Abstract
Aeromonas are bacteria broadly spread in the environment, particularly in aquatic habitats and can induce human infections. Several virulence factors have been described associated with bacterial pathogenicity, such as the Type VI Secretion System (T6SS). This system translocates effector proteins into target cells through a bacteriophage-like contractile structure encoded by tss genes. Here, a total of 446 Aeromonas genome sequences were screened for T6SS and the proteins subjected to in silico analysis. The T6SS-encoding locus was detected in 243 genomes and its genes are encoded in a cluster containing 13 core and 5 accessory genes, in highly conserved synteny. The amino acid residues identity of T6SS proteins ranges from 78 to 98.8%. In most strains, a pair of tssD and tssI is located upstream the cluster (tssD-2, tssI-2) and another pair was detected distant from the cluster (tssD-1, tssI-1). Significant variability was seen in TssI (VgrG) C-terminal region, which was sorted in four groups based on its sequence length and protein domains. TssI containing ADP-ribosyltransferase domain are associated exclusively with TssI-1, while genes coding proteins carrying DUF4123 (a conserved domain of unknown function) were observed downstream tssI-1 or tssI-2 and escort of possible effector proteins. Genes coding proteins containing DUF1910 and DUF1911 domains were located only downstream tssI-2 and might represent a pair of toxin/immunity proteins. Nearly all strains display downstream tssI-3, that codes for a lysozyme family domain protein. These data reveal that Aeromonas T6SS cluster synteny is conserved and the low identity observed for some genes might be due to species heterogeneity or its niche/functionality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-020-00405-y.
Keywords: Aeromonas, tss genes, Type VI Secretion System, Comparative genomic analysis
Importance
Aeromonas presents great relevance to human health due to its ability to induce infections and several virulence factors are known in these bacteria, including the Type VI Secretion System (T6SS). Moreover, this system is distributed in gram-negative bacteria and plays an important role in bacterial interactions, since it facilitates the colonization of infection sites by allowing more competitiveness of pathogenic bacteria related to remaining microbiota. In order to achieve that, the T6SS translocates effector proteins into target cells, dependent on a bacteriophage-like contractile structure designed by tss genes. In Aeromonas, this system was firstly described in A. hydrophila, and it is related to biofilm formation and evasion of the immune system in some strains.
Introduction
Currently, six major secretion systems have been identified in gram-negative bacteria, named Secretion System Types I to VI, which differs in their structural components, secreted substrates, delivered proteins (environment or into cells), and operating mechanism [1, 2].
The Type VI Secretion System (T6SS) is a puncturing device resembling a bacteriophage tail-like structure anchored in the bacterial cell envelope via a membrane-associated complex that translocates multiple lethal effector proteins directly into target cells (eukaryotic or bacterial) in a contact-dependent manner [3, 4].
Genomic studies revealed that T6SS genes are encoded in a cluster containing at least 13 highly conserved genes (tssA–M), which codify the structural components, and accessory genes, essential for proper assembly or regulation in different systems [3, 5]. Briefly, the T6SS structure is assembled in an orderly manner starting with the membrane complex, a trans-envelope structure composed by TssJ, TssL, and TssM proteins, allowing the assembling of the baseplate [3, 6]; and a cytoplasmic complex, composed by TssI, TssE, TssF, TssG, and TssK proteins. Once the baseplate is docked to the membrane complex, the contractile sheath formed by TssBC and the TssD inner tube will assemble forming the extended tail, with TssA requirement in this process [6]. It was attested, in Escherichia coli, that TssA plays a critical role as a regulator of T6SS tail biogenesis, priming and coordinating tail tube and sheath biogenesis [7, 8]. TssH induces the disassembly of the TssBC tubes after the “firing” process allowing recycling of the T6SS machinery [3].
The effectors’ translocation is dependent of the puncturing device set via the tube of TssD (Hcp) coated by a TssI (VgrG) spike which can be extended through a final tip of PAAR (proline-alanine-alanine-arginine) domain–containing protein [4, 9]. Different effector proteins can be translocated fused to or by noncovalent interaction with one of the components of the expelled tube [3].
Additionally, to this widespread and well-characterized proteobacterial T6SS, also designed T6SSi, two evolutionarily divergent subtypes of T6SS have been described. One, designated T6SSii, is found on the Francisella pathogenicity island, while the other, designed as T6SSiii, is restricted to the phylum Bacteroidetes; they have distinct components but a common overall mode of action when compared to the canonical T6SSi [10, 11].
T6SS was first linked to virulence, when it was simultaneously identified in Vibrio cholerae and Pseudomonas aeruginosa. T6SS was described in V. cholerae as a secretion apparatus transporting proteins lacking the N-terminal hydrophobic leader sequences, claimed for cytotoxicity toward amoeba Dictyostelium and J774 macrophages, to the cytosol of eukaryotic target cells by a contact-dependent mechanism [12]. In P. aeruginosa, the T6SS was associated with a virulence locus codifying a secretion device essential in chronic rat lung infection model and also for the secretion of Hcp-1 (TssD-1) protein during chronic infection of cystic fibrosis patients [13].
Later, antibacterial T6SS targeting competitor bacterial cells were reported, indicating that additionally to the role in virulence of anti-eukaryotic T6SS these secretion systems also participate in interbacterial interactions [14]. Currently, it is comprehended that T6SS are commonly found in environmental, commensal, and pathogenic bacteria from animals or plants [12–18]. Also, this system is involved in several bacterial activities besides virulence, like commensal or mutualistic relationships between bacteria and eukaryotes, or to mediate cooperative or competitive interactions between bacteria, action against microbial fungi, and scavenging of scarce metal ions [11, 19]. Exceptionally, more than one T6SS are observed in some bacteria [20, 21] and they shall mediate interactions with multiple hosts, predators, cooperators, or competitors [19].
Aeromonas relates to Gammaproteobacteria and consists of 36 species, including some recent reclassifications [22–24]. These bacteria are gram-negative rods widely disseminated in the environment, especially in aquatic habitats, and can also be isolated from fish, foods (including seafood, raw milk, meats, poultry, and vegetables), and several animals [24, 25]. Some species can cause a sort of infections in humans such as septicemia, wound infections, and intra-abdominal or respiratory infections, but they are most often associated with diarrhea [24–27]. They are also important pathogens of fish, causing important financial losses in the aquaculture industry [24].
The virulence of Aeromonas is multifactorial and numerous characteristics correlated with pathogenicity have been reported, such as pili [28], toxins like hemolysins [29], cytotonic and cytotoxic enterotoxins [30, 31], extracellular enzymes, S layer, polar and lateral flagella, and siderophores, among others [25, 26, 32]. Additionally, Secretion Systems Type II [33], Type III [34], and Type VI [15] have also been associated with Aeromonas virulence, since these systems are likely to be involved in the delivery of protein effectors to the extracellular environment or directly into target cells.
In Aeromonas, the first evidence of T6SS was discovered in A. hydrophila ATCC 7966 genome where homolog genes of V. cholerae T6SS were identified [35], supported by their description in A. dhakensis SSU [15]. A recent study [36] investigated the distribution of secretion systems in 55 A. hydrophila, including virulent strains associated with disease in catfish, strains associated with other fish disease, human clinical isolates, and environmental strains, from distinct geographical origins. It was shown that all of the evaluated A. hydrophila genomes encode either the entire T6SS operon or remnants of it, represented by hcp1 (tssD-1), tssH, and vgrG1 (tssI-1). Interestingly, the deletion of hcp1 and vgrG1 (tssI-1) in the strain ML09-119 affected its virulence significantly, indicating that these genes contribute to ML09-119 virulence in catfish despite the absence of a complete T6SS [36].
Considering the clinical and economical importance of Aeromonas, the vast number of genome sequences available, and the role of T6SS in virulence and bacterial interactions, we conducted an in silico comparative analysis of T6SS to determine its distribution and gene organization in the genus. Additionally, we also attempted to identify potential effector proteins by bioinformatic approach.
Results
Distribution of T6SS among Aeromonas
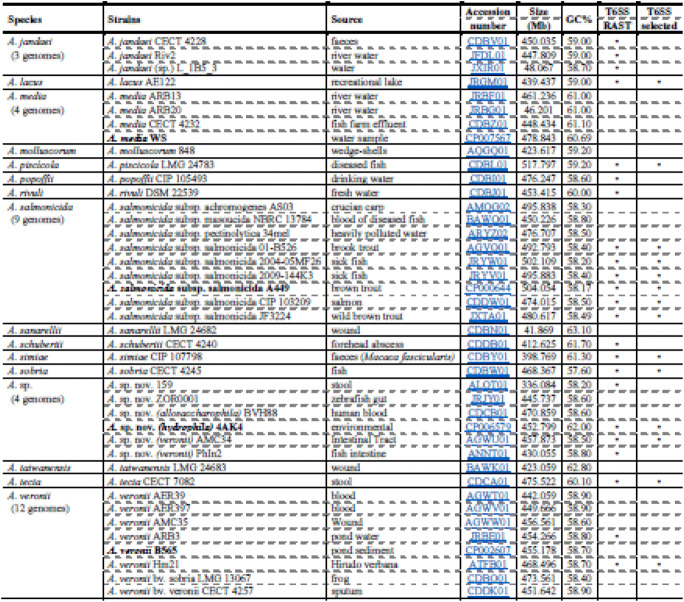
The Aeromonas genome sequences available in NBCI Genome database, release 210 (October 15, 2015), were analyzed for presence of T6SS. A total of 31 species (Table 1) including 114 genome sequences, 16 complete and 98 draft genomes, were found.
Table 1.
Distribution of Type VI Secretion System (T6SS) among Aeromonas spp. genome sequences (release 210)
Genome sequences available in NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome), release 210; in bold the Aeromonas strains with complete genomes. The “•” in the T6SS RAST column indicates strains where T6SS was identified by RAST annotation (https://rast.nmpdr.org); in T6SS selected column “•” indicate strains selected for sequence analysis. The strain previous identification is under parenthesis. The new identification is based on TYGS and [22]
The last version of NCBI Genome Database, release 238, comprehends 446 genomes of 32 species. Two-hundred forty-three genomes have the T6SS but 146 were used for comparison analysis (for complete results, see Supplementary Table I).
We used the Type (Strain) Genome Server to verify the identification of these species. Results (Table 2) indicate that 11 strains were misidentified, 4 strains previously considered Aeromonas sp. were identified at species level, and 6 strains considered potential new species; in total 31 species were represented (Table 1).
Table 2.
Updated identification of Aeromonas by Type (Strain) Genome Server (TYGS)
| Aeromonas strain | Conclusion | Identification result |
|---|---|---|
| Aeromonas sp. HZM | Belongs to known species | Aeromonas caviae |
| Aeromonas sp. ZOR0002 | Belongs to known species | Aeromonas caviae |
| Aeromonas veronii CIP 107763 | Belongs to known species | Aeromonas culicicola |
| Aeromonas enteropelogenes LK14 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila 116 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila 14 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila 145 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila 187 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila 259 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila L14f | Belongs to known species | Aeromonas dhakensis |
| Aeromonas hydrophila YL17 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas jandaei L14h | Belongs to known species | Aeromonas dhakensis |
| Aeromonas sp. MDS8 | Belongs to known species | Aeromonas dhakensis |
| Aeromonas veronii CECT 4486 | Belongs to known species | Aeromonas ichthiosmia |
| Aeromonas sp. L_1B5_3 | Belongs to known species | Aeromonas jandaei |
| Aeromonas allosaccharophila BVH88 | Potential new species | |
| Aeromonas hydrophila 4AK4 | Potential new species | |
| Aeromonas sp. 159 | Potential new species | * |
| Aeromonas sp. ZOR0001 | Potential new species | * |
| Aeromonas veronii AMC34 | Potential new species | * |
| Aeromonas veronii PhIn2 | Potential new species | * |
Analysis of annotated sequences indicated the presence of T6SS coding genes in 22 species, including 71 genomes (62.3%), of which 9 were complete and 62 draft genomes (Table 1). Thirty-one draft genome sequences were excluded from analyses due to absence of one or more protein sequences of the T6SS main cluster.
The remaining 40 genomes covering 14 species including A. dhakensis (11), A. hydrophila (7), A. salmonicida (6), A. caviae (4), A. veronii (2), A. bestiarum, A. bivalvium, A. encheleia, A. eucrenophila, A. lacus, A. piscicola, A. simiae, A. sobria, and A. tecta, one each; and additionally six strains, which represents potential new Aeromonas species (Table 2) [22], were used in further comparative analyses.
Gene content and organization of T6SS cluster of Aeromonas
In Aeromonas, the T6SS-associated genes are organized in a cluster (Fig. 1) which contains 17 conserved genes. Thirteen genes (tssA to tssM) encode T6SS “core” proteins, and 5 are “accessory” genes coding for VasH, the sigma-54-dependent transcriptional regulator; the PAAR protein, part of the tip of the expelled spike; Fha, involved in post-translational regulation [3, 5]; a hypothetical protein containing ImpA_N and VasL superfamily domains; and a VasI homolog, both with unknown function.
Fig. 1.
T6SS gene cluster synteny in Aeromonas. On the left are indicated the names of strains analyzed; for draft genome sequences, the contig containing T6SS cluster is indicated within brackets; the T6SS gene names are shown at the top and homolog genes in Aeromonas genomes represented by squares at the correspondent column; the cluster structure is based on RAST annotation followed by manual curation of tssD, tssI, tssL, PAAR, and conserved hypothetical genes; only amino acid sequences containing phage late D protein (GPD) domain were considered TssI; T6SS-associated genes located outside the cluster were not considered. The number sign indicates gene coding for PAAR domain–containing protein
Nonetheless, the regions flanking those 17 genes are variable, containing elements such as tRNAs, transposases/integrases, putative transmembrane proteins, or hypothetical proteins, and a pair of tssD-2 and tssI-2 genes (tssDI-2) is located upstream to the cluster in 28 strains (70%) (Fig. 1). A gene coding for a conserved hypothetical protein was detected between tssDI-2 in A. eucrenophila CECT 4224, A. sp. nov. 4AK4, and A. tecta CECT 7082.
In five strains (A. dhakensis strains 14, 116, CECT 7289, LK14, and A. hydrophila NF-1), a second pair of tssDI-2 is also present in the upstream region. In A. dhakensis strains LK14 and 116, a gene coding for a PAAR protein was found between this second pair of tssDI-2.
In A. salmonicida, there are some deletions/insertions or potential frameshifts affecting tssA, tssM, and tssC. Additionally, all A. salmonicida strains lack tssD-2 and tssI-2 upstream T6SS gene cluster (Fig. 1). These alterations observed in the sequence analyses (deletions/insertions and frameshifts) suggest that T6SS may not be functional in this species, which can only be proven experimentally. Potential frameshifts are also found in nucleotide sequences of fha and tssI-2 genes of A. caviae FDAARGOS 72, according to RAST.
For six strains with draft genome sequences, the T6SS cluster is located at contig ends, and it was not possible to determine the presence of additional pairs of tssD and tssI (tssDI). Finally, in 7 strains, a second pair of tssI-1 and tssD-1 was also observed in a genome region distant from the T6SS cluster (determined from complete genome sequences, as indicated in Table 3).
Table 3.
Nucleotide distance of tssI–tssD copies to T6SS cluster genes in Aeromonas complete genomes
The A. hydrophila strains AH10 and NJ-35 present two tssDI-1 pairs, then the distance A was calculated from the pair closest to RhlR coding gene. Data suggest that these distances, A and B, vary according to species (Table 3). For A. hydrophila, both distances are similar among the strains, except for strain 4AK4, previously considered A. hydrophila but that represents a potential new Aeromonas species as indicated by multilocus phylogenetic analysis and pairwise average nucleotide identity [22] and by TYGS analysis as well (Table 2).
In A. caviae, the distance A (tssI-1 to tssD-2) is twice that observed for A. hydrophila, while the distance B (tssI-2 to cluster) is variable, indicating diversity among strains. Strain 4AK4 presents characteristics of both species (Table 3) concerning these distances.
The identity of protein sequence of the T6SS cluster
The identity percentage (%ident.) of amino acid sequences indicated that T6SS components are conserved among Aeromonas species (Fig. 2).
Fig. 2.
Boxplot of identities (%) in the TSS6 proteins. The boxplot represents the identity percentage median of the amino acid sequences of the T6SS components. The number sign indicates gene coding for PAAR domain–containing protein
The identity percentage median ranged from 78.0 to 98.8%, and lower identity values were observed for TssI (unclassified), VasI, TssA, and Fha (Fig. 3). The TssI identity results were intriguing, and further investigation of protein domains showed a high diversity at C-terminal portion of these protein sequences. Thus, these proteins were deeply investigated (Fig. 3).
Fig. 3.
Conservation in amino acid sequences of T6SS components. Numbers below gene boxes are amino acid identity percentages from a multiple sequence alignment including all homologs of each protein. hypot. = hypothetical protein. The number sign indicates gene coding for PAAR domain–containing protein
The analysis with the updated genome dataset demonstrated that T6SS genes content and organization in the additional strains follows the same pattern as predicted in the release 210 analysis, adding no new information to our model and can be classified within the previous patterns (Supplementary Table IV).
The lowest results were found in a second copy of TssI-2 (A. hydrophila PAQ091014-1, PAQ091014-21, NF1) and in TssI-1 (A. bestiarum GA97-22; A. hydrophila 2014-10509-28-27, AHNIH1, MGYG-HGUT-02526, PAQ091014-12, PAQ091014-9, TN-97-08, AH10; A. salmonicida 3012STDY7122732; A. veronii AK241, AMC 25, BVH37, BVH46, Hm22, ML09-123, MS 17-88, X12) proteins. These proteins are highly variable in Aeromonas as shown in our previously submitted study.
The lowest query cover was a second copy of A. hydrophila TN-97-08 TssI-1 (55%) and the lowest identity was also a second copy of A. hydrophila AHNIH1 and A. hydrophila MGYG-HGUT-02526 (64.37%). The remaining T6SS proteins were highly conserved with the lowest identity found for A. allosaccharophila TTU2014-159ASC (74.13%) when A. dhakensis SSU was used as reference.
These results demonstrated that our model and classification generated using the original dataset was enough to cover all existing patterns in Aeromonas T6SS.
TssI comparative analysis
The comparative analysis of the Aeromonas TssI amino acid sequences indicates at least four distinct groups, designated from A to D, based on its conserved domains and size (Fig. 4). The length of TssI sequences range from 215 to about 930 amino acids, and the B and C groups range from 677 to 785 and 617 to 683 amino acids, respectively (Fig. 4). This variability is mainly associated with the presence/absence of family domains in the C-terminal region, since the N-terminal region is conserved and contains a VI_Rhs_Vgr domain.
Fig. 4.

Classification and distribution of T6SS structural protein TssI among Aeromonas strains. The VI_Rhs_Vgr domain includes the phage late gene D protein (GPD) domain; TssI was classified through group A to D according to amino acids sequence, length, and presence of protein family domains; the number of sequences of each TssI type according to tssI genomic location is also indicated. Seven draft genomes (A. bivalvium CECT 7113, A. caviae FDAARGOS_72, A. hydrophila 187, A. simiae CIP 107798, A. sobria CECT 4245, A. veronii AMC34, A. veronii Hm21) were not considered for the TssI distribution analysis since it was not possible to identify sequences of TssI-1 and TssI-2
TssI group A evolved an ADP-ribosyltransferase domain (TssI/ADRT) associated with the anti-eukaryotic activity [37]. This domain was observed in 11 strains belonging to five species and detected exclusively with TssI-1.
TssI group B contains a domain of unknown function (TssI/DUF2345) near the C-terminal region; this domain is present virtually in all TssI-3, is very common among TssI-2, and uncommon in TssI-1 (Fig. 4). It was suggested that this domain plays a role in virulence promoting adhesion to host cell [5]; however, it may be involved in the anchorage of TssI to the effector proteins, modulating the function of TssI as structural component of T6SS and the transport of effector proteins [38].
In group C TssI proteins, we identified no conserved domain (TssI/NID) at the C-terminal region in TssI-1, 2, and 3 (Fig. 4). Finally, TssI group D presents a VI_Rhs_Vgr incomplete domain, fused with an InsA element, as already described for strain A. salmonicida A449 [39] and it is associated with TssI-3 of all A. salmonicida (TssI/AS).
When the co-occurrence of TssI copies into each strain was analyzed, it was observed that it varies, with a clear predominance of 2 copies per strain. The most frequent combinations were [TssI-1/ADRT][TssI-2/DUF2345][TssI-3/DUF2345] and [TssI-1/NID][TssI-2/DUF2345][TssI-3/DUF2345]. Presence of only one TssI copy was observed only in A. lacus (TssI-1,2,3/NID). In strains with two or more TssI-1 (A. dhakensis CIP 107500; A. hydrophila strains 259, 277, AH-10, NJ-35, KOR1, and A. piscicola LMG 24783 which contains 4 tssI-1 copies) or two TssI-2 (Fig. 1), both homologs are conserved, as in A. hydrophila AH-10, or one may be a representative of another TssI type like in A. hydrophila NJ-35.
tssDI genetic neighborhood
Functional classification and domain search were conducted in amino acid sequences of coding regions flanking tssD and tssI genes in order to identify possible effector proteins (Fig. 5). For complete analysis, see Supplementary Table V.
Fig. 5.
TssI paralog copies of coding sequences and its genomic neighborhood. The first column represents each TssI paralog–encoding gene, regarding its genomic position to the main cluster; the second column show protein-coding genes found upstream tssD; the third column indicates the TssI-coding gene group classification (from A to D) according to amino acids sequence, length, and presence of protein family domains; the fourth column represents the protein families codified downstream of tssI
Analysis of regions flanking the tssDI-1 pair revealed the presence of sequence coding for transcriptional regulator RhlR upstream of tssD-1 in most of the strains (30/40). Exceptions were observed for all A. salmonicida strains, in which those genes were absent, as well as A. bivalvium, A. lacus, A. simiae, and A. veronii Hm21, for which only draft genome sequences were making precise identification of genes impossible. In P. aeruginosa, RhlR is part of the quorum sensing (QS) regulatory system and regulates the expression of biofilm and other QS-dependent virulence factors [40–42]. Moreover, RhlR also participates in the expression of T6SS systems (H2- and H3-T6SS operons) in strain PAO1 [21]. However, the identity of Aeromonas and Pseudomonas RhlR was only 27%, but its possible role as a transcriptional regulator on the pair tssD-1/tssI-1 expression deserves to be further investigated.
On the other hand, in most strains (25/40), downstream tssI-1 were found genes coding for hypothetical proteins containing the DUF 4123 domain (Fig. 5). It was shown that proteins containing this domain are observed in T6SS gene clusters of numerous bacterial species, often co-occurring with tssD, tssI, or PAAR coding genes and found upstream of genes coding T6SS effectors. Proteins containing DUF 4123 were recognized as adaptors required for the translocation of specific effectors that act binding the effector and loading it onto the secretion apparatus, but are not secreted [43–45].
It was pointed out that in A. dhakensis SSU, a gene coding for protein containing DUF4123 is found downstream of tssI-1 and upstream of the genes coding for TseC, which carries a colicin domain and has antibacterial activity, and TsiC, which is the cognate immunity protein to TseC. In our analyses, BLASTp search revealed TseC homologs in 13 out of 15 species of Aeromonas analyzed with identity ranging from 80 to 98% (data not shown). However, for the cognate immunity protein, the identity was around 60%, and it was absent in some strains analyzed. In most of these strains, putative membrane protein and/or hypothetical proteins are detected following proteins with the DUF 4123 domain.
Interestingly, genes coding for proteins containing other conserved domain of unknown function, named DUF 1795, were observed in few strains where the protein with DUF 4123 domain was lacking. This domain was also correlated with proteins recognized as adaptors required for secretion of effectors in Serratia marcescens and P. aeruginosa [45].
Furthermore, genes coding for proteins containing DUF 2235 or DUF 2931 were detected downstream tssI-1, following hypothetical proteins or proteins with DUF 4123 (Fig. 5). Proteins containing DUF 2235 were associated with toxic activities, as Tox-AB hydrolase 1 (a toxin containing Rhs repeats that act on lipids [46]), lipases T6SS antibacterial effectors (described in Vibrio proteolyticus [20]), and associated with MIX (marker for type six effectors) domains (observed in several proteins with bactericidal and virulent activities) which were proposed to be T6SS effectors [47].
It is possible that in Aeromonas, the proteins containing DUF 2235 (putative lipase) and DUF 2931 may represent a pair of toxin/immunity proteins, as seen in V. proteolyticus in which genes coding for lipases are followed by putative immunity genes [20]. In E. coli, the Tle1 toxin, which possesses a DUF 2235, and presents phospholipase activities, is encoded with the following tli1 gene that encodes its cognate immunity protein, an outer membrane lipoprotein, that has a DUF 2931 [48].
Analysis of tssD-2 gene neighborhood revealed an upstream coding sequence for tRNA-Ser-GGA in most of the strains, although mobile elements may also be found in some strains. Downstream tssI-2, there are mainly genes coding for proteins with DUF 4123 or DUF 1910 and DUF 1911 domain (Fig. 5). Interestingly, the putative transmembrane protein–containing DUF 1910 and DUF 1911 domains were seen only related to TssI-2 group B (TssI-2/DUF 2345), while proteins with DUF 4123 domain are associated with TssI-1 and TssI-2, regardless the group. DUF 1910 and DUF 1911 domains were associated with immunity protein Imm-PA2201 which confers protection against effects of toxins Tox-REase-1 and Tox-AHH [46].
Additionally, genes coding for proteins containing these domains follows hypothetical proteins, suggesting they may represent pairs of effector/immunity which are usually located in tandem in genomes. As indicated above, proteins containing DUF 4123 domain are chaperones, or adaptors, required for effector delivery by binding it to TssI and are encoded upstream of their cognate effector genes [43, 44].
In A. dhakensis SSU, downstream tssI-2, there is a gene coding DUF 4123 domain–containing protein and a TC toxin. These toxins are virulence factors of many bacteria such as Yersinia pestis and Photorhabdus luminescens, an insect pathogen [43]. Homologs of the TC toxin were detected in 16 strains, of 9 distinct species (data not shown).
In upstream of all tssI-3, there is a gene coding for PAAR proteins (Fig. 1 and Fig. 5), on the other hand, in downstream tssI-3, there is a gene coding for hypothetical protein. Detailed analysis of these hypothetical proteins revealed they are diverse and contain domains for lysozyme-like superfamily (LysM), N-acetylmuramic alanine amidase, pesticin superfamily, and chitinase, which could represent possible T6SS effectors.
Mobile elements (DDE_Tnp_IS1 superfamily) were also seen in all A. salmonicida genome sequences analyzed. In a few strains, it was observed a gene coding DUF 4123 domain–containing proteins downstream to TssI-3 coding gene.
Discussion
The T6SS is a complex structure, present in many gram-negative bacteria, that resemble a bacteriophage tail anchored to the bacterial cell envelope. It delivers a range of protein effectors directly into the prokaryotic or eukaryotic target cell through contraction of a sheath-like structure that propels an inner tube terminated by a membrane-puncturing spike toward the target [3, 9]. The effector proteins can be incorporated into the spike complex fused or as independent proteins binding noncovalently to the T6SS components TssI, TssD, or PAAR [3, 49, 50]. TssD and TssI were the first proteins secreted by T6SS to be identified [12] and beyond their role as structural T6SS components, it was shown that they also have effector activities [12, 13, 15, 37, 51, 52].
The presence of T6SS in Aeromonas was first detected in A. hydrophila ATCC 7966 [35] and followed by the description of T6SS main cluster organization in A. hydrophila ATCC 7966 and A. dhakensis SSU, as well as the presence of three copies of TssI in ATCC 7966. It was shown the importance of VasH and TssK in the expression of T6SS and translocation of effectors, and that TssD is translocated and induces apoptosis in HeLa cells [15].
A proteomic analysis of culture supernatants of A. hydrophila indicated the existence of TssI-1 with its gene located outside the cluster. It was also shown that TssI-1 has a C-terminal extension with actin-ADP ribosylating activity and induces apoptosis in HeLa cells [37]. Next, it was revealed that TssD inhibited phagocytosis of SSU strains by macrophages, playing a role in modulating the innate immunity [52]. Followed by the identification of tssI-1 and tssD-1 outside of the T6SS cluster in the genome of A. dhakensis SSU and that TssD and TssI paralogs within and outside of the cluster had the distinct influence on bacterial physiology [51].
Recent studies with A. hydrophila strains NF1 and NF2, recovered from mixed infection leading to necrotizing fasciitis, provide evidence that T6SS mediates interaction between NF1 and NF2, shown both in vitro and in vivo [53, 54]. Although both strains present T6SS, only NF1 has the T6SS effector-immunity pair TseC/TsiC. It was shown that TseC was involved in contact-dependent killing of NF2 in vitro, as well as in its elimination at the intramuscular injection site in vivo during mixed infection, with overall reduced mouse mortality.
The contact-dependent killing of NF2 by NF1 was shown to be mediated by T6SS and its effector TseC, since deletion of tseC from NF1 significantly increased survival of NF2 in mixed NF1 and NF2 cultures in vitro, and also and the expression of tsiC in trans provided protection to NF2 against killing by NF1. In the in vivo assays, it was observed increased animal survival in groups with NF1 tseC mutants, which could be related to the overall reduced virulence of the T6SS mutants.
Additionally, it was shown that T6SS of NF1 modulates phagocytosis and enhances survival in macrophages, similar to the observed strain SSU [54]. Also, it was evidenced the T6SS presence in other Aeromonas, including A. dhakensis, A. salmonicida, and A. jandaei [55], and A. veronii [56].
In the present investigation, genomic analysis revealed that T6SS is spread in Aeromonas and was located in 22 species including clinical and environmental isolates. T6SS components and five accessory proteins are coded by a single cluster in Aeromonas (Fig. 1) with tssDI genes present in multiple copies, a part of the main cluster (Table 3) in the majority of the species/strains analyzed. Furthermore, two accessory components are proteins of unknown function, VasI and a hypothetical protein. The latter contain ImpA and VasL domains at N- and C-terminal regions, respectively.
An ImpA domain is also present at the N-terminal region of TssA homolog proteins of Aeromonas, enteroaggregative E. coli (EAEC), and P. aeruginosa. Nevertheless, EAEC and P. aeruginosa TssA are different at the C-terminal end and do not share functional properties [8]. EAEC TssA presents a VasJ domain (PF16989) at its C-terminal region [8], as verified in Aeromonas. Therefore, we hypothesize they share the same role as a critical component of the T6SS biogenesis.
A third class of TssA-like proteins, comprising a N-terminal ImpA domain with C-terminal extensions containing a hydrophobic region and a domain of unknown function (VasL, PF12486), was described and is probably an accessory component of the T6SS, named TagA (Type VI secretion accessory gene with ImpA domain) [8]. Apparently, this component represents the hypothetical protein detected in the present work for the Aeromonas T6SS analyzed.
Regions flanking the main cluster and the tssDI-1 genes vary among the genomes (Fig. 1 and Fig. 5). Variability was observed regarding the copy number of tssDI-1 gene pairs, which were absent in some strains (e.g., A. salmonicida A449), a single copy in most strains, two copies in few strains, or four copies in A. piscicola LMG2478 (Fig. 6). Interestingly, a possible transcriptional regulator–coding gene, rhlR, was often detected upstream of a tssDI-1 pair. It was suggested that the regulation of TssI-1 is independent of VasH since its coding gene location occurs outside the cluster, and the protein was obtained in the pellet fraction of act vasH mutants of strain SSU [37]; also further conclusive results are required to elucidate the role of RhlR.
Fig. 6.
Aeromonas T6SS gene cluster and associated protein-coding genes. The protein-coding genes organization is shown in Aeromonas spp. genomes as columns; for tss genes, the number of copies are indicated and the tssI group classification is represented as the color code:  type A,
type A,  type B,
type B,  type C, and
type C, and  type D; protein-coding genes upstream and downstream are also indicated, relative to tss cluster or tssDI copies. The number sign indicates gene coding for PAAR domain–containing protein
type D; protein-coding genes upstream and downstream are also indicated, relative to tss cluster or tssDI copies. The number sign indicates gene coding for PAAR domain–containing protein
In all A. salmonicida, four genes of the T6SS main cluster (tssC, tssA, tssM, and tssI-3) are disrupted, although most of them are draft genomes and no T6SS-associated genes were located in other contigs or chromosome regions (Fig. 1 and Fig. 5), though these results are in agreement with data of Reith et al. [39] which found 16 proteins showing high similarity to T6SS in A. salmonicida A449 genome. They noticed that the gene encoding TssM contains deletions and is fused to the upstream coding sequence in the operon. Also, a partial TssI is fused to a transposon subunit, although a complete tssI gene is encoded on pAsa4; tssD also encoded in the plasmid is interrupted by an insertion sequence [39]. Therefore, T6SS is unlikely to be functional in this species, what should be studied experimentally.
In general, the T6SS homologous proteins show a high level of identity (Fig. 3). However, a great diversity was observed for TssI for which three different copies and four different types were found (Fig. 4 and Fig. 5), with most of the variation occurring in the C-terminal region, and its role fused to effectors or as a carrier for distinct effectors (Fig. 4). It was indicated that TssI C-terminal region is associated with transport specificity and is required for binding with adaptor-effector complex [57].
The DUF 2345 domain is usually related to TssI-3, but also with TssI-2, and rarely with TssI-1; it was proposed that it might be involved in the anchorage of the TssI to the effector proteins [5, 38], since the genetic neighborhood is distinct for each TssI/DUF 2345 coding-pair genes, with lysozyme and LysM encoded downstream tssI-3.
Genes coding for hypothetical proteins followed by genes coding putative transmembrane proteins containing DUF 1910 and DUF 1911 domains after tssI-2, which may represent a pair toxin/immunity protein [46], each one could transport specific effectors which may play different roles. Moreover, proteins containing the DUF 2345 domain were associated with MIX motifs proposed to be polymorphic T6SS effectors. Many of the MIX-encoding genes occur with TssI in genome neighborhoods [47] and together with colicins, pyocins, proteins containing domains LysM and DUF 4132, among others [47].
Interestingly, both tssI-1 and tssI-2 genes are most frequently neighbored by genes coding for DUF 4123 domain–containing proteins, which are recognized as effector chaperones or adaptors [43, 44], and could connect possible effectors encoded downstream of them to the respective TssI transporter. Nevertheless, in the tssI-1 region, downstream genes coding for proteins containing DUF 4123 are essentially hypothetical, putative membrane proteins or proteins containing DUF 2235 or DUF 2931.
Lastly, TssI-1 may also present the TssI evolved type (TssI-1/ADRT) which has anti-eukaryotic activity [37]. In the genetic neighborhood of this, TssI type–coding gene are observed genes coding for DUF 4123 domain–containing hypothetical protein generally followed by proteins containing DUF 2235 or DUF 2931 domains, implying that other effectors may also be loaded to the transporter beyond the anti-eukaryotic fused to TssI-1 C-terminal region [38].
These data indicate that there are distinct TssI-1 and TssI-2 types (Fig. 5), and suggest that each one may play different functions, as also TssI-3, thus enabling greater variability in the number and types of effectors translocated by T6SS. No correlation was recognized between the TssI sequences and Aeromonas species or source of isolates.
In conclusion, this investigation brings new information on the Aeromonas T6SS (Fig. 6). The cluster is most similar to V. cholerae [58], T6SS genes are highly conserved synteny, and the amino acid identity is greater than 78%. In most strains, a pair of tssD-2 tssI-2 is located in the upstream region of the cluster commonly with variable elements. Other T6SS-related genes (tssD-1 and tssI-1) were located in another genome region.
At least four TssI types were recognized, one of which is probably nonfunctional, what should be checked with experimental studies, and associated with A. salmonicida. They are placed in a different genetic neighborhood with possible effectors or pair effector/immunity protein–coding genes located downstream them. The different TssI possibly have a distinct role and are a source of diversity of T6SS.
Methodology
Aeromonas genome sequences and T6SS retrieval
Complete and draft genome sequences of Aeromonas spp. were obtained in the public genome database at NCBI (National Center for Biotechnology Information – http://www.ncbi.nlm.nih.gov), [59]. The original genome sequence dataset, containing 114 genome sequences, was retrieved from database release 210 (October 2015) from where T6SS clusters and associated genes were identified and the coded proteins were retrieved and further used for comparative analysis, as described below.
An update in the dataset and some analyses was attempted using database release 238 (June 2020). In this updated version of the analysis, 332 new genome sequences were analyzed for the presence of T6SS and associated genes and for those genomes presenting the T6SS, all predicted proteins were extracted and analyzed in order to check its identity and classification in groups previously determined by former analyses using the original dataset.
The genome sequences were submitted to strain identification using Type (Strain) Genome Server (TYGS) version 222 [60]. Since some genomes were not automatically annotated, all genome sequences were subject to annotation using RAST system version 2.0, following the default parameters (RASTtk - for the current modular customizable production RAST pipeline and automatically fix errors - such as gene candidates overlapping RNAs, or genes embedded inside other genes) [61–63], even those already annotated, in order to standardize the annotation process and those presenting T6SS coding genes were selected for comparative analyses.
Additionally, a manual check for presence and absence of T6SS genes was performed using T6SS protein sequences extracted from the genome sequence of A. dhakensis SSU (accession numbers JH815591, DQ667172, and JX646703) against a predicted proteome database built for each target genome, using the similarity search algorithm BLASTp [64], (identity > 70%) to ensure the accuracy of the data. Since genes coding for TssD, TssL, and PAAR and a conserved hypothetical protein were not included in the T6SS RAST subsystem, these protein sequences were manually verified by BLASTp alignment against the reference proteins of A. dhakensis SSU.
The T6SS nomenclature described by Shalom [3, 65, 66] was adopted (Table 4) to assign different copies of tssD or tssI paralog genes, according to its relative genomic position to the main cluster. Genes were assigned to tssD-1/tssI-1 if placed far from the cluster, tssD-2/tssI-2 if detected upstream of the cluster, and tssI-3 if located at the end of the cluster [37].
Table 4.
Variation in nomenclature, orthologous groups, and function of the T6SS genes in Aeromonas
| COG ID | Gene namea | RAST | A. dhakensis SSUb | Functionc | |
|---|---|---|---|---|---|
| COG3516 | tssB | impB | HMPREF1171_00930 | Outer sheath | Core |
| COG3517 | tssC | impC | HMPREF1171_00931 | ||
| COG3518 | tssE | VCA0109 | HMPREF1171_00932 | Baseplate | |
| COG3519 | tssF | impG/vasA | HMPREF1171_00933 | ||
| COG3520 | tssG | impH/vasB | HMPREF1171_00934 | ||
| COG3522 | tssK | impJ/vasE | HMPREF1171_00937 | ||
| COG3521 | tssJ | vasD | HMPREF1171_00936 | Membrane complex | |
| COG3455 | *tssL | impK/vasF, opmA/motB | HMPREF1171_00938 | ||
| COG3523 | tssM | icmF | HMPREF1171_00943 | ||
| COG0542 | tssH | clpB | HMPREF1171_00939 | Sheath recycling | |
| COG3515 | tssA | impA | HMPREF1171_00942 | Tail biogenesis regulator | |
| COG3157 | *tssD | hcp | Inner tube | ||
| COG3501 | *tssI | vgrG |
HMPREF1171_00946 |
Spike complex § | |
| COG4104 | *#PAAR | Uropathogenic specific protein | HMPREF1171_00945 | Accessory | |
| COG3829 | vasH | sigma-54-dependent transcriptional regulator | HMPREF1171_00940 | Transcriptional regulator a | |
| COG3456 | fha | impI/vasC | HMPREF1171_00935 | Post-translational regulation | |
| COG3515 | – | Hypothetical protein with impA/vasL domains | HMPREF1171_00944 | Unknown | |
| NOG245466 | – | vasI | HMPREF1171_00941 | Unknown |
aGene names used in this work
*Genes manually annotated
§tssI also participates in the baseplate formation
#Gene coding for PAAR domain–containing protein
T6SS sequence analysis
Amino acid sequences of homologous proteins for each T6SS element were compared through a multiple sequence alignment conducted in MEGA X version 10.1.8 [67, 68], applying the MUSCLE algorithm and applying the default (gap open − 2.9; gap extend 0.00; hydrophobicity multiplier 1.20; cluster method UPGMA, min length 24) [69, 70].
The identity percentage of each protein was estimated by the formula %identity = (1 − p-distance) * 100. The range of the identity percentage of the strains was illustrated using a boxplot using the Stata Statistical Software: Release 15 [71].
Additionally, the amino acid sequences of tssD and tssI neighbor genes were extracted from genome sequences and submitted to protein conserved domain search at NCBI conserved domain database (CDD), employing the default parameters of batch web CD-search tool (e-value threshold: 0.01, search against database: CDD – 52910 PSSMs, maximum number of hits value: 500) [72].
Supplementary information
(PDF 1358 kb)
(PDF 103 kb)
(PDF 130 kb)
(PDF 2177 kb)
(XLSX 237 kb)
Acknowledgments
We thank CAPES/Ciências sem Fronteiras for scholarships.
Funding
This work was financially supported by Fundação Araucária, Brazilian Program of National Institutes of Science and Technology–INCT/Brazilian Research Council–CNPq/MCT, and CAPES.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hayes CS, Aoki SK, Low DA. Bacterial contact-dependent delivery systems. Annu Rev Genet. 2010;44:71–90. doi: 10.1146/annurev.genet.42.110807.091449. [DOI] [PubMed] [Google Scholar]
- 2.Tseng TT, Tyler BM, Setubal JC. Protein secretion systems in bacterial-host associations, and their description in the Gene Ontology. BMC Microbiol. 2009;9(Suppl 1):S2. doi: 10.1186/1471-2180-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cianfanelli FR, Monlezun L, Coulthurst SJ. Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 2016;24(1):51–62. doi: 10.1016/j.tim.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A. 2007;104(39):15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics. 2009;10:104. doi: 10.1186/1471-2164-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet YR, Zoued A, Boyer F, Douzi B, Cascales E. The Type VI Secretion TssEFGK-VgrG phage-like baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as assembly platform for tail tube/sheath polymerization. PLoS Genet. 2015;11(10):e1005545. doi: 10.1371/journal.pgen.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoued A, Durand E, Brunet YR, Spinelli S, Douzi B, Guzzo M, Flaugnatti N, Legrand P, Journet L, Fronzes R, Mignot T, Cambillau C, Cascales E. Priming and polymerization of a bacterial contractile tail structure. Nature. 2016;531(7592):59–63. doi: 10.1038/nature17182. [DOI] [PubMed] [Google Scholar]
- 8.Zoued A, Durand E, Santin YG, Journet L, Roussel A, Cambillau C et al (2017) TssA: the cap protein of the Type VI secretion system tail. Bioessays 39(10). 10.1002/bies.201600262 [DOI] [PubMed]
- 9.Cianfanelli FR, Alcoforado Diniz J, Guo M, De Cesare V, Trost M, Coulthurst SJ. VgrG and PAAR proteins define distinct versions of a functional type VI secretion system. PLoS Pathog. 2016;12(6):e1005735. doi: 10.1371/journal.ppat.1005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abby SS, Cury J, Guglielmini J, Néron B, Touchon M, Rocha EPC. Identification of protein secretion systems in bacterial genomes. Sci Rep. 2016;6:23080. doi: 10.1038/srep230801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulthurst S. The Type VI secretion system: a versatile bacterial weapon. Microbiology. 2019;165:503–515. doi: 10.1099/mic.0.000789. [DOI] [PubMed] [Google Scholar]
- 12.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006;103(5):1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312(5779):1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood RD, Singh P, Hsu F, Guvener T, Carl MA, Trinidad RR, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7(1):25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez G, Sierra JC, Sha J, Wang S, Erova TE, Fadl AA, et al. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2008;44(4):344–361. doi: 10.1016/j.micpath.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernal P, Llamas MA, Filloux A. Type VI secretion systems in plant-associated bacteria. Environ Microbiol. 2018;20(1):1–15. doi: 10.1111/1462-2920.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Zou Y, She P, Wu Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol Res. 2015;172:19–25. doi: 10.1016/j.micres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Sana TG, Lugo KA, Monack DM. T6SS: the bacterial “fight club” in the host gut. PLoS Pathog. 2017;13(6):e1006325. doi: 10.1371/journal.ppat.1006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jani AJ, Cotter PA. Type VI secretion: not just for pathogenesis anymore. Cell Host Microbe. 2010;8(1):2–6. doi: 10.1016/j.chom.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray A, Schwartz N, de Souza Santos M, Zhang J, Orth K, Salomon D. Type VI secretion system MIX-effectors carry both antibacterial and anti-eukaryotic activities. EMBO Rep. 2017;18(11):1978–1990. doi: 10.15252/embr.201744226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sana TG, Soscia C, Tonglet CM, Garvis S, Bleves S. Divergent control of two type VI secretion systems by RpoN in Pseudomonas aeruginosa. PLoS One. 2013;8(10):e76030. doi: 10.1371/journal.pone.0076030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaz-Hidalgo R, Hossain MJ, Liles MR, Figueras MJ. Strategies to avoid wrongly labelled genomes using as example the detected wrong taxonomic affiliation for Aeromonas genomes in the GenBank database. PLoS One. 2015;10(1):e0115813. doi: 10.1371/journal.pone.0115813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parte AC. LPSN - list of prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol. 2018;68(6):1825–1829. doi: 10.1099/ijsem.0.002786. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Bravo A, Figueras MJ. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020;8:129. doi: 10.3390/microorganisms8010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker JL, Shaw JG. Aeromonas spp. clinical microbiology and disease. J Inf Secur. 2011;62(2):109–118. doi: 10.1016/j.jinf.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Teunis P, Figueras MJ. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front Microbiol. 2016;7:1395. doi: 10.3389/fmicb.2016.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirov SM, Barnett TC, Pepe CM, Strom MS, Albert MJ. Investigation of the role of type IV Aeromonas pilus (Tap) in the pathogenesis of Aeromonas gastrointestinal infection. Infect Immun. 2000;68(7):4040–4048. doi: 10.1128/IAI.68.7.4040-4048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuzenroeder MW, Wong CY, Flower RL. Distribution of two hemolytic toxin genes in clinical and environmental isolates of Aeromonas spp.: correlation with virulence in a suckling mouse model. FEMS Microbiol Lett. 1999;174(1):131–136. doi: 10.1111/j.1574-6968.1999.tb13559.x. [DOI] [PubMed] [Google Scholar]
- 30.Chopra AK, Peterson JW, Xu XJ, Coppenhaver DH, Houston CW. Molecular and biochemical characterization of a heat-labile cytotonic enterotoxin from Aeromonas hydrophila. Microb Pathog. 1996;21(5):357–377. doi: 10.1006/mpat.1996.0068. [DOI] [PubMed] [Google Scholar]
- 31.Xu XJ, Ferguson MR, Popov VL, Houston CW, Peterson JW, Chopra AK. Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants. Infect Immun. 1998;66(8):3501–3509. doi: 10.1128/IAI.66.8.3501-3509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen-Ivey CR, Figueras MJ, McGarey D, Liles MR. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front Microbiol. 2016;7:1337. doi: 10.3389/fmicb.2016.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenzweig JA, Chopra AK. Modulation of host immune defenses by Aeromonas and Yersinia species: convergence on toxins secreted by various secretion systems. Front Cell Infect Microbiol. 2013;3:70. doi: 10.3389/fcimb.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilches S, Urgell C, Merino S, Chacon MR, Soler L, Castro-Escarpulli G, et al. Complete type III secretion system of a mesophilic Aeromonas hydrophila strain. Appl Environ Microbiol. 2004;70(11):6914–6919. doi: 10.1128/AEM.70.11.6914-6919.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seshadri R, Joseph SW, Chopra AK, Sha J, Shaw J, Graf J, et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: jack of all trades. J Bacteriol. 2006;188(23):8272–8282. doi: 10.1128/JB.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tekedar HC, Abdelhamed H, Kumru S, Blom J, Karsi A, Lawrence ML. Comparative genomics of Aeromonas hydrophila secretion systems and mutational analysis of hcp1 and vgrG1 genes from T6SS. Front Microbiol. 2019;9:3216. doi: 10.3389/fmicb.2018.03216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suarez G, Sierra JC, Erova TE, Sha J, Horneman AJ, Chopra AK. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J Bacteriol. 2010;192(1):155–168. doi: 10.1128/JB.01260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Maayer P, Venter SN, Kamber T, Duffy B, Coutinho TA, Smits TH. Comparative genomics of the Type VI secretion systems of Pantoea and Erwinia species reveals the presence of putative effector islands that may be translocated by the VgrG and Hcp proteins. BMC Genomics. 2011;12:576. doi: 10.1186/1471-2164-12-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reith ME, Singh RK, Curtis B, Boyd JM, Bouevitch A, Kimball J, et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics. 2008;9:427. doi: 10.1186/1471-2164-9-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouwer S, Pustelny C, Ritter C, Klinkert B, Narberhaus F, Haussler S. The PqsR and RhlR transcriptional regulators determine the level of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa by producing two different pqsABCDE mRNA isoforms. J Bacteriol. 2014;196(23):4163–4171. doi: 10.1128/JB.02000-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekimpe V, Deziel E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology. 2009;155(Pt 3):712–723. doi: 10.1099/mic.0.022764-0. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee S, Moustafa D, Smith CD, Goldberg JB, Bassler BL. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathog. 2017;13(7):e1006504. doi: 10.1371/journal.ppat.1006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang X, Moore R, Wilton M, Wong MJ, Lam L, Dong TG. Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc Natl Acad Sci U S A. 2015;112(29):9106–9111. doi: 10.1073/pnas.1505317112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unterweger D, Kostiuk B, Otjengerdes R, Wilton A, Diaz-Satizabal L, Pukatzki S. Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae. EMBO J. 2015;34(16):2198–2210. doi: 10.15252/embj.201591163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unterweger D, Kostiuk B, Pukatzki S. Adaptor proteins of type VI secretion system effectors. Trends Microbiol. 2017;25(1):8–10. doi: 10.1016/j.tim.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salomon D, Kinch LN, Trudgian DC, Guo X, Klimko JA, Grishin NV, et al. Marker for type VI secretion system effectors. Proc Natl Acad Sci U S A. 2014;111(25):9271–9276. doi: 10.1073/pnas.1406110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flaugnatti N, Le TT, Canaan S, Aschtgen MS, Nguyen VS, Blangy S, et al. A phospholipase A1 antibacterial Type VI secretion effector interacts directly with the C-terminal domain of the VgrG spike protein for delivery. Mol Microbiol. 2016;99(6):1099–1118. doi: 10.1111/mmi.13292. [DOI] [PubMed] [Google Scholar]
- 49.Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, Leiman PG. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature. 2013;500(7462):350–353. doi: 10.1038/nature12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, et al. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun. 2014;5:3549. doi: 10.1038/ncomms4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sha J, Rosenzweig JA, Kozlova EV, Wang S, Erova TE, Kirtley ML, et al. Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis. Microbiology. 2013;159(Pt 6):1120–1135. doi: 10.1099/mic.0.063495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suarez G, Sierra JC, Kirtley ML, Chopra AK. Role of Hcp, a type 6 secretion system effector, of Aeromonas hydrophila in modulating activation of host immune cells. Microbiology. 2010;156(Pt 12):3678–3688. doi: 10.1099/mic.0.041277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponnusamy D, Kozlova EV, Sha J, Erova TE, Azar SR, Fitts EC, Kirtley ML, Tiner BL, Andersson JA, Grim CJ, Isom RP, Hasan NA, Colwell RR, Chopra AK. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. PNAS. 2016;113:722–727. doi: 10.1073/pnas.1523817113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Bravo A, Kilgore PB, Andersson JA, Blears E, Figueras MJ, Hasan NA, Colwell RR, Sha J, Chopra AK. T6SS and ExoA of flesh-eating Aeromonas hydrophila in peritonitis and necrotizing fasciitis during mono- and polymicrobial infections. PNAS. 2019;116(48):24084–24092. doi: 10.1073/pnas.1914395116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grim CJ, Kozlova EV, Ponnusamy D, Fitts EC, Sha J, Kirtley ML, et al. Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl Environ Microbiol. 2014;80(14):4162–4183. doi: 10.1128/AEM.00486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tekedar HC, Kumru S, Blom J, Perkins AD, Griffin MJ, Abdelhamed H, Karsi A, Lawrence ML. Comparative genomics of Aeromonas veronii: identification of a pathotype impacting aquaculture globally. PLoS One. 2019;14(8):e0221018. doi: 10.1371/journal.pone.0221018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bondage DD, Lin JS, Ma LS, Kuo CH, Lai EM. VgrG C terminus confers the type VI effector transport specificity and is required for binding with PAAR and adaptor-effector complex. Proc Natl Acad Sci U S A. 2016;113(27):E3931–E3940. doi: 10.1073/pnas.1600428113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi A, Kostiuk B, Rogers A, Teschler J, Pukatzki S, Yildiz FH. Rules of engagement: the type VI secretion system in Vibrio cholerae. Trends Microbiol. 2017;25(4):267–279. doi: 10.1016/j.tim.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44(D1):D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome- based taxonomy. Nat Commun. 2019;10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, et al. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep. 2015;5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42(Database issue):D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 65.Coulthurst SJ. The Type VI secretion system - a widespread and versatile cell targeting system. Res Microbiol. 2013;164(6):640–654. doi: 10.1016/j.resmic.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153(Pt 8):2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stecher G, Tamura K, Kumar S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol Biol Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.StataCorp . Stata Statistical Software: Release 15. College Station: StataCorp LLC; 2017. [Google Scholar]
- 72.Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45(D1):D200–D2D3. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1358 kb)
(PDF 103 kb)
(PDF 130 kb)
(PDF 2177 kb)
(XLSX 237 kb)