Summary
Background
The number of individuals with vision impairment worldwide is increasing because of an ageing population. We aimed to systematically identify studies describing the association between vision impairment and mortality, and to assess the association between vision impairment and all-cause mortality.
Methods
For this systematic review and meta-analysis, we searched MEDLINE (Ovid), Embase, and Global Health database on Feb 1, 2020, for studies published in English between database inception and Feb 1, 2020. We included prospective and retrospective cohort studies that measured the association between vision impairment and all-cause mortality in people aged 40 years or older who were followed up for 1 year or more. In a protocol amendment, we also included randomised controlled trials that met the same criteria as for cohort studies, in which the association between visual impairment and mortality was independent of the study intervention. Studies that did not report age-adjusted mortality data, or that focused only on populations with specific health conditions were excluded. Two reviewers independently assessed study eligibility, extracted the data, and assessed risk of bias. We graded the overall certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluations framework. We did a random-effects meta-analysis to calculate pooled maximally adjusted hazard ratios (HRs) for all-cause mortality for individuals with a visual acuity of <6/12 versus those with ≥6/12; <6/18 versus those with ≥6/18; <6/60 versus those with ≥6/18; and <6/60 versus those with ≥6/60.
Findings
Our searches identified 3845 articles, of which 28 studies, representing 30 cohorts (446 088 participants) from 12 countries, were included in the systematic review. The meta-analysis included 17 studies, representing 18 cohorts (47 998 participants). There was variability in the methods used to assess and report vision impairment. Pooled HRs for all-cause mortality were 1·29 (95% CI 1·20–1·39) for visual acuity <6/12 versus ≥6/12, with low heterogeneity between studies (n=15; τ2=0·01, I2=31·46%); 1·43 (1·22–1·68) for visual acuity <6/18 versus ≥6/18, with low heterogeneity between studies (n=2; τ2=0·0, I2=0·0%); 1·89 (1·45–2·47) for visual acuity <6/60 versus ≥6/18 (n=1); and 1·02 (0·79–1·32) for visual acuity <6/60 versus ≥6/60 (n=2; τ2=0·02, I2=25·04%). Three studies received an assessment of low risk of bias across all six domains, and six studies had a high risk of bias in one or more domains. Effect sizes were greater for studies that used best-corrected visual acuity compared with those that used presenting visual acuity as the vision assessment method (p=0·0055), but the effect sizes did not vary in terms of risk of bias, study design, or participant-level factors (ie, age). We judged the evidence to be of moderate certainty.
Interpretation
The hazard for all-cause mortality was higher in people with vision impairment compared with those that had normal vision or mild vision impairment, and the magnitude of this effect increased with more severe vision impairment. These findings have implications for promoting healthy longevity and achieving the Sustainable Development Goals.
Funding
Wellcome Trust, Commonwealth Scholarship Commission, National Institutes of Health, Research to Prevent Blindness, the Queen Elizabeth Diamond Jubilee Trust, Moorfields Eye Charity, National Institute for Health Research, Moorfields Biomedical Research Centre, Sightsavers, the Fred Hollows Foundation, the Seva Foundation, the British Council for the Prevention of Blindness, and Christian Blind Mission.
Introduction
Over half a billion people are blind or have distance vision impairment worldwide.1 Blindness and vision impairment are most common among adults aged 50 years and older, who account for more than 80% of people with vision loss.2 As populations continue to age, the prevalence of vision impairment and blindness are projected to more than double over the next 30 years.1 The impacts of vision impairment and blindness are wide-reaching, including an increased risk of falls, cognitive impairment and dementia, depression, disability, and loss of independence.2, 3 Some studies have also reported that vision impairment and blindness are associated with an increased risk of mortality.4
Research in context.
Evidence before this study
We searched PubMed on Feb 1, 2020, for primary research articles published in English from database inception up to Feb 1, 2020. A full list of the search terms used can be found in the appendix (pp 2–5). We identified a single meta-analysis of the association between vision impairment and mortality. This study analysed the association between vision impairment (measured by use of objective clinical instruments, self-reported visual difficulty, and administrative claims) and all-cause mortality. The results showed a significant association between mortality and the highest degree of vision impairment when compared with no vision impairment. The study did not include a narrative review of the literature, an assessment of the risk of bias in included studies, or an overall grading of the certainty of the evidence. We also found that, since the publication of this meta-analysis, several additional primary research articles had been published, and that some of these articles were from regions of the world, including sub-Saharan Africa and east Asia, that were previously not well represented in the literature.
Added value of this study
This systematic review and meta-analysis adds to the existing literature by including newly published articles investigating the association between vision impairment and all-cause mortality in adults worldwide. By conducting a full systematic review, we have identified opportunities for standardisation of data collection and reporting, and we found additional studies on the topic that could not be included in the meta-analysis due to the choice of vision impairment thresholds, the analytic methods used, or both. Additionally, we used the Quality in Prognostic Studies tool to assess the risk of bias in included studies, and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework to judge the overall certainty of the evidence. We also did meta-analyses comparing the hazard of all-cause mortality in participants with and without specified levels of visual acuity impairment. We found that the hazard of mortality was higher among participants with mild vision impairment (visual acuity <6/12) compared with those who had no vision impairment (≥6/12), and was higher in those with moderate vision impairment (<6/18) compared with those with no vision impairment or mild vision impairment (≥6/18). Among people with severe vision impairment or blindness (visual acuity <6/60), the hazard for mortality was higher than for those with normal vision or mild vision impairment. However, no association between vision impairment and mortality was observed when participants with a visual acuity of worse than 6/60 were compared with those with visual acuity of better than 6/60, probably because the reference group (ie, those with a visual acuity of ≥6/60) comprised a heterogeneous group of participants with moderate vision impairment, mild vision impairment, and normal vision. We assessed the robustness of our findings by examining heterogeneity in our effect estimates, performing meta-regressions, and testing for publication bias; we found little heterogeneity in our estimates and no evidence of publication bias. However, studies reported a significantly larger effect size if they assessed the association between mortality and best-corrected visual acuity rather than the association between mortality and presenting visual acuity.
Implications of all the available evidence
Our systematic review and meta-analysis highlights the prevailing finding that vision impairment is associated with a higher hazard of age-adjusted all-cause mortality in adults across diverse global settings and populations. Using the GRADE framework, we are moderately confident that the mortality risk associated with vision impairment reported in this study is likely to be close to the true value, but there is a possibility that the true hazard might be substantially different. Future research should focus on assessing the association between mortality and other clinical measures of vision (eg, visual field or contrast sensitivity) that have been shown to affect functioning, quality of life, and health outcomes. In addition, no studies on this topic have been conducted in eastern Europe, Latin America, the Caribbean, north Africa, or the Pacific islands, and data from these regions is important to improve the generalisability of study findings. Future calculations of disability-adjusted life-years might include years of life lost due to vision impairment, which could provide a more complete estimate of the overall global burden of vision impairment. As most vision impairment and blindness is avoidable or correctable, this study has important implications for optimising healthy longevity for populations worldwide, and for achieving the Sustainable Development Goals (SDGs), particularly SDG3, which aims to “ensure healthy lives and promote well-being for all at all ages”.
In a previous meta-analysis, Zhang and colleagues4 examined 29 studies that measured the association between vision impairment and mortality. Among these studies, 15 used objective measures of vision (eg, visual acuity), whereas others relied on self-reported visual difficulty, or vision impairment defined by International Classification of Diseases (ICD) codes. The risk of bias in these studies and the overall quality of evidence was not assessed.4 Since this meta-analysis was published in 2016, several additional primary studies have been published,5, 6, 7, 8, 9, 10 including those done in previously under-represented regions, such as sub-Saharan Africa5 and east Asia.6
An improved understanding of the association between vision impairment and mortality is needed to inform public policy, public health planning, and allocation of limited health-care resources. As part of The Lancet Global Health Commission on Global Eye Health,11 we therefore did an updated systematic review and meta-analysis to assess the extent, strength, and quality of evidence on the association between vision impairment and age-adjusted all-cause mortality in adults worldwide. To provide a comprehensive assessment of the current state of scientific knowledge, we also examined the potential causes of variation in this association.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched MEDLINE (Ovid), Embase, and Global Health database on Feb 1, 2020, for studies published in English between database inception and Feb 1, 2020. We included prospective and retrospective cohort studies that measured the association between vision impairment and all-cause mortality in people aged 40 years or older, who were followed up for 1 year or more. In a protocol amendment, we included randomised controlled trials (RCTs), as long as the reported association between vision impairment and mortality was independent of the study intervention; we also included RCTs and cohort studies with participants younger than 40 years if more than 50% of participants were aged 40 years or older. We assessed the effect of these protocol amendments on effect estimates in meta-regression analyses. Conference abstracts and grey literature were not included. We identified additional studies by searching the reference lists of included studies. The searches were done by an information specialist (IG), and the search strategy and full list of search terms used are provided in the appendix (pp 2–5).
We intended to include studies in which vision was assessed by use of any objective clinical measure of vision and in which age-adjusted all-cause mortality was reported. We only included studies in the meta-analysis that assessed visual acuity, as few studies reported associations with other measures of vision (eg, contrast sensitivity or visual fields). In studies that used best-corrected visual acuity and presenting visual acuity as vision assessment methods, data on best-corrected visual acuity were included in the primary analysis. Studies that did not report age-adjusted mortality, or that focused only on populations with specific health conditions (eg, diabetes or stroke) were excluded, as in such cases, age and systemic disease might have a strong confounding effect.
The internet-based systematic review management software, Covidence (Veritas Health Innovation, Melbourne, VIC, Australia), was used to screen titles and abstracts, assess full-text articles, and extract summary estimates from included studies. All titles, abstracts, and full-text articles were screened independently by pairs of investigators (one of JREh, JR, and JREv paired with one of HB, CNL, JHZ, or WW). Disagreements were resolved through discussion and adjudication by a third investigator (JREh, JR, or JREv), as needed.
This study was done as part of The Lancet Global Health Commission on Global Eye Health.11 The complete study protocol was registered prospectively at the Open Science Framework Registries, and has been published previously.12 Amendments to the initial study protocol are noted herein. We used the PROGRESS prognosis research strategy13 to develop the protocol for this study, which is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (appendix pp 6–7).14
Data analysis
The following data were extracted from each publication: study setting; study timing; study design; sample size; age, gender, and ethnicity of study participants; follow-up time; definition of vision impairment; methods and eyes used for vision assessment; methods of mortality assessment; statistical modelling approach; and effect size estimates. Three pairs of investigators (JREh and CNL, JR and JHZ, and JREv and HB) independently extracted data from each article, guided by the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies framework.15 Disagreements were resolved through discussion and adjudication by a third investigator (JREh, JR, or JREv), as needed. When duplicate data were available from multiple published studies, preference was given to the study with the longest follow-up. Many studies reported results from models with different combinations of covariates. When estimates were reported with more than one level of adjustment, we extracted two estimates: (1) the age-adjusted estimate with the fewest additional covariates (minimally adjusted); and (2) the age-adjusted estimate with the greatest number of additional covariates (maximally adjusted). When multiple publications contained data from a single cohort, data were extracted from the publication with the longest follow-up. When data on multiple cohorts were presented in a single publication, each cohort was separately eligible for inclusion.
The risk of bias in included studies was assessed independently by three pairs of investigators (JREh and CNL, JR and JHZ, and JREv and HB) using the Quality in Prognostic Studies (QUIPS) tool.16 The following domains were assessed: study participation, attrition, prognostic factor measurement, outcome measurement, confounding, and statistical analysis and reporting. Risk of bias was defined as high if a study received a high rating in one or more domains; low if it received a low rating in all six domains; or moderate if it did not meet criteria for low or high risk or bias. Disagreements on risk of bias ratings were resolved through discussion and adjudication by a third investigator (JREh, JR, or JREv).
We classified vision impairment according to WHO reporting standards: mild vision impairment (visual acuity <6/12 to 6/18); moderate vision impairment (<6/18 to 6/60); and severe vision impairment or blindness (<6/60). We compared the following visual acuity thresholds in the meta-analysis: (1) <6/12 versus ≥6/12; (2) <6/18 versus ≥6/18; (3) <6/60 versus ≥6/18; and (4) <6/60 versus ≥6/60. Note that some studies classified visual acuities at the category threshold as vision impairment, whereas others did not (eg, ≤6/12 vs 6/12).
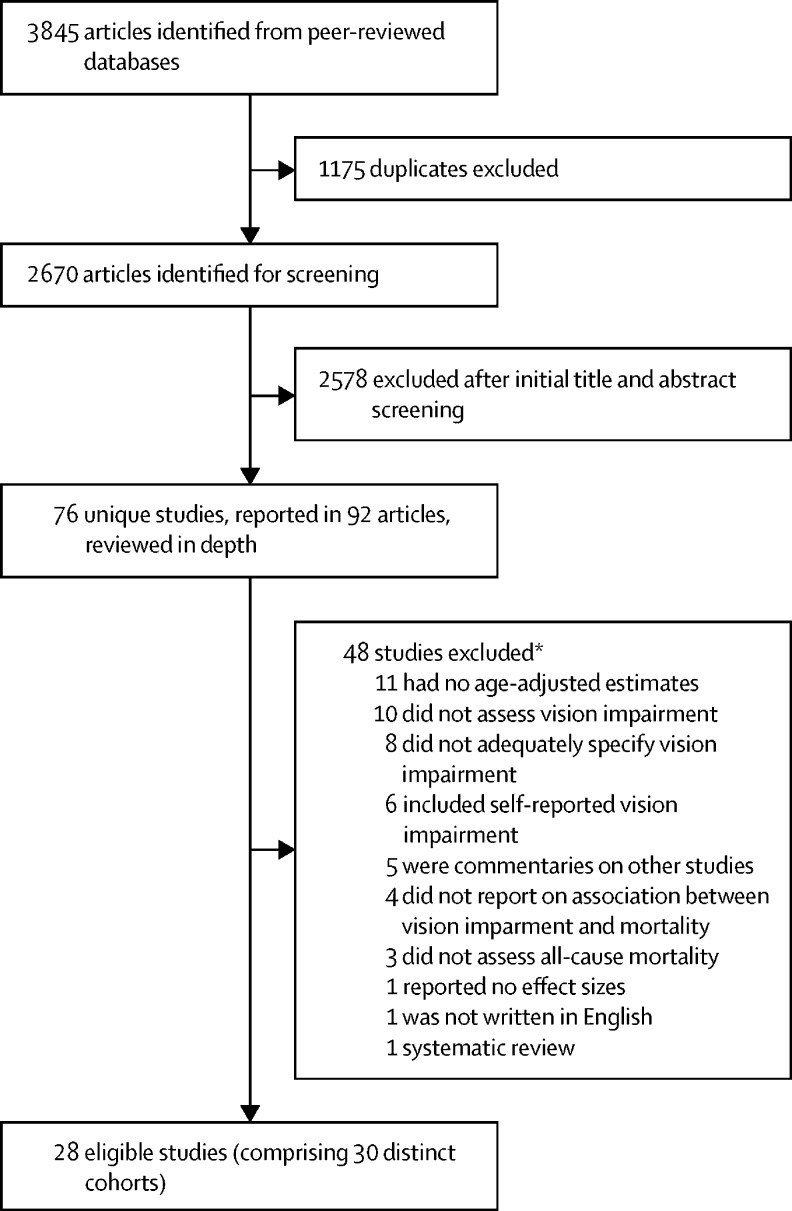
Figure 1.
Study selection
*Some studies were excluded for more than one reason.
We did a random-effects meta-analysis to generate a pooled effect estimate, reported as the hazard ratio (HR) with 95% CIs, for the association between vision impairment and age-adjusted, all-cause mortality. Between-study heterogeneity was assessed with I2 and τ2 statistics. The primary analysis used maximally adjusted estimates, and the sensitivity analysis used minimally adjusted estimates. For studies in which only one estimate was available, the same estimate was used in both the maximally adjusted and minimally adjusted models.
We did meta-regression analyses to test whether effect estimates varied by the following factors: risk of bias, type of visual acuity chart, analysis of better-eye data (compared with other definitions), the use of best-corrected visual acuity or presenting visual acuity as the vision assessment method, follow-up duration, a lower age limit (ie, participants aged <50 years vs those aged ≥50 years), and study design. We only did meta-regression analyses for studies that reported on the association between mortality and vision impairment, defined as a visual acuity of <6/12, as there were too few studies to do meta-regression analyses for other vision impairment categories. The results are not reported by Global Burden of Disease Study super-region17 as planned because all studies with a visual acuity <6/12 group were done in high-income countries. We assessed publication bias using Egger's test (threshold for significance p<0·05) and by inspection of funnel plots. All analyses were done with Stata software, version 16.0.
Two investigators (JREh and JREv) graded the overall certainty of the evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework, modified for prognostic studies.18 This instrument is used to rate the certainty of evidence by considering risk of bias, imprecision, inconsistency, indirectness, and publication bias.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We identified 3845 articles through electronic database searches. After removing duplicate references, we screened the titles and abstracts of 2670 articles. Of these, we identified 76 unique studies in 92 articles for full-text review, during which 48 studies were excluded, leaving 28 studies5, 6, 7, 8, 9, 10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 that met the inclusion criteria. Two of the studies each included two distinct cohorts; therefore, these 28 studies comprised 30 distinct cohorts (446 088 participants) from 12 countries. 25 were observational cohort studies,5, 6, 8, 9, 10, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 and three were RCTs7, 19, 20 reporting an association between vision impairment and mortality independent of the trial interventions.
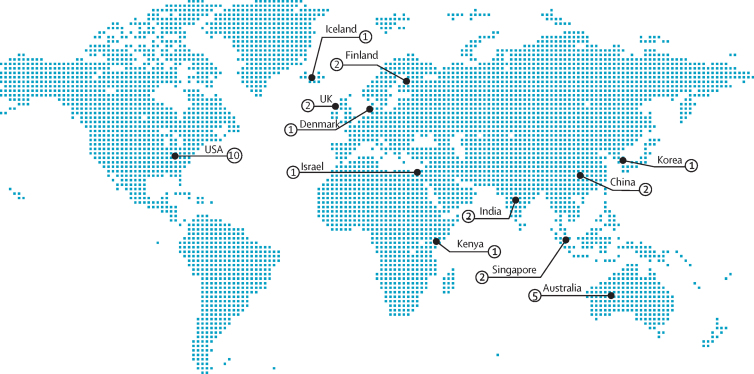
The characteristics of each cohort are reported in table 1. The global distribution of the included cohorts is shown in figure 2; there were no cohorts from eastern Europe, Latin America, the Caribbean, north Africa, or the Pacific islands.
Table 1.
Summary of included cohorts
| Studies using the same cohort | Country | Sample size | Age, years | Ethnic group | Study design | Baseline assessment period | Mean follow-up, months | Visual acuity assessment instrument | Vision assessment method (eye) | Vision impairment definition | Mortality assessment method | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agrawal et al (2011)34 | NA | India | 1422 (687 [48%] men and 735 [52%] women) | ≥60 | NR | Cohort | 2008–09 | 17 | Snellen chart | PVA (better eye) | <6/60 | Death registry maintained by local health workers |
| Anstey et al (2001)31 | NA | Australia | 1947 (1039 [53%] men and 908 [47%] women) | ≥70 | NR | Cohort | 1992–93 | 72 | Snellen chart | BCVA (eye NR) | Various categories | Follow-up with participants and death certificates |
| Buch et al (2005)38 | NA | Denmark | 946 (462 [49%] men and 484 [51%] women) | 60–80 | NR | Cohort | 1986–88 | 168 | Snellen Tumbling E chart | BCVA (better eye) | ≤6/12 | National death registry |
| Clemons et al (2004)20 | NA | USA | 4753 (2099 [44%] men and 2654 [56%] women) | 55–81 | 4546 (95%) white and 207 (5%) other | RCT | 1992–98 | 78 | ETDRS chart | BCVA (either eye) | <6/12 | Death certificates and hospital records |
| Crewe et al (2015)23 | NA | Australia | 3452 (1350 [39%] men and 2102 [61%] women) | ≥65 | 3231 (94%) non-Indigenous Australians, 15 (<1%) Indigenous Australians, and 208 (6%) unknown | Cohort | 2003–10 | 123 | NR | BCVA (better eye) | <6/60 | National death registry |
| Fisher et al (2014)39 | NA | Iceland | 4926 (2121 [43%] men and 2805 [57%] women) | 66–96 | NR | Cohort | 2002–06 | 64 | Nidek ARK 760 | PVA (better eye) | <6/12 | National death registry |
| Foong et al (2008)29 | NA | Singapore | 1225 (553 [45%] men and 672 [55%] women) | 40–79 | NR | Cohort | 1997–98 | 80 | logMAR | BCVA (better eye) | <6/12 | National death registry |
| Freeman et al (2005)37 | Christ et al (2014)41 | USA | 1991 (836 [42%] men and 1155 [58%] women) | 65–84 | 497 (26%) African American and 1494 (74%) white | Cohort | 1993–2003 | 118 | ETDRS chart | PVA (eye NR) | Per unit change | Follow-up with participants |
| Jacobs et al (2005)33 | Stessman et al (2005)42 | Israel | 452 (245 [54%] men and 207 [46%] women) | 70 | NR | Cohort | 1990 | 96 | Snellen equivalent | BCVA (better eye) | ≤6/12 | National death registry |
| Karpa et al (2009)30 | Gopinath et al (2013),43 Wang et al (2001),44 and Cugati et al (2007)45 | Australia | 3654 (1569 [43%] men, 2054 [57%] women, and 31 [1%] NR) | 49–97 | NR | Cohort | 1991 and 1993 | 156 | logMAR | PVA (better eye) | <6/12 | National death registry |
| Khanna et al (2013)27 | NA | India | 4188 (1964 [47%] men and 2224 [53%] women) | 30–70 | NR | Cohort | 1996–2000 | 132 | logMAR | PVA (eye NR) | <6/18, <6/60 | Key informants |
| Kim et al (2019)6 | NA | South Korea | 359 984 (195 052 [54%] men and 164 932 [46%] women) | ≥40 | NR | Cohort | 2009–10 | 48 | NR | BCVA (various) | <6/30, <6/300 | National death registry |
| Knudtson et al (2006)36 | Schubert et al (2017),46 Klein et al (2006),47 and Klein et al (1995)48 | USA | 4897 (42% men and 58% women)* | 43–84 | 99% white and 1% NR* | Cohort | 1987–88 | 158 | ETDRS chart | BCVA (better eye) | ≤6/12 | National death registry and follow-up with participants |
| Kulmala et al (2008)21 | Era et al (1997)49 | Finland | 223 (80 [36%] men and 143 [64%] women) | 75 | Finnish | Cohort | 1989–90 | 106 | Landolt C ring | PVA (better eye) | Various categories | National death registry and follow-up with participants |
| Kulmala et al (2008)21 | Era et al (1997)49 | Finland | 193 (56 [29%] men and 137 [71%] women) | 80 | Finnish | Cohort | 1989–90 | 89 | Landolt C ring | PVA (better eye) | Various categories | National death registry and follow-up with participants |
| Kuper et al (2019)5 | NA | Kenya | 3441 (1656 [48%] men and 1785 [52%] women) | ≥50 | Kikuyu and Kalenjin | Cohort | 2007–14 | 72 | logMAR | PVA (better eye) | Various categories | Key informants |
| Lee et al (2003)40 | NA | USA | 245 (109 [44%] men and 136 [56%] women) | 25–74 | African American | Cohort | 1974–75 | 210 | Sloan chart | PVA (binocular) | <6/12 | National death registry, death certificates, hospital records, insurance records, key informants, and follow-up with participants |
| Lee et al (2003)40 | NA | USA | 2571 (1115 [43%] men and 1456 [57%] women) | 25–74 | Non-Hispanic white | Cohort | 1974–75 | 210 | Sloan chart | PVA (binocular) | <6/12 | National death registry, death certificates, hospital records, insurance records, key informants, and follow-up with participants |
| Li et al (2011)26 | NA | China | 5057 (2383 [47%] men and 2674 [53%] women) | 50–96 | NR | Cohort | 2006–10 | 48 | logMAR | BCVA (better eye) | <6/18, <6/60 | Follow-up with participants and death certificates |
| Liao et al (2019)8 | Zheng et al (2014)50 | USA | 2550 (1257 [49%] men and 1293 [51%] women) | ≥60 | 1543 (61%) non-Hispanic white, 370 (15%) non-Hispanic Black, 487 (19%) Mexican American, and 156 (6%) other | Cohort | 1999–2011 | 119† | Nidek ARK 760 | PVA (better eye) | <6/12 | National death registry |
| Loprinzi et al (2016)9 | Zheng et al (2014)50 | USA | 1658 (800 [48%] men and 858 [52%] women) | 40–85 | 76 (5%) Mexican American, 40 (2%) other Hispanic, 1313 (79%) non-Hispanic white, 154 (9%) non-Hispanic Black, and 75 (5%) other | Cohort | 2003–04 | 92 | Nidek ARK 760 | BCVA (better eye) | <6/12 | National death registry |
| Lott et al (2010)25 | NA | USA | 900 (414 [46%] men and 486 [54%] women) | 58–102 | NR | Cohort | 1992–95 | 120 | Bailey-Lovie chart | PVA (binocular) | <6/12, <6/20 | National death registry, local obituaries, and key informants |
| Ng et al (2018)10 | Liu et al (2017)51 and Estevez et al (2018)52 | Australia | 1257 (476 [38%] men and 781 [62%] women) | ≥40 | Indigenous Australian | Cohort | 2005–08 | 120 | Snellen Tumbling E chart | PVA (better eye) | <6/12 | National death registry |
| Papudesu et al (2018)7 | NA | USA | 4203 (1816 [43%] men and 2387 [57%] women) | 50–80 | 3997 (95%) non-Hispanic white and 206 (5%) other | RCT | 2006–08 | 60† | ETDRS chart | BCVA (left eye) | <6/12 | NR |
| Pedula et al (2006)22 | Swindell et al (2010)53 | USA | 4932 (all women) | ≥65 | White | Cohort | 1986–88 | 144 | Bailey-Lovie chart | PVA (binocular) | ≤6/12 | Follow-up with participants and death certificates |
| Siantar et al (2015)28 | Foong et al (2007)54 | Singapore | 3273 (1573 [48%] men and 170 [52%] women) | 40–79 | Malay | Cohort | 2004–12 | 87† | logMAR | BCVA (better eye) | <6/12 | National death registry |
| Taylor et al (2000)35 | McCarty et al (2001)55 | Australia | 3271 (1511 [46%] men and 1760 [54%] women) | 40–98 | NR | Cohort | 1992–94 | 60 | ETDRS chart | BCVA (better eye) | ≤6/12 | National death registry |
| Thiagarajan et al (2005)19 | NA | UK | 13 569 (5279 [39%] men and 8290 [61%] women) | ≥75 | NR | RCT | 1995–99 | 73† | Glasgow cards | PVA (binocular) | Various categories | National death registry |
| Thompson et al (1989)24 | NA | UK | 469‡ | ≥75 | NR | Cohort | 1981–87 | 60 | Snellen chart | BCVA (better eye) | Various categories | Local vital statistics registry |
| Wang et al (2014)32 | Xu et al (2009)56 | China | 4439 (1928 [44%] men and 2511 [56%] women) | 40–101 | Han Chinese | Cohort | 2001–11 | 120 | Snellen chart | BCVA (worse eye) | Per unit change | Follow-up with participants, key informants, physicians, and hospital records |
ARK=auto refractometer or keratometer. BCVA=best-corrected visual acuity. ETDRS=Early Treatment Diabetic Retinopathy Study. logMAR=logarithm of the minimum angle of resolution. NA=not applicable. NR=not reported. PVA=presenting visual acuity. RCT=randomised controlled trial.
The exact number of male, female, white, and non-white participants included in vision impairment analyses in this study was NR.
Median follow-up.
Number of men and women NR.
Figure 2.
Global distribution of included studies
The map shows the number of unique study cohorts from each country that were included in the systematic review.
Studies collected data between the 1970s and 2012. The findings from six cohorts had been published since 2015.5, 6, 7, 8, 9, 10 The duration of follow-up among included studies ranged from 17 months to 210 months, with a mean of 103·3 (SD 46·4) months. Sample sizes ranged from 193 participants in Finland21 to 359 984 participants in Korea.6 24 cohorts contained an approximately equal number of male and female participants. However, the study by Pedula and colleagues22 included female participants only, and five other cohorts comprised less than 40% male participants.10, 19, 21, 23 None of the included studies had less than 40% female participants, although one study did not report on the gender distribution of participants.24
20 publications reported the HR as an effect estimate. Eight publications reported odds ratios or risk ratios of death for a given follow-up period. Given the high mortality rates in most cohorts, these estimates of effect size could not be considered as equivalent. Thus, meta-analyses were done only with studies reporting HRs or incident rate ratios.
Measures of vision other than visual acuity were not commonly used. Several studies measured visual fields,6, 23, 25 contrast sensitivity,22, 25 colour vision,25 and stereopsis.25 In this subset of studies, contrast sensitivity impairment, peripheral field loss, and stereoacuity impairment were all significantly associated with an increased hazard for mortality in adjusted models. However, because of the small number of studies that assessed vision using these tests, only visual acuity was considered in this report.
Studies used a wide variety of instruments to assess visual acuity. The most commonly used vision charts were logarithm of the minimum angle of resolution (n=6),5, 26, 27, 28, 29, 30 Snellen charts (n=5),24, 31, 32, 33, 34 and Early Treatment Diabetic Retinopathy Study charts (n=5),7, 20, 35, 36, 37 whereas two studies did not specify the instrument used.6, 23 There was also considerable heterogeneity in the methods used to define vision impairment. 15 (54%) studies used best-corrected visual acuity to define vision impairment, and 17 (61%) studies defined vision impairment based on visual acuity in the better-seeing eye.
Definitions of vision impairment also varied between studies. Two studies reported the association between mortality and a continuous measure of visual acuity.32, 37 Six other studies (comprising seven cohorts) compared a reference group of participants with good vision with groups of participants with various non-overlapping vision impairment categories.5, 19, 21, 24, 26, 31 The remaining studies compared participants with visual acuity better than and worse than one or more visual acuity thresholds.
Studies used various strategies to assess mortality, and were included regardless of the methods used because official death registries might not have been available or provided high-quality data in many low-income and middle-income countries (LMICs).57 Most studies (n=24) searched official vital records, with some (n=12) also relying on other methods, including following up with participants, key informants, or both.
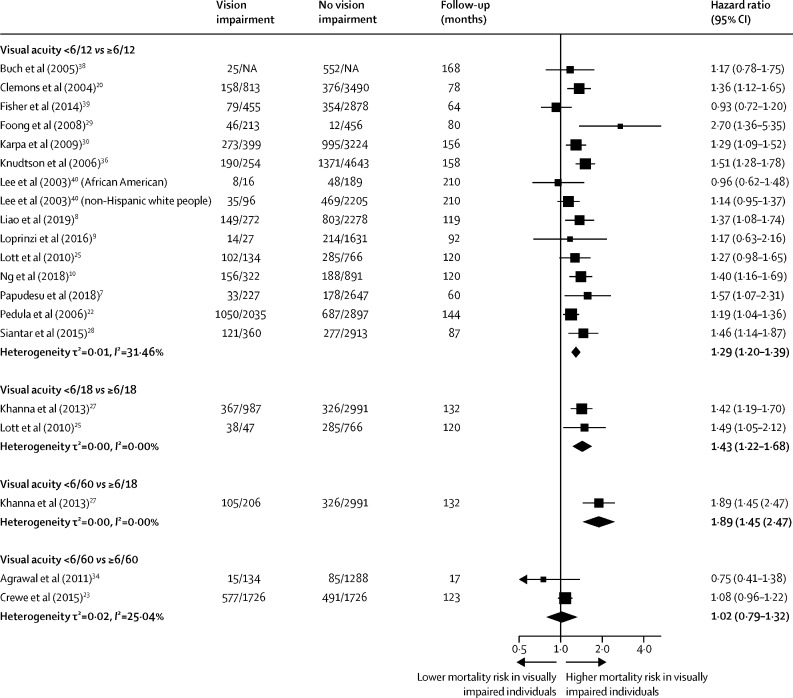
The pooled maximally adjusted HRs for mortality in adults with vision impairment compared with those who had better vision are shown in figure 3. The 18 cohorts included in the meta-analysis comprised 47 998 participants. The remaining 12 cohorts identified in the systematic review were not included in the meta-analysis for one or more of the following reasons: they used a vision impairment threshold that could not be aggregated with other studies;6 they reported results per unit difference in visual acuity;32, 37 they reported measures of effect that could not be pooled with HRs;5, 19, 24, 26, 31, 32, 33, 35 or they compared a reference category of participants with good vision to participants with various non-overlapping vision impairment categories.5, 19, 21, 24, 26, 31 The associations between vision impairment and mortality among these 12 cohorts are shown in table 2.
Figure 3.
Random-effects meta-analysis results
For each study, the number of participants who died out of the total number of participants in the study is shown. Data are the maximally adjusted pooled hazard ratios of mortality in adults with mild vision impairment or worse (visual acuity <6/12) versus those with a visual acuity of ≥6/12; moderate vision impairment or worse (visual acuity <6/18) versus those with a visual acuity of ≥6/18; and severe vision impairment or blindness (visual acuity <6/60) versus those with a visual acuity of ≥6/18 and ≥6/60. NA=not applicable.
Table 2.
Results of studies not included in the meta-analysis
| Reason for exclusion from the meta-analysis | Vision impairment definition (vision assessment method, eye) | Comparison group | Minimally adjusted effect estimates (95% CI)* | Maximally adjusted effect estimates (95% CI) | |
|---|---|---|---|---|---|
| Anstey et al (2001)31 | Categorical analysis | 6/9 (BCVA, eye NR); 6/12 (BCVA, eye NR); and 6/18 to 6/60 (BCVA, eye NR) | 6/6 | 6/9, RR 0·95 (0·67–1·33); 6/12, 1·16 (0·84–1·59); and 6/18 to 6/60, 1·10 (0·80–1·53) | 6/9, RR 0·89 (0·63–1·25); 6/12, 1·10 (0·80–1·52); and 6/18 to 6/60, 1·01 (0·72–1·39) |
| Freeman et al (2005)37 | Effect estimate per unit change in visual acuity | Mild vision loss, 2–3 lines (PVA, eye NR); moderate vision loss, ≥3 lines (PVA, eye NR); and vision gain, ≥2 lines (PVA, eye NR) | No change in visual acuity | Mild vision loss, HR 0·92 (0·61–1·37); moderate vision loss, 2·23 (1·43–3·46); and vision gain, HR 0·47 (0·23–0·96) | Mild vision loss, HR 0·91 (0·61–1·36); moderate vision loss, 2·26 (1·45–3·52); and vision gain, 0·47 (0·23–0·95) |
| Jacobs et al (2005)33 | Did not report HRs | ≤6/12 (BCVA, better eye) | NA | OR 2·84 (1·48–5·46) | |
| Kim et al (2019)6 | Non-standard vision impairment thresholds | Mild vision loss, 6/30 to 6/100 (BCVA, better eye) or ≤6/300 (BCVA, worse eye); and severe vision loss, ≤6/300 (BCVA, better eye) | >6/30 | Mild vision loss, HR 1·17 (0·81–1·69); and severe vision loss, 1·90 (1·08–3·35) | Mild vision loss, HR 1·16 (0·81–1·67); and severe vision loss, 1·87 (1·06–3·29) |
| Kulmala et al (2008)21; 75-year-old cohort | Categorical analysis | ≤6/12 to ≥6/18 (PVA, better eye); and <6/18 (PVA, better eye) | >6/12 | ≤6/12 to ≥6/18, HR 1·98 (1·25–3·13); and <6/18, 1·90 (1·12–3·20) | ≤6/12 to ≥6/18, HR 2·11 (1·27–3·48); and <6/18, 1·34 (0·75–2·39) |
| Kulmala et al (2008)21; 80-year-old cohort | Categorical analysis | ≤6/12 to ≥6/18 (PVA, better eye); and <6/18 (PVA, better eye) | >6/12 | ≤6/12 to ≥6/18, HR 1·13 (0·74–1·72); and <6/18, 0·92 (0·47–1·78) | ≤6/12 to ≥6/18, HR 0·77 (0·48–1·26); and <6/18, 0·75 (0·33–1·67) |
| Kuper et al (2019)5 | Categorical analysis and did not report HRs | <6/12 to ≥6/18 (PVA, better eye); <6/18 to ≥6/60 (PVA, better eye); and <6/60 (PVA, better eye) | ≥6/12 | <6/12 to ≥6/18, RR 0·92 (0·57–1·50); <6/18 to ≥6/60, 1·75 (1·28–2·40); and <6/60, 1·98 (1·04–3·80) | <6/12 to ≥6/18, RR 0·82 (0·48–1·41); <6/18 to ≥6/60, 1·56 (1·14–2·15); and <6/60, 1·46 (0·80–2·68) |
| Li et al (2011)26 | Categorical analysis and did not report HRs | <6/18 to ≥3/60 (BCVA, better eye); <3/60 (BCVA, better eye) | ≥6/18 | NA | <6/18 to ≥3/60, OR 3·1 (1·5–6·4); and <3/60, 3·9 (2·1–7·2) |
| Taylor et al (2000)35 | Did not report HRs | ≤6/12 (BCVA, better eye) | >6/12 | NA | OR 2·42 (1·07–5·43) |
| Thiagarajan et al (2005)19 | Categorical analysis and did not report HRs | <6/6 to ≥6/9 (PVA, binocular); <6/9 to ≥6/18 (PVA, binocular); and <6/18 (PVA, binocular) | ≥6/6 | <6/6 to ≥6/9, RR 1·10 (1·01–1·19); <6/9 to ≥6/18, 1·32 (1·22–1·42); and <6/18, 1·60 (1·47–1·74) | <6/6 to ≥6/9, RR 1·06 (0·97–1·16); <6/9 to ≥6/18, 1·24 (1·14–1·35); and <6/18, 1·52 (1·39–1·66) |
| Thompson et al (1989)24 | Categorical analysis and did not report HRs | ≤6/7·5 to ≥6/9 (BCVA, better eye); ≤6/12 to ≥6/18 (BCVA, better eye); ≤6/24 to ≥6/60 (BCVA, better eye); and <6/60 (BCVA, better eye) | ≥6/6 | NA | ≤6/7·5 to ≥6/9, RR 1·62 (0·87–3·01); ≤6/12 to ≥6/18, 1·83 (0·93–3·63); ≤6/24 to ≥6/60, 1·72 (0·77–3·84); and <6/60, 0·35 (0·08–1·57) |
| Wang et al (2014)32 | Effect estimate per unit difference in visual acuity and did not report HRs | (BCVA, worse eye) | NA | OR 1·76 (1·35–2·29) | NA |
The table shows effect estimates of studies that were excluded from meta-analysis, with reasons for exclusion and definitions of vision impairment. BCVA=best-corrected visual acuity. HR=hazard ratio. NA=not applicable. NR=not reported. OR=odds ratio. PVA=presenting visual acuity. RR=risk ratio.
All estimates are, at minimum, adjusted for age.
A total of 14 studies (comprising 15 cohorts) compared the hazard for mortality in participants with a visual acuity of <6/12 versus those with a visual acuity of ≥6/12; the adjusted HR estimate for mortality was 1·29 (95% CI 1·20–1·39) and heterogeneity between studies was low (τ2=0·01, I2=31·46%), suggesting a consistent effect across studies (figure 3). Two studies compared the hazard for mortality among participants with a visual acuity <6/18 versus those with a visual acuity of ≥6/18; the adjusted estimated HR for mortality was 1·43 (1·22–1·68) and heterogeneity between studies was low (τ2=0·00, I2=0·00%). Only one study compared the hazard for mortality in participants with a visual acuity of <6/60 versus those with a visual acuity of ≥6/18, with a HR for mortality of 1·89 (1·45–2·47). Two studies compared the hazard for mortality in participants with a visual acuity of <6/60 versus those with a visual acuity of ≥6/60; the adjusted pooled HR for mortality was 1·02 (0·79–1·32; τ2=0·02, I2=25·04%).
The pooled minimally adjusted HR for mortality among participants with a visual acuity of <6/12 versus those with a visual acuity of ≥6/12 is shown in the appendix (p 8). In this analysis, the pooled minimally adjusted HR for mortality was 1·41 (95% CI 1·29–1·53).
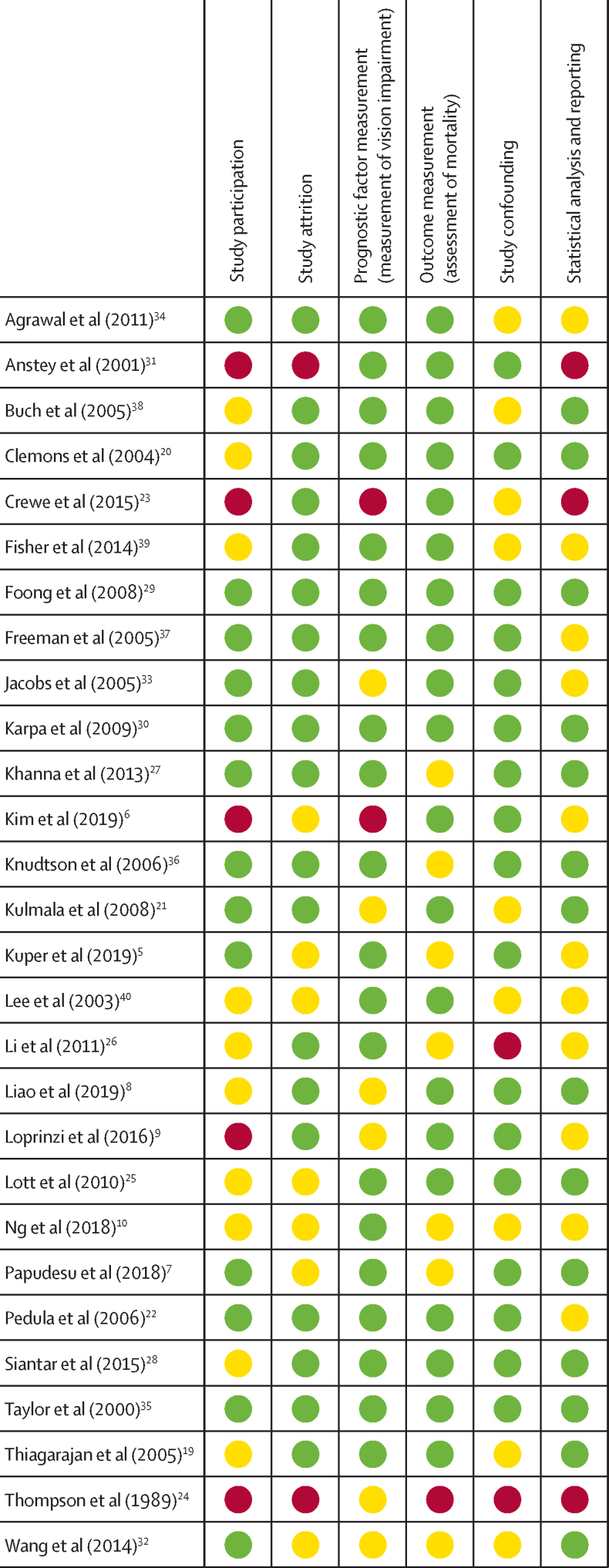
For risk of bias assessment using the QUIPS tool,16 only three studies received an assessment of low risk of bias across all six domains (figure 4).29, 30, 35 Six studies were assessed as having a high risk of bias in one or more domains.6, 9, 23, 24, 26, 31
Figure 4.
Risk of bias assessment
Risk of bias was assessed by use of the Quality in Prognostic Studies tool.16 Green represents low risk of bias, yellow represents a moderate risk of bias, and red represents high risk of bias.
Funnel plots were reviewed for studies comparing all-cause mortality in participants with a visual acuity of <6/12 with those that had a visual acuity of ≥6/12, and no evidence of publication bias was identified (p=0·63; appendix p 9). Meta-regression analysis of studies comparing all-cause mortality between these two groups of participants revealed no evidence that the estimated effect size differed by risk of bias, the type of vision chart used, the eye assessed, follow-up duration, a lower age limit (ie, participants aged <50 years vs those aged ≥50 years), or study design (table 3). However, studies that used best-corrected visual acuity as the vision assessment method reported a significantly higher hazard for mortality (HR 1·45 [95% CI 1·31–1·60]) than those using presenting visual acuity (1·22 [1·13–1·31]; p=0·0055).
Table 3.
Meta-regression analysis results
| Number of studies | HR (95% CI) | p value | ||
|---|---|---|---|---|
| Risk of bias | .. | .. | 0·76 | |
| Low | 2 | 1·73 (0·85–3·50) | .. | |
| Moderate | 12 | 1·28 (1·18–1·39) | .. | |
| High | 1 | 1·17 (0·63–2·16) | .. | |
| Vision chart | .. | .. | 0·082 | |
| ETDRS chart | 3 | 1·46 (1·29–1·64) | .. | |
| Snellen Tumbling E chart | 2 | 1·35 (1·14–1·61) | .. | |
| logMAR* | 5 | 1·28 (1·17–1·40) | .. | |
| Other or not reported | 5 | 1·12 (0·95–1·31) | .. | |
| Eye | .. | .. | 0·24 | |
| Better eye | 9 | 1·34 (1·19–1·50) | .. | |
| Other | 6 | 1·22 (1·12–1·33) | .. | |
| Vision assessment method | .. | .. | 0·0055 | |
| Best-corrected visual acuity | 7 | 1·45 (1·31–1·60) | .. | |
| Presenting visual acuity | 8 | 1·22 (1·13–1·31) | .. | |
| Follow-up duration, years | .. | .. | 0·58 | |
| <10 | 7 | 1·35 (1·13–1·60) | .. | |
| ≥10 | 8 | 1·27 (1·17–1·39) | .. | |
| Lower age limit, years | .. | .. | 0·27 | |
| <50 | 7 | 1·35 (1·19–1·54) | .. | |
| ≥50 | 8 | 1·24 (1·15–1·34) | .. | |
| Study design | .. | .. | 0·38 | |
| Cohort | 13 | 1·27 (1·17–1·39) | .. | |
| Randomised controlled trial | 2 | 1·40 (1·18–1·66) | .. | |
Estimated HRs for mortality in people with a visual acuity of <6/12 versus those with a visual acuity of ≥6/12, subcategorised by seven variables, with p values from the meta-regression analysis. ETDRS=Early Treatment Diabetic Retinopathy Study. HR=hazard ratio. logMAR=logarithm of minimum angle of resolution.
Includs Bailey-Lovie charts.
Using the GRADE framework, we judged the evidence to be of moderate certainty overall, downgrading half a level for risk of bias and half a level for inconsistency. Even though only three of the 28 studies were judged as having a low risk of bias in all domains, meta-regression analyses suggested that the effect estimates were not associated with risk of bias. Measured inconsistency or heterogeneity between studies was not high, but there was some variation in study results. Using this framework, we are moderately confident that the mortality risk associated with vision impairment reported in this study is likely to be close to the true value.18
Discussion
This systematic review and meta-analysis summarises the existing evidence on the association between vision impairment and the risk of mortality among adults from 12 countries across five continents. The results support the existence of a consistent association between poor vision and mortality across different study settings, thereby reinforcing the specific importance of vision and eye health to Sustainable Development Goal (SDG) 3, which aims to “ensure healthy lives and promote well-being for all at all ages”, as well as to the SDGs more generally.58
This study builds on a previous meta-analysis that considered studies on the association between vision impairment and mortality published before 2015.4 This previous meta-analysis provided evidence that vision impairment could be associated with an increased risk of mortality; however, the study also had several key limitations that we sought to address in the current report. First, the meta-analysis included studies that not only assessed vision with objective clinical measures (eg, visual acuity), but also self-reported visual difficulty, and administrative billing codes. Because of the heterogeneous data, the study compared participants with the highest level of vision impairment to those with no vision impairment. This approach could have resulted in misclassification bias, since poor visual acuity, self-reported visual difficulty, and ICD codes could represent distinct constructs. In addition, this approach could have overestimated effect sizes by only including participants in the best and worst vision categories. However, in the meta-regression analyses, the effect size was similar for the 15 studies that assessed vision with visual acuity charts (relative risk 1·36 [95% CI 1·16–1·59]), for the three studies that used ICD codes (1·55 [1·15–2·09]), and for the seven studies that used self-reported visual acuity (1·44 [1·34–1·56]). Another limitation of this previous meta-analysis was not assessing the risk of bias in included studies or not including an overall assessment of the certainty of the evidence.
The meta-analyses in our study help to quantify the magnitude of the association between vision impairment and mortality. The hazard for mortality was 29% higher for participants with a visual acuity of <6/12 versus those with a visual acuity of ≥6/12, 43% higher for participants with a visual acuity of <6/18 versus those with a visual acuity of ≥6/18, and 89% higher for participants with a visual acuity of <6/60 versus those with a visual acuity of ≥6/18. However, there was no significant difference in the hazard for mortality between participants with a visual acuity of <6/60 versus those with a visual acuity of ≥6/60, probably because the reference group (visual acuity ≥6/60) in these studies contained participants with a substantial degree of vision impairment. Data from 12 cohorts identified in the systematic review could not be included in the meta-analyses because they were not comparable with other included studies in terms of their analytical methods and categorisation of vision impairment. Among these heterogeneous cohorts, most (n=9) reported a significant association between vision impairment and increased mortality.
Notably, there was considerable variability in the methods used to assess and report visual acuity and mortality among included studies, which could affect interpretation of the findings. However, results of meta-regression analyses showed that the hazard for mortality was not significantly affected by the eye chart used to assess visual acuity or the eye used to assess the level of vision impairment (eg, the better-seeing eye). Nonetheless, the high degree of variability in the measurement and reporting of visual acuity data does highlight the need for widespread adoption of standard definitions and protocols to promote comparability across cohorts in future studies. The Lancet Global Health Commission on Global Eye Health has proposed visual acuity measurement and reporting standards for epidemiological studies, which are described in detail in the main Commission report.11
Meta-regression analysis revealed that the hazard for mortality was significantly higher in studies reporting best-corrected visual acuity compared with those reporting presenting visual acuity as the vision assessment method. This finding suggests that, compared with uncorrected refractive error, non-refractive causes of vision impairment might have a stronger association with mortality. This association could be due to common risk factors for non-refractive vision loss and mortality (eg, stroke or diabetes). Non-refractive vision impairment could also have a greater effect on factors that mediate the association with mortality (eg, physical activity). It is also possible that some study participants with vision impairment due to uncorrected refractive error received glasses during the course of the study, which could have decreased their risk of vision-impairment-related mortality. Additionally, some causes of vision impairment, such as cataract, glaucoma, and age-related macular degeneration, have been referred to as markers of ageing and might therefore indicate accelerated biological ageing.36
This systematic review and meta-analysis was limited by the wide variation in how studies adjusted for potential confounding variables, which could have biased the findings. Nonetheless, both maximally adjusted and minimally adjusted pooled effect estimates showed a significant association between vision impairment (visual acuity <6/12) and all-cause mortality. Since age is a strong common risk factor for both vision impairment and mortality, studies were only included if they reported, at a minimum, age-adjusted mortality. Studies also adjusted for other important factors that could confound the association between vision impairment and mortality. For example, socioeconomic deprivation, poor access to health care, diabetes, and stroke are a few of the well documented common risk factors for vision impairment and mortality,3 for which models were adjusted in many studies. Some studies, however, might have over-adjusted their statistical models, including for variables that might lie on the causal pathway between vision impairment and mortality. Adjusting for variables hypothesised to be on this causal pathway could bias study results toward the null hypothesis (ie, no effect).
Most included studies were from high-income countries, and additional evidence from regions not represented in the literature would contribute to a more complete understanding of this topic to inform policy. Future studies could also consider adopting standardised measurement and reporting guidelines, as outlined in the main Commission report.11 Furthermore, there is a need for studies to consider the risk of mortality associated with other types of vision impairment that are less commonly assessed, such as contrast sensitivity impairment and peripheral field loss. Mediating pathways between vision impairment and mortality, which could include shared risk factors, such as physical inactivity, social isolation, and disability,3, 59, 60, 61 should be investigated. Finally, future calculations of disability-adjusted life-years might include years of life lost due to vision impairment, which could provide a more complete estimate of the overall global burden of vision impairment.
The current study has several key strengths. First, we included multiple additional studies published in 2016–19,5, 6, 7, 8, 9, 10 including those from regions of the world that were not well represented in the previous meta-analysis,4 such as sub-Saharan Africa (Kenya)5 and east Asia (South Korea).6 Second, we only included studies that assessed visual acuity. Even though this strategy might have resulted in some well designed studies being excluded, it served to strengthen the internal validity of our meta-analysis and to limit misclassification bias. The current investigation also included an assessment of the risk of bias in included studies using the well described QUIPS tool.16 Even though only three included studies were considered to have a low risk of bias across all domains, there was no evidence from the meta-regression analyses that the estimated association was affected by risk of bias. Finally, by use of the GRADE framework, an overall assessment of the strength of the evidence was described.
The results of this study have important implications for policy and practice. Worldwide, more than 80% of people with vision impairment and blindness live in LMICs, and 55% are women and girls.1 Four of five cases of vision impairment and blindness are preventable or correctable. In fact, the leading causes of vision impairment and blindness worldwide are cataract and uncorrected refractive error,62 both of which are readily treatable with inexpensive, cost-effective interventions.63 Therefore, there is an important opportunity to promote not only health and wellbeing, but also longevity by correcting, rehabilitating, and preventing avoidable vision loss. Policies and strategies for achieving this are outlined in the main Commission report,11 and have the potential to make important contributions to achieving the SDGs.
Data sharing
The protocol for this study has been published previously. As this study is a systematic review and meta-analysis, there are no primary data to be shared.
Acknowledgments
Acknowledgments
We acknowledge funding support for The Lancet Global Health Commission on Global Eye Health from the Queen Elizabeth Diamond Jubilee Trust, Commonwealth Scholarship Commission, National Institutes of Health, Research to Prevent Blindness, Moorfields Eye Charity (GR001061), National Institute for Health Research Moorfields Biomedical Research Centre, Wellcome Trust, Sightsavers, the Fred Hollows Foundation, the Seva Foundation, the British Council for the Prevention of Blindness, and Christian Blind Mission.
Contributors
JREh, JR, BKS, NC, MB, and JREv designed the study. JREh, JR, HB, CNL, JHZ, WW, and JREv collected the data, and DM analysed the data and generated the figures. IG did the literature search. JREh and JREv interpreted the data and drafted the manuscript. JR, DM, HB, CNL, JHZ, WW, BKS, IG, NC, and MB critically revised the manuscript. JREv and JREh accessed and verified all data in the study and JREh, JR, DM, and JREv had full access to all the data in the study. JREh had final responsibility for the decision to submit for publication.
Declaration of interests
MB reports receiving grants from the Wellcome Trust (207472/Z/17/Z). JR reports grants from the Buchanan Charitable Foundation, New Zealand. BKS reports grants from the National Institutes of Health (K01AG052640). JREh reports grants from the National Institutes of Health (K23EY027848); and an unrestricted grant from Research to Prevent Blindness awarded to the University of Michigan Kellogg Eye Center. NC reports being a Director of Research for Orbis International, an organisation working on eye health issues in underserved areas. All other authors declare no competing interests.
Supplementary Material
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden Of Disease Study. Lancet Glob Health. 2021;9:e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2019. World report on vision. [Google Scholar]
- 3.National Academies of Sciences, Engineering, and Medicine . National Academies Press; Washington, DC: 2016. Making eye health a population health imperative: vision for tomorrow. [PubMed] [Google Scholar]
- 4.Zhang T, Jiang W, Song X, Zhang D. The association between visual impairment and the risk of mortality: a meta-analysis of prospective studies. J Epidemiol Community Health. 2016;70:836–842. doi: 10.1136/jech-2016-207331. [DOI] [PubMed] [Google Scholar]
- 5.Kuper H, Mathenge W, Macleod D. Mortality during 6 years of follow-up in relation to visual impairment and eye disease: results from a population-based cohort study of people aged 50 years and above in Nakuru, Kenya. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim K-N, Park SJ, Kim W. Modification of the association between visual impairment and mortality by physical activity: a cohort study among the Korean national health examinees. International J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study 2 Research Group. Papudesu C, Clemons TE, Agron E, Chew EY. Association of mortality with ocular diseases and visual impairment in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 report number 13. Ophthalmology. 2018;125:512–521. doi: 10.1016/j.ophtha.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao H, Zhu Z, Wang H, Rong X, Young CA, Peng Y. Cognitive performance concomitant with vision acuity predicts 13-year risk for mortality. Front Aging Neurosci. 2019;11:65. doi: 10.3389/fnagi.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loprinzi PD, Crush E. Sensory impairment, functional balance and physical activity with all-cause mortality. J Phys Act Health. 2016;13:980–987. doi: 10.1123/jpah.2015-0692. [DOI] [PubMed] [Google Scholar]
- 10.Ng SK, Kahawita S, Andrew NH, Henderson T, Craig JE, Landers J. Association of visual impairment and all-cause 10-year mortality among Indigenous Australian individuals within central Australia: the Central Australian Ocular Health Study. JAMA Ophthalmol. 2018;136:534–537. doi: 10.1001/jamaophthalmol.2017.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton MJ, Ramke J, Marques AP. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. Lancet Glob Health. 2021 doi: 10.1016/S2214-109X(20)30488-5. published online Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich JR, Ramke J, Macleod D. Association between vision impairment and mortality: protocol for a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemingway H, Croft P, Perel P. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346 doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 15.Moons KGM, de Groot JAH, Bouwmeester W. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Medicine. 2014;11 doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 17.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foroutan F, Guyatt G, Zuk V. GRADE guidelines 28: use of GRADE for the assessment of evidence about prognostic factors: rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol. 2020;121:62–70. doi: 10.1016/j.jclinepi.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Thiagarajan M, Evans JR, Smeeth L, Wormald RP, Fletcher AE. Cause-specific visual impairment and mortality: results from a population-based study of older people in the United Kingdom. Arch Ophthalmol. 2005;123:1397–1403. doi: 10.1001/archopht.123.10.1397. [DOI] [PubMed] [Google Scholar]
- 20.Clemons TE, Kurinij N, Sperduto RD, AREDS Research Group Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS report no. 13. Arch Ophthalmol. 2004;122:716–726. doi: 10.1001/archopht.122.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulmala J, Era P, Tormakangas T, Parssinen O, Rantanen T, Heikkinen E. Visual acuity and mortality in older people and factors on the pathway. Ophthalmic Epidemiol. 2008;15:128–134. doi: 10.1080/09286580701840388. [DOI] [PubMed] [Google Scholar]
- 22.Pedula KL, Coleman AL, Hillier TA. Visual acuity, contrast sensitivity, and mortality in older women: study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1871–1877. doi: 10.1111/j.1532-5415.2006.00983.x. [DOI] [PubMed] [Google Scholar]
- 23.Crewe JM, Spilsbury K, Morlet N. Health service use and mortality of the elderly blind. Ophthalmology. 2015;122:2344–2350. doi: 10.1016/j.ophtha.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JR, Gibson JM, Jagger C. The association between visual impairment and mortality in elderly people. Age Ageing. 1989;18:83–88. doi: 10.1093/ageing/18.2.83. [DOI] [PubMed] [Google Scholar]
- 25.Lott LA, Schneck ME, Haegerstrom-Portnoy G, Brabyn JA. Non-standard vision measures predict mortality in elders: the Smith-Kettlewell Institute (SKI) study. Ophthalmic Epidemiol. 2010;17:242–250. doi: 10.3109/09286586.2010.498660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Sun D, Liu P, Zhang L, Bai J, Cui H. Visual impairment and mortality in a rural adult population (the Southern Harbin eye study) Ophthalmic Epidemiol. 2011;18:54–60. doi: 10.3109/09286586.2010.545503. [DOI] [PubMed] [Google Scholar]
- 27.Khanna RC, Murthy GVS, Giridhar P. Cataract, visual impairment and long-term mortality in a rural cohort in India: the Andhra Pradesh Eye Disease Study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siantar RG, Cheng C-Y, Gemmy Cheung CM. Impact of visual impairment and eye diseases on mortality: the Singapore Malay Eye Study (SiMES) Sci Rep. 2015;5 doi: 10.1038/srep16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foong AWP, Fong CW, Wong TY, Saw S-M, Heng D, Foster PJ. Visual acuity and mortality in a Chinese population. The Tanjong Pagar Study. Ophthalmology. 2008;115:802–807. doi: 10.1016/j.ophtha.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 30.Karpa MJ, Mitchell P, Beath K. Direct and indirect effects of visual impairment on mortality risk in older persons. Arch Ophthalmol. 2009;127:1347–1353. doi: 10.1001/archophthalmol.2009.240. [DOI] [PubMed] [Google Scholar]
- 31.Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychol Aging. 2001;16:3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Wang YX, Zhang JS, You QS, Xu L, Jonas JB. Ocular diseases and 10-year mortality: the Beijing Eye Study 2001/2011. Acta Ophthalmol. 2014;92:e424–e428. doi: 10.1111/aos.12370. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs JM, Hammerman-Rozenberg R, Maaravi Y, Cohen A, Stessman J. The impact of visual impairment on health, function and mortality. Aging Clin Exp Res. 2005;17:281–286. doi: 10.1007/BF03324611. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal N, Kalaivani M, Gupta SK, Misra P, Anand K, Pandav CS. Association of blindness and hearing impairment with mortality in a cohort of elderly persons in a rural area. Indian J Community Med. 2011;36:208–212. doi: 10.4103/0970-0218.86522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor HR, McCarty CA, Nanjan MB. Vision impairment predicts five-year mortality. Trans Am Ophtalmol Soc. 2000;98:91–99. [PMC free article] [PubMed] [Google Scholar]
- 36.Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–249. doi: 10.1001/archopht.124.2.243. [DOI] [PubMed] [Google Scholar]
- 37.Freeman EE, Egleston BL, West SK, Bandeen-Roche K, Rubin G. Visual acuity change and mortality in older adults. Invest Ophthalmol Vis Sci. 2005;46:4040–4045. doi: 10.1167/iovs.05-0687. [DOI] [PubMed] [Google Scholar]
- 38.Buch H, Vinding T, la Cour M, Jensen GB, Prause JU, Nielsen NV. Age-related maculopathy: a risk indicator for poorer survival in women: the Copenhagen City Eye Study. Ophthalmology. 2005;112:305–312. doi: 10.1016/j.ophtha.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Fisher D, Li C-M, Chiu MS. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing. 2014;43:69–76. doi: 10.1093/ageing/aft122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DJ, Gómez-Marín O, Ma F, Lam BL. Distance visual acuity impairment and survival in African Americans and non-Hispanic whites. Ethn Dis. 2003;13:485–491. [PubMed] [Google Scholar]
- 41.Christ SL, Zheng DD, Swenor BK. Longitudinal relationships among visual acuity, daily functional status, and mortality: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2014;132:1400–1406. doi: 10.1001/jamaophthalmol.2014.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stessman J, Hammerman-Rozenberg R, Maaravi Y, Azoulai D, Cohen A. Strategies to enhance longevity and independent function: the Jerusalem Longitudinal Study. Mech Ageing Dev. 2005;126:327–331. doi: 10.1016/j.mad.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual sensory impairment in older adults increases the risk of mortality: a population-based study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang JJ, Mitchell P, Simpson JM, Cumming RG, Smith W. Visual impairment, age-related cataract, and mortality. Arch Ophthalmol. 2001;119:1186–1190. doi: 10.1001/archopht.119.8.1186. [DOI] [PubMed] [Google Scholar]
- 45.Cugati S, Cumming RG, Smith W, Burlutsky G, Mitchell P, Wang JJ. Visual impairment, age-related macular degeneration, cataract, and long-term mortality: the Blue Mountains Eye Study. Arch Ophthalmol. 2007;125:917–924. doi: 10.1001/archopht.125.7.917. [DOI] [PubMed] [Google Scholar]
- 46.Schubert CR, Fischer ME, Pinto AA. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:710–715. doi: 10.1093/gerona/glw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Klein R, Klein BE, Moss SE. Age-related eye disease and survival. The Beaver Dam Eye Study. Arch Ophthalmol. 1995;113:333–339. doi: 10.1001/archopht.1995.01100030089026. [DOI] [PubMed] [Google Scholar]
- 49.Era P, Rantanen T. Changes in physical capacity and sensory/psychomotor functions from 75 to 80 years of age and from 80 to 85 years of age–a longitudinal study. Scand J Soc Med Suppl. 1997;53:25–43. [PubMed] [Google Scholar]
- 50.Zheng DD, Christ SL, Lam BL. Visual acuity and increased mortality: the role of allostatic load and functional status. Invest Ophthalmol Vis Sci. 2014;55:5144–5150. doi: 10.1167/iovs.14-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu E, Ng SK, Kahawita S, Andrew NH. Ten-year all-cause mortality and its association with vision among Indigenous Australians within Central Australia: the Central Australian Ocular Health Study. Clin Exp Ophthalmol. 2017;45:348–356. doi: 10.1111/ceo.12880. [DOI] [PubMed] [Google Scholar]
- 52.Estevez J, Kaidonis G, Henderson T, Craig JE, Landers J. Association of disease-specific causes of visual impairment and 10-year mortality amongst Indigenous Australians: the Central Australian Ocular Health Study. Clin Exp Ophthalmol. 2018;46:18–24. doi: 10.1111/ceo.13009. [DOI] [PubMed] [Google Scholar]
- 53.Swindell WR, Ensrud KE, Cawthon PM. Indicators of “healthy aging” in older women (65-69 years of age). A data-mining approach based on prediction of long-term survival. BMC Geriatr. 2010;10:55. doi: 10.1186/1471-2318-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foong AW, Saw SM, Loo JL. Rationale and methodology for a population-based study of eye diseases in Malay people: the Singapore Malay eye study (SiMES) Ophthalmic Epidemiol. 2007;14:25–35. doi: 10.1080/09286580600878844. [DOI] [PubMed] [Google Scholar]
- 55.McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85:322–326. doi: 10.1136/bjo.85.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu L, Wang YX, Wang J, Jonas JJ. Mortality and ocular diseases: the Beijing Eye Study. Ophthalmology. 2009;116:732–738. doi: 10.1016/j.ophtha.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Byass P, Sankoh O, Tollman SM, Högberg U, Wall S. Lessons from history for designing and validating epidemiological surveillance in uncounted populations. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.UN Transforming our world: the 2030 agenda for sustainable development. 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld/publication
- 59.Bansback N, Czoski-Murray C, Carlton J. Determinants of health related quality of life and health state utility in patients with age related macular degeneration: the association of contrast sensitivity and visual acuity. Qual Life Res. 2007;16:533–543. doi: 10.1007/s11136-006-9126-8. [DOI] [PubMed] [Google Scholar]
- 60.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R, Los Angeles Latino Eye Study Group Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:941–948. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramulu PY, Maul E, Hochberg C, Chan ES, Ferrucci L, Friedman DS. Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology. 2012;119:1159–1166. doi: 10.1016/j.ophtha.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.GBD 2019 Blindness and Vision Impairment Collaborators Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busbee BG, Brown MM, Brown GC, Sharma S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology. 2002;109:606–612. doi: 10.1016/s0161-6420(01)00971-x. discussion 612–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol for this study has been published previously. As this study is a systematic review and meta-analysis, there are no primary data to be shared.