rev Inst Med Trop S Paulo. 1989;31:351–358. doi: 10.1590/s0036-46651989000500010. [DOI] [PubMed] [Google Scholar]
- 13.Marques SA, Franco SR, de Camargo RM, Dias LD, Haddad Junior V, Fabris VE (1993) Sporotrichosis of the domestic cat (Felis catus): human transmission. Rev Inst Med Trop S Paulo 35:327-330 [PubMed]
- 14.De Beurmann L, Gougerot H. Les sporotrichoses. Paris: Librarie Félix Alcan; 1912. [Google Scholar]
- 15.Luriei HI. Sporotrichosis. In: Uehlinger E, Baker RGD, editors. The pathologic anatomy of mycoses human infection· with fungi actinomycetes and algae. Berlin-Heidelberg-New York: Springer-Verlag; 1971. pp. 614–675. [Google Scholar]
- 16.Barros MBL, Paes RA, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathogens. 2017;19:e1006077. doi: 10.1371/journal.ppat.1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues AM, De Hoog GS, De Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:e1005638. doi: 10.1371/journal.ppat.1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues A M, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP (2020) The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 10.1007/s11046-020-00425-0 [DOI] [PubMed]
- 21.Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, Feng P, Yang L, Chen M, Deng S, Li S, Liao W, Li R, Li F, Meis JF, Guarro J, Teixeira M, Al-Zahrani HS, Pires de Camargo Z, ZhangL, de Hoog GS (2015) Phylogeograpy and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia 35: 1-20 [DOI] [PMC free article] [PubMed]
- 22.Oliveira MM, Almeida-Paes R, Muniz MM, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia. 2011;172:257–267. doi: 10.1007/s11046-011-9437-3. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues AM, Teixeira MM, Hoog GS, Schubach TMP, Pereira SA, Fernandes GF, Bezerra LML, Felipe MS, Camargo ZP Santos PO. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montenegro H, Rodrigues AM, Dias MAG, Silva EA, Bernardi F, Camargo ZP (2014) Feline sporotrichosis due to Sporothrix brasiliensis: an emerging animal infection in São Paulo, Brazil. BMC Vet Res 10, 10(1) [DOI] [PMC free article] [PubMed]
- 25.Boechat JS, Oliveira MME, Almeida-Paes R, Gremião IDF, Machado ACS, Oliveira RVC, Figueiredo ABF, Rabello VBS, Silva KBL, Zancopé-Oliveira RM, Schubach TMP, Pereira SA. Feline sporotrichosis: associations between clinical-epidemiological profiles and phenotypic-genotypic characteristics of the etiological agents in the Rio de Janeiro epizootic area. Mem Inst Oswaldo Cruz. 2018;113:185–196. doi: 10.1590/0074-02760170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacerda Filho AM, Cavalcante CM, Da Silva AB, Inácio CP, de Lima-Neto RG, de Andrade MCL, Magalhães OMC, Dos Santos FAG, Neves RP. High-virulence cat-transmitted ocular sporotrichosis. Mycopathologia. 2019;184:547–549. doi: 10.1007/s11046-019-00347-6. [DOI] [PubMed] [Google Scholar]
- 27.Falcão EMM, de Lima Filho JB, Campos DP, Valle ACFD, Bastos FI, Gutierrez-Galhardo MC, Freitas DFS (2019) Hospitalizations and deaths related to sporotrichosis in Brazil (1992-2015) Cad Saude Publica 35:e00109218 [DOI] [PubMed]
- 28.Mialski R, De Almeida JN Jr, da Silva LH, Kono A, Pinheiro RL, Teixeira MJ, Gomes RR, de Queiroz-Telles F, Pinto FG, Benard G (2018) Chronic meningitis and hydrocephalus due to Sporothrix brasiliensis in immunocompetent adults: a challenging entity. Open Forum Infect Dis 28:ofy081 [DOI] [PMC free article] [PubMed]
- 29.Poester VR, Mattei AS, Madrid IM, Pereira JTB, Klafke GB, Sanchotene KO, Brandolt TM, Xavier MO. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health. 2018;65:815–821. doi: 10.1111/zph.12504. [DOI] [PubMed] [Google Scholar]
- 30.Da Rocha RFDB, Schubach TMP, Pereira SA, Dos Reis ÉG, Carvalho BW, Gremião IDF. Refractory feline sporotrichosis treated with itraconazole combined with potassium iodide. J Small Anim Pract. 2018;59:720–721. doi: 10.1111/jsap.12852. [DOI] [PubMed] [Google Scholar]
- 31.De Souza EW, Borba CM, Pereira SA, Gremião IDF, Langohr IM, Oliveira MME, De Oliveira RVC, Da Cunha CR, Zancopé-Oliveira RM, Miranda LHM, Menezes RC. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Sci Rep. 2018;8:9074. doi: 10.1038/s41598-018-27447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eyer-Silva WA, Rosa da Silva GA, Martins CJ. A challenging case of disseminated subcutaneous mycosis from inner Rio de Janeiro state, Brazil. Am J Trop Med Hyg. 2017;97:1280–1281. doi: 10.4269/ajtmh.17-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida K, de Castro RA, Borba Dos Santos LP, Quintella LP, Lopes-Bezerra LM, Rozental S. Amphotericin B, alone or followed by itraconazole therapy, is effective in the control of experimental disseminated sporotrichosis by Sporothrix brasiliensis. Med Mycol. 2015;53:34–41. doi: 10.1093/mmy/myu050. [DOI] [PubMed] [Google Scholar]
- 34.Fernández-Silva F, Capilla J, Mayayo E, Guarro J. Modest efficacy of voriconazole against murine infections by Sporothrix schenckii and lack of efficacy against Sporothrix brasiliensis. Mycoses. 2014;57:121–124. doi: 10.1111/myc.12112. [DOI] [PubMed] [Google Scholar]
- 35.Orofino-Costa R, Unterstell N, Carlos Gripp A, de Macedo PM, Brota A, Dias E, de Melo Teixeira M, Felipe MS, Bernardes-Engemann AR, Lopes-Bezerra LM. Pulmonary cavitation and skin lesions mimicking tuberculosis in a HIV negative patient caused by Sporothrix brasiliensis. Med Mycol Case Rep. 2013;2:65–71. doi: 10.1016/j.mmcr.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro RA, Kubitschek-Barreira PH, Teixeira PA, Sanches GF, Teixeira MM, Quintella LP, Almeida SR, Costa RO, Camargo ZP, Felipe MS, de Souza W, Lopes-Bezerra LM. Differences in cell morphometry, cell wall topography and gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS One. 2013;8:e75656. doi: 10.1371/journal.pone.0075656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes GF, dos Santos PO, Rodrigues AM, Sasaki AA, Burger E, de Camargo ZP. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence. 2013;4:241–249. doi: 10.4161/viru.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva-Vergara ML, de Camargo ZP, Silva PF, Abdalla MR, Sgarbieri RN, Rodrigues AM, dos Santos KC, Barata CH, Ferreira-Paim K. Disseminated Sporothrix brasiliensis infection with endocardial and ocular involvement in an HIV-infected patient. Am J Trop Med Hyg. 2012;86:477–480. doi: 10.4269/ajtmh.2012.11-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siew HH. The current status of feline sporotrichosis in Malaysia. Med Mycol J. 2017;58:E107–E113. doi: 10.3314/mmj.17.014. [DOI] [PubMed] [Google Scholar]
- 40.Kano R, Okubo M, Siew HH, Kamata H, Hasegawa A. Molecular typing of Sporothrix schenckii isolates from cats in Malaysia. Mycoses. 2015;58:220–224. doi: 10.1111/myc.12302. [DOI] [PubMed] [Google Scholar]
- 41.Duangkaew L, Yurayart C, Limsivilai O, Chen C, Kasorndorkbua C. Cutaneous sporotrichosis in a stray cat from Thailand. Med Mycol Case Rep. 2018;23:46–49. doi: 10.1016/j.mmcr.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makri N, Paterson GK, Gregge F, Urquhart C, Nuttall T. First case of cutaneous sporotrichosis (Sporothrix species) in a cat in the UK. J Feline Med Surg. 2020;6:2055116920906001. doi: 10.1177/2055116920906001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubach TM, Schubach A, Okamoto T, Barros M, Figueiredo FB, Cuzzi T, Fialho-Monteiro PC, Reis RS, Perez MA, Wanke B. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998–2001) J Am Vet Med Assoc. 2004;224:1623–1629. doi: 10.2460/javma.2004.224.1623. [DOI] [PubMed] [Google Scholar]
- 44.Pereira SA, Gremião ID, Kitada AA, Boechat JS, Viana PG, Schubach TM. The epidemiological scenario of feline sporotrichosis in Rio de Janeiro, state of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2014;47:392–393. doi: 10.1590/0037-8682-0092-2013. [DOI] [PubMed] [Google Scholar]
- 45.Sanchotene KO, Madrid IM, Klafke GB, Bergamashi M, Della Terra PP, Rodrigues AM, de Camargo ZP, Xavier MO. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–658. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- 46.Macêdo-Sales PA, Souto SRL, Destefani CA, Lucena RP, Machado RLD, Pinto MR, Rodrigues AM, Lopes-Bezerra LM, Rocha EM, Baptista ARS. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: a comparison between infected and non-infected populations. BMC Vet Res. 2018;14:1–10. doi: 10.1186/s12917-018-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han HS, Kano R, Chen C, Noli C. Comparison of two in vitro antifungal sensitivity tests and monitoring during therapy of Sporothrix schenckii sensu stricto in Malaysian cats. Vet Dermatol. 2017;28:156–e32. doi: 10.1111/vde.12417. [DOI] [PubMed] [Google Scholar]
- 48.Schubach TM, Schubach Ade O, Cuzzi-Maya T, Okamoto T, Reis RS, Monteiro PC, Gutierrez-Galhardo MC, Wanke B. Pathology of sporotrichosis in 10 cats in Rio de Janeiro. Vet Rec. 2003;152:172–175. doi: 10.1136/vr.152.6.172. [DOI] [PubMed] [Google Scholar]
- 49.Leme LR, Schubach TM, Santos IB, Figueiredo FB, Pereira SA, Reis RS, Mello MF, Ferreira AM, Quintella LP, Schubach AO. Mycological evaluation of bronchoalveolar lavage in cats with respiratory signs from Rio de Janeiro, Brazil. Mycoses. 2007;50:210–214. doi: 10.1111/j.1439-0507.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 50.Miranda LHM, Silva JN, Gremião IDF, Menezes RC, Almeida-Paes R, Dos Reis ÉG, de Oliveira RVC, de Araujo DSDA, Ferreiro L, Pereira SA. Monitoring fungal burden and viability of Sporothrix spp. in skin lesions of cats for predicting antifungal treatment response. J Fungi (Basel) 2018;4:1–11. doi: 10.3390/jof4030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubach TM, de Oliveira Schubach A, dos Reis RS, Cuzzi-Maya T, Blanco TC, Monteiro DF, Barros BM, Brustein R, Zancopé-Oliveira RM, Monteiro PCF, Wanke B (2002) Sporothrix schenckii isolated from domestic cats with and without sporotrichosis in Rio de Janeiro, Brazil. Mycopathologia 153:83-86 [DOI] [PubMed]
- 52.Souza LL, Nascente PS, Nobre MO, Meinerz ARM, Meireles MCA. Isolation of Sporothrix schenckii from the nails of healthy cats. Braz J Microbiol. 2006;37:372–374. [Google Scholar]
- 53.Schubach TM, Schubach A, Okamoto T, Barros MB, Figueiredo FB, Cuzzi T, Pereira SA, dos Santos IB, Paes RA, et al. Canine sporotrichosis in Rio de Janeiro, Brazil: clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998-2003) Med Mycol. 2006;44:87–92. doi: 10.1080/13693780500148186. [DOI] [PubMed] [Google Scholar]
- 54.Ramos ACMO, Oliveira IVPM, Reis-Lima RK, de Paula VV, Filgueira KD. Zoonotic transmission of canine sporotrichosis in northeastern Brazil. Acta Vet Bras. 2017;11:79–84. [Google Scholar]
- 55.Barros MBL, Schubach AO, do Valle AC, Galhardo MCG, Conceição-Silva F, Schubach TM, Reis RS, Wanke B, Marzochi KB, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 56.Teixeira MM, de Almeida LG, Kubitschek-Barreira P, Alves FL, Kioshima ES, Abadio AK, Fernandes L, Derengowski LS, Ferreira KS, et al. Comparative genomics of the major fungal agents of human and animal Sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genomics. 2014;15:943. doi: 10.1186/1471-2164-15-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freitas DC, Migliano MF, Neto LZ. Esporotricose - Observação de caso espontâneo em gato doméstico (F. catus) Rev Fac Med Vet Univ S Paulo. 1956;5:601–604. [Google Scholar]
- 58.Freitas DC, Moreno G, Saliba AM, Bottino JA, Mós EM. Esporotricose em cães e gatos. Rev Fac Med Vet S Paulo. 1965;7:381–387. [Google Scholar]
- 59.Cruz LCH, Rosa CAR, Baffa MC, Campos SG. Anais do 12° Congresso Brasileiro de Microbiologia e 9. São Paulo: Congresso Latino-Americano de Microbiologia; 1983. Isolamento do Sporothrix schenckii de gatos com lesões ulcerativas. [Google Scholar]
- 60.Nogueira RHG, Guedes RMC, Cassali GD, Gheller VA, Moreira YK. Relato de esporotricose felina (Sporothrix schenckii) com transmissão para o homem: aspectos clínicos, microbiológicos e anatomopatológicos [article in portuguese] Arq Bras Med Vet Zootec. 1995;47:43–51. [Google Scholar]
- 61.Baroni FA, Campos SG, Direito GM. Esporotricose em gato (descrição de um caso) [article in portuguese] Rev Bras Med Vet. 1998;20:25–27. [Google Scholar]
- 62.Viana PG, Gremião IDF, Figueiredo ABF, Miranda LHM, Antonio IMS, Figueiredo FB, Pereira SA (2015) Clinical and epidemiological aspects of the largest epidemic of sporotrichosis in dogs: 203 cases [2004-2014]. In: Proceeding of the 7th Trends in Medical Mycology. Mycoses. 58: (Suppl.4), p.145.
- 63.Araujo ML, Rodrigues AM, Fernandes GF, de Camargo ZP, de Hoog GS. Human sporotrichosis beyond the epidemic front reveals classical transmission types in Espírito Santo, Brazil. Mycoses. 2015;58:485–490. doi: 10.1111/myc.12346. [DOI] [PubMed] [Google Scholar]
- 64.Lecca LO, Paiva MT, de Oliveira CSF, Morais MHF, de Azevedo MI, Bastos CVE, Keller KM, Ecco R, Alves MRS, Pais GCT, Salvato LA, Xaulim GMD, Barbosa DS, Brandão ST, Soares DFM (2020) Associated factors and spatial patterns of the epidemic sporotrichosis in a high density human populated area: A cross-sectional study from 2016 to 2018. Prev Vet Med 176:104939. [DOI] [PubMed]
- 65.Oliveira DC, Lopes PG, Spader TB, Mahl CD, Tronco-Alves GR, Lara VM, Santurio JM, Alves SH. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047–3049. doi: 10.1128/JCM.00255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Da Silva E, Plahinsce CRS, Poleto APCM, Morikawa VM. Ações de vigilância da esporotricose felina em Curitiba [article in portuguese] Ação & informação. 2019;01:7–9. [Google Scholar]
- 67.Colodel MM, Jark PC, Ramos CJR, Martins VMV, Schneider AF, Pilati C. Cutaneous feline sporotrichosis in Santa Catarina, Brazil: cases report [article in portuguese] Vet Foco. 2009;7:18–27. [Google Scholar]
- 68.Nobre MO, Castro AP, Caetano D, Souza LL, Meireles MCA, Ferreiro L. Recurrence of sporotrichosis in cats with zoonotic involvement. Rev Iberoam Micol. 2001;18:137–140. [PubMed] [Google Scholar]
- 69.Nobre MO, Meireles MCA, Caetano DT, Faé F, Cordeiro JMC, Meireles RM, Appelt CE, Ferreiro L. Esporotricose zoonótica na região sul do Rio Grande do Sul (Brasil) e revisão da literatura brasileira [artigo em português] Rev Bras Ciên Vet. 2002;9:36–41. [Google Scholar]
- 70.Xavier MO, Nobre MO, Sampaio DP, Jr, Antunes TA, Nascente PS, Soria FBA, Meireles MCA. Esporotricose felina com envolvimento humano na cidade de Pelotas, RS, Brasil. Cien Rural. 2004;34:1961–1963. [Google Scholar]
- 71.Madrid IM, Mattei A, Martins A, Nobre M, Meireles M. Feline sporotrichosis in the southern region of Rio Grande do Sul, Brazil: clinical, zoonotic and therapeutic aspects. Zoonoses Public Health. 2010;57:151–154. doi: 10.1111/j.1863-2378.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 72.Madrid IM, Mattei AS, Fernandes CG, Nobre Mde O, Meireles MC. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265–273. doi: 10.1007/s11046-011-9509-4. [DOI] [PubMed] [Google Scholar]
- 73.Fernandes CGN, Moura ST, Dantas AFM, Fernandes MCSB. Feline Sporotrichosis - Clinical and Epidemiological Aspects: Case Reports (Cuiabá, Mato Grosso, Brazil) [article in portuguese] MedVep Rev Cient Med Vet Peq An. 2004;2:39–43. [Google Scholar]
- 74.Cordeiro FN, Bruno CB, Paula CD, Motta JO. Familial occurrence of zoonotic sporotrichosis. An Bras Dermatol. 2011;86:121–124. doi: 10.1590/s0365-05962011000700032. [DOI] [PubMed] [Google Scholar]
- 75.Filgueira KD. (2009) Esporotricose na espécie canina: Relato de um caso na cidade de Mossoró. RN [article in portuguese] Cienc Anim Bras. 2009;10:673–677. [Google Scholar]
- 76.Nunes GDL, Carneiro RS, Filgueira KD, Filgueira FGF, Fernandes THT. Esporotricose felina no município de Itaporanga, estado da Paraíba, Brasil: relato de um caso. Arq Cienc Vet Zool Unipar. 2011;14:157–161. [Google Scholar]
- 77.Araújo AKL, Leal CAS. Esporotricose felina no município de Bezerros, Agreste Pernambucano: relato de caso [article in Portuguese] PubVet. 2016;10:816–820. [Google Scholar]
- 78.Silva GM, Howes JCF, Leal CAS, Mesquita EP, Pedrosa PM, Oliveira AAF, Silva LBG, Mota RA. Surto de esporotricose felina na região metropolitana do Recife. Pesq Vet Bras. 2018;38:1767–1771. [Google Scholar]
- 79.Marques-Melo EH, Lessa DFS, Nunes ACBT, Chaves KP, Porto WJN, Notomi MK, Garrido LHA. Felino doméstico como agente transmissor de esporotricose para humano: relato de primeiro caso no estado de Alagoas [article in portuguese] Rev Baiana Saúde Pública. 2014;38:490–498. [Google Scholar]
- 80.Pereira SA, Gremião IDF, Menezes RC (2015) Sporotrichosis in Animals: Zoonotic Transmission. In: Carlos IZ (ed) Sporotrichosis. New Developments and Future Prospects, Springer, pp 83–102
- 81.Rosser E, Dunstan R. Sporotrichosis. In: Greene CE, editor. Infectious diseases of the dog and cat. 3. Philadelphia: Saunders Elsevier; 2006. pp. 608–612. [Google Scholar]
- 82.Gremião ID, Schubach TM, Pereira SA, Rodrigues AM, Chaves AR, Barros MB. Intralesional amphotericin B in a cat with refractory sporotrichosis. J Feline Med Surg. 2009;1:720–723. doi: 10.1016/j.jfms.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gremião ID, Schubach TM, Pereira SA, Rodrigues AM, Honse CO, Barros MB. Treatment of refractory feline sporotrichosis with a combination of intralesional amphotericin B and oral itraconazole. Aust Vet J. 2011;89:346–351. doi: 10.1111/j.1751-0813.2011.00804.x. [DOI] [PubMed] [Google Scholar]
- 84.Gremião IDF, Menezes RC, Schubach TMP, Figueiredo AB, Cavalcanti MC, Pereira SA. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15–21. doi: 10.1093/mmy/myu061. [DOI] [PubMed] [Google Scholar]
- 85.Pereira SA, Passos SR, Silva JN, Gremião ID, Figueiredo FB, Teixeira JL, Monteiro PCF, Schubach TMP. Response to azolic antifungal agents for treating feline sporotrichosis. Vet Rec. 2010;166:290–294. doi: 10.1136/vr.166.10.290. [DOI] [PubMed] [Google Scholar]
- 86.Werner AH, Werner BE. Sporotrichosis in man and animal. Int J Dermatol. 1994;33:692–700. doi: 10.1111/j.1365-4362.1994.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 87.Dunstan RW, Langham RF, Reinmann KA, Wakenell PS. Feline sporotrichosis: a report of five cases with transmission to humans. J Am Acad Dermatol. 1986;15:37–45. doi: 10.1016/s0190-9622(86)70139-4. [DOI] [PubMed] [Google Scholar]
- 88.Schubach TM, Schubach A, Okamoto T, Pellon IV, Fialho-Monteiro PC, Reis RS, Barros MBL, Andrade-Perez M, Wanke B. Haematogenous spread of Sporothrix schenckii in cats with naturally acquired sporotrichosis. J Small Anim Pract. 2003;44:395–398. doi: 10.1111/j.1748-5827.2003.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 89.Miranda LHM, Santiago MA, Schubach TMP, Morgado FN, Pereira SA, Oliveira RVC, Conceição-Silva F. Severe feline sporotrichosis associated with an increased population of CD8low cells and a decrease in CD4+ cells. Med Mycol. 2016;54:29–39. doi: 10.1093/mmy/myv079. [DOI] [PubMed] [Google Scholar]
- 90.Reis ÉG, Schubach TM, Pereira SA, Silva JN, Carvalho BW, Quintana MS, Gremião ID. Association of itraconazole and potassium iodide in the treatment of feline sporotrichosis: a prospective study. Med Mycol. 2016;54:684–690. doi: 10.1093/mmy/myw027. [DOI] [PubMed] [Google Scholar]
- 91.Miranda LHM, Meli M, Conceição-Silva F, Novacco M, Menezes RC, Pereira SA, Sugiarto S, Dos Reis ÉG, Gremião IDF, Hofmann-Lehmann R. Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis. PLoS One. 2018;13:e0207644. doi: 10.1371/journal.pone.0207644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schubach TM, Menezes RC, Wanke B. Sporotrichosis. In: Greene CE, editor. Infectious diseases of the dog and cat. 4. Philadelphia: Saunders Elsevier; 2012. pp. 645–650. [Google Scholar]
- 93.Lopes-Bezerra LM, Schubach A, Costa RO. Sporothrix schenckii and sporotrichosis. An Acad Bras Cienc. 2006;78:293–308. doi: 10.1590/s0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 94.Pereira SA, Menezes RC, Gremião ID, Silva JN, Honse CO, Figueiredo FB, da Silva DT, Kitada AAB, Reis EG, Schubach TM. Sensitivity of cytopathological examination in the diagnosis of feline sporotrichosis. J Feline Med Surg. 2011;13:220–223. doi: 10.1016/j.jfms.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silva JN, Passos SRL, Menezes RC, Gremião I, Schubach T, Oliveira J, Figueiredo A, Pereira S. Diagnostic accuracy assessment of cytopathological examination of feline sporotrichosis. Med Mycol. 2015;53:880–884. doi: 10.1093/mmy/myv038. [DOI] [PubMed] [Google Scholar]
- 96.Silva GM, Howes JCF, Leal CAS, Mesquita EP, Pedrosa CM, Oliveira AAF, Silva LBG, LBG MRA. Surto de esporotricose felina na região metropolitana do Recife [article in portuguese] Pesq Vet Bras. 2018;38:1767–1771. [Google Scholar]
- 97.Macêdo-Sales PA, Souto SRLSS, Destefani CA, Lucena RP, Rocha EMSR, Baptista ARS. Laboratory diagnosis of feline sporotrichosis in samples from Rio de Janeiro state, Brazil: imprint cytopathology limitations. [article in Portuguese] Rev Pan-Amaz Saúde. 2018;9:13–19. [Google Scholar]
- 98.Gonsales FF, Fernandes NCCA, Mansho W, Montenegro H, Guerra JM, de Araújo LJT, da Silva SMP, Benites NR. Feline Sporothrix spp. detection using cell blocks from brushings and fine-needle aspirates: performance and comparisons with culture and histopathology. Vet Clin Pathol. 2019;48:143–147. doi: 10.1111/vcp.12708. [DOI] [PubMed] [Google Scholar]
- 99.Carson FL, Hladick C. Histotechnology: a self-instructional text. 3. Chicago: ASCP Press; 2009. [Google Scholar]
- 100.Miranda LH, Conceição-Silva F, Quintella LP, Kuraiem BP, Pereira SA, Schubach TM. Feline sporotrichosis: histopathological profile of cutaneous lesions and their correlation with clinical presentation. Comp Immunol Microbiol Infect Dis. 2013;36:425–432. doi: 10.1016/j.cimid.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 101.Bernardes-Engemann AR, de Lima Barros M, Zeitune T, Russi DC, Orofino-Costa R, Lopes-Bezerra LM (2015) Validation of a serodiagnostic test for sporotrichosis: a follow-up study of patients related to the Rio de Janeiro zoonotic outbreak. Med Mycol:28–33 [DOI] [PubMed]
- 102.Orofino-Costa R, Macedo PM, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandes GF, Lopes-Bezerra LM, Bernardes-Engemann AR, Schubach TMP, Dias MAGD, Pereira SA, Camargo ZP. Serodiagnosis of sporotrichosis infection in cats by enzyme-linked immunosorbent assay using a specific antigen, SsCBF, and crude exoantigens. Vet Microbiol. 2010;147:445–449. doi: 10.1016/j.vetmic.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monti F (2019) Diagnostic and therapeutic approach to sporotrichosis in domestic cats (Felis catus domesticus) Thesis. Pontifícia Universidade Católica do Paraná (PUC-PR)
- 105.Baptista VS, Mothé GB, Santos GMP, CSI M, Santos TO, Virginio ED, Macêdo-Sales PA, Pinto MR, RLD M, EMS R, Lopes-Bezerra LM, ARS B Promising application of the SsCBF ELISA test to monitor the therapeutic response of feline sporotrichosis caused by Sporothrix brasiliensis from Brazilian epidemics. Braz. J. of Microbiol. 2020. 10.1007/s42770-020-00362-6. (ahead of print) [DOI] [PMC free article] [PubMed]
- 106.Kano R, Watanabe K, Murakami M, Yanai T, Hasegawa A (2005) Molecular diagnosis of feline sporotrichosis. Vet Rec 156:484–485 [DOI] [PubMed]
- 107.Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLOS Neg Trop Dis. 2015;9:1–22. doi: 10.1371/journal.pntd.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodrigues AM, de Hoog GS, de Camargo ZP. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn Microbiol Infect Dis. 2014;78:383–387. doi: 10.1016/j.diagmicrobio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Rodrigues AM, Najafzadeh MJ, de Hoog GS, de Camargo ZP. Rapid identification of emerging human-pathogenic Sporothrix species with rolling circle amplification. Front Microbiol. 2015;6:1–14. doi: 10.3389/fmicb.2015.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaves AR, Campos MP, Barros MBL, Carmo CN, Gremião IDF, S A Pereira SA, Schubach TPM. Treatment Abandonment in Feline Sporotrichosis – Study of 147 Cases. Zoonoses Public Health. 2013;60:149–153. doi: 10.1111/j.1863-2378.2012.01506.x. [DOI] [PubMed] [Google Scholar]
- 111.Brilhante RS, Silva NF, Marques FJ, Castelo-Branco DS, de Lima RA, Malaquias AD, Caetano EP, Barbosa GR, de Camargo ZP, et al. In vitro inhibitory activity of terpenic derivatives against clinical and environmental strains of the Sporothrix schenkii complex. Med Mycol. 2015;53:93–98. doi: 10.1093/mmy/myu085. [DOI] [PubMed] [Google Scholar]
- 112.Almeida-Paes R, Brito-Santos F, Figueiredo-Carvalho MHG, Machado ACS, Oliveira MME, Pereira SA, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz. 2017;112:376–381. doi: 10.1590/0074-02760160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, et al. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother. 2017;61:1–8. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rossi CN, Odaguiri J, Larsson CE. Retrospective assessment of the treatment of sporotrichosis in cats and dogs using itraconazole. Acta Sci Vet. 2013;41:1–5. [Google Scholar]
- 115.Crothers SL, White SD, Ihrke PJ, Affolter VK. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007) Vet Dermatol. 2009;20:249–259. doi: 10.1111/j.1365-3164.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 116.Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, Nucci M, Lopes-Bezerra LM, Ishida K, et al. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles and terbinafine. Med Mycol. 2015;53:178–188. doi: 10.1093/mmy/myu056. [DOI] [PubMed] [Google Scholar]
- 117.Mawby DI, Whittemore JC, Genger S, Papich MG. Bioequivalence of orally administered generic, compounded, and innovator-formulated itraconazole in healthy dogs. J Vet Intern Med. 2014;28:72–77. doi: 10.1111/jvim.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Renschler J, Albers A, Sinclair-Mackling H, Wheat LJ. Comparison of compounded, generic and innovator-formulated itraconazole in dogs and cats. J Am Anim Hosp Assoc. 2018;54:195–200. doi: 10.5326/JAAHA-MS-6591. [DOI] [PubMed] [Google Scholar]
- 119.Reis EG, Gremião IDF, Kitada AAB, Rocha RF, Castro VS, Barros MB, Menezes RC, Pereira SA, Schubach TM. Potassium iodide capsule in the treatment of feline sporotrichosis. J Fel Med Surg. 2012;14:399–404. doi: 10.1177/1098612X12441317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greene CE. Antifungal chemotherapy In: Greene CE (ed) Infectious diseases of the dog and cat 4rd edn. Philadelphia: Saunders Elsevier; 2012. pp. 579–588. [Google Scholar]
- 121.Francesconi G, Valle AC, Passos S, Reis R, Galhardo MC. Terbinafine (250 mg/day): effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273–1276. doi: 10.1111/j.1468-3083.2009.03306.x. [DOI] [PubMed] [Google Scholar]
- 122.Francesconi G, Francesconi do Valle AC, Passos SL, de Lima Barros MB, de Almeida Paes R, Curi AL, Liporage J, Porto CF, Galhardo MC. Comparative study of 250 mg/day terbinafine and 100 mg/day itraconazole for the treatment of cutaneous sporotrichosis. Mycopathologia. 2011;171:349–354. doi: 10.1007/s11046-010-9380-8. [DOI] [PubMed] [Google Scholar]
- 123.Viana PG, Figueiredo ABF, Gremião IDF, de Miranda LHM, da Silva Antonio IM, Boechat JS, de Sá Machado AC, de Oliveira MME, Pereira SA. Successful treatment of canine sporotrichosis with terbinafine: case reports and literature review. Mycopathologia. 2018;183:471–478. doi: 10.1007/s11046-017-0225-6. [DOI] [PubMed] [Google Scholar]
- 124.Honse CO, Rodrigues AM, Gremiao ID, Pereira SA, Schubach TM. Use of local hyperthermia to treat sporotrichosis in a cat. Vet Rec. 2010;13:208–209. doi: 10.1136/vr.b4768. [DOI] [PubMed] [Google Scholar]
- 125.Gremião IDF, Pereira SA, Rodrigues AM, Figueiredo FB, Nascimento A, Jr, Santos IB, Schubach TMP. Combination of surgical treatment and conventional antifungal therapy in feline sporotrichosis. Acta Sci Vet. 2006;34:221–223. [Google Scholar]
- 126.Hirano M, Watanabe K, Murakami M, Kano R, Yanai T, Yamazoe K, Fukata T, Kudo T. A case of feline sporotrichosis. J Vet Med Sci. 2006;68:283–284. doi: 10.1292/jvms.68.283. [DOI] [PubMed] [Google Scholar]
- 127.Souza CP, Lucas R, Ramadinha RH, Pires TB. Cryosurgery in association with itraconazole for the treatment of feline sporotrichosis. J Feline Med Surg. 2016;18:137–143. doi: 10.1177/1098612X15575777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Webster CR, Cooper J. Therapeutic use of cytoprotective agents in canine and feline hepatobiliary disease. Vet Clin North Am Small Anim Pract. 2009;39:631–652. doi: 10.1016/j.cvsm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 129.Avizeh R, Najafzadeh H, Razijalali M, Shirali S. Evaluation of prophylactic and therapeutic effects of silymarin and nacetylcysteine in acetaminophen-induced hepatotoxicity in cats. J Vet Pharmacol Ther. 2010;33:95–99. doi: 10.1111/j.1365-2885.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 130.Moudgal VV, Sobel JD. Antifungal drugs in pregnancy: a review. Expert Opin Drug Saf. 2003;2:475–483. doi: 10.1517/14740338.2.5.475. [DOI] [PubMed] [Google Scholar]
- 131.Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother. 2015;70:14–22. doi: 10.1093/jac/dku355. [DOI] [PubMed] [Google Scholar]
- 132.Silva DT, Menezes RC, Gremião ID, Schubach TM, Boechat JS, Pereira SA. Zoonotic sporotrichosis: biosafety procedures. Acta Sci Vet. 2012;40:1–10. [Google Scholar]
- 133.World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), World Organization for Animal Health (OIE) A Tripartite Guide to Addressing Zoonotic Diseases in Countries. 2019. Taking a multisectoral, one health approach. [Google Scholar]
- 134.Cabañes FJ. Sporotrichosis in Brazil: Animals+humans=one health. Rev Iberoam Micol. 2020;S1130-1406(20):30003–30006. doi: 10.1016/j.riam.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 135.Cruz SA, Moutinho BFF (2018) NOTVET: Aplicativo para notificações de enfermidades via telefone móvel e tablet. https://proceedings.science/saude-coletiva-2018/papers/notvet%2D%2Daplicativo-para-notificacoes-de-enfermidades-via-telefone-movel-e-tablet
- 136.Rodrigues AM, Fernandes GF, Araujo LM, Della Terra PP, dos Santos PO, Pereira SA, Schubach TMP, Burger E, Lopes-Bezerra LM, Camargo ZP. Proteomics-Based Characterization of the humoral immune response in sporotrichosis: toward discovery of potential diagnostic and vaccine antigens. PLoS Negl Trop Dis. 2015;9(8):e0004016. doi: 10.1371/journal.pntd.0004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nascimento RC, Espíndola NM, Castro RA, Teixeira PA, Loureiro y Penha CV, Lopes-Bezerra LM, Almeida SR. Passive immunization with monoclonal antibody against a 70-kDa putative adhesin of Sporothrix schenckii induces protection in murine sporotrichosis. Eur J Immunol. 2008;38(11):3080–3089. doi: 10.1002/eji.200838513. [DOI] [PubMed] [Google Scholar]
- 138.Almeida SR. Advances in vaccine development against sporotrichosis. Curr Trop Med Rep. 2019;6:126–131. doi: 10.1007/s40475-019-00183-0. [DOI] [Google Scholar]
- 139.de Almeida JRF, Jannuzzi GP, Kaihami GH, Breda LCD, Ferreira KS, de Almeida SR. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci Rep. 2018;8:4192. doi: 10.1038/s41598-018-22709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen F, Jiang R, Wang Y, Zhu M, Zhang X, Dong S, Shi H, Wang L. Recombinant phage elicits protective immune response against systemic S. globosa infection in mouse model. Sci Rep. 2017;7:42024. doi: 10.1038/srep42024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Portuondo DL, Dores-Silva PR, Ferreira LS. Immunization with recombinant enolase of Sporothrix spp. (rSsEno) confers effective protection against sporotrichosis in mice. Sci Rep. 2019;9:17179. doi: 10.1038/s41598-019-53135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]