Abstract
Cryptococcosis is a life-threatening fungal infection caused by the Cryptococcus neoformans/Cryptococcus gattii species complex. Most cases are recorded in patients suffering from HIV/AIDS (human immunodeficiency virus/acquired immunodeficiency syndrome). However, this infection also occurs in non-HIV patients with a proportion of 10–30% of all cases. The study aimed at the clinical and molecular characterization of non-HIV patients diagnosed with cryptococcosis at the Tropical Medicine Foundation (FMT-HVD) from July 2016 to June 2019. Medical records of respective patients were analyzed to describe the course of cryptococcosis in non-HIV patients. In addition, multi-locus sequence typing (MLST) was applied to identify the sequence types of the isolated Cryptococcus strains, to perform phylogenetic analysis, and to evaluate the isolates’ genetic relationship to global reference strains. Antifungal susceptibility profiles to amphotericin B, fluconazole, and itraconazole were assessed by broth microdilution. From a total of 7 patients, 4 were female, the age range varied between 10 and 53 years (median of 36.3 years). Cryptococcal meningitis was the common clinical manifestation (100%). The period between onset of symptoms and confirmed diagnosis ranged from 15 to 730 days (mean value of 172.9 days), and the observed mortality was 57.1%. Of note, comorbidities of the assessed cryptococcosis patients comprised hypertension, diabetes mellitus, and intestinal tuberculosis. Genotyping applying PCR-RFLP of the URA5 gene identified all clinical isolates as C. gattii genotype VGII. Using MLST, it was possible to discriminate the sequence types ST20 (n = 4), ST5 (n = 3), and the newly identified sequence type ST560 (n = 1). The antifungals amphotericin B, fluconazole, and itraconazole showed satisfactory inhibitory activity (microdilution test) against all C. gattii VGII strains.
Keywords: Amazon, Cryptococcus gattii, HIV-negative, MLST, Case series, Cryptococcal meningitis, VGII genotype
Introduction
Cryptococcosis is an infection of global importance with significant attributable mortality [1]. Involvement of the central nervous system (CNS), usually as meningoencephalitis, is the most common manifestation [2, 3]. Worldwide, cryptococcal meningitis is typically associated with human immunodeficiency virus (HIV) infection. In high-income countries, however, the disease is increasingly recognized in HIV-negative patients as well. Of note, HIV-negative patients with cryptococcosis are more frequently diagnosed with considerable delay and show higher mortality rates compared with HIV-infected patients [4–6].
A few studies suggest that cryptococcosis in immunocompromised hosts without association to HIV may even outnumber HIV-associated cases, potentially indicating a shift in epidemiology [4, 5, 7, 8]. Particularly endangered patient groups comprise solid organ transplant recipients; patients with rheumatic diseases, liver disease, cancer, and hematopoietic diseases; as well as those receiving immunosuppressive therapies [4, 9–12]. Notably, disease also occurs in immunologically competent hosts [13–17]. In addition, the species of the causative agent, i.e., Cryptococcus neoformans or Cryptococcus gattii, is associated with distinct epidemiological features [18].
This article focuses on a case series of HIV-negative patients with cryptococcal meningitis caused by C. gattii in Northern Brazil. The aim of the study was the characterization of the local epidemiology, potential risk factors of infection, clinical complications, and diagnostic as well as typing approaches (multi-locus sequence typing (MLST) and antifungal susceptibility).
Methods
Clinical isolates and patient population
We performed a prospective study assessing patients with cryptococcal meningitis hospitalized from July 2016 to June 2019 at the Tropical Medicine Foundation Dr. Heitor Vieira Dourado [Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD)] in Manaus, Amazonas State (AM), Brazil. A total of 7 clinical Cryptococcus strains were isolated from cerebrospinal fluid (CSF) obtained from 7 HIV-negative patients. All isolates were stored in Sabouraud dextrose agar tubes at 4 °C at the Medical Mycology Laboratory at FMT-HVD. The strains were purified twice on niger seed plates. Subsequently, only one isolated colony was randomly selected for further analysis.
Collection of epidemiological and laboratory data
Clinical, epidemiological, and laboratory records of all patients were accessed from the online database of FMT-HVD. The data collected for further analyses included age; gender; geographic location; initial symptoms and subsequent sequelae; HIV infection status; laboratory tests; clinical outcome (death or survival); need for surgical intervention and hospitalization in the intensive care unit (ICU) due to complications, clinical forms, time, and number of hospitalizations; and the amount of positive cultures recovered at the time of initial diagnosis as well as during treatment.
Ethical clearance
This study was approved by the FMT-HVD Human Research Ethical Committee (CAAE 90749718.3.0000.0005). Patients enrolled in the study provided their written informed consent, and data were analyzed anonymously.
Molecular typing by URA5-RFLP
DNA extraction was performed using the phenol/chloroform/isoamyl-alcohol method [19]. The major molecular types were initially identified applying URA5-RFLP (restriction fragment length polymorphism) analysis with the enzymes Sau96I and HhaI (Thermo Scientific, Waltham, USA) as described by Meyer et al. (2003) [20]. The genotypes were assigned by comparison with respective reference strains: WM 148 (serotype A, VNI), WM 626 (serotype A, VNII), WM 628 (serotype AD, VNIII), WM 629 (serotype D, VNIV), WM 179 (serotype B, VGI), WM 178 (serotype B, VGII), WM 161 (serotype B, VGIII), and WM 779 (serotype C, VGIV).
MLST and phylogenetic analysis
MLST (multi-locus sequence typing) analysis was performed by amplification and Sanger sequencing of six housekeeping genes CAP59, GPD1, LAC1, PLB1, SOD1, and URA5 as well as the IGS1 region according to protocols published previously by the ISHAM (International Society for Human & Animal Mycology) [21]. The PCR products were purified with a modified method taken from the literature using polyethylene-glycol/NaCl [22] and were bidirectionally Sanger-sequenced on an ABI3130 DNA Analyzer with BigDye Terminators v3.1 (Applied Biosystems, Foster City, California, USA) at the Laboratory of Functional Genomic and Bioinformatics (Fiocruz, Rio de Janeiro, Brazil). The sequences were manually edited using the software Sequencher 5.4.6 (Gene Codes Corporation, Ann Arbor, MI, USA), and the contigs were aligned using the Muscle algorithm linked to the software Mega v.10.0.2 [23]. All sequences were assessed by MLST applying the scheme for C. gattii (database: http://mlst.mycologylab.org) to determine allelic numbers and associated sequence types (ST).
Applying the abovementioned algorithm, the DNA sequences of seven MLST loci from clinical isolates were aligned with the sequences of VGII STs available in the Fungal MLST Database. To verify the genetic and evolutionary relationship among the STs from the HIV-negative patients of this study and the STs previously identified in Northern Brazil, a phylogenetic tree was constructed based on the neighbor-joining (NJ) model with a bootstrap analysis using 1000 replicates. Pairwise distances and the related substitution parameters were estimated by maximizing the composite likelihood. The evolutionary distances were computed using the p-distance and all gaps were eliminated [24, 25].
Antifungal susceptibility testing
Antifungal susceptibility testing was performed using the microdilution method in RPMI (Roswell Park Memorial Institute) broth according to the M27-A3 guideline of the Clinical and Laboratory Standards Institute (CLSI) [26]. The assessment was performed in duplicate within the following ranges: 0.125–64 μg/mL for fluconazole (Sigma Aldrich, Saint Louis, USA) and 0.03–16 μg/mL for amphotericin B (Sigma Aldrich, Saint Louis, USA) as well as itraconazole (Sigma Aldrich, Saint Louis, USA).
Cryptococcus isolates were sub-cultured on Sabouraud dextrose agar and incubated for 48 h at 35 °C. The yeast colonies were transferred to 5-mL sterile saline solution (0.85%) and adjusted to a density equivalent to the 0.5 McFarland standard scale. The inoculum was adjusted to 2.5 × 103 cells in 10 mL of RPMI medium (Sigma Aldrich, Saint Louis, USA) by counting in a Neubauer chamber. The 96-well microplates were incubated at 35 °C for 72 h. The MIC of amphotericin B was determined as the lowest concentration that completely inhibited fungal growth (100%), while for the azoles, the lowest concentration allowing partial reduction (50%) of growth compared with the growth control wells was chosen.
Statistical analysis
A study database was filled with all assessed clinical, epidemiological, and laboratory information using the Microsoft Office Excel® software version 2019 16.0.6742.2048 (Microsoft, Redmond, Washington, USA). Statistical analyses (relative frequency, mean, and standard deviation) were performed applying the software R version 3.3.1 (https: //www.r-project).
Results
Clinical and epidemiological data
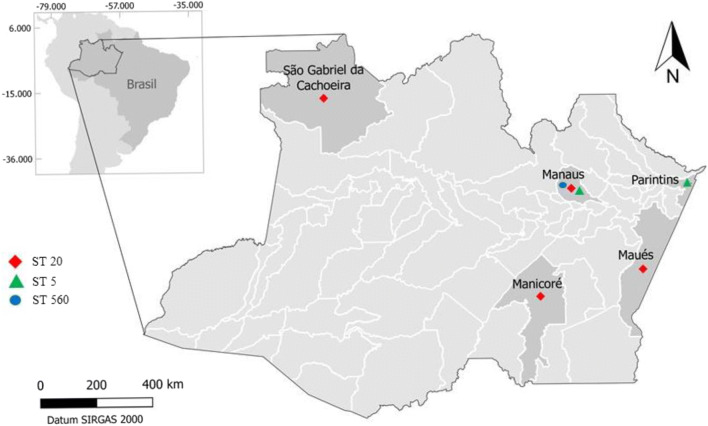
The most patients described in this case series were from Manaus (3/7) while the other ones lived in the municipalities Manicore (1/7), Maues (1/7), Parintins (1/7), and Sao Gabriel da Cachoeira (1/7) (Fig. 1).
Fig. 1.
Map of Brazil showing the origin of the 7 patients assessed and the sequence types of the Cryptococcus gattii stains isolated from them (Quantum GIS version 2.18)
The majority of patients were female (4/7). The median age was 36.3 years with a range between 10 and 53 years of age (Table 1). Hypertension (3/7) and diabetes (2/7) were the most frequently observed comorbidities among the HIV-negative cryptococcosis patients. The most common initial signs and symptoms comprised meningitis-associated symptoms like headache (100%), vomiting (71.4%), and photophobia (71.4%), but also weight loss (57.1%). During hospitalization and treatment of the patients, constipation (57.1%), convulsion (57.1%), decreased level of consciousness (57.1%), and delirium (42.9%) were among the most frequently recorded symptoms. The most frequent neurological sequelae comprised decrease of visual acuity (5/7), hearing deficit (1/7), motor deficit (1/7), facial palsy (1/7), cerebral edema/vasculitis (1/7), and dysphagia (1/7). Moreover, disease-associated death was observed for four hospitalized patients during the first 20 days after admission (Table 1).
Table 1.
Epidemiological and laboratory features of HIV-negative patients with cryptococcosis hospitalized in the period of time between July 2016 and June 2019 at the Tropical Medicine Foundation Dr. Heitor Vieira Dourado (Manaus-Amazonas-Brazil)
| Anonymized patient number | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Gender | M | F | F | M | F | F | M |
| Age (in years) | 50 | 23 | 41 | 25 | 52 | 10 | 53 |
| Initial symptoms (before diagnosis) | Headache, fever, vomiting, ocular pain, asthenia, photophobia, weight loss | Headache, photophobia, nausea, vomiting, diplopia, irritability | Headache, stiff neck, vomiting, photophobia, night sweats, face palsy, weight loss | Headache, stiff neck, fever, seizure, photophobia, weight loss | Headache, seizure, photophobia, ocular pain, stiff neck, lethargy | Headache, fever, vomiting, hyporexia, weight loss, asthenia | Headache, fever, vomiting, hyporexia, asthenia, weight loss |
| Symptoms developed during the hospitalization | Intestinal constipation, decrease of consciousness, spikes of HBP, weight loss | Paresis IM, seizures, decrease of consciousness | Intestinal constipation, seizures, anemia, delirium, asthenia, weight loss | Decrease of consciousness, paresis ISM, dysphagia, anemia, dysarthria, fever, peripheral neuropathy | Intestinal constipation, decrease of consciousness, tongue palsy, seizures | Intestinal constipation, seizures, delirium, strabismus | Decrease of consciousness, stroke, delirium, weight loss |
| Duration of initial symptoms until diagnosis (days) | 180 | 150 | 30 | 15 | 730 | 90 | 15 |
| Occupation | Construction worker | Housewife | Teacher | Farmer | Housewife | Student | Commercial manager |
| Medical history | Depression, pericardial tuberculosis | – | Ganglion tuberculosis | Malaria (5 times) | – | – | – |
| Comorbidities | – | Hypertension | Intestinal tuberculosis | – | Diabetes, hypertension | – | Diabetes, hypertension |
| CSF level of proteins (mg/dL) | 25.0 | 109.0 | 59.0 | 52.0 | 22.1 | 29.0 | 26.7 |
| CSF level of glucose (mg/dL) | 47.0 | 3.0 | 59.0 | 44.0 | 102.0 | 61.0 | 46.0 |
| Clinical form | CM | CM | CM | CM | CM | CM | CM |
| Treatment | AmB + FLU | AmB + FLU | LAmB-L+ FLU | AmB + FLU | AmB + FLU | AmB + FLU | AmB + FLU |
| Time of hospitalization in the ICU (days) | 4 | 5 | – | 26 | – | – | 5 |
| Sequelae* | Ocular choroiditis (R) | Loss of vision (R, L) | Decrease of visual and hearing acuity | Facial palsy (R), dysarthria, motor dysfunction | Decrease of visual | Loss of vision (R), decrease of vision acuity | – |
| Time of hospitalization (days) | 18 | 20 | 57 | 100 | 13 | 85 | 15 |
| Clinical outcome | Death | Death | Recovered | Recovered | Death | Recovered | Death |
| Microorganism isolated | C. gattii (VGII/ST20) | C. gattii (VGII/ST5) | C. gattii (VGII/ST5) | C. gattii (VGII/20) | C. gattii (VGII/ST20) | C. gattii (VGII/ST20) | C. gattii (VGII/ST560) |
HBP high blood pressure, CM cryptococcal meningitis, ICU intensive care unit, ISM inferior and superior members, AmB amphotericin B, LAmB liposomal amphotericin B, FLU fluconazole; (˗)=not reported; F female, M male, CFS cerebrospinal fluid, R right, L left. *In the present work, sequels were considered pathologies that persisted after the end of hospitalization and antifungal treatment
MLST and phylogenetic analysis
URA5-RFLP analysis identified all isolates as C. gattii genotype VGII. In addition, MLST analysis assigned these 7 strains to three different STs (sequence types). The STs responsible for the observed infections in HIV-negative patients comprised ST20 in Manaus, Maues, Manicore, and Sao Gabriel da Cachoeira; ST5 in Parintins and Manaus; as well as ST560 as a newly described ST in the city of Manaus (Fig. 1), (Table 2).
Table 2.
Molecular types of C. gattii isolates and the numerical sequences of the alleles in MLST
| Isolate | Genotype | Alleles in MLST | ST | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CAP59 | GPD1 | IGS1 | LAC1 | PBL1 | SODCG | URA5 | |||
| P1FMT-66 | VGII | 1 | 1 | 4 | 4 | 1 | 14 | 7 | 20 |
| P2FMT-103 | VGII | 3 | 16 | 15 | 4 | 9 | 23 | 2 | 5 |
| P3FMT-111 | VGII | 3 | 16 | 15 | 4 | 9 | 23 | 2 | 5 |
| P4FMT-215 | VGII | 1 | 1 | 4 | 4 | 1 | 14 | 7 | 20 |
| P5FMT-339 | VGII | 1 | 1 | 4 | 4 | 1 | 14 | 7 | 20 |
| P6FMT-346 | VGII | 1 | 1 | 4 | 4 | 1 | 14 | 7 | 20 |
| P7FMT-829 | VGII | 2 | 6 | 27 | 4 | 1 | 104 | 2 | 560 |
P patient, FMT Fundacao de Medicina Tropical, ST sequence type
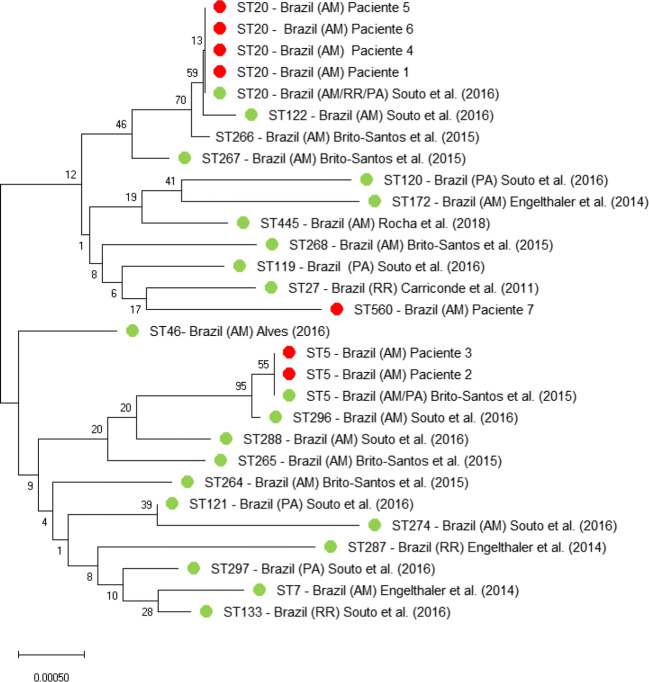
A monophyletic tree was calculated to verify the ancestral relationship of the seven isolates with C. gattii strains formerly isolated in the Northern Region of Brazil. The obvious genetic relationship between the sequences of the seven clinical isolates from this study (red color) with the sequences of other C. gattii isolates from the Northern Region of Brazil (green color) obtained from the fungal MLST database is shown in Fig. 2. The red dots indicate the STs responsible for the infections in HIV-negative patients from this study (Fig. 2).
Fig. 2.
Unrooted neighbor-joining (NJ) tree constructed applying the software Mega v.10.0.2 with the concatenated data set of seven MLST loci (CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and URA5), showing the genetic relatedness of clinical isolates of C. gattii VGII from the Northern Region of Brazil with those obtained from the fungal MLST database (http://mlst.mycologylab.org) with known geographic origin. Abbreviations: AM (Amazonas), RR (Roraima), and PA (Para). References: [24, 25, 27–30]
MIC results
Antifungal susceptibility testing was performed with one isolate per patient (n = 7). The antifungals amphotericin B, fluconazole, and itraconazole showed satisfactory inhibitory activity against all C. gattii genotype VGII strains. It was observed that amphotericin B and itraconazole were associated with MIC less than 1 μg/mL and fluconazole showed MIC between 1 and 8 μg/mL. The detailed MIC ranges of each drug tested are depicted in Table 3.
Table 3.
Minimal inhibitory concentration of clinical C. gattii isolates from Amazonas, Brazil, to antifungals
| Antifungal | Cryptococcus gattii isolates | ||||||
|---|---|---|---|---|---|---|---|
| P1FMT-66 | P2FMT-103 | P3FMT-111 | P4FMT-215 | P5FMT-339 | P6FMT-346 | P7FMT-829 | |
| Amphotericin B MIC (μg/mL) | 0.03 | 0.03 | 0.03 | 0.25 | 0.03 | < 0.03 | 0.03 |
| Fluconazole MIC (μg/mL) | 4 | 8 | 4 | 2 | 4 | 4 | 1 |
| Itraconazole MIC (μg/mL) | 0.12 | 0.12 | 0.62 | 0.12 | 0.12 | 0.12 | < 0.03 |
P patient, FMT Fundacao de Medicina Tropical, MIC minimum inhibitory concentration
Among the assessed cryptococcosis cases in HIV-negative patients from Amazonas (Northern Brazil), we have chosen to exemplarily describe the clinical case of a co-infection with Cryptococcus gattii and Mycobacterium spp. (patient 3) due to its interesting clinical features.
Exemplary case report
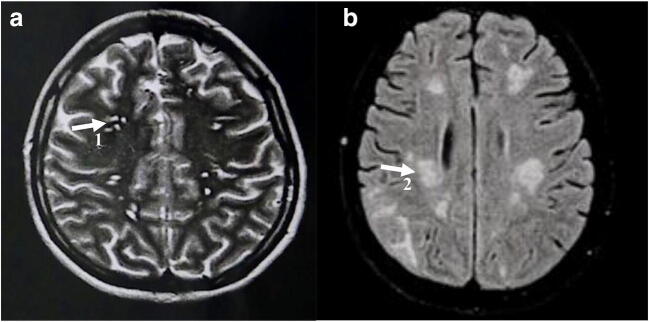
A 41-year-old female public school teacher, who had been diagnosed 2 years earlier with ganglionic tuberculosis, suffered from severe headache, fever, neck stiffness, vomiting, and weakness. After 2 months of continuous headaches, the weak and unconscious patient was sent to the emergency department of a local hospital. Clinically diagnosed with cryptococcosis, she was transferred to the reference hospital for the treatment of cryptococcosis Fundacao de Medicina Tropical Dr. Heitor Vieira Dourado. Screening for HIV antibodies and antigen showed negative results. Cryptococcus antigen (CRAG) testing was positive in cerebrospinal fluid (CSF). Additional assessed CSF parameters comprised a glucose level of 59.0 mg/dL, a protein level of 59.0 mg/dL, and cytometry indicating 67 cells per mm3. Nankin ink staining showed a high density of Cryptococcus cells in the budding process. After 48 h, Cryptococcus growth was observed on the culture media bird seed agar, heart brain infusion (BHI) agar, and Sabouraud agar. Treatment was initiated with liposomal amphotericin B; however, due to renal dysfunction, it had to be changed to intravenous fluconazole. During hospitalization, the patient developed acute progressive anemia (Hb (hemoglobin): 5.91/Ht (hematocrit): 17.17), ocular pain, weight loss (12 kg), constipation, jaundice, chronic headache, and episodes of seizures. Neurological imaging showed multiple lesions in the nucleo-capsular region (Fig. 3). Since the patient had been diagnosed with ganglion tuberculosis 2 years prior to the diagnosis cryptococcosis, tuberculosis screening was also performed including imaging (chest X-ray) and mycobacterial culture that resulted in the growth of Mycobacterium tuberculosis in fecal samples. The patient was diagnosed with intestinal tuberculosis, and combination therapy with rifampicin (150 mg per day), isoniazid (75 mg per day), pyrazinamide (400 mg per day), and ethambutol (275 mg per day) was initiated. After 57 days of hospitalization, she was dismissed from hospital. Finally, 12 months after treatment with fluconazole, the patient was in a good clinical condition and returned to work.
Fig. 3.
Magnetic resonance imaging of the patient’s brain. (A) Axial T2-weighted turbo-spin-echo image after 1 month of treatment showing multiple lesions in the nucleo-capsular region as well as potentially related gelatinous pseudo-cysts (1) due to cryptococcosis. (B) Image with the turbo-spin-echo technique in T2 in the axial plane after 7 months of treatment showing signs of alteration of the subcortical white substance of the cerebral and cerebellar hemispheres, particularly abundant in the parieto-occipital regions (2), signs of a breakdown of the blood-brain barrier, and an inflammatory process resulting from meningoencephalitis
Discussion
This work is among the first studies dedicated to the clinical, epidemiological, and laboratory-based characterization of HIV-negative patients with cryptococcal meningitis in the Northern Region of Brazil. Of note, the assessed HIV-negative patients with cryptococcosis had very diverse clinical and epidemiological conditions prior to cryptococcal infection. Cryptococcal meningitis and neurological sequelae (mainly decrease of visual acuity) dominated. C. gattii genotype VGII was the causative agent in case of all seven infections. Thereby, three out of these seven isolates belonged to the sequence type ST20, an ST which is well known to be associated with severe neurological manifestations and high mortality rates [31–33].
Cryptococcosis predominantly affected female patients with a median age of 36.3 years and an age spectrum ranging from 10 to 53 years. George et al. (2018) reported an average of 58 years with a range from 18 to 98 years in NTNH patients in the USA [34]. Spec et al. (2016), in contrast, reported a higher frequency of male patients with 66% of all documented cases [7]. Various studies indicated that HIV-negative patients with cryptococcosis are significantly older compared with HIV-positive cryptococcosis patients [5, 7, 35].
In the present work, most of the patients reported a long period of time between the initial symptoms and the diagnosis of cryptococcal meningitis. In particular, this was true for the patients 1, 2, and 5. The medical history prior to the diagnosis was provided by the patients in the course of interviews specifically addressing this topic. Necessarily, subjective experience influenced the patients’ descriptions of symptoms and comorbidities regarding this period. As a common feature, patients 1, 2, and 5 described headache and photophobia, suggesting rather mild infections.
All 7 HIV-negative patients in this study were diagnosed with manifestations of cryptococcal meningitis. This clinical form is predominant in this region as also indicated by the results of a previous study carried out by Rocha et al. (2018) [27]. This finding is in contrast to the observations in resource-rich industrialized countries. In the USA, only 50% out of a total of 300 HIV-negative patients with cryptococcosis showed CNS (central nervous system) involvement. Most likely, the considerable delay in diagnosing cryptococcosis in this study is a reason for this difference associated with the risk of severe clinical courses.
Another important finding of the study was the description of the sequelae, which were mostly related to optical (papilledema and retinal hemorrhage) and auditory impairment. As described previously, neurological sequelae are more prominent in HIV-negative patients and can cause cognitive impairment in up to 78% of the reported cases [7]. The observed mortality in the present study was 57.2% (4/7) and thus higher than the mortality rates of 45.0% (10/22) as reported by Aye et al. (2016), 20.7% (304/1470) as reported by George et al. (2018), and 41.1% (65/108) as observed by Hevey et al. (2019) [11, 34, 35]. The high mortality rate in the here-described study may have been a consequence of late diagnosis. Such an association has been shown in a study conducted at the University of Alabama, which suggested that the prolonged time to diagnosis is responsible for increased 90 days mortality [4, 5]. Of note, the comparably low number of only 7 patients was associated with high impact of single fatal courses on the mortality rate.
Another aim of the study was the correlation of comorbidities with the acquisition of cryptococcosis. From the 7 patients assessed, the recorded comorbidities comprised arterial hypertension (n = 3), diabetes mellitus (n = 2), and tuberculosis (n = 1). Diabetes mellitus has been considered a risk factor for cryptococcosis previously [12]. Hyperglycemia can lead to a decline of the number of immune cells, a likely explanation for the association between cryptococcosis and diabetes mellitus [36]. Arterial hypertension can be associated with stroke as shown in the case of patient 7 (Table 1), and cerebral infarction in patients with neurocryptococcosis is associated with high mortality [37].
Cases with cryptococcosis and tuberculosis co-infection are common in places where tuberculosis is endemic. According to the health surveillance secretariat’s report in 2018, a total of 72,788 new tuberculosis cases were recorded in Brazil. Amazonas is the state with the most tuberculosis cases in Brazil with an incidence rate of 72.9 cases per 100,000 inhabitants [38]. There is evidence that both cryptococcosis and tuberculosis show immunomodulatory activity in the host and that one disease may act as a gateway for the other, because they act synergistically in the dysregulation of the host’s immune response [39]. The case report as detailed in the present work shows that the patient had ganglionic tuberculosis 2 years prior to the diagnosis of cryptococcosis and suggests that cryptococcosis may have possibly contributed to the reactivation of tuberculosis. Cryptococcus spp. isolates from the C. neoformans/C. gattii complex produce melanin and capsule polysaccharide (GXM) that cause suppression of immune cells by inactivating T cells, by preventing the migration of T lymphocytes, and by causing apoptosis of macrophages. All these mechanisms predispose patients to active tuberculosis or reactivation of the disease [40–42].
Infections by C. gattii of genotype VGII were observed in this study without exemption. This genotype is widely dispersed in Brazil and was also responsible for the Vancouver outbreak in Canada [2, 43, 44]. In addition, C. gattii infections have been described as common in HIV-negative hosts in Australia and South America [45]. The sequence types among the isolates in the present study were ST20 (VGIIa), followed by ST5, and the new sequence type ST560. VGIIa strains are associated with high virulence, rapid reproducibility at 37 °C, and higher melanin production compared with other genotypes [46]. In particular, ST20 clades (VGIIa) have been responsible for the Vancouver outbreak in Canada in 1999. Probably, Canadian and South American ST20 strains have a common ancestor, suggesting that this clonal lineage originally came from South America [28, 47, 48]. Further, there have been descriptions of the occurrence of ST5 in Amazonas isolated from household dust, mainly in wooden houses [25].
Antifungal susceptibility testing indicated low amphotericin B and itraconazole MICs for the seven clinical isolates, ranging from < 0.62–0.25 μg/mL to < 0.62–0.12 μg/mL, respectively. Resistance to these antifungals is uncommon, although there has been a report on a clinical C. gattii VGII stain with a MIC of 2 μg/mL for amphotericin B [48]. All isolates were susceptible to fluconazole, with MICs ranging from 1 to 8 μg/mL. Lee et al. (2019) also described reliable clinical susceptibility of clinical strains of the clonal lineages VNI, VNII, VGI, and VGII that did not show high MICs in microdilution testing. In Lee’s study, MIC variation of the strains of the VGII genotype was 0.5–0.5 μg/mL for amphotericin B, 2–4 μg/mL for fluconazole, and 0.015–0.03 μg/mL for itraconazole, respectively [49]. However, higher MICs against fluconazole ranging from 2 to 64 μg/mL have been described for individual strains of the VGII genotype [27, 48, 50]. In the presented study, none of the seven patients suffered from recurrent cryptococcosis, a condition with known association with resistance to antifungal drugs [51].
Conclusion
In this study, seven cases of cryptococcal meningitis in HIV-negative patients in Northern Brazil were characterized. The patients’ age ranged about several decades (10–53 years of age), observed comorbidities were only indirectly related or unrelated to the immune status, and the main sequelae were neurological ones. We highlighted the delay in diagnosing cryptococcosis with the risk of severe clinical courses and rapid dying even of HIV-negative patients. C. gattii, mainly of the molecular type VGIIa, was the only observed etiologic agent. This genotype is widely dispersed in Brazil and was also responsible for the Vancouver outbreak in Canada. Multi-locus sequencing typing identified the sequence types ST20, ST5, and a newly described sequence type ST560. The antifungals amphotericin B, fluconazole, and itraconazole showed satisfactory inhibitory activity in microdilution testing against all C. gattii VGII strains.
Acknowledgments
We thank the medical staff of the FMT-HVD and all participating patients for providing the clinical and epidemiological data. We thank the Mycology and Mycobacteriology laboratories of the National Research Institute of Amazonia (INPA) for assistance with equipment and reagents. In addition, we are also grateful to the Laboratory of Functional Genomics and Bioinformatics of Oswaldo Cruz Institute (Fiocruz, RJ) for the indispensable support regarding the DNA sequencing procedures and to Dr. Jaidson Nandi Becker for designing the map used in this publication.
Funding
We wish to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) program for financial support and the Amazonas Research Foundation (FAPEAM) for funding this research: Public Notice N. 001/2017 – PPSUS.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SCA, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev. 2014;27:980–1024. doi: 10.1128/CMR.00126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, Harrison TS. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2016;13:13–24. doi: 10.1038/nrneurol.2016.167. [DOI] [PubMed] [Google Scholar]
- 4.Bratton EW, El Husseini N, Chastain CA, Lee MS, Poole C, Stürmer T, Juliano JJ, Weber DJ, Perfect JR (2012) Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One. 10.1371/journal.pone.0043582 [DOI] [PMC free article] [PubMed]
- 5.Brizendine KD, Baddley JW, Pappas PG (2013) Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One. 10.1371/journal.pone.0060431 [DOI] [PMC free article] [PubMed]
- 6.Zhu LP, Wu JQ, Xu B, Ou XT, Zhang QQ, Weng XH (2010) Cryptococcal meningitis in non-HIV-infected patients in a Chinese tertiary care hospital, 1997–2007. Med Mycol. 10.3109/13693780903437876 [DOI] [PubMed]
- 7.Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis. 2016;3:1–5. doi: 10.1093/ofid/ofw195.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas PG, Perfect JR, Cloud GA et al (2001) Cryptococcosis in human immunodeficiency virus–negative patients in the era of effective azole therapy. Clin Infect Dis. 10.1086/322597 [DOI] [PubMed]
- 10.Yuchong C, Fubin C, Jianghan C, Fenglian W, Nan X, Minghui Y, Yalin S, Zhizhong Z. Cryptococcosis in China (1985-2010): review of cases from Chinese database. Mycopathologia. 2012;173:329–335. doi: 10.1007/s11046-011-9471-1. [DOI] [PubMed] [Google Scholar]
- 11.Hevey MA, George IA, Raval K, Powderly WG, Spec A (2019) Presentation and mortality of cryptococcal infection varies by predisposing illness: a retrospective cohort study. Am J Med. 10.1016/j.amjmed.2019.04.026 [DOI] [PMC free article] [PubMed]
- 12.Beardsley J, Sorrell TC, Chen SC-A. Central nervous system cryptococcal infections in non-HIV infected patients. J Fungi. 2019;5:71. doi: 10.3390/jof5030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St-Pierre J, Dufresne PJ, Carignan A, Lévesque É, Bernard F, Longtin J, LeBlanc L. Case series: report of the first two human indigenous cases of Cryptococcus gattii infection in eastern Canada. Mycopathologia. 2018;183:399–406. doi: 10.1007/s11046-017-0215-8. [DOI] [PubMed] [Google Scholar]
- 14.Bauer M, Wickenhauser C, Haak A et al (2018) Case report: a fatal case of cryptococcosis in an immunocompetent patient due to Cryptococcus deuterogattii (AFLP6/VGII). JMM Case Reports. 10.1099/jmmcr.0.005168 [DOI] [PMC free article] [PubMed]
- 15.Aldemir Kocabaş B, Emin Parlak M, Özhak Baysan B, Karaali K, Bingöl A, Haspolat Ş. Disseminated cryptococcosis with severe increased intracranial pressure complicated with cranial nerve palsy in a child. Pediatr Infect Dis J. 2018;37:373–375. doi: 10.1097/INF.0000000000001765. [DOI] [PubMed] [Google Scholar]
- 16.Correa-Forero V, Pinilla-Monsalve GD, Valderrama-Chaparro JA, Amaya-Gonzalez P. Cryptococcal meningitis presenting as acute flaccid paralysis: a case report. J Infect Public Health. 2020;13:143–148. doi: 10.1016/j.jiph.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Andreou M, Cogliati M, Kolonitsiou F, Stroumpos C, Stamouli V, Ravazoula P, Siagris D, Papadogeorgaki H, Christofidou M, Lekkou A. Cryptococcus gattii infection in an immunocompetent host in Greece. Med Mycol Case Rep. 2020;27:1–3. doi: 10.1016/j.mmcr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung KJ, Bennett JE, Wickes BL, et al. The case for adopting the “species complex” nomenclature for the etiologic agents of cryptococcosis. mSphere. 2017;2:1–7. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrer C, Colom F, Frasés S, Mulet E, Abad JL, Alió JL. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J Clin Microbiol. 2001;39:2873–2879. doi: 10.1128/JCM.39.8.2873-2879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer W, Castañeda A, Jackson S et al (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 10.3201/eid0902.020246 [DOI] [PMC free article] [PubMed]
- 21.Meyer W, Aanensen DM, Boekhout T, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47:561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn IS, Blattner FR. Charons 36 to 40: multi enzyme, high capacity, recombination deficient replacement vectors with polylinkers and polystuffers. Nucleic Acids Res. 1987;15:2677–2698. doi: 10.1093/nar/15.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souto ACP, Bonfietti LX, Ferreira-Paim K, et al. Population genetic analysis reveals a high genetic diversity in the Brazilian Cryptococcus gattii VGII population and shifts the global origin from the Amazon rainforest to the semi-arid desert in the northeast of Brazil. PLoS Negl Trop Dis. 2016;10:1–19. doi: 10.1371/journal.pntd.0004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brito-Santos F, Barbosa GG, Trilles L, Nishikawa MM, Wanke B, Meyer W, Carvalho-Costa FA, Dos Santos Lazéra M (2015) Environmental isolation of Cryptococcus gattii VGII from indoor dust from typical wooden houses in the deep Amazonas of the Rio Negro basin. PLoS One. 10.1371/journal.pone.0115866 [DOI] [PMC free article] [PubMed]
- 26.Clinical and Laboratory Standards Institute (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd edn. CLSI M27-A3. Clinical and Laboratory Standards Institute, Wayne
- 27.Rocha DFS, Cruz KS, Santos CSDS, Menescal LSF, Neto JRDS, Pinheiro SB, Silva LM, Trilles L, Braga de Souza JV. MLST reveals a clonal population structure for Cryptococcus neoformans molecular type VNI isolates from clinical sources in Amazonas, northern-Brazil. PLoS One. 2018;13:1–15. doi: 10.1371/journal.pone.0197841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelthaler DM, Hicks ND, Gillece JD, et al. Cryptococcus gattii in north American Pacific northwest: whole-population genome analysis provides insights into species evolution and dispersal. MBio. 2014;5:1–18. doi: 10.1128/mBio.01464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carriconde F, Gilgado F, Arthur I, Ellis D, Malik R, van de Wiele N, Robert V, Currie BJ, Meyer W (2011) Clonality and α-a recombination in the Australian Cryptococcus gattii VGII population - an emerging outbreak in Australia. PLoS One. 10.1371/journal.pone.0016936 [DOI] [PMC free article] [PubMed]
- 30.Alves GSB (2016) Genotipagem De Cryptococcus neoformans E C. gattii Isolados De Poeira Domiciliar E Avaliação Da Susceptibilidade a antifúngicos E Da Presença Do Aantígeno Em Moradores De Uma Comunidade Rural Do Amazonas. [Monografia] Universicade Fed do Amaz 1–71
- 31.Dos Santos WRA, Meyer W, Wanke B, et al. Primary endemic Cryptococcosis gattii by molecular type VGII in the state of Pará, Brazil. Mem Inst Oswaldo Cruz. 2008;103:813–818. doi: 10.1590/S0074-02762008000800012. [DOI] [PubMed] [Google Scholar]
- 32.Khell Da Silva B, Freire AK, Dos Santos Bentes A, De Lima Sampaio I, Oliveira Santos L, Silva Dos Santos M, De Souza JV. Characterization of clinical isolates of the Cryptococcus neoformans-Cryptococcus gattii species complex from the Amazonas state in Brazil. Rev Iberoam Micol. 2012;29:40–43. doi: 10.1016/j.riam.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Trilles L, Lazéra MDS, Wanke B, Oliveira RV, Barbosa GG, Nishikawa MM, Morales BP, Meyer W. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem Inst Oswaldo Cruz. 2008;103:455–462. doi: 10.1590/S0074-02762008000500008. [DOI] [PubMed] [Google Scholar]
- 34.George IA, Spec A, Powderly WG, Santos CAQ (2018) Comparative epidemiology and outcomes of human immunodeficiency virus (HIV), non-HIV non-transplant, and solid organ transplant associated cryptococcosis: a population-based study. Clin Infect Dis. 10.1093/cid/cix867 [DOI] [PMC free article] [PubMed]
- 35.Aye C, Henderson A, Yu H, Norton R. Cryptococcosis—the impact of delay to diagnosis. Clin Microbiol Infect. 2016;22:632–635. doi: 10.1016/j.cmi.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Lin KH, Chen CM, Chen TL, Kuo SC, Kao CC, Jeng YC, Ho MW. Diabetes mellitus is associated with acquisition and increased mortality in HIV-uninfected patients with cryptococcosis: a population-based study. J Inf Secur. 2016;72:608–614. doi: 10.1016/j.jinf.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Lan S-H. Cerebral infarction in chronic meningitis: a comparison of tuberculous meningitis and cryptococcal meningitis. Qjm. 2001;94:247–253. doi: 10.1093/qjmed/94.5.247. [DOI] [PubMed] [Google Scholar]
- 38.Ministério da Saúde Brasil Brasil Livre da Tuberculose: evolução dos cenários epidemiológicos e operacionais da doença. Bol Epidemiológico. 2019;50:1–18. [Google Scholar]
- 39.Van Tongeren L, Shaipanich T, Fleetham JA. Coinfection with Cryptococcus gattii and Mycobacterium tuberculosis in an otherwise healthy 18-year-old woman. Can Respir J. 2011;18:62–64. doi: 10.1155/2011/812345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huffnagle GB, Chen GH, Curtis JL, McDonald RA, Strieter RM, Toews GB. Downregulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 41.Decote-Ricardo D, LaRocque-de-Freitas IF, Rocha JDB, Nascimento DO, Nunes MP, Morrot A, Freire-de-Lima L, Previato JO, Mendonça-Previato L, Freire-de-Lima CG. Immunomodulatory role of capsular polysaccharides constituents of Cryptococcus neoformans. Front Med. 2019;6:1–8. doi: 10.3389/fmed.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajima K, Yamanaka D, Ishibashi K, Adachi Y, Ohno N. Solubilized melanin suppresses macrophage function. FEBS Open Bio. 2019;9:791–800. doi: 10.1002/2211-5463.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, MacDougall L, Boekhout T, Kwon-Chung KJ, Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilas-Bôas AM, Andrade-Silva LE, Ferreira-Paim K, Mora DJ, Ferreira TB, Santos D de A, Borges AS, Melhem M de SC, Silva-Vergara ML (2020) High genetic variability of clinical and environmental Cryptococcus gattii isolates from Brazil. Med Mycol:1–12 [DOI] [PubMed]
- 45.Phillips P, Galanis E, Macdougall L, et al. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin Infect Dis. 2015;60:1368–1376. doi: 10.1093/cid/civ041. [DOI] [PubMed] [Google Scholar]
- 46.Ngamskulrungroj P, Serena C, Gilgado F, Malik R, Meyer W (2011) Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clin Microbiol Infect. 10.1111/j.1469-0691.2010.03222.x [DOI] [PubMed]
- 47.Science E, Engelthaler DM, Casadevall A. Northwest: ballast tanks , tsunamis. and Black Swans MBio. 2019;10:1–10. doi: 10.1128/mBio.02193-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trilles L, Meyer W, Wanke B, Guarro J, Lazéra M. Correlation of antifungal susceptibility and molecular type within the Cryptococcus neoformans/C. gattii species complex. Med Mycol. 2012;50:328–332. doi: 10.3109/13693786.2011.602126. [DOI] [PubMed] [Google Scholar]
- 49.Lee GA, Arthur I, Merritt A, Leung M (2019) Molecular types of Cryptococcus neoformans and Cryptococcus gattii in Western Australia and correlation with antifungal susceptibility . Med Mycol 1–7 [DOI] [PubMed]
- 50.Chowdhary A, Randhawa HS, Sundar G, Kathuria S, Prakash A, Khan Z, Sun S, Xu J. In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from North-Western India. J Med Microbiol. 2011;60:961–967. doi: 10.1099/jmm.0.029025-0. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Goncer I, Bongomin F, Doran HM, Novak-Frazer L, Masania R, Moore CB, Richardson MD. A case of pulmonary cryptococcoma due to Cryptococcus gattii in the United Kingdom. Med Mycol Case Rep. 2018;21:23–25. doi: 10.1016/j.mmcr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]