Abstract
Purpose
Congenital hypopituitarism (CH) can cause significant morbidity or even mortality. In the majority of patients, the etiology of CH is unknown. Understanding the etiology of CH is important for anticipation of clinical problems and for genetic counselling. Our previous studies showed that only a small proportion of cases have mutations in the known ‘CH genes’. In the current project, we present the results of SNP array based copy number variant analysis in a family with unexplained congenital hypopituitarism.
Methods
DNA samples of two affected brothers with idiopathic CH and their mother were simultaneously analyzed by SNP arrays for copy number variant analysis and Whole Exome Sequencing (WES) for mutation screening. DNA of the father was not available.
Results
We found a 6 Mb duplication including GPR101 and SOX3 on the X-chromosome (Xq26.2-q27.1) in the two siblings and their mother, leading to 2 copies of this region in the affected boys and 3 copies in the mother. Duplications of GPR101 are associated with X-linked acrogigantism (the phenotypic ‘opposite’ of the affected brothers), whereas alterations in SOX3 are associated with X-linked hypopituitarism.
Conclusion
In our patients with hypopituitarism we found a 6 Mb duplication which includes GPR101, a gene associated with X- linked gigantism, and SOX3, a gene involved in early pituitary organogenesis that is associated with variable degrees of hypopituitarism. Our findings show that in duplications containing both GPR101 and SOX3, the growth hormone deficiency phenotype is dominant. This suggests that, if GPR101 is duplicated, it might not be expressed phenotypically when early patterning of the embryonic pituitary is affected due to SOX3 duplication. These results, together with the review of the literature, shed a new light on the role of GPR101 and SOX3 in pituitary function.
Electronic supplementary material
The online version of this article (10.1007/s11102-020-01101-8) contains supplementary material, which is available to authorized users.
Keywords: Pituitary gland, Transcription factors, Gene duplication, Acromegaly, G-protein coupled receptor
Introduction
Normal development and function of the pituitary gland is crucial for several important physiological processes in the human body, such as growth, reproduction, lactation, response to stress, blood pressure, energy management and metabolism [1, 2]. Congenital hypopituitarism (CH) is a rare disorder with an estimated incidence of 1:3000–1:4000 live births. It is characterized by the diminished production or secretion of one or more of the pituitary hormones [3, 4].
Growth hormone deficiency (GHD) is the most common form of hypopituitarism. Both children and adults with GHD may present with short stature, increased fat mass and decreased lean body mass, delayed skeletal maturation, truncal obesity, abnormal glucose and lipid metabolism and an increased risk of cardiovascular disease [3, 4]. GHD can either be isolated (IGHD) or combined with other pituitary hormone deficiencies (CPHD). CPHD is defined as any combination of two or more pituitary hormone deficiencies, whereas in panhypopituitarism all pituitary hormones are deficient [5].
The vast majority of GHD cases are idiopathic. Up to 30% of cases are familial, which suggests a genetic etiology [6, 7]. As a result of dedicated genetic studies, such as the Dutch HYPOPIT study, our knowledge about the genetic etiology of GHD has drastically improved. Frequent causes of IGHD are mutations in the Growth Hormone 1 (GH1) gene and the Growth Hormone Releasing Hormone Receptor (GHRHR) gene [4, 7]. In Dutch CPHD patients we have previously studied several known disease related genes encoding pituitary transcription factors, such as PROP1 [MIM 601,538), HESX1 (MIM 601802), POU1F1 (MIM 173110), LHX3 (MIM 600577), LHX4 (MIM 602146), OTX2 (MIM 600037), SHH (MIM 600725), HHIP (MIM 606178) and GLI2 (MIM 165230). However, we found a genetic explanation in only 10% of the patients, leaving the majority of cases unsolved [8–12]. When candidate gene analysis has turned out negative, array based copy number variation analysis and Next Generation Sequencing (NGS) is a next step. In this study we present the surprising results of NGS in two brothers with idiopathic CH.
Material and methods
Genetic analysis
DNA isolation
Genomic DNA of the two brothers and their mother was extracted from peripheral whole blood samples according to standard procedures. The samples were subsequently analyzed by SNP array and Whole Exome Sequencing (WES).
Copy number variant analysis by SNP array
DNA was hybridized to Illumina Human CytoSNP850K SNP arrays according to standard protocol. Copy number analysis was performed using Nexus 8.0 from BioDiscovery.
WES
Genomic DNA was fragmented into 200–400 base pairs (bp) fragments using Covaris Adaptive Focused Acoustics shearing according to the manufacturer’s instructions (Covaris, Inc., Woburn, MA). Illumina TruSeq DNA Library preparation (Illumina, Inc., San Diego, CA) was performed on a Caliper Sciclone NGS workstation (Caliper Life Sciences, Hopkinton, MA), followed by exome capture using the Nimblegen SeqCap EZ V2 kit (Roche Nimblegen, Inc., Madison, WI). This capture targets 44 Mb of exonic regions covering 30,246 coding genes, 329,028 exons and 710 miRNAs. Paired-end 2 × 100 bp sequencing was performed at 6 samples per lane on Illumina HiSeq2000 sequencer using Illumina TruSeq V3, resulting in 6 Gb of sequencing data.
Literature search
In order to explain the phenotype of the two brothers, we performed an extensive literature search of all genes included in the duplicated region using OMIM, NCBI, MGI and Pubmed online databases. PubMed search was carried out using the names of all duplicated genes, combined with the terms [‘hypopituitarism’ OR ‘growth retardation’ OR ‘growth hormone deficiency’ OR ‘growth hormone’ OR ‘combined pituitary hormone deficiency’] AND ‘congenital’.
Results
Clinical data
The index case, an Italian male with idiopathic CH, presented with growth retardation late in life, with a height SDS of—2.1 at the age of 16 years. BMI was normal. The GH peak during a GH stimulation test was 2.16 µg/L (ref > 6.66 µg/L). Apart from GHD, he was diagnosed with central hypothyroidism, hypogonadism and hypocortisolism. Magnetic resonance imaging (MRI) revealed anterior pituitary hypoplasia (APH) and an ectopic posterior pituitary (EPP). His brother also had growth retardation. Despite the fact that he did not present until the age of 21 years, he had always been small (height—3 SDS). The GH peak during his GH test was 1.86 µg/L. Although the original laboratory values and MRI images of both brothers were not available, the medical files reported that all pituitary hormone concentrations as well as IGF-I and IGFBP3 were low. Both brothers had normal cognition and no other birth defects. The unaffected mother had no pituitary hormone deficiencies and a height of 153.5 cm (− 1.5 SDS). Clinical data of the father were not available.
Genetic analysis
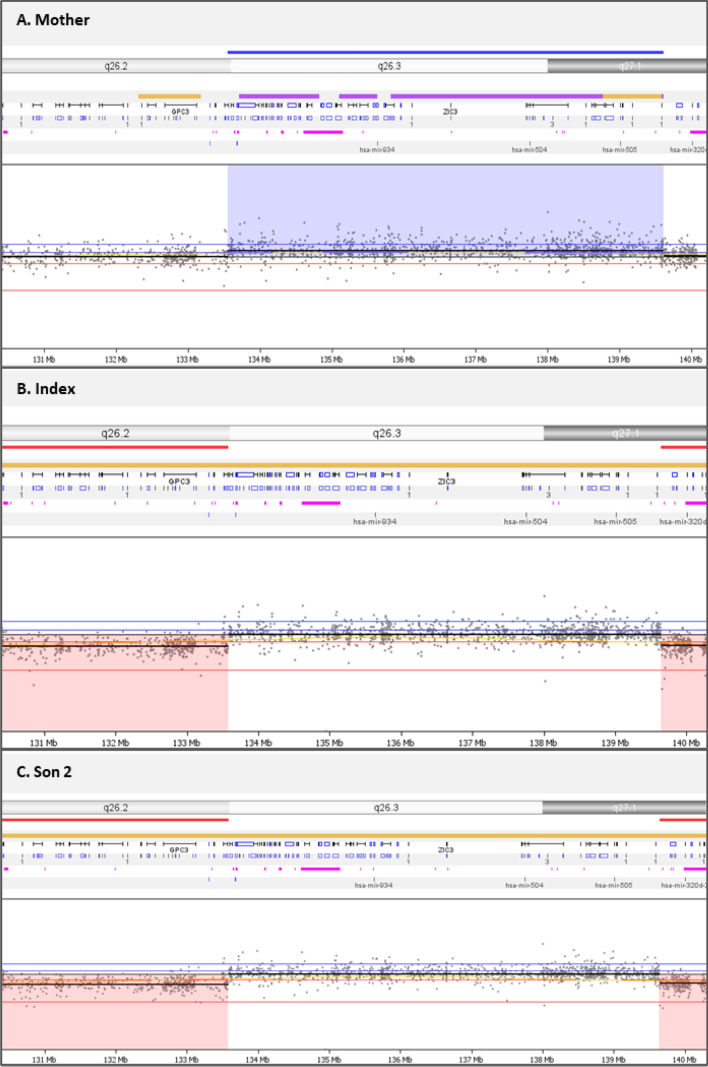
SNP array data analysis revealed a 6 Mb duplication of chromosome Xq26.2-q27.1 in all 3 subjects. Figure 1 shows the 6 Mb duplication that results in two copies of part of the X-chromosome in the affected brothers and 3 copies in the mother. The duplicated region (chrX: 133.553.751–139,613,851; build 37) contains 70 genes (Fig. 2a). Table 1 shows the phenotypes associated with defects in these genes. The duplicated region includes GPR101 (MIM 300393), a gene previously described in patients with X-linked acrogigantism (X-LAG) and acromegaly, which is the opposite clinical phenotype of our patients.
Fig. 1.
SNP array results of the two brothers and their mother. LogR ratios and B-allele frequencies (BAF) are indicated. Duplications are shown between vertical bars for a the mother, b and c the affected brothers
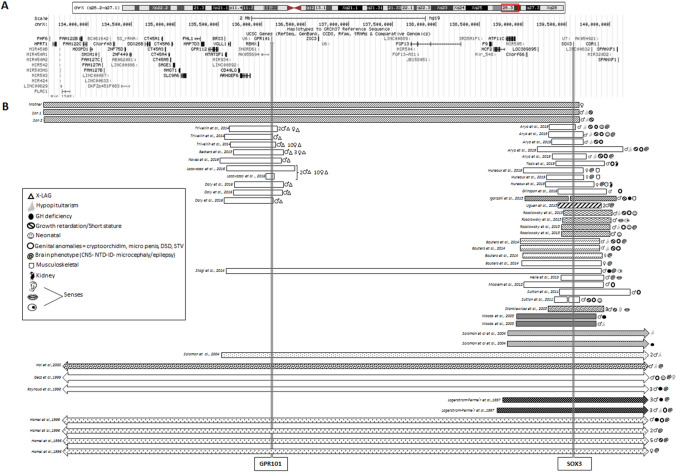
Fig. 2.
a Overview of 70 genes included in the duplicated region. b All duplications and deletions found in the literature involving GPR101 and SOX3
Table 1.
Genes included in the duplicated region of the two brothers, clustered by disease association
| Gene symbol | Gene description | Disease association | |
|---|---|---|---|
| Acromegaly Gigantism | GPR101 | G protein-coupled receptor 101 | X-linked acro-gigantism (X-LAG),pituitary adenoma, excessive GH secretion and rapid growth beginning in early childhood |
| Short stature | MOSPD1 | Motile sperm domain containing protein 1 | Short stature and abnormal right ventricle development |
| Cancer |
LINC00629 CXorf48 |
Long intergenic non-protein coding RNA 629 Chromosome X open reading frame 48 |
Gastric cancer Chronic myeloid leucemia |
| LINC00086 | Long intergenic non-protein coding RNA 86 | Gastric cancer | |
| CT45A1 | Cancer/testis antigen family 45, member A1 | ||
| CT45A3 | Cancer/testis antigen family 45, member A3 | ||
| CT45A4 | Cancer/testis antigen family 45, member A4 | ||
| CT45A5 | Cancer/testis antigen family 45, member A5 | ||
| CT45A6 | Cancer/testis antigen family 45, member A6 | ||
| SAGE1 | Sarcoma antigen 1 | Glioma and urothelial Cancer | |
| VGLL1 | Vestigial like 1 (Drosophila) | ||
| Intellectual disability | PHF6 | PHD finger protein | Borjeson-Forssman-Lehmann syndrome (BFLS), a disorder characterized by intellectual disability (ID), epilepsy, hypogonadism, hypometabolism, obesity, swelling of subcutaneous tissue of the face, narrow palpebral fissures, and large ears |
| FGF13 | Fibroblast growth factor 13 | Borjeson-Forssman-Lehmann syndrome (BFLS) | |
| SLC9A6 | Solute carrier family 9, subfamily A | Intellectual disability (ID), X-linked syndromic cognitive disability, Christianson type | |
| RBMX | RNA binding motif protein, X-linked | X-linked intellectual disability syndrome | |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 | Lesch-Nyhan syndrome (neurological and behavioural abnormalities and the overproduction of uric acid) | |
| SOX3 | SRY (sex determining region Y)-box 3 | X-linked intellectual disability (ID) with growth hormone deficiency (GDH), X-linked hypopituitarism, 46,XX, Disorder of Sex Development (DSD) and neural tube defects (NTD) | |
| Reproduction | MIR503HG | MIR503 host gene (non-protein coding) | Pre-Eclampsia |
| PLAC1 | Placenta-specific 1 (PLAC1) | Pre-Eclampsia | |
| Mir_1302 | Rfam model RF00951 hit found at contig region AL672032.6/126123-126174 | Recurrent embryo implantation failure (RIF), male infertility | |
| Hematologic diseases | F9 | Coagulation factor IX (F9) | Factor IX deficiency, also called haemophilia B or Christmas disease |
| ATP11C | ATPase, class VI, type 11C | Congenital haemolytic anaemia | |
| Other | MIR503 | microRNA 503 | Tumor suppression |
| CD40LG | CD40 ligand (CD40LG) | Hyper-IgM syndrome | |
| ZIC3 | Zic family member 3 | Heterotaxy and congenital heart disease | |
| DDX26B | DEAD/H box polypeptide 26B | Autism spectrum disorder | |
| FHL1 | Four and a half LIM domains 1 | Emery-Dreifuss muscular dystrophy, Reducing body myopathy (RBM), Uruguay faciocardiomusculoskeletal syndrome |
The duplication also includes SOX3 (MIM 313430), a gene associated with variable degrees of X-linked hypopituitarism and GHD, sometimes combined with intellectual disability (ID). Additional information of all genes located in the duplicated region is documented in Supplementary Table 1. WES data of the brothers and their mother returned negative.
Literature search
GPR101 and SOX3 duplications and deletions published to date, with the associated clinical findings are shown in Fig. 2b. Of all previously reported X-LAG cases, 68% were females with germline duplications and 31% were males carrying somatic duplications. For SOX3 duplications, there was a male predominance with 89.1% males and 8.9% females, respectively. SOX3 duplications were associated with variable phenotypes, ranging from hypopituitarism, GHD only, intellectual disability (ID), neural tube defect (NTD), Disorders of Sex Development (DSD), or a combination of these phenotypes (Fig. 2b). Of the reported males with SOX3 duplications, 42% had hypopituitarism, 28% had GHD and 48% had ID. Among males with duplications that included SOX-3, DSD was present in 32% and NTD in 8%. Eighty percent of affected females had a NTD, of which 60% were fetuses of terminated pregnancies. Sixty-nine percent of the described SOX3 cases were of familial origin, where the carrier mother transmitted the duplication to the affected offspring. Table 2 shows the endocrine phenotypes that have been reported in the literature in patients with SOX3 duplications. Table 3 shows non-endocrine features associated with SOX3 duplications. Although less frequent, SOX3 point mutations and poly alanine tract mutations have also been reported. Pituitary MRI findings and hormone deficiencies associated with these mutations are shown in Table 4.
Table 2.
Endocrine phenotypes of SOX3 duplications
| Refs | Case | Sex | Growth | Gonads | Hormone deficiencies | Neonatal | MRI |
|---|---|---|---|---|---|---|---|
| [38] | F1 | F | NA | Chiari II malformation | |||
| [38] | F2 | F | NA | Chiari II malformation, voluminous AP, absent PP | |||
| [38] | F3 | F | NA | Chiari II malformation | |||
| [39] | F IV | ? | Hydrocephalus | ||||
| [39] | F | F | hydrocephalus | ||||
| [40] | III.2 | F | SS/GR | ||||
| [40] | II.2 | F | SS/GR | ||||
| [40] | III.4 | F | SS/GR | ||||
| [32] | I | M | FTT | Micropenis, hypoplastic scrotum | GH, LH/FSH, TSH, ACTH | Jaundice, hypoglycemia | APH, EPP |
| [32] | II | M | SS/GR | Micropenis and STV | GH, ACTH, TSH, LH/FSH | Hypoglycemia | ACC, hydrocephalus |
| [32] | III | M | SS/GR | Slender phallus and STV | LH/FSH | Thin CC, hydrocephalus | |
| [32] | IV | M | SS/GR | GH, TSH | APH, EPP | ||
| [32] | V | M | SS/GR | Micropenis and STV, hypogonadism, pubertal delay | GH, LH/FSH | Partial ACC, absent SP, heterotopic grey matter | |
| [21] | M | STV and coronal hypospadias | NA | ||||
| [41] | M | Hypospadias, cryptorchidism, ovarian tissue and primary follicles | Testosterone, AMH | ||||
| [42] | Index | M | Mild SS/GR | mild GH | |||
| [43] | F2 | M | |||||
| [43] | F3 | M | |||||
| [44] | C1 | M | SS/GR | Microphallus and undescended testes | Hypopituitarism,GH, testosterone | Hypoglycemia, Jaundice | CVP, shallow pituitary fossa |
| [44] | C2 | M | Thin CC, poorly developed pituitary gland | ||||
| [44] | C3 | M | Microphallus, small penis, undescended testis, underdeveloped scrotum | All including diabetes insipidus | Hypoglycemia | Absent pituitary gland and stalk | |
| [44] | C4 | M | Hypoglycemia, | APH, hypoplastic pituitary stalk | |||
| [37] | M | GH | |||||
| [39] | III.7 | M | Growth delay | Undescended testicle, pubertal delay | GH, LH/ FSH | ||
| [39] | III.1 | M | SS | Small testicles | GH, LH/ FSH | Temporal brain atrophy | |
| [45] | M | ||||||
| [46] | M | Bifid scrotum and penoscrotal hypospadias | |||||
| [47] | A | M | SRY, 46, XX negative | ||||
| [24] | 2 | M | GH, TSH, ACTH, LH/FSH | APH, undescended PP, absent infundibulum | |||
| [24] | 1 | M | GH | APH, undescended hypoplastic infundibulum | |||
| [28] | A II.1 | M | GH, TSH | ||||
| [28] | A II.2 | M | GH | ||||
| [28] | B II.1 | M | GH, TSH | ||||
| [28] | B II.2 | M | GH, TSH | ||||
| [48] | M | Cryptorchidism with a small penis, hypogonadism | Hypoglycaemia Microcephaly | ||||
| [30] | M | Pan-hypopituitarism | |||||
| [29] | 1 | M | GH | ||||
| [29] | 2 | M | GH | ||||
| [29] | 3 | M | GH | ||||
| [27] | IV | M | GH, NA | ||||
| [27] | IV.4 | M | GH | ||||
| [27] | IV.5 | M | GH | ||||
| [27] | II.5 | M | Hypogonadal | GH, TSH, PRL | |||
| [27] | III.3 | M | GH, TSH, PRL | ||||
| [27] | III.9 | M | Hypogonadal | GH, TSH, PRL | |||
| [31] | III.9 | M | Mild gynaecomastia | GH | |||
| [31] | II.6 | M | NA | ||||
| [31] | II.7 | M | NA | ||||
| [31] | II.8 | M | SS | ||||
| [31] | III.3 | M | GR | ||||
| [31] | III.6 | M | SS | ||||
| [31] | III.7 | M | SS | ||||
| [31] | IV.6 | M | SS | ||||
| Present case | M | SS/GR | All pituitary hormones | APH, EPP | |||
| Present case | M | SS/GR | All pituitary hormones | NA |
AP anterior pituitary, APH anterior pituitary hypoplasia, EPP ectopic posterior pituitary, EP ectopic pituitary, NA not assessed, ACC agenesis corpus callosum, CC corpus callosum, SP septum pellucidum, FTT failure to thrive, STV small testicle volume, SS/GR short stature or growth retardation
Table 3.
Non- endocrine phenotypes of SOX3 duplications
| Refs | Case | Sex | ID | Myelum | Senses | Speech | Musculo-skeletal | Kidney | Additional findings |
|---|---|---|---|---|---|---|---|---|---|
| [39] | F IV | ? | − | Lumbosacral MMC | |||||
| [39] | F | F | − | Lumbosacral MMC and myeloschisis | |||||
| [40] | III.2 | F | − | Hearing impairment | Dyslalia | ||||
| [40] | II.2 | F | − | Hearing impairment | Dyslalia | Premature aging, Epilepsy, aneurysm | |||
| [40] | III.4 | F | − | Hearing impairment | Dyslalia | ||||
| [31] | III.2 | F | + | ||||||
| [38] | F1 | F | − | MMC | Clubfeet, calf muscle atrophy, 3 sacral vertebrae | ||||
| [38] | F2 | F | − | Lumbosacral MMC | Varus feet | ||||
| [38] | F3 | F | − | Lumbosacral MMC | Calf muscle hypotrophy | Bilateral kidney hypertrophy | |||
| [32] | I | M | ++ | Feeding difficulties | |||||
| [32] | II | M | ++ | Other complex disabilities | |||||
| [32] | III | M | − | Lumbral MMC (repaired after birth) | |||||
| [32] | IV | M | + | ||||||
| [32] | V | M | + | Hyposmia, dysgeusia | |||||
| [21] | M | − | Right kidney hypoplasia | ||||||
| [42] | Index | M | − | Madelung deformity of the forearm, hypoplastic tibia and fibula, clubfeet | |||||
| [43] | F2 | M | − | MMC | |||||
| F3 | M | − | MMC | ||||||
| [44] | C1 | M | − | ||||||
| [44] | C2 | M | − | Ocular abnormalities | Raspy voice language delay | ||||
| [44] | C3 | M | + | ||||||
| [44] | C4 | M | − | ||||||
| [39] | III.7 | M | + | High pitched voice | |||||
| [39] | III.1 | M | Obesity | ||||||
| [37] | M | + | Ocular dyspraxia | ||||||
| [45] | M | + | Hyperphagia | Dysarthria | Behavior problems, minor facial anomalies | ||||
| [46] | M | − | |||||||
| [47] | A | M | − | ||||||
| [47] | C | M | − | ||||||
| [24] | 1 | M | − | ||||||
| [24] | 2 | M | − | ||||||
| [28] | A II.1 | M | − | ||||||
| [28] | A II.2 | M | − | ||||||
| [28] | B II.1 | M | − | ||||||
| [28] | B II.2 | M | − | ||||||
| [30] | M | − | Spina bifida | ||||||
| [48] | M | + | Conductive hearing loss | Single kidney | Feeding difficulties | ||||
| [29] | 1 | M | + | ||||||
| [29] | 2 | M | + | ||||||
| [29] | 3 | M | + | ||||||
| [27] | IV | M | + | ||||||
| [27] | IV.4 | M | + | ||||||
| [27] | IV.5 | M | + | ||||||
| [27] | II.5 | M | + | ||||||
| [27] | III.3 | M | + | ||||||
| [27] | III.9 | M | + | ||||||
| [31] | III.9 | M | + | ||||||
| [31] | II.6 | M | + | ||||||
| [31] | II.7 | M | + | ||||||
| [31] | II.8 | M | + | ||||||
| [31] | III.3 | M | + | ||||||
| [31] | III.6 | M | + | ||||||
| [31] | III.7 | M | + | Postaxial polydactyly of both hands | |||||
| [31] | IV.6 | M | Truncal obesity and puffy face | ||||||
| Present case | M | − | |||||||
| Present case | M | − |
Table 4.
Single nucleotide variants, poly alanine tract insertions and deletions of SOX3 reported in literature and corresponding clinical findings
| Refs | Sex | ID | Clinical findings | Affected pituitary hormones | MRI findings | SOX 3 mutation | Functional relevance |
|---|---|---|---|---|---|---|---|
| Single nucleotide variants | |||||||
| [34] | 1 | Mild ID | GH | Small AP; EPP | c.424C > A; p.142T | Predicted as disease-causing transcription activation | |
| [34] | 2 | GH | APH | c.424C > A; p.142T | |||
| [35] | M1 | Mild ID | GH, THD, LH/FSH | AP | c.449C > A; p.S150Y | Predicted as disease-causing | |
| [35] | M2 | Mild ID | GH, THD, LH/FSH | DP | c.449C > A; p.S150Y | ||
| [35] | M3 | Mild ID | GH, LH/FSH | EP | c.449C > A; p.S150Y | ||
| [36] | M | Severe SS/GR | GH, LH and FSH | APH | c.14G > A; p.R5Q | No functional effect, benign likely benign | |
| [24] | c.127G > A; p.A43T | polymorphism | |||||
| Poly alanine tract variants | |||||||
| [25] | M | Short stature | GH, TSH, ACTH | APH; EPH | p.239-245del7 | Increased transcription activity | |
| [36] | F | Normal intelligence | SS/GR | GH, TSH, LH/FSH | Enlarged AP; NPP | p.243-248del6 | Transcription activation; Repress β-catenin |
| [37] | M | Normal intelligence | SS/GR | All | NA | p.A240-241ins7 | Loss of transcriptional activity; Reduced nuclear transport unable to repress β-catenin |
| [37] | M | Learning difficulties | SS/GR | GH | APH; EPP | ||
| [49] | M | SS/GR | GH | Normal AP; EPP | p.A240-241ins7 | Loss of transcriptional activity; Reduced nuclear transport unable to repress β-catenin | |
| [49] | M | Normal intelligence | GH | APH; EPP | |||
| [24] | M | Normal intelligence | SS/GR | GH, TSH, LH/FSH, ACTH | NA; AHI | p.A240-241ins7 | Loss of transcriptional activity; Reduced nuclear transport unable to repress β-catenin |
| [16] | M | Normal intelligence | GH, TSH, LH/FSH, ACTH | APH; EPP, AHI | |||
| [16] | M | SS/GR | GH, TSH, LH/FSH, ACTH | APH; EPP, AHI | |||
| [33] | M | X-linked ID | SS/GR | GH | NA | p.A234-245ins11 | |
| [33] | M | Severe ID | SS/GR | – | NA | p.240-248del9 | Transcription activation; Repress β-catenin |
PA polyalanine, ID intellectual disability, APH anterior pituitary hypoplasia, EPP ectopic posterior pituitary, AHI absent or hypoplastic infundibulum, AP absent pituitary gland, DP dysplastic pituitary gland, EP ectopic pituitary gland, NA not available (adapted from Tagaki et al. 2013)
Discussion
We performed SNP array analysis and WES in a family with unexplained hypopituitarism. SNP array data revealed a 6 Mb duplication of Chromosome X at position Xq26.2-q27.1. The duplication included GPR101, a single exon gene that has been associated with X-LAG and acromegaly. GPR101 encodes an Orphan G-protein Coupled Receptor (GPCR) that is strongly expressed in the hypothalamus in rodents [13–15]. In humans, high expression of GPR101 is seen during fetal development of the pituitary gland while expression is low in the adult pituitary, suggesting that GPR101 is predominantly active during proliferation and maturation of the pituitary. Overexpression of GPR101 leads to increased Growth Hormone-Releasing Hormone (GHRH) expression, which causes hyperplasia of the pituitary and leads to increased GH and IGF-I concentrations [14].
Xq26.3 (micro) duplications including GPR101 have been described in patients with X-linked acro-gigantism (X-LAG). X-LAG is characterized by early age pediatric-onset gigantism associated with mixed GH-PRL secreting pituitary adenomas, or hyperplasia that leads to GH and IGF-I overexpression resulting in gigantism [14, 16–19]. In 2014, the smallest region of overlap (SRO) was reported, which was shared by all X-LAG patients, and which contains four genes: CD40LG (MIM 300386), ARHGEF6 (MIM 300267), RBMX (MIM 300199) and GPR101 (MIM 300393). Of these four genes, only GPR101 was overexpressed in pituitary samples of X-LAG patients [16]. Two years later, a smaller duplication including GPR101 only was reported in a patient with X-LAG, thereby supporting the causative role of GRP101 [16]. In addition, GPR101 variants have been identified in pituitary adenoma samples of patients with sporadic acromegaly [16].
The duplication of an acrogigantism gene in the two boys with the phenotypic opposite (hypopituitarism) was unexpected. However, the duplication also included SOX3, also a single exon gene, which has been associated with variable degrees of hypopituitarism. SOX3 belongs to the SOX (SRY-related high mobility group- box) family of transcription factors that is expressed in neuro-epithelial progenitor and stem cells in the earliest stages of embryogenesis [20–23]. SOX3 protein is required for normal development of the brain, pituitary and face in mice and humans. Correct gene dosage of SOX3 is critical for the development of the hypothalamo-pituitary axis and for cognitive development [24–26].
Duplications including SOX3 have been associated with variable clinical phenotypes, including X-linked intellectual disability (ID), GHD, X-linked hypopituitarism (XH), SRY-negative 46,XX disorders of sex development (DSD) and neural tube defects (NTD) [27–33]. The severity of the phenotype is not dependent on the size of the duplication. Hypopituitarism is the most frequently reported phenotype among males with SOX3, duplication, followed by GHD. Most of the reported cases were of familial origin with transmission of the duplicated region from mother to son. Transmission to females often resulted in a NTD phenotype, and in most cases elective termination of pregnancies. However females with SOX3 duplications can also have a normal phenotype, which is probably due to non-random X-inactivation of the affected X chromosome. Normal X-inactivation is a random process which is thought to have arisen during the differentiation of mammalian sex chromosomes to achieve an equal dosage of X chromosome genes in females and males (as males only possess a single copy of the X chromosome). Non-random X inactivation might explain the presence or absence of a X-LAG phenotype. In females with GPR101 duplications, non-random inactivation of the affected allele can lead to a normal phenotype. When inactivation of the affected X does not occur at a high rate, leaving expression of the affected copy, females with GPR101 duplications do have the X-LAG phenotype. This mechanism is likely also true for females with SOX3 duplications. These females often have a normal phenotype, due to the non-random inactivation of the affected allele. Only few females with SOX3 duplications are clinically affected, probably due to the lack of inactivation of the affected allele.
Apart from SOX3 duplications, SOX3 single nucleotide substitutions (three point variants and one polymorphism) have also been described. Two variants (p.S150Y and p.142T), predicted as pathogenic, were found in patients with pituitary anomalies with GHD or hypopituitarism with ID [24, 34–36]. Several insertions and deletions found in the first poly-alanine tract of SOX3 have been described in patients with short stature with IGHD, with and without cognitive impairment [5, 24, 25, 33]
Although rare, large duplications and deletions including both GPR101 and SOX3 have previously been reported [28, 30, 31, 37]. A Xq26.1–q27.3 duplication was reported in 2 male patients with hypopituitarism only [28], whereas a deletion of this region was reported in a male patient with panhypopituitarism who also had ID, spina bifida (NTD), and growth retardation [30]. Another Xq26.3–27.3 duplication was reported in a male patient with severe growth retardation, ocular abnormalities, hypotonia, seizures and developmental delay [37]. Hamel et al. reported the largest duplication (Xq24–q27.3) containing GPR101 and SOX3 in a male patient with ID, GHD and growth retardation [31].
These data support our current finding that, in duplications containing both GPR101 and SOX3, the GHD phenotype is dominant. This is probably explained by the timing of GPR101 and SOX3 expression. SOX proteins are crucial for the patterning and morphogenetic processes occurring in the early embryo. During early embryogenesis, cells are organized by tissue patterning. This means that induction of fate-determining genes is spatially controlled to generate patterns for cell differentiation and maturation. GPR101 is predominantly active during maturation of the pituitary [14], which take place at a later stage. As SOX3 is affected, and patterning is thus already disturbed, the later possible effects of GPR101 overexpression in the pituitary might be overruled. We cannot disregard the possibility that dysregulation of other genes in this duplicated region might contribute to the suppression of GPR101.
In conclusion We found a 6 Mb duplication of Xq26.2-q27.1 in two brothers with hypopituitarism, which included GPR101, a gene associated with the phenotypic opposite: X-linked acrogigantism. Additional analysis showed that the duplication also included SOX3, a gene involved in early pituitary organogenesis which is associated with variable degrees of hypopituitarism. Our findings, supported by the literature, show that in duplications containing both GPR101 and SOX3, the GHD phenotype is dominant. This suggests that GPR101 duplication is overruled when early patterning of the embryonic pituitary is affected due to SOX3 duplication. The fact that the mother (carrying the same duplication as the two boys) was unaffected, is probably due to non-random X inactivation. Our results, combined with our genotype–phenotype analysis, sheds a new light on the genetic background of both hypopituitarism and gigantism.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PNG 75 kb). Supplemental figure 1: Overview of previously described point mutations in GPR101 (3A) and SOX3 (3B). A. Overview of GPR101 gene mutations described to date in literature and corresponding clinical findings. B. Overview of previously described point mutations found in the 1st poly-alanine tract of SOX3 protein with the corresponding clinical findings. Allele frequency in normal population is shown below nucleotide and protein sequence
Electronic supplementary material 2 (PDF 69 kb)
Acknowledgements
We thank Pfizer for supporting WES and SNP array analysis.
Abbreviations
- ACTH
Adrenocorticotrophic hormone
- ARHGEF6
Rho guanine nucleotide exchange factor 6
- AVP
Vasopressin
- CD40LG
CD40 ligand
- CH
Congenital hypopituitarism
- PHD
Pituitary hormone deficiency
- IGHD
Isolated growth hormone deficiency
- CPHD
Combined pituitary hormone deficiency
- FSH
Follicle stimulating hormone
- GH
Growth hormone
- GH1
Growth hormone 1 gene
- GHD
Growth hormone deficiency
- GHRH(R)
Growth hormone releasing hormone (receptor)
- GLI2
Glioma-associated oncogene family zinc finger 2
- GPCR
G protein coupled receptor
- GPR101
G protein-coupled receptor 101
- HESX1
HESX homeobox 1
- HHIP
Hedgehog interacting protein
- HPRT
Hypoxanthine phosphoribosyltransferase 1
- ID
Intellectual disability
- IGF-1
Gene encoding insulin-like growth factor -1
- IGF-I
Insulin-like growth factor -1 (protein)
- IGFBP
Insulin-like growth factor binding protein
- LH
Luteinizing horrmone
- LHX3
LIM homeobox 3
- LHX4
LIM homeobox 4
- MRI
Magnetic resonance imaging
- NTD
Neural tube defect
- OTX2
Orthodenticle homeobox 2
- OXY
Oxytocin
- POU1F1
POU domain, class 1 transcription factor 1 (PIT1)
- PRL
Prolactin
- PROP1
Prophet of POU1F1
- RBMX
RNA-binding motif protein, X chromosome
- SOX3
Sex determining region Y box 3
- TSH
Thyroid stimulating hormone
- X-LAG
X-linked acromegaly
Author contributions
ME, AV, AHK, JV, JA, RP, SN and JV have nothing to declare. LdG received an Investigator Initiated Research Grant from Pfizer.
Funding
For this project, we received financial support from Pfizer Netherlands.
Compliance with ethical standards
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We obtained approval from the medical ethics committees of all participating hospitals.
Informed consent
Informed consent was obtained from the individuals participating in this study and their parents if they were younger than 18 years.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Melitza S. M. Elizabeth and Annemieke J. M. H. Verkerk contributed equally to this manuscript.
References
- 1.Meazza C, et al. Metabolic parameters and adipokine profi le in growth hormone deficient (GHD) children before and after 12-month GH treatment. Horm Metab Res. 2013;45:1–5. doi: 10.1055/s-0033-1358730. [DOI] [PubMed] [Google Scholar]
- 2.Youngblood JL, Coleman TF, Davis SW. Regulation of pituitary progenitor differentiation by beta-catenin. Endocrinology. 2018;159(9):3287–3305. doi: 10.1210/en.2018-00563. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY. Diagnosis and treatment of hypopituitarism. Endocrinol Metab (Seoul) 2015;30(4):443–455. doi: 10.3803/EnM.2015.30.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Graaff L (2008) Genetic and non-genetic causes of isolated growth hormone deficiency and combined pituitary hormone deficiency: results of the HYPOPIT study
- 5.Alatzoglou KS, et al. Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocr Rev. 2014;35(3):376–432. doi: 10.1210/er.2013-1067. [DOI] [PubMed] [Google Scholar]
- 6.Castinetti F, et al. GPR101 mutations are not a frequent cause of congenital isolated growth hormone deficiency. Horm Metab Res. 2016;48(6):389–393. doi: 10.1055/s-0042-100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks JS. Congenital hypopituitarism. Clin Perinatol. 2018;45(1):75–91. doi: 10.1016/j.clp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Castinetti F, et al. Mechanisms in endocrinology: an update in the genetic aetiologies of combined pituitary hormone deficiency. Eur J Endocrinol. 2016;174(6):R239–R247. doi: 10.1530/EJE-15-1095. [DOI] [PubMed] [Google Scholar]
- 9.Gorbenko del Blanco D, et al. Single-nucleotide variants in two Hedgehog genes, SHH and HHIP, as genetic cause of combined pituitary hormone deficiency. Clin Endocrinol (Oxf) 2013;78(3):415–423. doi: 10.1111/cen.12000. [DOI] [PubMed] [Google Scholar]
- 10.Gorbenko Del Blanco D, et al. A novel OTX2 mutation in a patient with combined pituitary hormone deficiency, pituitary malformation, and an underdeveloped left optic nerve. Eur J Endocrinol. 2012;167(3):441–452. doi: 10.1530/EJE-12-0333. [DOI] [PubMed] [Google Scholar]
- 11.de Graaff LC, et al. PROP1, HESX1, POU1F1, LHX3 and LHX4 mutation and deletion screening and GH1 P89L and IVS3+1/+2 mutation screening in a Dutch nationwide cohort of patients with combined pituitary hormone deficiency. Horm Res Paediatr. 2010;73(5):363–371. doi: 10.1159/000308169. [DOI] [PubMed] [Google Scholar]
- 12.Elizabeth M, et al. Genetic screening of regulatory regions of pituitary transcription factors in patients with idiopathic pituitary hormone deficiencies. Pituitary. 2018;21(1):76–83. doi: 10.1007/s11102-017-0850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135(3):561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DK, et al. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene. 2001;275(1):83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- 15.Bates B, et al. Characterization of Gpr101 expression and G-protein coupling selectivity. Brain Res. 2006;1087:1–14. doi: 10.1016/j.brainres.2006.02.123. [DOI] [PubMed] [Google Scholar]
- 16.Trivellin G, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363–2374. doi: 10.1056/NEJMoa1408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckers A, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22(3):353–367. doi: 10.1530/ERC-15-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacovazzo D, et al. Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol Commun. 2016;4(1):56. doi: 10.1186/s40478-016-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacovazzo D, Korbonits M. Gigantism: X-linked acrogigantism and GPR101 mutations. Growth Horm IGF Res. 2016;30–31:64–69. doi: 10.1016/j.ghir.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Pevny LH, Lovell-Badge R. Sox genes find their feet. Curr Opin Genet Dev. 1997;7(3):338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- 21.Tasic V, et al. Duplication of the SOX3 gene in an sry-negative 46, XX male with associated congenital anomalies of kidneys and the urinary tract: case report and review of the literature. Balkan J Med Genet. 2019;22(1):81–88. doi: 10.2478/bjmg-2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collignon J, et al. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development. 1996;122(2):509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- 23.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16(4):182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 24.Woods KS, et al. Over- and underdosage of SOX3 is associated with infundibular hypoplasia and hypopituitarism. Am J Hum Genet. 2005;76(5):833–849. doi: 10.1086/430134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi M, et al. A novel mutation in SOX3 polyalanine tract: a case of Kabuki syndrome with combined pituitary hormone deficiency harboring double mutations in MLL2 and SOX3. Pituitary. 2014;17(6):569–574. doi: 10.1007/s11102-013-0546-5. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre V, et al. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39(12):2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagerström-Fermér M, et al. X-linked recessive panhypopituitarism associated with a regional duplication in Xq25-q26. Am J Hum Genet. 1997;60(4):910–916. [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon NM, et al. Increased gene dosage at Xq26-q27 is associated with X-linked hypopituitarism. Genomics. 2002;79(4):553–559. doi: 10.1006/geno.2002.6741. [DOI] [PubMed] [Google Scholar]
- 29.Raynaud M, et al. X-linked mental retardation with isolated growth hormone deficiency is mapped to Xq22-Xq27.2 in one family. Am J Med Genet. 1998;76(3):255–261. [PubMed] [Google Scholar]
- 30.Hol FA, et al. Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics. 2000;69(2):174–181. doi: 10.1006/geno.2000.6327. [DOI] [PubMed] [Google Scholar]
- 31.Hamel BC, et al. Familial X-linked mental retardation and isolated growth hormone deficiency: clinical and molecular findings. Am J Med Genet. 1996;64(1):35–41. doi: 10.1002/(SICI)1096-8628(19960712)64:1<35::AID-AJMG5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Arya VB, et al. Xq27.1 Duplication encompassing SOX3: variable phenotype and smallest duplication associated with hypopituitarism to date - a large case series of unrelated patients and a literature review. Horm Res Paediatr. 2019;6:1–8. doi: 10.1159/000503784. [DOI] [PubMed] [Google Scholar]
- 33.Laumonnier F, et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet. 2002;71(6):1450–1455. doi: 10.1086/344661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu T, et al. Increased transactivation and impaired repression of beta-catenin-mediated transcription associated with a novel SOX3 missense mutation in an X-linked hypopituitarism pedigree with modest growth failure. Mol Cell Endocrinol. 2018;478:133–140. doi: 10.1016/j.mce.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Jelsig AM, et al. A complex phenotype in a family with a pathogenic SOX3 missense variant. Eur J Med Genet. 2018;61(3):168–172. doi: 10.1016/j.ejmg.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Alatzoglou KS, et al. Increased transactivation associated with SOX3 polyalanine tract deletion in a patient with hypopituitarism. J Clin Endocrinol Metab. 2011;96(4):E685–E690. doi: 10.1210/jc.2010-1239. [DOI] [PubMed] [Google Scholar]
- 37.Stagi S, et al. A SOX3 (Xq26.3–27.3) duplication in a boy with growth hormone deficiency, ocular dyspraxia, and intellectual disability: a long-term follow-up and literature review. Hormones (Athens) 2014;13(4):552–560. doi: 10.14310/horm.2002.1523. [DOI] [PubMed] [Google Scholar]
- 38.Hureaux M, et al. SOX3 duplication: a genetic cause to investigate in fetuses with neural tube defects. Prenat Diagn. 2019;39(11):1026–1034. doi: 10.1002/pd.5523. [DOI] [PubMed] [Google Scholar]
- 39.Bauters M, et al. Evidence for increased SOX3 dosage as a risk factor for X-linked hypopituitarism and neural tube defects. Am J Med Genet A. 2014;164:1947. doi: 10.1002/ajmg.a.36580. [DOI] [PubMed] [Google Scholar]
- 40.Stankiewicz P, et al. Duplication of Xq26.2-q27.1, including SOX3, in a mother and daughter with short stature and dyslalia. Am J Med Genet A. 2005;138(1):11–17. doi: 10.1002/ajmg.a.30910. [DOI] [PubMed] [Google Scholar]
- 41.Grinspon R, et al. 46, XX ovotesticular DSD associated with a SOX3 gene duplication in a SRY -negative boy. Clin Endocrinol. 2016;85:673. doi: 10.1111/cen.13126. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi M, et al. SOX3 overdosage permits normal sex development in females with random X inactivation. Sex Dev. 2015;9(3):125–129. doi: 10.1159/000377653. [DOI] [PubMed] [Google Scholar]
- 43.Uguen A, et al. Duplication of SOX3 (Xq27) may be a risk factor for neural tube defects. Am J Med Genet A. 2015;167(7):1676–1678. doi: 10.1002/ajmg.a.37072. [DOI] [PubMed] [Google Scholar]
- 44.Rosolowsky ET, et al. Marked phenotypic variable expression among brothers with duplication of Xq27.1 involving the SOX3 gene. J Pediatr Endocrinol Metab. 2020;33(3):443–447. doi: 10.1515/jpem-2015-0131. [DOI] [PubMed] [Google Scholar]
- 45.Helle JR, et al. Hyperphagia, mild developmental delay but apparently no structural brain anomalies in a boy without SOX3 expression. Am J Med Genet A. 2013;161(5):1137–1142. doi: 10.1002/ajmg.a.35823. [DOI] [PubMed] [Google Scholar]
- 46.Moalem S, et al. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am J Med Genet A. 2012;158(7):1759–1764. doi: 10.1002/ajmg.a.35390. [DOI] [PubMed] [Google Scholar]
- 47.Sutton E, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121(1):328–341. doi: 10.1172/JCI42580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gecz J, et al. Fibroblast growth factor homologous factor 2 (FHF2): gene structure, expression and mapping to the Börjeson-Forssman-Lehmann syndrome region in Xq26 delineated by a duplication breakpoint in a BFLS-like patient. Hum Genet. 1999;104(1):56–63. doi: 10.1007/s004390050910. [DOI] [PubMed] [Google Scholar]
- 49.Burkitt Wright EMM, et al. X-linked isolated growth hormone deficiency: expanding the phenotypic spectrum of SOX3 polyalanine tract expansions. Clin Dysmorphol. 2009;18(4):218–221. doi: 10.1097/MCD.0b013e32832d06f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PNG 75 kb). Supplemental figure 1: Overview of previously described point mutations in GPR101 (3A) and SOX3 (3B). A. Overview of GPR101 gene mutations described to date in literature and corresponding clinical findings. B. Overview of previously described point mutations found in the 1st poly-alanine tract of SOX3 protein with the corresponding clinical findings. Allele frequency in normal population is shown below nucleotide and protein sequence
Electronic supplementary material 2 (PDF 69 kb)