Abstract
Due to the severity of infections caused by P. aeruginosa and the limitations in treatment, it is necessary to find new therapeutic alternatives. Thus, the use of silver nanoparticles (AgNPs) is a viable alternative because of their potential actions in the combat of microorganisms, showing efficacy against Gram-positive and Gram-negative bacteria, including multidrug-resistant microorganisms (MDR). In this sense, the aim of this work was to conduct a literature review related to the antibacterial and antibiofilm activity of AgNPs against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. The AgNPs are promising for future applications, which may match the clinical need for effective antibiotic therapy. The size of AgNPs is a crucial element to determine the therapeutic activity of nanoparticles, since smaller particles present a larger surface area of contact with the microorganism, affecting their vital functioning. AgNPs adhere to the cytoplasmic membrane and cell wall of microorganisms, causing disruption, penetrating the cell, interacting with cellular structures and biomolecules, and inducing the generation of reactive oxygen species and free radicals. Studies describe the antimicrobial activity of AgNPs at minimum inhibitory concentration (MIC) between 1 and 200 μg/mL against susceptible and MDR P. aeruginosa strains. These studies have also shown antibiofilm activity through disruption of biofilm structure, and oxidative stress, inhibiting biofilm growth at concentrations between 1 and 600 μg/mL of AgNPs. This study evidences the advance of AgNPs as an antibacterial and antibiofilm agent against Pseudomonas aeruginosa strains, demonstrating to be an extremely promising approach to the development of new antimicrobial systems.
Keywords: AgNPs, Antibacterial activity, Antibiofilm activity, Pseudomonas aeruginosa, Multidrug-resistant bacteria, Biofilm
Introduction
Bacterial infections represent one of the world’s major public health problems, mainly due to increased persistence of these infections, treatment failure, and consequently, high rates of morbidity and mortality. It is estimated that 14% of hospital admissions are due to bacterial infections [1]. This problem is characterized mainly by bacterial resistance, which refers to the ability of bacteria to survive even after exposure to antimicrobials, and can happen by mechanisms such as reduced cellular permeability, enzymatic inactivation, production of flow pump, and alteration of binding site, in addition to biofilm production, which offers greater stability and safety to the microorganism [2].
In particular, the bacterium Pseudomonas aeruginosa has been increasingly gaining resistance to various antimicrobials, such as piperacillin/tazobactam, carbapenems, fluoroquinolones, ceftazidime, aminoglycosides, and polymyxins, and are already present in almost all continents of the world [3–8].
P. aeruginosa is a γ-proteobacterium, Gram-negative, non-fermentative, responsible for infection of a wide variety of organisms, including plants, animals and humans. Because it is an opportunistic pathogen, it is responsible for causing bacteremia, otitis, soft tissue infection, urinary tract, and respiratory infections [9]. Immunocompromised patients with pulmonary infection or burns are considered the risk groups for their colonization. In addition, there is still the ability to colonize implanted medical devices such as catheter [10, 11]. Once infection is established, P. aeruginosa progresses to a growth mode characterized as biofilm, by the formation of an extracellular matrix composed by exopolysaccharides, proteins and extracellular DNA [12, 13].
Due to adaptation, and exposure to various antibiotics, some strains become multiresistant to the therapies currently employed, especially carbapenems, and also adapt to the biofilm condition [14]. Thus, it is necessary to develop new therapeutic approaches able to exert not only antibacterial activity, but also antibiofilm since the latter is considered a challenge for eradication [15, 16]. Therefore, the use of materials on a nanometric scale is a viable strategy for carrying biomolecules and drugs, as it provides advantages such as increased half-life time and systemic circulation time, greater contact between the compound and the pathogen, better bioavailability, and greater absorption, resulting in a better adherence to therapy and more efficient treatment [17, 18].
Among the nanosystems, silver nanoparticles (AgNPs) are shown to be a potential application, because they present relevant physicochemical characteristics necessary to combat microorganisms, such as stability, colloidal state, and good chemical interaction [19]. AgNPs are small reduced particles of silver metal with high potential for biological application, and can present several forms, such as spherical, flat, triangular, tetrahedral, prismatic, cubic, octahedral and irregular, and variable size, with a range between 1 and 100 nm [20, 21]. Therefore, research related to the development or modifications of compounds with antibacterial and antibiofilm activities, especially against P. aeruginosa strains, is an area that interests and growth. In this sense, the present work aims to conduct a literature review related to the antibacterial and antibiofilm activity of AgNPs against pathogenic strains of P. aeruginosa.
This is a descriptive study of the literature review based on the following stages: identification of the theme and development of the guiding question; establishment of inclusion and exclusion criteria, analysis, and selection of studies; interpretation of data and results; presentation of the review. The guiding question was “What are the benefits of silver nanoparticles for the treatment of infections caused by P. aeruginosa?” The literary search took place from articles indexed in international virtual libraries, U.S. National Library of Medicine (PubMed), ScienceDirect, and Scientific Electronic Library Online (SciELO).
The inclusion criteria adopted were complete studies, published in English that were related to the proposed theme and are indexed in these databases, and published between 2011 and 2020. In turn, repeated studies, studies that do not address the proposed theme, incomplete studies, duplicates, monographs, and publications of events were excluded. The following descriptors were used: silver nanoparticles, AgNPs, antimicrobial properties/activity, antibiofilm properties/activity, and P. aeruginosa. From the search in the databases, 100 articles were selected using the inclusion and exclusion criteria. The analysis of the selected studies made it possible to identify variables, observations, and data that gathered the knowledge about the use of AgNPs against P. aeruginosa. Both the analysis and the relationship of the data extracted from the articles were developed descriptively, making it possible to count, observe, describe, and classify them, with the purpose of organizing the knowledge generated about silver nanoparticles.
Synthesis of AgNPs
AgNPs can be synthesized by different methods and present different characteristics according to these methods. The most used approaches are the synthesis through chemical methods that uses organic solvents and inorganic reducing agents. The main reagents are sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, Tollens’ and N, N-dimethylformamide (DMF) reagent, and stabilizing agents such as vinyl alcohol, polyvinylpyrrolidone, polyethylene glycol, polyacidomethacrylic, and polymethylmethacrylate [22, 23]. This method is based on the reduction of metal ions for the formation of atoms, and then these are aggregated in a controlled manner [22, 24, 25]. However, this synthesis can result in AgNPs with chemicals sedimented in their surfaces. Some of these chemicals can be toxic and harmful, and can increase AgNPs toxicity to human cells, making its use unfeasible [26].
The methodologies that involve the synthesis of AgNPs by physical methods include the technique of laser ablation and evaporation-condensation. In laser ablation, silver is introduced into a liquid environment that, in turn, undergoes radiation from a pulsed laser, which results in the formation of AgNPs [27]. However, the wavelength that the laser reaches the metallic target, the pulsation period of lasers, and the liquid medium are some of the factors that determine the effectiveness and characteristics of the nanoparticles. This technique is one of the methods that results in silver nanoparticles without using chemical reagents [28, 29].
Another approach used is evaporation-condensation. In this method, the silver is evaporated from the center of a tube furnace to a gas phase route, allowing the synthesis of AgNPs at atmospheric pressure. Then, the products are handled on a nanometric scale, and through physical processes, the particles are broken down to the nanoscale. However, the tube oven occupies a large space and consumes high energy, thereby raising the ambient temperature of the metal source and requiring a long duration to maintain thermal stability [25, 29]. Besides that, this method results in imperfections on the surface structure of the AgNPs, which affects AgNPs potential, plus it produces low amounts of AgNPs [30]. In addition, electrochemical synthesis is also gaining space and is characterized by the formation of a reduced intermediate metal salt at the cathode in the presence of a stabilizing agent using an electronic device containing electrolytic cells with silver plate electrodes [31].
Among all methods to obtain AgNPs, biosynthesis is the most economical and ecologically viable alternative, as it increases stability and avoids the use of organic solvents and toxic reagents [32]. Biosynthesis is a less harmful method, and a relevant practice in the field of nanotechnology. They can be produced enzymatically and non-enzymatically, under pressure and at room temperature, without using external stabilizing agents. The reducing and stabilizing agents used in this type of synthesis are molecules produced by proteins, carbohydrates, plants, algae, bacteria, yeasts, and fungi [33, 34].
The use of plants for the synthesis of AgNPs is one of the most viable methods, as it is considered faster compared to other routes, reliable, non-toxic, and ecologically correct [35–37]. The synthesis of nanoparticles by methodologies that use biological routes has been extensively researched. The first microorganism, reported in the literature, used for this purpose was Pseudomonas stutzeri, and later actinomycetes, fungi, cyanobacteria, and other plant materials such as fruits, peels, and roots [36, 37].
Antimicrobial mechanism of action of AgNPs
In the last two decades, the number of bacterial infections caused by multidrug-resistant pathogenic microorganisms (MDR) has increased sharply, mainly due to the indiscriminate use of antimicrobials in clinical practice and in agriculture. Thus, there is a need for the development of new therapies that act on MDR stains, in which AgNPs have been gaining prominence [38]. The antibacterial activity attributed to AgNPs can be explained by the large surface area of nanoparticles, which allows greater contact with the microorganism, causing its death even in low concentrations [39, 40].
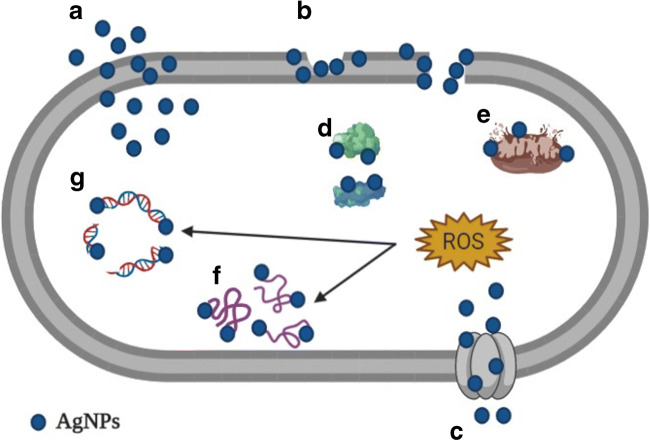
AgNPs adhere to the cytoplasmic membrane and cell wall of microorganisms, penetrating them and, consequently, affecting vital cell function, since they interact with cellular structures and biomolecules, such as ribosomes, mitochondria, proteins, lipids, and DNA, inducing the generation of reactive oxygen species (ROS) and free radicals, as well as in the modulation of microbial signal transduction pathways (Fig. 1) [39–41]. Another mechanism of antibacterial action occurs through the oxidation of AgNPs that leads to silver toxicity in bacterial cells. Toxicity depends on the presence of oxygen and is linked to the release of silver ions that are released by AgNPs when they come into contact with water [42].
Fig. 1.
(A) AgNPs attach to cell wall and penetrate membrane; (B) AgNPs damage the cell wall and membrane; (C) Entry of AgNPs by porin proteins; (D) AgNPs cause ribosome disassembly; (E) AgNPs cause mitochondrial dysfunction; (F) AgNPs cause protein denaturation; (G) AgNPs cause DNA damage; (H) AgNPs cause ROS production and oxidative stress
Antibacterial activity of AgNPs against Pseudomonas aeruginosa
The use of silver as a therapeutic alternative for several diseases has been reported since ancient times. Prior to the advent of antibiotics, silver was used for antibacterial purposes in the treatment of patients who had open wounds and burns [43]. After the development of antibiotics, and later the use of nanotechnology as a carrier of bioactive products, silver started to be used in the form of nanoparticles. Although AgNPS have antibacterial activity against both Gram-negative and Gram-positive bacteria, it is reported that Gram-negative strains are more sensitive to AgNPs than Gram-positive strains, mainly due to the greater ease of crossing the cell wall [44–46].
Gram-negative bacteria have an outer layer of lipopolysaccharides and a thin layer of peptidoglycan. This layer of lipopolysaccharides is composed of lipids covalently that are linked to polysaccharides, having a negative charge, electrostatically attracting the positive charge of AgNPs, facilitating the adhesion of AgNPs. In the Gram-positive, the interaction of AgNPs occurs, but due to the thick layer of peptidoglycan on the cell wall, AgNPs are stationary, impairing the release and uptake of proteins, ions, sugars, among others, essential for vital cellular activities, such as the production of energy. However, regardless of the composition of the bacterial cell wall, the penetration of AgNPs will occur, and it will act against Gram-positive and Gram-negative [41, 47].
The different toxicity profiles of AgNPs against Gram-positive and Gram-negative bacteria are also observed in other microorganisms. In the case of viruses, such as HIV-1 and hepatitis B, AgNPs can inactivate viruses by inhibiting their binding to host cells, interacting with glycoproteins and thus blocking the viral entry phase, besides, in other studies, it is believed that AgNPs also cause inhibition of replication phases [48]. In bacteria, it is suggested that toxicity depends on the constitution of the microorganism’s cell wall and, as observed in fungi, it interferes both in the metabolism with the generation of ROS, and through interactions with the membrane constituents that lead to cell lysis [49, 50]. Toxicity to more complex organisms, such as human cells, is possibly different due to structural and physiological differences, as well as defense mechanisms that allow exposure to high concentrations of AgNPs [48].
Antibacterial activity against P. aeruginosa isolates is generally considered to be more effective in AgNPs with reduced size, because they have a surface area that allows greater interaction with bacterial cells, and therefore have a promising antibacterial activity compared to larger size (Table 1). In addition, the bacterial cell membrane is negatively charged, and AgNPs are positively charged, causing them to accumulate in the membrane, causing structural changes, and making it more permeable [37].
Table 1.
Antibacterial activity of AgNPs against Pseudomonas aeruginosa
| Silver nanoparticles size | Silver nanoparticles shape | Type of synthesis | Pseudomonas aeruginosa strains | Antibacterial activity | References |
|---|---|---|---|---|---|
| 30 nm | Spherical | Chemical | NS | MIC = 2 μg/mL | [51] |
| 22 nm | Spherical | Biosynthesis - Tribulus terrestris | MDR | Zone of inhibition = 9.25 mm | [52] |
| 20 nm | Spherical | Chemical | MDR |
MIC = 100 μg/mL MBC = 200 μg/mL |
[53] |
| 20 nm | Spherical |
Biosynthesis - Solanum tricobatum |
NS | Zone of inhibition = 12 mm | [54] |
| 47 nm | NS | Chemical | PA01 | 10 μg/mL caused a − 5 log reduction. | [55] |
| 15 nm | NS | Biosynthesis - Punica granatum | ATCC | MIC = 45 μg/mL | [56] |
| NE | NS | Chemical | Susceptible; MDR; ATCC 27853 | Inhibition rate = 67% | [57] |
| 36 ± 9 nm | Spherical | Biosynthesis - Tinospora cordifolia | Clinical isolate | MIC = 6.25–200 μg/mL | [58] |
| NS | NS | NS | ATCC 10145 | MIC = 1 mg/mL | [59] |
| 15.7, 24 ± 8 nm | Spherical | Biosynthesis - Phyllanthus amarus | Clinical isolate | MIC = 6.25–12.5 μg/mL | [60] |
| 7 nm | Spherical | Chemical | MDR | MIC = 11.25 μg/mL | [61] |
| 20 nm | Spherical | Biosynthesis - Justicia adhatoda | MTCC 741 | Inhibition zone = 8–10 mm | [62] |
| 40 nm | Spherical |
Biosynthesis - Psidium Guajava |
NS | Inhibition zone = 8–10 mm | [63] |
| 121 nm | Hexagonal and Spherical | Biosynthesis - Rheum palmatum | ATCC 27853 | MIC = 15 μg/mL | [64] |
| 11 nm | Spherical | Chemical | MDR | MIC = 1 μg/mL | [65] |
| 10 nm | NS | NS |
INCQS 0230; ATCC 27853; PA01 PA02 |
Bacterial reduction at a concentration of 1.25 and 0.156 μg/mL | [66] |
| 12 nm | Spherical | Biosynthesis - Azadirachta indica | NS | Inhibition zone = 6 mm | [67] |
| 26.95 nm | Spherical | Biosynthesis - Punica granatum | ATCC 27584 | Inhibition zone = 10 mm | [68] |
| 20–40 nm | Spherical | Biosynthesis - Cannabis sativa | PA01 susceptible |
MIC = 6.25 μg/mL; MBC = 12.5 μg/mL |
[69] |
| 5–20 nm | Spherical | Chemical | MDR |
MIC = 1.406–5.625 μg/mL MBC = 2.813–5.625 μg/mL. |
[11] |
| 14–48 nm | Spherical | Biosynthesis - Piper betle | PA01 | MIC = 12.5 μg/mL | [70] |
| 20 ± 3 nm | Spherical | Chemical | PA14 |
MIC = 10 μg/mL; MBC = 20 μg/mL |
[71] |
NS, not specified; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; PA, Pseudomonas aeruginosa; ATCC, American Type Culture Collection; MTCC, Microbial Type Culture Collection; INCQS, National Institute of Quality Control in Health
As we can see in Table 1, the size of the AgNPs directly influences the activity of the nanoparticles, because smaller sizes increase the surface contact area of AgNPs with microorganisms, specifically P. aeruginosa. Arokiyaraj et al. [64] analyzed the activity of AgNPs with a size of 121 nm, obtaining MIC results of 15 μg/mL against P. aeruginosa strains, while Shah et al. [70] using nanoparticles with a size of 48 nm obtained MIC value of 12.5 μg/mL. Singh et al. [69] developed AgNPs with particle size of 20 and 40 nm, observing differences in activity against P. aeruginosa strains with MIC values of 6.25 and 12.5 μg/mL, respectively.
Values less than MIC were observed in the studies by Yuan, Peng, and Gurunathan [65] and Liao et al. [11] who obtained AgNPs with sizes of 11 and 5 nm, respectively, and tested their activity in P. aeruginosa strains. Yuan, Peng, and Gurunathan [65] obtained MIC value of 1 μg/mL, while Liao et al. [11] obtained an MIC value of 1.406 μg/mL.
Jasuja et al. [56] used extracts from the bark of Punica granatum L. (Lythraceae), to synthesize AgNPs. The results showed that the MIC was 45 μg/mL against P. aeruginosa ATCC, and the mechanism of action of AgNPs was possibly due to the decrease of the stiffness of the cell wall polysaccharides, inactivate the transport of enzymes, which in turn generated H202 resulting in bacterial death. The same mechanism of action is reported by Arokiyaraj et al. [64] who used as source of AgNPs the root of the plant Rheum palmatum L. (Polygonaceae), reaching an inhibition halo of 13 mm and MIC of 15 μg/mL against P. aeruginosa ATCC 27853.
Singh et al. [58] used twenty MDR P. aeruginosa strains isolated from burned patients to investigate the antibacterial activity of AgNPs synthetized by the bark of the plant Tinospora cordifolia (Thunb.) Miers (Menispermaceae). The evaluation of the antibacterial activity of these AgNPs at a concentration of 12.5 to 200 μg/mL of Ag+ by the diffusion disc method showed an inhibition zone of 10 ± 0.58 to 21 ± 0.25 mm, and MIC from 6.25 to 200 μg/mL. Another study developed by Singh et al. [60] produced AgNPs using the aqueous extract of the plant Phyllanthus amarus Schum. & Thonn (Phyllanthaceae). The antibacterial activity of these AgNPs was evaluated against fifteen MDR P. aeruginosa strains also isolated from patients who suffered burns. Using the same methods, the results of the inhibition zone in this study ranged from 10 ± 0.53 to 21 ± 0.11 mm in concentrations at 12.5 to 100 μg/mL and MIC from 6.25 to 12.5 μg/mL, where, according to the authors, MIC values are equivalent to those of standard antibiotics.
Through these results, it was possible to describe that the main mechanism considered for the release of Ag+ from AgNPs may occur due to the phytochemical composition of plants, where terpenoids, organic acids, and flavonoids are the main mediators of reduction. In addition, both extracts also exhibit therapeutic potential against MDR P. aeruginosa strains, and can act in synergism with Ag+ as therapeutic agents against bacterial infections [58].
Singh et al. [69] explored the synthesis of AgNPs from the aqueous extract of the plant Cannabis sativa L. (Cannabaceae). These AgNPs exhibit MIC of 6.25 μg/mL and MBC of 12.5 μg/mL against PA01 (chloramphenicol-resistant P. aeruginosa). Due to the small size of the AgNPs (20–40 nm), they easily entered the bacterial intracellular environment, which, in turn, managed to cause oxidation of cellular components through the generation of ROS. Shah et al. [70] used AgNPs synthetized from Piper betle L. (Piperaceae) against the isolate PA01. The MIC was 12.5 μg/mL, and, according to the authors, it was due to the antibacterial nature of silver ions.
In the study conducted by Kora and Arunachalam [51], the AgNPs were synthesized in a solution containing 1 mM of silver nitrate with 1.6 mM of sodium dodecyl sulfate and 0.85 M of ethanol, obtaining nanoparticles with 30 nm in spherical format, highly stable and with a double layer of sodium dodecyl sulfate on its surface that demonstrated antimicrobial activity against antibiotic-sensitive P. aeruginosa (MIC = 2 μg/mL). However, Amirulhusni et al. [53] produced AgNPs by chemical synthesis and obtained nanoparticles with 20 nm in spherical format that exhibit antibacterial activity against ten MDR P. aeruginosa strains (MIC and MBC = 100 μg/mL and 200 μg/mL, respectively).
During the research performed by Yuan, Peng, and Gurunathan [65], AgNPs were produced by the chemical synthesis using quercetin, a flavonoid with five hydroxyl. These spherical AgNPs exhibit an average size of 11 nm and MIC of 1 μg/mL in cultures with MDR P. aeruginosa, isolated from goat’s milk, unlike the results found in the study of Liao et al. [11] who obtained MIC of 2.596 ± 1.126 μg/mL and MBC of 3.246 ± 1.056 μg/mL using AgNPs synthesized chemically with 5–20 nm in spherical format against MDR clinical isolates of P. aeruginosa. Silva et al. [71] performed chemical synthesis of AgNPs covered with sodium citrate. The nanoparticles exhibit size of 20 ± 3 nm and spherical format. According to the authors, light excites the local plasmonic resonance of the surface in AgNPs. From that, they verified the activity of AgNPs in bright and dark light. AgNPs present MIC of 10 μg/mL and MBC of 20 μg/mL in dark light and MIC of 5 μg/mL and MBC of 10 μg/mL in bright light.
The synergistic effect between antimicrobial drugs and AgNPs has already been tested, and it was proved that it potentiates antimicrobial action against antibiotic-sensitive and MDR P. aeruginosa strains. Studies have associated AgNPs with chloramphenicol, kanamycin, vancomycin, ciprofloxacin, and polymyxin B. In this sense, AgNPs optimize and facilitate the infiltration of antibiotics, allowing maximum efficiency, as well as promoting damage to the microorganism [72–74].
In the work of Ghosh et al. [72], numerous antibiotics were tested against P. aeruginosa, but chloramphenicol, kanamycin, and vancomycin showed better results when tested together with AgNPs. AgNPs mechanism of action is not fully elucidated in the literature, but it is known that they have a selective approach towards the cell membrane, thus destabilizing it. Chloramphenicol acts on the bacterial 50S ribosomal subunit, binding to it, thereby prevents transfer of amino acids and peptides formation. Therefore, AgNps could facilitate chloramphenicol diffusion in the bacteria. Kanamycin is an aminoglycoside and it also interferes in protein formation, binding to bacterial 30S ribosomal subunit. So, the mechanism of this synergistic antibacterial effect could be the same as chloramphenicol and AgNps together. Vancomycin inhibits cell wall synthesis, preventing further elongation of the peptidoglycan matrix, so together with AgNPs, the damage to the cell wall could increase, resulting in better antibacterial results [72, 75–78].
The association of polymyxin B with AgNPs has shown to be more effective than either of these agents alone. Jasim et al. [73] reported that this combination results in greater increase of ROS production and greater morphological changes in the outer membrane, leading to cytosolic green fluorescent protein (GFP) release. Polymyxins mechanism of action is based on the interaction of this drug with the lipid A component in the lipopolysaccharide of the outer membrane of Gram-negative bacteria; thus, AgNPs can also interact with the outer membrane, causing disruption and leading to ROS production. So, the antibacterial synergy of this combination may involve their combined membrane disruption activity and their respiratory chain poisoning activity [73].
In this sense, the choice of the antibiotic according to its therapeutic target is important when wants to associate its effects with those of AgNPs. By destabilizing the membrane, AgNPs can facilitate the entry of antibiotics whose target is intracellular and thereby facilitate its therapeutic effect. Thus, the association of AgNPs with drugs that have an intracellular target is more interesting, as it could provide synergistic effects, than drugs that have an extracellular target [78]. Brasil et al. [79] used surface-enhanced Raman scattering spectroscopy (SERS) to assess the association of AgNPs, chitosan, and the antibiotics azithromycin, levofloxacin, or tetracycline against Gram-negative and Gram-positive bacteria strains, noting that the combination promoted a reduction of 37–97% in the minimum inhibitory concentration of drugs. Deng et al. [80] observed the interaction of AgNPs with the antibiotics enoxacin, neomycin and tetracycline, showing through Raman’s technique that they form complex with AgNPs and may exhibit a synergistic effect against microorganisms.
Antibiofilm mechanism of action of AgNPs
Biofilms are communities of microorganisms connected to a surface or to other microorganisms, forming aggregates wrapped in an extracellular matrix of polysaccharides, proteins, and glycoproteins. Hence, a favorable environment is formed, and the biofilm is established to act as a protector against exogenous stress [81, 82]. Biofilms may increase risks of infection, prolong hospitalizations, and raise the costs for health services, since there is a great difficulty to eradicate them. The mechanisms that are related to defense and survival of the biofilm include resistance to antibiotic therapy and gene exchanges between microorganisms, which help the pathogen to avoid host immune responses, thereby establishing chronic infections [83, 84].
In the attempt to overcome bacterial resistance, researchers are looking for new antibiotic alternatives that not only prevent the development of resistance, but also reduce the use of conventional antibiotics allowing the effective destruction of biofilms [85]. Among them, the use of AgNPs has gained a lot of attention due to its antibacterial and antibiofilm activity, since breaking through the biofilm barrier is a challenge. Thus, new strategies using AgNPs were developed in order to interrupt biofilm growth or to degrade it [47, 86, 87].
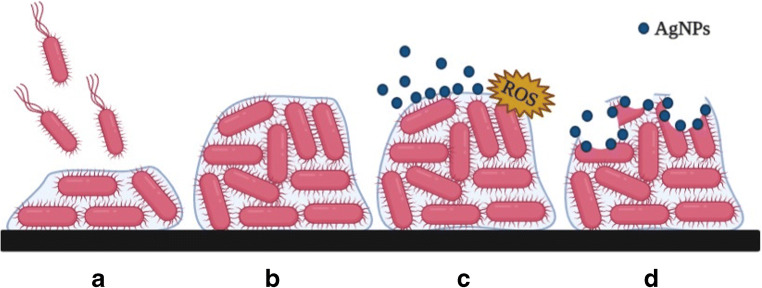
Some studies suggest that the main mechanism of biofilm destruction occurs through the binding of AgNPs in the exopolysaccharide matrix, disrupting the biofilm structure by recognizing the peptidoglycan structure present in bacterial membranes, causing physical damage, ion release, ROS production, leading to oxidative stress, and DNA damage (Fig. 2) [88, 89]. When bacteria is treated with AgNPs, morphological changes are revealed in biofilm’s architecture, such as uneven cell surface, suggesting cell lysis [70], relevant morphological damages in the cell wall, membrane corrugation damage, changes in membrane polarization and/or permeability [90], and distinct EPS-matrix formation surrounding the bacterial strains [91]. Moreover, electrostatic interactions between AgNPs and bacterial membranes cause them to rupture, so that AgNPs can penetrate into the mature biofilm [86, 92].
Fig. 2.
(A) Attachment of cells to form biofilm; (B) Mature biofilm; (C) AgNPs bind to the exopolysaccharide matrix of biofilm and cause ROS production; (D) disruption of biofilm
Antibiofilm activity of AgNPs against Pseudomonas aeruginosa
The AgNPs have demonstrated biological activity against several pathogens. Thus, their actions were tested against different biofilm-producing microorganisms. Among numerous published works, many evaluate AgNPs’ activity against biofilm produced by P. aeruginosa (Table 2).
Table 2.
Antibiofilm activity of AgNPs against Pseudomonas aeruginosa
| Silver nanoparticles size | Silver nanoparticles shape | Type of synthesis | Pseudomonas aeruginosa strains | Antibiofilm activity | References |
|---|---|---|---|---|---|
| 30 nm | Spherical | Chemical | NS | 1 μg/mL of AgNPs inhibited biofilm formation by 95 ± 0.62%. | [51] |
| 47 nm | NS | Chemical | PA01 | 10 μg/mL of AgNPs caused a 3 log inactivation of biofilm cells. | [55] |
| 7–70 nm | Spherical, pseudo-spherical and a few with a cylindrical shape. | Chemical | PA01 | 600 μg/mL of AgNPs (8 nm) resulted in approximately 90% of biofilm detachment. | [93] |
| 7 nm | Spherical | Chemical | ESBL, MBL and NON-ESBL clinical isolates | 60 μg/mL of AgNPs completely blocked biofilm formation. | [61] |
| 8.3 ± 1.9 nm | Spherical | Biosynthesis | PA01 | A decrease of the bacterial mass in the biofilm was seen when ~ 10 μg/mL of AgNPs was used. | [94] |
| NS | NS | Chemical | ATCC 27853; Susceptible and MDR clinical isolates. | 20 μg/mL of AgNPs inhibited the growth of biofilm from the sensitive strain in 67% and from the MDR strain in 56%. | [57] |
| 2–10 nm | Spherical | Biosynthesis - Allophylus cobbe | NS | 0.5 μg/mL of AgNPs decreased biofilm activity by more than 90%. | [95] |
| 14 nm | Predominantly spherical, but a few had an oval shape | Biosynthesis - Lagerstroemia speciosa | Clinical isolate | 50 μg/mL of AgNPs inhibited 86.73 ± 28%. | [96] |
| 20–40 nm | Spherical | Biosynthesis - Cannabis sativa | PA01 | 50 μg/mL of AgNPs inhibited more than 80% of biofilm formation. | [69] |
| 15–30 nm | Spherical | Biosynthesis - Rhodiola rosea | PA01 | 50 μg/mL of AgNPs inhibited more than 80% of biofilm formation. | [97] |
| 55.6 ± 2.9 nm | Quasi-spherical | Electrochemical | DIN1 | 17 μg/mL of AgNPs reduced biofilm viability in more than 90%. | [90] |
| 10–15 nm | Spherical | Biosynthesisl -Nardostachys jatamansi | NS | 64 μM of AgNPs prevented biofilm formation. | [98] |
| 14–48 nm | Spherical | Biosynthesis - Piper betle | PA01 | 8 μg/mL of AgNPs reduced biofilm formation in 78.20 ± 3.1%. | [70] |
| 31.49 ± 2.48 nm | Spherical | Chemical | CCM 3955 | 18 μg/mL of AgNPs completely inhibited biofilm growth. | [91] |
| 32–85 nm | Spherical | Biosynthesis - Black peel pomegranate | ATCC 10662 | 100 to 500 μg/mL of AgNPs inhibited biofilm formation significantly. | [99] |
NS, not specified; PA, Pseudomonas aeruginosa; MDR, multidrug-resistant; ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase; ATCC, American Type Culture Collection; CCM, Czech Collection of Microorganisms
In the work of Palanisamy et al. [57], 20 μg/mL of AgNPs inhibited the growth of biofilm from a sensitive strain with an inhibition rate of 67%. However, AgNPs inhibited the formation of biofilm of the MDR strain with an inhibition rate of 56%, indicating that MDR strains need a higher concentration of AgNPs to inhibit biofilm growth. Ansari et al. [61] tested AgNPs’ antibiofilm activity against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing P. aeruginosa strains, and biofilm formation was ceased when the biofilm was exposed to 60 μg/mL of AgNPs, but when AgNPs were tested against P. aeruginosa PA01 strain’s biofilm, only 10 μg/mL was necessary to decrease biofilm growth significantly [94], showing once more that MDR strains need a higher concentration of AgNPs to get inhibited.
The type of synthesis can also interfere in AgNPs antibiofilm potential, especially since it can affect AgNPs’ size and surface area [100]. Loo et al. [93] tested AgNPs with distinct sizes against P. aeruginosa PA01 biofilm and obtained different results using the same amount of AgNPs. They used AgNPs at the sizes of 8 nm, 20 nm, and 35 nm, and the 8 nm AgNPs showed better results in biofilm detachment in all different concentrations, especially due to its bigger surface area to volume which translates to a higher availability of surface area for oxidation and consequently, silver ions release, once AgNPs are exposed to liquids. In the work of Radzig et al. [94], they tested AgNPs at the size of 8.3 ± 1.9 nm against P. aeruginosa PA01 biofilm and a concentration of 10 μg/mL was enough to decrease biofilm growth significantly. However, when Habibipour, Moradi-Haghgou and Farmany [99] tested AgNPs with size ranged of 32–85 nm against P. aeruginosa PA01 biofilm, concentrations between 100 and 500 μg/mL were necessary to inhibit biofilm growth significantly. Showing once more that smaller AgNps have better activity, and demand a smaller concentration of AgNPs in the treatment.
AgNPs may be used against biofilm as pre-treatment to inhibit biofilm formation or post-treatment to reduce biomass and destroy biofilm formed by P. aeruginosa. In pre-treatment, it can inhibit biofilm growth in a dose-dependent manner, as Shah et al. [70] and Habibipour, Moradi-Haghgou, and Farmany [99] indicated. In the studies of Shah et al. [70], they reported that AgNPs synthesized from Piper betle, inhibited 14.33 ± 4.6% of P. aeruginosa PA01 biofilm formation at a concentration of 2 μg/mL, 36.10 ± 5.4% at a concentration of 4 μg/mL, 55.09 ± 2.62% at a concentration of 6 μg/mL, and 78.20 ± 3.1% at a concentration of 8 μg/mL. Habibipour, Moradi-Haghgou, and Farmany [99] tested different concentrations of AgNPs (0.05 mg/mL to 0.5 mg/mL), and when they using concentrations that were higher than 0.1 mg/mL, the AgNPs were able to decrease the biofilm formation.
Used as post-treatment therapy, AgNPs were able to reduce P. aeruginosa CCM 3955 biofilm viability by 46.28%, 65.50%, and 92.43% and reduce biomass by 5.69%, 37.87%, and 67.52%, after treatment with concentrations at 2, 6, and 12 μg/mL, respectively [91]. Singh et al. [69] evaluated the antibiofilm activity of AgNPs produced from Cannabis sativa against P. aeruginosa PA01 and as a result showed a decrease in biofilm’s viability at a concentration at 50 μg/mL. Pompilio et al. [90] tested AgNPs synthesized electrochemically and also showed a reduced biofilm’s viability, achieving biofilm erradication at a concentration of 17 μg/mL.
Furthermore, AgNPs can also work as an enhancer of antimicrobials action against biofilm. AgNPs work in a synergic form with tobramycin against P. aeruginosa PA01 biofilm, causing extensive cellular changes, including altered cellular morphology and cytoplasmic clearing, and eliminating biofilm formed by this strain [14]. AgNPs combined with polymyxin B has also presented an increase in its antibiofilm activity against antibiotic-sensitive and MDR P. aeruginosa clinical isolates even in low concentrations, when compared to its action alone [101]. Aztreonam can also work in synergism with AgNPs, reducing P. aeruginosa PA01 biofilm biomass and viability in a dose-dependent manner, as well as reducing biofilm thickness and causing cellular [102]. Ampicillin was also tested together with AgNPs and the results were about three times more effective comparing to AgNPs antibiofilm action alone [95].
Conclusions
AgNPs are rapidly obtained through green synthesis with the use of plants and/or microorganisms without the development of toxic waste to the handler and the environment. More and more studies show the antimicrobial activity of AgNPs and their importance in the insertion of antibacterial therapy. AgNPs exhibit potential against gram-negative bacteria, with antimicrobial activity and promising antibiofilm activities against Pseudomonas aeruginosa resistance profile multidrug-resistant strains, due to the small concentrations capable of promoting rapid cytotoxicity in the microorganism and, consequently, death. It is worth mentioning that the size of the obtained AgNPs is important because the surface area of contact with the microorganism is greater in smaller nanoparticles (20–40 nm), thereby enhancing its antimicrobial effect. In vivo studies should be developed to better assess the safety of administering AgNPs.
Code availability
Not applicable.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iago Dillion Lima Cavalcanti, Email: iagodillion@hotmail.com.
Isabella Macário Ferro Cavalcanti, Email: isabella.cavalcanti@ufpe.br.
References
- 1.World health organization (2017) Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016–2017. Geneva. Available on: https://apps.who.int/iris/bitstream/handle/10665/259744/9789241513449-eng.pdf;jsessionid=DF103DA3FE5FA323F5E291D17C64FF65?sequence=1. Accessed 3 June 2020
- 2.Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectr 4. 10.1128/microbiolspec.VMBF-0016-2015 [DOI] [PMC free article] [PubMed]
- 3.McCracken M, Mataseje LF, Loo V, Walkty A, Adam HJ, Hoban DJ, Zhanel GG, Mulvey MR. Characterization of Acinetobacter baumannii and meropenem-resistant Pseudomonas aeruginosa in Canada: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69(3):335–341. doi: 10.1016/j.diagmicrobio.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Yong D, Toleman MA, Bell J, Ritchie B, Pratt R, Ryley H, Walsh TR. Genetic and biochemical characterization of an acquired subgroup B3 metallo-β-lactamase gene, blaAIM-1, and its unique genetic context in Pseudomonas aeruginosa from Australia. Antimicrob Agents Chemother. 2012;56(12):6154–6519. doi: 10.1128/AAC.05654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MJ, Bae IK, Jeong SH, Kim SH, Song JH, Choi JY, Yoon SS, Thamlikitkul V, Hsueh PR, Yasin RM, Lalitha MK. Dissemination of metallo-β-lactamase-producing Pseudomonas aeruginosa of sequence type 235 in Asian countries. J Antimicrob Chemother. 2013;68(12):2820–2824. doi: 10.1093/jac/dkt269. [DOI] [PubMed] [Google Scholar]
- 6.Mudau M, Jacobson R, Minenza N, Kuonza L, Morris V, Engelbrecht H, Nicol MP, Bamford C. Outbreak of multi-drug resistant Pseudomonas aeruginosa bloodstream infection in the haematology unit of a South African Academic Hospital. PLoS One. 2013;8(3):e55985. doi: 10.1371/journal.pone.0055985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labarca JA, Salles MJ, Seas C, Guzmán-Blanco M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbio. 2016;42(2):276–292. doi: 10.3109/1040841X.2014.940494. [DOI] [PubMed] [Google Scholar]
- 8.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S (2019) Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 32(4) pii: e00031-19. 10.1128/CMR.00031-19 [DOI] [PMC free article] [PubMed]
- 9.Chen Q, Shah KN, Zhang F, Salazar AJ, Shah PN, Li R, Sacchettini JC, Wooley KL, Cannon CL. Minocycline and silver dual-loaded polyphosphoester-based nanoparticles for treatment of resistant Pseudomonas aeruginosa. Mol Pharm. 2019;16(4):1606–1619. doi: 10.1021/acs.molpharmaceut.8b01288. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Chen Y, Hu P, Zhou T, Xu X, Pei X. Risk assessment of infected children with Pseudomonas aeruginosa pneumonia by combining host and pathogen predictors. Infec Genet Evol. 2018;57:82–87. doi: 10.1016/j.meegid.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Liao S, Zhang Y, Pan X, Zhu F, Jiang C, Liu Q, Cheng Z, Dai G, Wu G, Wang L, Chen L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int J Nanomedicine. 2019;14:1469–1487. doi: 10.2147/IJN.S191340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36(4):893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingemans J, Al-Feghali RE, Lau GW, Sauer K. Controlling chronic Pseudomonas aeruginosa infections by strategically interfering with the sensory function of SagS. Mol Microbiol. 2019;111(5):1211–1228. doi: 10.1111/mmi.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habash MB, Goodyear MC, Park AJ, Surette MD, Vis EC, Harris RJ, Khursigara CM (2017) Potentiation of tobramycin by silver nanoparticles against Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 61(11). pii: e00415-17. 10.1128/AAC.00415-17 [DOI] [PMC free article] [PubMed]
- 15.Cavalcanti IM, Pontes-Neto JG, Kocerginsky PO, Bezerra-Neto AM, Lima JL, Lira-Nogueira MC, Maciel MA, Neves RP, Pimentel MF, Santos-Magalhães NS. Antimicrobial activity of β-lapachone encapsulated into liposomes against meticillin-resistant Staphylococcus aureus and Cryptococcus neoformans clinical strains. J Glob Antimicrob Resist. 2015;2:103–108. doi: 10.1016/j.jgar.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Lima R, Del Fiol FS, Balcão VM. Prospects for the use of new technologies to combat multidrug-resistant bacteria. Front Pharmacol. 2019;10:692. doi: 10.3389/fphar.2019.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quezada CQ, Azevedo CS, Charneau S, Santana JM, Chorilli M, Carneiro MB, Bastos IM. Advances in nanocarriers as drug delivery systems in Chagas disease. Int J Nanomedicine. 2019;14:6407–6424. doi: 10.2147/IJN.S206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamburowicz-Klimkowska M, Poplawska M, Grudzinski IP. Nanocomposites as biomolecules delivery agents in nanomedicine. J Nanobiotechnology. 2019;17(1):48. doi: 10.1186/s12951-019-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Ouay B, Stellacci F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10(3):339–354. doi: 10.1016/j.nantod.2015.04.002. [DOI] [Google Scholar]
- 20.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52(6):1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 22.Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- 23.Güzel R, Erdal G. Synthesis of silver nanoparticles. In: Maaz K, editor. Silver nanoparticles—fabrication. London: Characterization and Applications. Intechopen; 2018. pp. 03–20. [Google Scholar]
- 24.Abbasi E, Milani M, Fekri Aval S, Kouhi M, Akbarzadeh A, Tayefi Nasrabadi H, Nikasa P, Joo SW, Hanifehpour Y, Nejati-Koshki K, Samiei M. Silver nanoparticles: synthesis methods, bio-applications and properties. Crit Rev Microbiol. 2014;42(2):173–180. doi: 10.3109/1040841X.2014.912200. [DOI] [PubMed] [Google Scholar]
- 25.Beyene HD, Werkneh AA, Bezabh HK, Ambaye TG. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain Mater Technol. 2017;13:18–23. doi: 10.1016/j.susmat.2017.08.001. [DOI] [Google Scholar]
- 26.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perito B, Giorgetti E, Marsili P, Muniz-Miranda M. Antibacterial activity of silver nanoparticles obtained by pulsed laser ablation in pure water and in chloride solution. Beilstein J Nanotechnol. 2016;7:465–473. doi: 10.3762/bjnano.7.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji T, Iryo K, Watanabe N, Tsuji M. Preparation of silver nanoparticles by laser ablation in solution: influence of laser wavelength on particle size. Appl Surf Sci. 2002;202(1–2):80–85. doi: 10.1016/S0169-4332(02)00936-4. [DOI] [Google Scholar]
- 29.Lee SH, Jun BH. Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):865. doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur P, Jha S, Ramteke S, Jain NK. Pharmaceutical aspects of silver nanoparticles. Artif Cell Nanomed. 2018;46(1):115–126. doi: 10.1080/21691401.2017.1414825. [DOI] [PubMed] [Google Scholar]
- 31.Scotti L, Angelini G, Gasbarri C, Bucciarelli T. Uncoated negatively charged silver nanoparticles: speeding up the electrochemical synthesis. Mater Res Express. 2017;4(10):105001. doi: 10.1088/2053-1591/aa8c39. [DOI] [Google Scholar]
- 32.Almadiy AA, Nenaah GE. Ecofriendly synthesis of silver nanoparticles using potato steroidal alkaloids and their activity against phytopathogenic fungi. Braz Arch Biol. 2018;61(1):1–14. doi: 10.1590/1678-4324-2018180013. [DOI] [Google Scholar]
- 33.Ge L, Li Q, Wang M, Ouyang J, Li X, Xing MM. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int J Nanomedicine. 2014;9(1):2399. doi: 10.2147/IJN.S55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojo OA, Oyinloye BE, Ojo AB, Afolabi OB, Peters OA, Olaiya O, Fadaka A, Jonathan J, Osunlana O. Green synthesis of silver nanoparticles (AgNPs) using Talinum triangulare (Jacq.) Willd. Leaf extract and monitoring their antimicrobial activity. J Bionanosci. 2017;11(4):292–296. doi: 10.1166/jbns.2017.1452. [DOI] [Google Scholar]
- 35.Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sc. 2016;9(3):217–227. doi: 10.1016/j.jrras.2015.10.002. [DOI] [Google Scholar]
- 36.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles—a review on biomolecules involved, characterisation and antibacterial activity. Chem Biol Interact. 2017;273:219–227. doi: 10.1016/j.cbi.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Kumar M, Curtis A, Hoskins C. Application of nanoparticle technologies in the combat against antimicrobial resistance. Pharmaceutics. 2018;10(1):11. doi: 10.3390/pharmaceutics10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):32. doi: 10.1186/2228-5326-2-32. [DOI] [Google Scholar]
- 40.Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 2016;58:36–43. doi: 10.1016/j.msec.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kędziora A, Speruda M, Krzyżewska E, Rybka J, Łukowiak A, Bugla-Płoskońska G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int J Mol Sci. 2018;19(2):444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassir N, Rolain JM, Brouqui P. A new strategy to fight antimicrobial resistance: the revival of old antibiotics. Front Microbiol. 2014;5:551. doi: 10.3389/fmicb.2014.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehnavi AS, Raisi A, Aroujalian A. Control size and stability of colloidal silver nanoparticles with antibacterial activity prepared by a green synthesis method. Synth React Inorg M. 2013;43(5):543–551. doi: 10.1080/15533174.2012.741182. [DOI] [Google Scholar]
- 45.Zhang M, Zhang K, De Gusseme B, Verstraete W, Field R. The antibacterial and anti-biofouling performance of biogenic silver nanoparticles by Lactobacillus fermentum. Biofouling. 2014;30(3):347–357. doi: 10.1080/08927014.2013.873419. [DOI] [PubMed] [Google Scholar]
- 46.Sadeghi B, Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. 2015;134:310–315. doi: 10.1016/j.saa.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 47.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20(5):8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, García-García M, Morales JD, Bogdanchikova N, Huerta-Saquero A (2017). Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicology letters 276:(1)11-20. 10.1016/j.toxlet.2017.05.007 [DOI] [PubMed]
- 49.Vazquez-Muñoz R, Avalos-Borja M, Castro-Longoria E (2014). Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS One 9(10) 1–10. 10.1371/journal.pone.0108876 [DOI] [PMC free article] [PubMed]
- 50.Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett. 2016;11(1):2–14. doi: 10.1186/s11671-016-1257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kora AJ, Arunachalam J. Assessment of antibacterial activity of silver nanoparticles on Pseudomonas aeruginosa and its mechanism of action. World J Microbiol Biotechnol. 2011;27(5):1209–1216. doi: 10.1007/s11274-010-0569-2. [DOI] [Google Scholar]
- 52.Gopinath V, MubarakAli D, Priyadarshini S, Priyadharsshini NM, Thajuddin N, Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B Biointerfaces. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Amirulhusni AN, Palanisamy NK, Mohd-Zain Z, Ping LJ, Durairaj R. Antibacterial effect of silver nanoparticles on multi drug resistant Pseudomonas aeruginosa. Int J Med Sci Public Health. 2012;6(7):291–294. doi: 10.5281/zenodo.1329579. [DOI] [Google Scholar]
- 54.Logeswari P, Silambarasan S, Abraham J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. Journal of Saudi Chemical Society. 2015;19(3):311–317. doi: 10.1016/j.jscs.2012.04.007. [DOI] [Google Scholar]
- 55.Park HJ, Park S, Roh J, Kim S, Choi K, Yi J, Kim Y, Yoon J. Biofilm-inactivating activity of silver nanoparticles: a comparison with silver ions. J Ind Eng Chem. 2013;19(2):614–619. doi: 10.1016/j.jiec.2012.09.013. [DOI] [Google Scholar]
- 56.Jasuja ND, Gupta DK, Reza M, Joshi SC. Green synthesis of AgNPs stabilized with biowaste and their antimicrobial activities. Braz J Microbiol. 2014;45(4):1325–1332. doi: 10.1590/s1517-83822014000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palanisamy NK, Ferina N, Amirulhusni AN, Mohd-Zain Z, Hussaini J, Ping LJ, Durairaj R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J nanobiotechnology. 2014;12:2. doi: 10.1186/1477-3155-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP. Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J Nanomed Nanotechnol. 2014;5:192. doi: 10.4172/2157-7439.1000192. [DOI] [Google Scholar]
- 59.Markowska K, Grudniak AM, Krawczyk K, Wróbel I, Wolska KI (2014) Modulation of antibiotic resistance and induction of a stress response in Pseudomonas aeruginosa by silver nanoparticles. J Med Microbiol 63(6):849-54. 10.1099/jmm.0.068833-0 [DOI] [PubMed]
- 60.Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP. Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Nanobiotechnol. 2014;12:40. doi: 10.1186/s12951-014-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansari MA, Khan HM, Khan AA, Cameotra SS, Saquib Q, Musarrat J. Gum arabic capped-silver nanoparticles inhibit biofilm formation by multi-drug resistant strains of Pseudomonas aeruginosa. J Basic Microbiol. 2014;54(7):688–699. doi: 10.1002/jobm.201300748. [DOI] [PubMed] [Google Scholar]
- 62.Bose D, Chatterjee S. Antibacterial activity of green synthesized silver nanoparticles using Vasaka (Justicia adhatoda L.) leaf extract. Indian J Microbiol. 2015;55(2):163–167. doi: 10.1007/s12088-015-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bose D, Chatterjee S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl Nanosci. 2016;6(6):895–901. doi: 10.1007/s13204-015-0496-5. [DOI] [Google Scholar]
- 64.Arokiyaraj S, Vincent S, Saravanan M, Lee Y, Oh YK, Kim KH (2017). Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif Cell Nanomed B 45(2):372–379 10.3109/21691401.2016.1160403 [DOI] [PubMed]
- 65.Yuan YG, Peng QL, Gurunathan S. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: an alternative approach for antimicrobial therapy. Int J Mol Sci. 2017;18(3):569. doi: 10.3390/ijms18030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salomoni R, Léo P, Montemor AF, Rinaldi BG, Rodrigues MFA. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol Sci Appl. 2017;10:115–121. doi: 10.2147/NSA.S133415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senthilkumar P, Rashmitha S, Veera P, Ignatious CV, Saipriya C, Samrot AV (2018) Antibacterial activity of neem extract and its green synthesized silver nanoparticles against Pseudomonas aeruginosa.. J Pure App Microbiol 12(2):969-974. 10.22207/JPAM.12.2.60
- 68.Devanesan S, AlSalhi MS, Balaji RV, Ranjitsingh AJ, Ahamed A, Alfuraydi AA, AlQahtani FY, Aleanizy FS, Othman AH. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale rese l. 2018;13(1):315. doi: 10.1186/s11671-018-2731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh P, Pandit S, Garnæs J, Tunjic S, Mokkapati VR, Sultan A, Thygesen A, Mackevica A, Mateiu RV, Daugaard AE, Baun A. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int J Nanomedicine. 2018;13:3571–3591. doi: 10.2147/IJN.S157958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah S, Gaikwad S, Nagar S, Kulshrestha S, Vaidya V, Nawani N, Pawar S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling. 2019;35:34–49. doi: 10.1080/08927014.2018.1563686. [DOI] [PubMed] [Google Scholar]
- 71.Silva RT, Petri MV, Valencia EY, Camargo PH, Torresi SI, Spira B (2020) Visible light plasmon excitation of silver nanoparticles against antibiotic-resistant Pseudomonas aeruginosa. BioRxiv. 10.1101/2020.01.10.902676 [DOI] [PubMed]
- 72.Ghosh S, Patil S, Ahire M, Kitture R, Kale S, Pardesi K, Cameotra SS, Bellare J, Dhavale DD, Jabgunde A, Chopade BA. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomedicine. 2012;7:483. doi: 10.2147/IJN.S24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jasim R, Schneider EK, Han M, Azad MAK, Hussein M, Nowell C, Baker MA, Wang J, Li J, Velkov T. A fresh shine on cystic fibrosis inhalation therapy: antimicrobial synergy of polymyxin B in combination with silver nanoparticles. J Biomed Nanotechnol. 2017;13(4):447–457. doi: 10.1166/jbn.2017.2355. [DOI] [PubMed] [Google Scholar]
- 74.Al-Obaidi H, Kalgudi R, Zariwala MG. Fabrication of inhaled hybrid silver/ciprofloxacin nanoparticles with synergetic effect against Pseudomonas aeruginosa. Eur J Pharm Biopharm. 2018;128:27–35. doi: 10.1016/j.ejpb.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Dinos GP, Athanassopoulos CM, Missiri DA, Giannopoulou PC, Vlachogiannis IA, Papadopoulos GE, Papaioannou D, Kalpaxis DL. Chloramphenicol derivatives as antibacterial and anticancer agents: historic problems and current solutions. Antibiotics. 2016;5(2):20. doi: 10.3390/antibiotics5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Wang J, Wu Q, Li L, Wang Y, Yang H. Determination of kanamycin by high performance liquid chromatography. Molecules. 2019;24(10):1902. doi: 10.3390/molecules24101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, da Luz Goncalves Pedreira M, Sorgini Peterlini MA, Azzalis LA, Campos Junqueira VB, Fonseca FL. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19(4):694–700. [PubMed] [Google Scholar]
- 78.Vazquez-Muñoz R, Meza-Villezcas A, Fournier PGJ, Soria-Castro E, Juarez-Moreno K, Gallego-Hernández AL, Bogdanchikova N, Vazquez-Duhalt R, Huerta-Saquero A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS One. 2019;14:e0224904. doi: 10.1371/journal.pone.0224904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brasil MSL, Filgueiras AL, Campos MB, Neves MSL, Eugênio M, Sena LA, Sant’Anna CB, Silva VL, Diniz CG, Sant’Ana AC (2018). Synergism in the antibacterial action of ternary mixtures involving silver nanoparticles, chitosan and antibiotics. Journal of the Brazilian Chemical Society 29(10):2026–2033. 10.21577/0103-5053.20180077
- 80.Deng H, McShan D, Zhang Y, Sinha SS, Arslan Z, Ray PC, Yu H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environmental Science & Technology. 2016;50(16):8840–8848. doi: 10.1021/acs.est.6b00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant SS, Hung DT. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence. 2013;4:273–283. doi: 10.4161/viru.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pérez-Díaz M, Alvarado-Gomez E, Magaña-Aquino M, Sánchez-Sánchez R, Velasquillo C, Gonzalez C, Martinez-Gutierrez F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater Sci Eng C. 2016;60:317–323. doi: 10.1016/j.msec.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 83.Seth AK, Geringer MR, Hong SJ, Leung KP, Mustoe TA, Galiano RD. In vivo modeling of biofilm-infected wounds: a review. J Surg Res. 2012;178:330–338. doi: 10.1016/j.jss.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 84.Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M. Surface functionalization of central venous catheter with mycofabricated silver nanoparticles and its antibiofilm activity on multidrug resistant Acinetobacter baumannii. Microb Pathog. 2020;138:103832. doi: 10.1016/j.micpath.2019.103832. [DOI] [PubMed] [Google Scholar]
- 85.Gondil VS, Chhibber S. Exploring potential of phage therapy for tuberculosis using model organism. Biomed Biotechnol Res J. 2018;2:9–15. doi: 10.4103/bbrj.bbrj_93_17. [DOI] [Google Scholar]
- 86.Barapatre A, Aadil KR, Jha H. Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour Bioprocess. 2016;3(1):3–8. doi: 10.1186/s40643-016-0083-y. [DOI] [Google Scholar]
- 87.Regí MV, González B, Barba II. Nanomaterials as promising alternative in the infection treatment. Int J Mol Sci. 2019;20(15):3806. doi: 10.3390/ijms20153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8:76. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bala Subramaniyan S, Senthilnathan R, Arunachalam J, Anbazhagan V. Revealing the significance of glycan binding property of butea monosperma seed lectin for enhancing the antibiofilm activity of silver nanoparticles against uropathogenic Escherichia coli. Bioconjug Chem. 2019;31(1):139–148. doi: 10.1021/acs.bioconjchem.9b00821. [DOI] [PubMed] [Google Scholar]
- 90.Pompilio A, Germiniani C, Bosco D, Rana R, Aceto A, Bucciarelli T, Scotti L, Bonaventura GD. Electrochemically synthesized silver nanoparticles are active against planktonic and biofilm cells of Pseudomonas aeruginosa and other cystic fibrosis-associated bacterial pathogens. Front Microbiol. 2018;9:1349. doi: 10.3389/fmicb.2018.01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo J, Quin S, Wei Y, Liu S, Peng H, Li Q, Luo L, Lv M. Silver nanoparticles exert concentration-dependent influences on biofilm development and architecture. Cell Prolif. 2019;52(4):e12616. doi: 10.1111/cpr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arya G, Sharma N, Mankamna R, Nimesh S. Antimicrobial silver nanoparticles: future of nanomaterials. In: Prasad R, editor. Microbial Nanobionics. Cham: Springer; 2019. pp. 89–119. [Google Scholar]
- 93.Loo CY, Young PM, Cavaliere R, Whitchurch CB, Lee WH, Rohanizadeh R. Silver nanoparticles enhance Pseudomonas aeruginosa PAO1 biofilm detachment. Drug Dev Ind Pharmacy. 2014;40(6):719–729. doi: 10.3109/03639045.2013.780182. [DOI] [PubMed] [Google Scholar]
- 94.Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, Khmel IA. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B Biointerfaces. 2013;102:300–306. doi: 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 95.Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against gram-negative and gram-positive bacteria. Nanoscale Res Lett. 2014;9:373. doi: 10.1186/1556-276X-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saraswathi VS, Kamarudheen N, BhaskaraRao KV, Santhakumar K. Phytoremediation of dyes using Lagerstroemia speciosa mediated silver nanoparticles and its biofilm activity against clinical strains Pseudomonas aeruginosa. J Photochem Photobiol B. 2017;168:107–116. doi: 10.1016/j.jphotobiol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Singh P, Pandit S, Beshay M, Mokkapati VR, Garnaes J, Olsson ME, Sultan A, Mackevica A, Mateiu RV, Lütken H, Daugaard AE (2018) Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif Cells Nanomed Biotechnol 46(sup3):S886-S899. 10.1080/21691401.2018.1518909 [DOI] [PubMed]
- 98.Muthuraman MS, Nithya S, Kumar VV, Christena LR, Vadivel V, Subramanian NS, Anthony SP. Green synthesis of silver nanoparticles using Nardostachys jatamansi and evaluation of its anti-biofilm effect against classical colonizers. Microb Pathog. 2019;126:1–5. doi: 10.1016/j.micpath.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 99.Habibipour R, Moradi-Haghgou L, Farmany A (2019) Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Inter J Nanomed 14:6891. 10.2147%2FIJN.S209912 [DOI] [PMC free article] [PubMed]
- 100.Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7(13):1701503. doi: 10.1002/adhm.201701503. [DOI] [PubMed] [Google Scholar]
- 101.Salman M, Rizwana R, Khan H, Munir I, Hamayun M, Rehman A, Amin K, Ahmed G, Khan M, Khan A, Amin FU. Synergistic effect of silver nanoparticles and polymyxin B against biofilm produced by Pseudomonas aeruginosa isolates of pus samples in vitro. Artif Cells Nanomed Biotechnol. 2019;47:2465–2472. doi: 10.1080/21691401.2019.1626864. [DOI] [PubMed] [Google Scholar]
- 102.Habash MB, Park AJ, Vis EC, Harris RJ, Khursigara CM. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob Agents Chemother. 2014;58(10):5818–5830. doi: 10.1128/AAC.03170-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.