Abstract
As a key precursor of vitamin C, 2-keto-L-gulonic acid (2-KLG) was mainly produced from L-sorbose by mixed fermentation of Ketogulonicigenium vulgare and a helper strain (Bacillus spp.) with a low conversion rate for decades. The aim of this study was to enhance the 2-KLG production by co-culturing K. vulgare and Bacillus megaterium using three-stage temperature control (TSTC) strategy. By investigating the temperature effect on the 2-KLG fermentation, the optimum temperatures for the growths of K. vulgare and B. megaterium were 32 °C and 29 °C, respectively, while the optimum temperature for 2-KLG production was 35 °C. We developed a TSTC process: the temperature was kept at 32 °C during the first 16 h of fermentation, then decreased to 29 °C for the following 14 h, and maintained at 35 °C to the end of fermentation. By using this new process, the productivity and yield of 2-KLG from L-sorbose were obtained at 2.19 ± 0.19 g/L/h and 92.91 ± 1.02 g/L in 20-L fermentors for 5 batches, respectively, which were 22.35% and 6.02% higher than that of the control treatment (the single temperature of 29 °C). The increased cell density of K. vulgare during the exponential phase and the enhanced SDH activity (increased by 25.18% at 36 h, 17.14% at 44 h) in the production stage might be the reasons for enhanced 2-KLG conversion rate and yield. Our results demonstrated the feasibility of the TSTC strategy for 2-KLG production.
Keywords: L-Ascorbic acid, Mixed fermentation, Fermentation process, L-Sorbose, Sorbose dehydrogenase
Introduction
2-Keto-L-gulonic acid (2-KLG), known as the key precursor of L-ascorbic acid (vitamin C), is mainly produced by a mixed fermentation process which had been established in China in the 1970s. In this process, 2-KLG is biotransformed from L-sorbose by a co-culture of Ketogulonigenium vulgare and Bacillus spp. at the second fermentation step [1, 2]. Many studies have identified that K. vulgare could complete the bioconversion from L-sorbose to 2-KLG, but the 2-KLG yields are very lower in monoculture [3, 4]. Bacillus spp. (Bacillus megaterium is the most popular strain for vitamin C industrial production), as the helper strain, can effectively promote the growth of K. vulgare and enhance 2-KLG production by releasing active substances to support K. vulgare’s growth and metabolism [5–7]. Therefore, the co-culture fermentation including two strains for 2-KLG production is necessary, and it has been used as the only fermentation process on the industrial scale. However, the mixed fermentation always results in poor fermentation stability and low productivity [3].
In order to improve the 2-KLG productivity in the two-strain-mixed fermentation system, a great deal of efforts have been made during the past few years, such as screening for efficient strains [8, 9], optimizing the fermentation conditions [10, 11], and constructing engineering strains [12, 13]. Among these endeavors, optimization of fermentation conditions is considered to be one of the most important and effective strategies [3, 14]. In recent years, the multiple-stage control strategies have been reported and applied for improving 2-KLG production [15, 16]. Zhang et al. [15] found that the optimum pH for growth of strains and 2-KLG biosynthesis are different. Based on their experimental results, they developed a three-stage pH control strategy and significantly enhanced the productivity of 2-KLG (1.38 g/L/h, increased by 33.2%) than that of the single pH control model. Likewise, Li et al. [16] developed a three-stage ventilation control method in flask fermentation, and the 2-KLG production was increased by 10.9% than control. These results showed that the multiple-stage control strategies can be more effective than the traditional single control models.
In the two-strain-mixed fermentation system of vitamin C, the influence of temperature on 2-KLG productivity is much significant than any other environment factors [10, 17], because the suitable temperature is essential not only to the growths of both helper strain and K. vulgare but also to the activities of key enzymes converting L-sorbose to 2-KLG. The optimum growth temperature for B. megaterium was 35–37 °C [18, 19]. However, K. vulgare as an extremely temperature-sensitive strain grows very well at 29–30 °C and stop growing when the temperature is above 35 °C [2]. In addition, the optimum enzyme activity of sorbose dehydrogenase (converting L-sorbose to 2-KLG) was 30–40 °C [20, 21]. Therefore, in the two-strain-mixed fermentation, the optimum temperature for strain growth, metabolism, and 2-KLG bioconversion are quite different. In order to improve the fermentation efficiency of L-sorbose to 2-KLG, the temperature should be controlled in multiple-stage to fully meet the needs of B. megaterium growth and sporulation, K. vulgare growth and metabolism, and SDH activities, respectively.
The objective of the present study was to investigate the effects of temperature on B. megaterium growth, K. vulgare growth, and the key enzyme activities in the two-strain-mixed fermentation system, respectively. According to these results, a three-stage temperature control (TSTC) strategy was proposed for the first time.
Materials and methods
Bacterial strains
Bacillus megaterium 29 (the helper strain) and Ketogulonicigenium vulgare 02 (2-KLG-producing strain) were used in this study. Both of them were obtained from Northeast Pharmaceutical Group Co., Ltd., China.
Media
Three kinds of media were used in this study, i.e., isolation medium, seed culture medium, and fermentation medium. The composition and preparation of these media were according to our previous literature [8].
Seed preparation
Firstly, about 1000 colonies of K. vulgare were collected from isolation medium plates (K. vulgare had been inoculated into the plates and incubated for 4 days at 29 °C) and suspended in 5 mL of sterile physiological saline. Secondly, one colony of B. megaterium from isolation medium plate (B. megaterium had been inoculated into the plate and incubated for 2 days at 35 °C) was mixed with 5 mL of sterile physiological saline. Thirdly, 0.1 mL of B. megaterium solution and 5 mL of K. vulgare solution were mixed. Then the mixture was inoculated into a 30-mL seed culture medium in a 250 flask. After shaking (220 rpm) for 18 h at 29 °C, the seed was obtained.
Fermentation in flasks
All the flask fermentations were conducted after inoculating every 15 mL of seed into each 500-mL flask containing a 135-mL fermentation medium. Except for the changed temperature for different treatments, the other fermentation conditions were the same: the flasks were cultivated on a shaking incubator at 220 rpm.
To investigate the fermentation process using the traditional constant temperature model, three inoculated flasks were cultured at 29 °C at 220 rpm for 48 h. The fermentation broth in each flask was sampled every 2 h. The OD values of B. megaterium and K. vulgare, the concentration of substrate L-sorbose, and product 2-KLG were determined, separately. When the concentration of L-sorbose in the broth was less than 1 g/L, the fermentation was ended.
To investigate the effects of temperature on the growths of both B. megaterium and K. vulgare, and the yields of 2-KLG in different fermentation stages, three independent experiments were performed. For the first one, at the B. megaterium growth stage (0–16 h), the flasks were cultured at 29, 32, and 35 °C, respectively. For the second one, the flasks were firstly cultured at 29 °C for 16 h and then were divided into three groups and cultured at 29, 32, and 35 °C at the K. vulgare growth stage (16–30 h), respectively. For the last one, the flasks were firstly cultured at 29 °C for 30 h and then were divided into three groups and cultured at 29, 32, and 35 °C, respectively, until the end of fermentation. The control treatment in any experiment was cultured at a constant temperature of 29 °C.
To verify the new three-stage temperature control (TSTC) strategy for enhancement of 2-KLG production, six flasks were allocated to 2 groups. One group was the control treatment, which was cultured at a constant temperature of 29 °C during the whole fermentation. The other group was the T treatment, in which the fermentation temperature was changed during the fermentation, i.e., 0–16 h, 32 °C; 16–30 h, 29 °C; and 30 h to the fermentation end, 35 °C. All treatments were performed in triplicate. The culture broth was sampled every 4 h from the flasks and the OD values of B. megaterium and K. vulgare, SDH activities, and the concentrations of L-sorbose and 2-KLG were determined and analyzed.
Fermentation in 20-L fermentor
To further verify the new three-stage temperature control strategy for enhanced 2-KLG yield, the experiment was further conducted in 20-L fermentors. Compared to the control that was cultured at a constant temperature of 29 °C, the T treatment changed the temperature during the fermentation by using the TSTC strategy. The other fermentation conditions were the same for both the control and T treatment. At 0 h, the concentration of L-sorbose substrate was 50 g/L, and at 16–30 h, 30% (w/v) L-sorbose solution was gradually added into the fermentor, so that the concentration of L-sorbose in the broth was lower than 55 g/L. The pH was kept at 6.7–7.0 by adding 40% (w/v) NaOH solutions; the ventilation was 40 L/min with the rotator shaking at 250 rpm. The culture broth in each fermentor was sampled every 4 h; the OD values of both K. vulgare and B. megaterium and the concentrations of L-sorbose and 2-KLG were determined.
Assay methods
The optical density values (OD, at 600 nm) of strains were determined according to previously published literatures [22]. Firstly, the concentration of the two mixed strains was measured: 9 mL of 0.1 mol/L HCl was added to 1 mL of fermentation broth, and then the OD value (OD1) was measured spectrophotometrically at 600 nm after mixing for 60 s, with 0.1 mol/L HCl as the blank. Secondly, the concentration of K. vulgare was determined: 9 mL of 0.1 mol/L HCl was added to 1 mL of fermentation broth, and centrifuged at 2000 r/min for 10 min. The OD value (OD2) of supernatant was measured spectrophotometrically at 600 nm, with 0.1 mol/L HCl as a blank. Finally, the concentration of B. megaterium was calculated: OD B. megaterium = OD1 – OD2.
2-KLG concentration was determined by iodometry [8]. 2 ml of fermentation broth and 2 ml of 7 mol/L H2SO4 was added into a glass tube, respectively. The tube was put into a water bath and incubated at 100 °C for 25 min, and then the mixture in the tube was transferred to a flask containing 100 mL of purified water. Taking 1% of starch as indicator, the solution was titrated to blue as the endpoint with 0.1 mol/L standard iodine solution. The consumption of standard iodine is proportional to the concentration of 2-KLG.
Sorbose concentration was determined according to the reports of Yemm and Willis [23]. After the fermentation broth was diluted 100 times with purified water, 1 mL of the diluent was fully mixed with 6 mL of anthrone solution, and the solution was placed at 25 °C for 10 min for color development. At the same time, 1 mL of purified water and 6 mL of anthrone solution were mixed and placed for 10 min at 25 °C as blank control. The optical density value was measured spectrophotometrically at 620 nm. The measured value is proportional to the sorbose concentration in the fermentation broth. The sorbose concentration was finally determined according to the standard curve.
L-Sorbose dehydrogenase (SDH) extractions and activity assays were performed using the literature’s method [24]. SDH activity was measured by reading the decrease in absorbance at 600 nm of 2,6-dichlorophenolindophenol (DCIP); one unit of enzyme activity was defined as the amount of enzyme that catalyzed the reduction of 1 μmol DCIP per minute at 25 °C. The protein concentration was determined using the Bio-Rad Protein Assay.
Data analysis and statistics
Student’s t tests were used for statistical analyses. Differences between two groups were regarded as statistically significant if p < 0.05.
Results
The dynamic changes of bacterial cells and 2-KLG production in two-strain co-culture fermentation
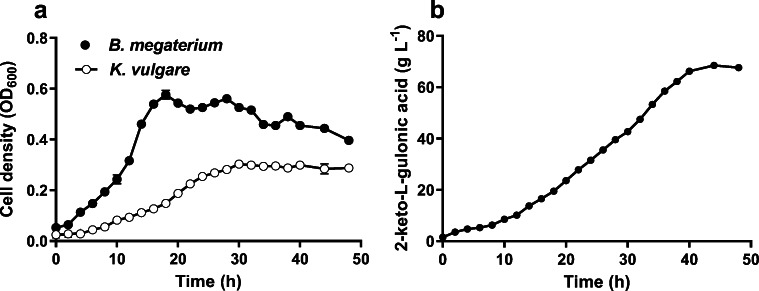
The growths of B. megaterium and K. vulgare and the 2-KLG yields with time courses during the flask fermentation were shown in Fig. 1(a, b). As results showed, B. megaterium, as the helper strain, got into the logarithmic growth phase at 10 h and its cell density reached the maximum at 18 h (Fig. 1a). Subsequently, the B. megaterium cells were ruptured and a large amount of spores were formed during the fermentation of 18 to 22 h. After that, a slight increase was found and a more rapid cell rupture has been observed, which led to the concentration of B. megaterium gradually decreased to the end of fermentation. Compared to B. megaterium, K. vulgare went into the logarithmic growth phase at 16 h. After about 14 h of propagation, K. vulgare increased to the maximum (The OD value was 0.303 ± 0.003). From 30 h to the end of fermentation, the concentration of K. vulgare was kept at 0.272–0.308. During the fermentation of 0–12 h, the 2-KLG production was fairly low with an average productivity of 0.72 ± 0.01 g/L/h. 2-KLG productivity began to increase rapidly at 16 h, when the K. vulgare got into the logarithmic growth phase. 2-KLG productivity by K. vulgare increased to1.87 ± 0.02 g/L/h during its growth stage. 2-KLG productivity continued increasing at 30–40 h with a maximum value of 2.35 ± 0.03 g/L/h (Fig. 1b). Therefore, the accumulation of 2-KLG was mainly at 30–40 h, which contributed 34.80 ± 0.36% of 2-KLG production during the fermentation period.
Fig. 1.
Time courses of cell density of B. megaterium and K. vulgare (a) and 2-KLG production (b) in two-strain mixed fermentation
According to the above results including both strains’ growths and 2-KLG production, the fermentation process could be divided into “three-stage fermentation,” i.e., growth stage 1, mainly for the growth of B. megaterium at 0–16 h; growth stage 2, mainly for the growth of K. vulgare at 16–30 h; and production stage, mainly for 2-KLG biosynthesis at 30 h to the end of fermentation.
The effect of temperature on the two strain growths at growth stage 1
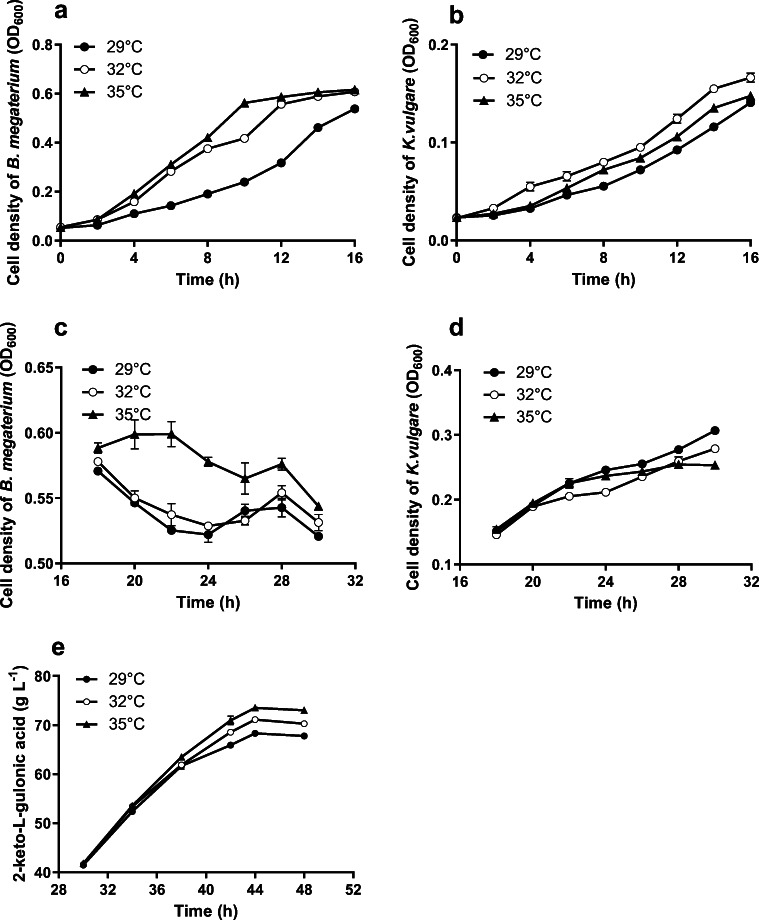
To investigate the temperature effect on the growths of both B. megaterium and K. vulgare at growth stage 1, the fermentations were conducted at 29 °C, 32 °C, and 35 °C, respectively. The strain concentrations (ODs) were determined (Fig. 2a, b).
Fig. 2.
Effects of temperature on cell density of B. megaterium and K. vulgare at growth stage 1 (a, b) and growth stage 2 (c, d), and on 2-KLG production (e) at production stage
The B. megaterium concentration was increased with the enhanced incubation temperature at growth stage 1 (Fig. 2a). Compared to the control treatment (cultured at 29 °C), fermentation conducted at 32 °C or 35 °C had shortened the lag phase (from 6 h at 29 °C to 4 h at 32 °C or 35 °C) obviously. During 4–16 h of fermentation, the concentration of B. megaterium cultured at 35 °C was higher than that at 32 °C, but showing no significant difference (p > 0.05).
However, the growth of K. vulgare showed different characteristics (Fig. 2b), when cultured at different temperatures at growth stage 1. Of all the treatments, K. vulgare showed the highest concentration at 32 °C, while it was the lowest at 29 °C. The concentration of K. vulgare at 35 °C was significantly higher than that at 29 °C, but significantly lower than that at 32 °C. The results showed that the optimum temperature for K. vulgare at growth stage 1 was 32 °C.
Comprehensively considering the effects of temperature on the two strain growths at growth stage 1, the optimum temperature was 32 °C.
The effect of temperature on the two strain growths at growth stage 2
At growth stage 2, the concentration of B. megaterium decreased with the time course when incubated at 29 °C or 32 °C. However, the number of B. megaterium incubated at 35 °C increased until 22 h (Fig. 2C). In contrast to B. megaterium, K. vulgare grew fast in any treatments and obtained the highest concentration at 30 h of fermentation (Fig. 2d). Of the three temperature treatments, K. vulgare cultured at 29 °C showed the fastest growth rate and the highest biomass at 30 h. Hence, the optimum temperature for K. vulgare growth at growth stage 2 was 29 °C.
The effect of temperature on 2-KLG production at production stage
As shown in the results in Fig. 2e, the 2-KLG productivity was increased accordingly with the temperature increased. At 35 °C, 73.00 ± 0.54 g/L of 2-KLG was finally obtained with a significantly higher (p < 0.05) conversion rate of 93.58 ± 0.69%, compared to the control treatment. The results indicated that the optimum temperature for 2-KLG production at production stage was 35 °C.
2-KLG production by using three-stage temperature control strategy in flask fermentation
A three-stage temperature control (TSTC) strategy was proposed. In this strategy, fermentation was initially conducted at 32 °C for the growth of helper strain at 0–16 h, and then the temperature decreased to 29 °C for K. vulgare growth at 16–30 h. Finally, the temperature was kept at 35 °C for 2-KLG production at 30 h to the end of fermentation.
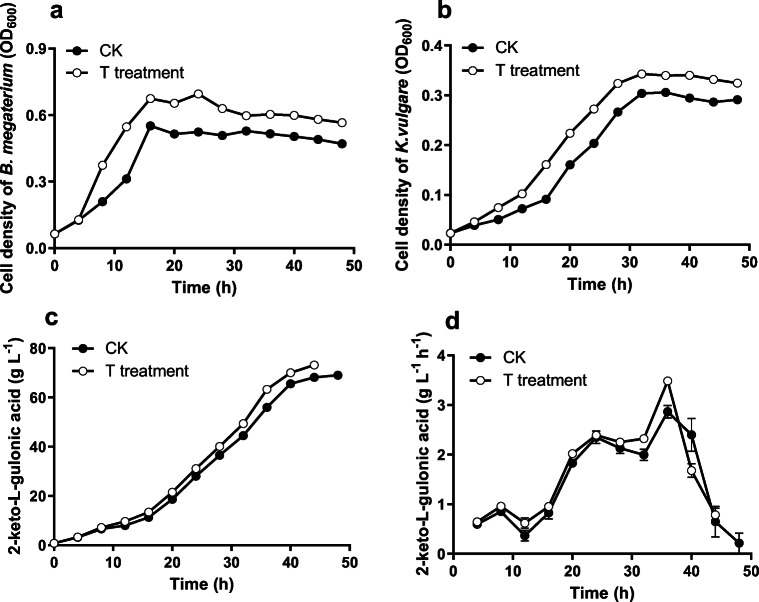
A flask fermentation test was conducted to verify the feasibility of the TSTC strategy in 2-KLG production. The results were shown in Fig. 3(a–d). Compared to the control treatment, the fermentation by using the TSTC strategy shorted the lag phase of the helper strain (decreased from 10 to 4 h) and enhanced its cell density during the fermentation of 8–44 h (Fig. 3a). The cell density of K. vulgare at T treatment got into the logarithmic growth phase at 12 h, which was ahead of 4 h than the control (Fig. 3b). In addition, the number of K. vulgare reached the maximum at 32 h with a significantly higher (p < 0.05) cell density of 0.333 ± 0.004 than control treatment (Fig. 3b). During the fermentation of 12–44 h, 2-KLG production in T treatment was significantly higher than the corresponding values of the control (p < 0.05) (Fig. 3c). At 36 h, the 2-KLG productivity in T treatment obtained the highest values (3.48 ± 0.05 g/L/h), 21.68% higher than the control (2.86 ± 0.13 g/L/h at 40 h) (Fig. 3d). The fermentation period was 44 h for T treatment and 48 h for control, respectively.
Fig. 3.
Kinetics of cell density of B. megaterium (a) and K. vulgare (b) and 2-KLG production (c, d) under two treatments (three-stage temperature control treatment and control treatment) in flask fermentation
The sorbose dehydrogenase (SDH) activity was determined at 12, 24, 36, and 44 h (Table 1). There were no significant differences on SDH activities at 12 and 24 h between the control and T treatments. However, the SDH activity of T treatment was significantly higher than the control treatment at 36 and 44 h, respectively (increased by 25.18% at 36 h, 17.14% at 44 h).
Table 1.
SDH activities of K. vulgare in different fermentation stages by using a three-stage temperature control strategy
| Fermentation time (h) | SDH activities of K. vulgare (U/mg protein) | |
|---|---|---|
| Control treatment | Three-stage temperature strategy | |
| 12 | 0.076 ± 0.006 | 0.085 ± 0.005 |
| 24 | 0.104 ± 0.003 | 0.107 ± 0.003 |
| 36 | 0.135 ± 0.009 | 0.169 ± 0.004* |
| 44 | 0.140 ± 0.005 | 0.164 ± 0.004* |
*p < 0.05
2-KLG production by using three-stage temperature control strategy in 20-L fermentor
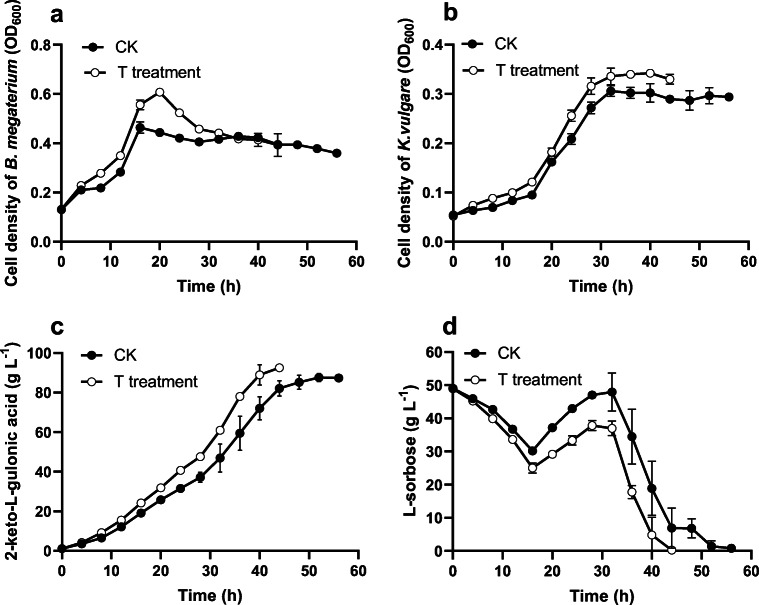
We investigated the fermentation process of 2-KLG by using the new strategy in 20-L fermentor. The results were shown in Table 2 and Fig. 4. By using the TSTC strategy, compared to the control treatment, the culture time was short for 6 h. In addition, the conversion rate of 2-KLG was 92.34 ± 1.20% with 2-KLG productivity of 2.19 ± 0.19 g/L/h, which was significantly higher than the corresponding values of control treatment (88.74 ± 1.44% for 2-KLG conversion rate and 1.79 ± 0.16 g/L/h for 2-KLG productivity).
Table 2.
Fermentation properties of 2-KLG production by using a three-stage temperature control strategy
| Parameters | Control strategy | |
|---|---|---|
| Constant temperature (29 °C) | Three-stage temperature strategy | |
| Culture time (h) | 49 ± 5 | 43 ± 3* |
| 2-KLG concentration (g/L) | 87.63 ± 1.19 | 92.91 ± 1.02* |
| 2-KLG conversion rate (%) | 88.74 ± 1.44 | 92.34 ± 1.20* |
| 2-KLG productivity (g/L/h) | 1.79 ± 0.16 | 2.19 ± 0.19* |
*p < 0.05
Fig. 4.
Time curves of cell density of B. megaterium (a) and K. vulgare (b), 2-KLG production (c), and L-sorbose concentration (d) under two treatments (three-stage temperature control treatment and control treatment) in 20-L fermentors
Discussion
In the 2-KLG fermentation process, the fermentation efficiency is not only affected by the transformation characteristics of the used strains but also influenced by several nutritional factors, such as glutathione [25], folate [26] and rare earth elements [27], and environmental factors, such as pH [15], dissolved oxygen [16], and temperature [10]. Among these factors, the temperature is one of the most important factors in influencing the fermentation efficiency, because the suitable temperature is essential to the growths of B. megaterium and K. vulgare, and to the transformation of L-sorbose to 2-KLG.
B. megaterium, the helper strain, plays a vital role in stimulating K. vulgare to grow more rapidly and to convert L-sorbose to 2-KLG more efficiently [5, 11, 28]. In the initial stage of fermentation, it is necessary to promote the growth of B. megaterium for providing adequate activators to K. vulgare [5]. Generally speaking, B. megaterium grows in a wide temperature range from 3 to 45 °C, with the optimum around 30 °C [29]. Some strains isolated from an Antarctic geothermal lake can grow when the temperature is up to 63 °C [29]. However, in our study, the optimum temperature for B. megaterium growth was 35 °C, at which the cell density was significantly higher than that at 29 °C. This may be attributed to different strains and incubation conditions.
In this two-strain mixed fermentation, K. vulgare is a more temperature-sensitive strain than the helper strain [10]. Although the increased temperature (35 °C) promoted the growth of B. megaterium, it inhibited K. vulgare growth. However, the cell density of both B. megaterium and K. vulgare was significantly increased at 32 °C than that at 29 °C. The results indicated that relative higher temperature (32 °C) promoted the growths of both strains at growth stage 1. Hence, by comprehensively considering the effects of temperature on both strains growths, the suitable temperature at growth stage 1was 32 °C.
Sorbose dehydrogenase (SDH) is the key enzyme in this mixed fermentation [8, 20]. It is a unique dehydrogenase that directly catalyzes the conversion of L-sorbose to 2-KLG using cofactor PQQ [30, 31]. This enzyme had been purified and characterized by Dong and Xiong et al. [30, 32], and the crystal structure had been determined by Han et al. [31]. The optimum enzyme activity occurred at 35 °C, and the relative enzyme activity of SDH at 35 °C was increased by 77.0% than that at 30 °C [30]. In this study, the SDH activity of the T treatment was higher than that of the control at growth stage 1, but it did not reach a significant level. This may be due to the low SDH expression of K. vulgare and the low release of companion substances of B. megaterium in the initial stage of fermentation, so the difference in temperature did not cause significant changes in SDH activity. At growth stage 2, the enzyme activity values were basically the same as the two treatments because of the same culture conditions such as temperature. However, at the 2-KLG production stage, the SDH activity of T treatment was significantly higher than that of the control (increased by 25.18% at 36 h, 17.14% at 44 h), which may be due to two reasons: on the one hand, the increase of temperature (from 29 °C to 35 °C) would significantly increase the enzyme activity [30]; on the other hand, B. megaterium in the T treatment might release more active substances, which could enhance the enzyme activity [11, 33]. The enhanced SDH activity may explain the increased 2-KLG production at production stage.
A comparison of the conversion rate and 2-KLG productivities in this study with other literature reported corresponding values indicates that the three-stage temperature control (TSTC) strategy competes favorably with other methods for process optimization. Many works have been reported to improve the 2-KLG yield using new regulation measures [15, 16, 33–35]. Zhang et al. [34] developed a new ecological regulation technology, by preparing high-quality seeds and optimizing the fermentation conditions. The conversion rate increased from 85 to 88%. Zhou et al. [35] further studied the interaction of K. vulgare and B. megaterium and investigated the effect of the environmental factor on the growth of strains and 2-KLG production. According to the optimum environmental conditions, the conversion rate had increased by 2.33% and the fermentation period shorted by 2.8 h. Zhang et al. [33] reported an adding lysozyme strategy to let helper strain secret more activators and stimulate K. vulgare growth. After 12-h co-culture in a 7-L fermentor, the growth of K. vulgare and 2-KLG productivity increased by 27.4 and 28.2%, respectively [33]. Zhang et al. [15] developed a multiple-stage pH control strategy; the 2-KLG productivity was achieved at 1.38 g/L/h, which exhibited 33.2% higher than that of the single pH control model. In our study, a TSTC strategy was proposed and verified in 20-L fermentor for 5 batches. The conversion rate was 92.34 ± 1.20%, increased by 3.60% than that at constant temperature control. Moreover, the 2-KLG productivity was 2.19 ± 0.19 g/L/h, increased by 22.35% than that of the control treatment. The conversion rate and 2-KLG productivity are much higher than those of the majority of literatures [11, 15, 33–35]. Compared with the currently reported highest fermentation efficiency [15], our results by using the TSTC strategy showed an increase in conversion rate (2.41%) and enhancement in productivity (58.70%), showing promising applications of TSTC in industrial fermentation processes.
In conclusion, the new proposed TSTC was found to be a promising strategy in improving the 2-KLG fermentation efficiency. The increased cell density during the exponential phase of K. vulgare and the enhanced SDH activity at the production stage might be the reasons for enhanced 2-KLG production with a high conversion rate. This TSTC process is a good candidate for 2-KLG production on an industrial scale.
Acknowledgments
The authors thank Mingyan Jiang and Litao Han for the technical contribution.
Funding
This work was supported by the Shenyang Municipal Science and Technology Project (No. Z17-7-011), the research project of high-level talents in Inner Mongolia Agricultural University (NDGCC2016-04).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mandlaa, Email: mandlaa@foxmail.com.
Hui Xu, Email: xuhui@iae.ac.cn.
References
- 1.Yin G, Tao Z, Yu L, Yan Z, Ning W, Wang C, Wang S, Jiang H, Zhang X, Feng X, Zhao Q, Wei W. Studies on the production of vitamin C precursor-2-keto-L-gulonic acid from L-sorbose by fermentation. I. Isolation, screening and identification of 2-keto-L-gulonic acid producing bacteria. Acta Microbiol Sin. 1980;20:246–251. [Google Scholar]
- 2.Urbance JW, Bratina BJ, Stoddard SF, Schmidt TM. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize L-sorbose to 2-keto-L-gulonic acid. Int J Syst Evol Microbiol. 2001;51:1059–1070. doi: 10.1099/00207713-51-3-1059. [DOI] [PubMed] [Google Scholar]
- 3.Zou W, Liu LM, Chen J. Structure, mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit Rev Microbiol. 2013;39:247–255. doi: 10.3109/1040841X.2012.706250. [DOI] [PubMed] [Google Scholar]
- 4.Fan S, Zhang Z, Zou W, Huang Z, Liu J, Liu L. Development of a minimal chemically defined medium for Ketogulonicigenium vulgare WSH001 based on its genome-scale metabolic model. J Biotechnol. 2014;169:15–22. doi: 10.1016/j.jbiotec.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Mandlaa M, Yang WC, Han LT, Wang ZY, Xu H. Two-helper-strain co-culture system: a novel method for enhancement of 2-keto-l-gulonic acid production. Biotechnol Lett. 2013;35:1853–1857. doi: 10.1007/s10529-013-1292-5. [DOI] [PubMed] [Google Scholar]
- 6.Jia N, Ding M, Gao F, Yuan Y. Comparative genomics analysis of the companion mechanisms of Bacillus thuringiensis Bc601 and Bacillus endophyticus Hbe603 in bacterial consortium. Sci Rep. 2016;6:28794. doi: 10.1038/srep28794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YH, Lin JY, Bai L, Huang M, Chen HQ, Yao S, Lyu SX. Antioxidant capacities of Bacillus endophyticus ST-1 and Ketogulonicigenium vulgare 25B-1 in vitamin C fermentation. Biotechnol Biotec Eq. 2018;32(1):1–10. doi: 10.1080/13102818.2017.1398051. [DOI] [Google Scholar]
- 8.Yang W, Han L, Mandlaa M, Chen H, Jiang M, Zhang Z, Xu H. Spaceflight-induced enhancement of 2-keto-L-gulonic acid production by a mixed culture of Ketogulonigenium vulgare and Bacillus thuringiensis. Lett Appl Microbiol. 2013;57:54–62. doi: 10.1111/lam.12083. [DOI] [PubMed] [Google Scholar]
- 9.Mandlaa SZ, Wang R, Han X, Xu H, Yang W. Enhanced 2-keto-l-gulonic acid production by applying l-sorbose-tolerant helper strain in the co-culture system. AMB Express. 2018;8(1):30. doi: 10.1186/s13568-018-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang Y, Zhao J, Yang W, Zhang H, Xu H, Sun C. Screening and conditions optimization of vitamin C bio-step fermentation by neo-comnination strain system B15-14. J Microbiol. 2008;28:35–38. [Google Scholar]
- 11.Yang W, Xu H. Industrial fermentation of vitamin C. In: Vandamme EJ, Revuelta JL, editors. Industrial biotechnology of vitamins, biopigments, and antioxidants. 1. Weinheim: Wiley-VCH Verlag GmbH & Co. KgaA; 2016. pp. 161–192. [Google Scholar]
- 12.Cai L, Yuan MQ, Li ZJ, Chen JC, Chen GQ. Genetic engineering of Ketogulonigenium vulgare for enhanced production of 2-keto-l-gulonic acid. J Biotechnol. 2012;157(2):320–325. doi: 10.1016/j.jbiotec.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Bai W, Song H, Yuan YJ. Combinational expression of sorbose/sorbosone dehydrogenases and cofactor pyrroloquinoline quinone increases 2-keto-L-gulonic acid production in Ketogulonigenium vulgare-Bacillus cereus consortium. Metab Eng. 2013;19:50–56. doi: 10.1016/j.ymben.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y, Hu ML, Lv YJ, Wang Y, Song H, Yuan YJ. Enhancement of 2-keto-gulonic acid yield by serial subcultivation of co-cultures of Bacillus cereus and Ketogulonigenium vulgare. Bioresour Technol. 2013;132:370–373. doi: 10.1016/j.biortech.2012.10.151. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhou J, Liu L, Liu J, Chen K, Du G, Chen J. Enhancement of 2-keto-L-gulonic acid production using three-stage pH control strategy. Chin J Biotechnol. 2010;26:1263–1268. [PubMed] [Google Scholar]
- 16.Li L, Li B, Lv S, Xu H, Yang W, Han L, Wang Z. Enhancement of 2-keto-L-gulonic acid production using three-stage ventilation control strategy. China Brewing. 2012;31:144–147. [Google Scholar]
- 17.Huang L, Lv S, Zhang L, Shao L, Zhang Z. Study on individual factor fermentation condition for new strain in two-step fermentation of Vc. J Anhui Agri Sci. 2005;33:2372–2375. [Google Scholar]
- 18.Jia X, Guo L, Liu Y, Jiao Q. Enzymic conversion of D-phenylalanine using Bacillus megaterium AS1.127. Chemical World. 2006;47(5):281–284. [Google Scholar]
- 19.Fan C, Li J, Wu H, Zhu X, Hao J, Ding L, Zhang L. Studies on fermentation conditions for penicillin G acylase production with a recombinant Bacillus megaterium system. J Cent South Univ For Technol. 2011;31(7):124–129,135. [Google Scholar]
- 20.Asakura A, Hoshino T. Isolation and characterization of a new quinoprotein dehydrogenase, L-sorbose/L-sorbosone dehydrogenase. Biosci Biotechnol Biochem. 1999;63:46–53. doi: 10.1271/bbb.63.46. [DOI] [PubMed] [Google Scholar]
- 21.Hao A, Jia Q, Wu H, Zhou H, Geng W, Gao W, Zhao J, He J. Isolation and characteristics research of L-sorbose dehydrogenase in Ketogulonigenium sp. WB0104. Indust Microbiol. 2008;38:10–14. [Google Scholar]
- 22.Liu LM, Chen KJ, Zhang J, Liu J, Chen J. Gelatin enhances 2-keto-L-gulonic acid production based on Ketogulonigenium vulgare genome annotation. J Biotechnol. 2011;156:182–187. doi: 10.1016/j.jbiotec.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Yemm E, Willis AJ. The estimation of carbohydrate in plant extracts by Anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning RF, Kahn MS (1992) Biosynthesis of 2-keto-L-gulonic acid. US Patent No. 5,082,785
- 25.Huang Z, Zou W, Liu J, Liu L. Glutathione enhances 2-keto-l-gulonic acid production based on Ketogulonicigenium vulgare model iWZ663. J Biotechnol. 2013;164:454–460. doi: 10.1016/j.jbiotec.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Leduc S, Troostembergh JCD, Lebeault JM. Folate requirements of the 2-keto-L-gulonic acid-producing strain Ketogulonigenium vulgare LMP P-20356 in L-sorbose/CSL medium. Appl Microb Biotechnol. 2004;65(2):163–167. doi: 10.1007/s00253-004-1562-1. [DOI] [PubMed] [Google Scholar]
- 27.Lyu S, Guo Z, Pan J, Yang Y, Yang W, Chen H, Zhang Z. Effect of rare earth elements on vitamin C fermentation by mixed cultures. Int J Agric Biol. 2014;16(6):1135–1140. [Google Scholar]
- 28.Lu S, Feng S, Zhang Z, Liu Y, Xie Z, An H. The effect of Bacillus megaterium in vitamin C two-step fermentation. Microbiology. 2001;28:10–13. [Google Scholar]
- 29.Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, et al. Bergey’s manual of systematic bacteriology. New York: Springer; 2009. [Google Scholar]
- 30.Dong F (2006) Study on key enzymes and its cofactor for converting L-sorbose to 2-keto-L-gulonic acid in the two-step fermentation of vitamin C. (Master Thesis). Xiamen University, Xiamen, China 26-35. (In Chinese)
- 31.Han X, Xiong X, Jiang D, Chen S, Huang E, Zhang W, Liu X. Crystal structure of l-sorbose dehydrogenase, a pyrroloquinoline quinone-dependent enzyme with homodimeric assembly, from Ketogulonicigenium vulgare. Biotechnol Lett. 2014;36:1001–1008. doi: 10.1007/s10529-013-1446-5. [DOI] [PubMed] [Google Scholar]
- 32.Xiong XH, Ge X, Zhao Y, Han XD, Wang JH, Zhang WC. Expression, purification and characterization of a quinoprotein L-sorbose dehydrogenase from Ketogulonicigenium vulgare Y25. Afr J Microbiol Res. 2013;7:3117–3124. doi: 10.5897/AJMR12.2280. [DOI] [Google Scholar]
- 33.Zhang J, Liu J, Shi ZP, Liu LM, Chen J. Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem. 2010;45:602–606. doi: 10.1016/j.procbio.2009.11.016. [DOI] [Google Scholar]
- 34.Zhang Z, Zhang C, Sun C, Zhang Z, Feng S, Zhang H, Zhu K, Li G, Zhang H, An H, Gao YT, Su ZC, Meng SD (1998) A mini-ecological regulation technology in two-step fermentation of Vc. CN patent No. 98114478.0
- 35.Zhou B, Li Y, Liu Y, Zhang Z, Zhu K, Liao D, Gao Y. Microbiological eco-regulation in Vc two-step fermentation. Chin J Appl Ecol. 2002;13:1452–1454. [PubMed] [Google Scholar]