Abstract
Sporotrichosis, a mycosis caused by pathogenic species of the genus Sporothrix, affects diverse species of mammals. Until 2007, Sporothrix schenckii was considered the unique etiologic agent of sporotrichosis. Canine sporotrichosis is a poorly reported disease, and the majority of cases are from Rio de Janeiro, Brazil. There are scarce studies on the characterization of canine isolates of Sporothrix schenckii complex, as well as few antifungal susceptibility data available. The aim of this study was to characterize the clinical isolates of Sporothrix from dogs from Brazil at species level and evaluate their antifungal susceptibility profile. Polyphasic taxonomy was used to characterization at species level (morphological, phenotypical characteristics, and molecular identification). Antifungal susceptibility profiles (amphotericin B, itraconazole, ketoconazole, posaconazole, and terbinafine) were determined using the Clinical and Laboratory Standards Institute broth microdilution method (M38-A2). According to phenotypic identification and molecular analysis, 46 isolates included in this study were identified as S. brasiliensis and one as S. schenckii. Amphotericin B presented the highest minimum inhibitory concentration values, and the other drugs showed effective in vitro antifungal activity. This is the first report of S. schenckii in dogs from Brazil, since S. brasiliensis is the only species that has been described in canine isolates from Rio de Janeiro to date. Nevertheless, no differences were observed in the antifungal susceptibility profiles between the S. brasiliensis and S. schenckii isolates, and it is important to continuously study new canine clinical isolates from Rio de Janeiro, Brazil.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00328-8) contains supplementary material, which is available to authorized users.
Keywords: Sporothrix species, Sporotrichosis, Dogs, Antifungal susceptibility, Molecular characterization
Introduction
Sporotrichosis is a subcutaneous mycosis caused by pathogenic species of the genus Sporothrix that affects humans and animal species, mainly cats [1, 2]. Canine sporotrichosis is an uncommon disease, and current knowledge is derived from a few case reports and case series [3]. Some canine cases have been documented in Italy [4], the USA [5], and Brazil [6–8], which has the highest number of reports at Rio de Janeiro since the late 1990s [9–12].
In dogs, the infection can be acquired during hunting activities when Sporothrix spp. are introduced through thorn injuries or wood splinters [13]. Another type of transmission of this fungus to dogs is through close contact with sick cats, which is the most frequent form of infection of Sporothrix spp. in the metropolitan region of Rio de Janeiro [6]. More importantly, despite the significant increase in case reports of zoonotic transmission involving sick cats in Brazil in the last two decades, especially in Rio de Janeiro [9–11, 14, 15], there is no evidence of dogs facilitating transmission of Sporothrix spp. to humans and other animals. Sporotrichosis in dogs is usually characterized by cutaneous lesions on the head, especially on the nasal region and thorax. Osteoarticular involvement and disseminated forms are rarely observed [16]. The most frequently observed lesions are nodules and ulcers [6]. In dogs, three clinical presentations have been reported: localized cutaneous or fixed cutaneous, cutaneous lymphatic, and disseminated forms [5, 13, 17]. In dogs, the lesions are neither painful nor pruritic, the fungus is difficult to find in the cytopathological examination of exudate from skin lesions, and generally, the animals are in good clinical condition [17].
Until 2007, Sporothrix schenckii was considered the unique etiologic agent of sporotrichosis in humans and animals [18]. Molecular studies performed since 2007 culminated in the description of eight pathogenic species within the genus Sporothrix. Sporothrix schenckii sensu stricto, S. brasiliensis, S. globosa, and S. luriei belong to the Sporothrix schenckii complex, while S. mexicana, S. pallida, S. humicola, and S. chilensis are members of Sporothrix pallida complex [19–24]. Currently, S. schenckii, S. brasiliensis, and S. globosa are considered to be the main species of clinical interest in humans [25–27], and S. brasiliensis and S. schenckii have been reported in cats and dogs [8, 28–32].
Sporothrix brasiliensis has been described as an emerging species that is highly pathogenic among humans and cats with a predominant geographic distribution in Brazil, although it was recently described in Argentina as well [19, 22, 28, 32–39]. Few studies have characterized canine isolates of the genus Sporothrix. In Brazil, 18 isolates from the southern region, 2 isolates from Rio de Janeiro, and 1 canine isolate from São Paulo were previously characterized as S. brasiliensis [8, 28, 40]. Sporothrix schenckii is considered the second most pathogenic species of the genus Sporothrix in murine experimental models of infection [33]; however, this species has not yet been reported in dogs [19, 41]. Sporothrix luriei has rarely appeared in cases of human and animal sporotrichosis, with only two human cases, including one case in Africa [20] and another case in India [42]; interestingly, this species has been characterized in a Brazilian canine case [34].
The most common drugs used in the treatment of canine sporotrichosis are itraconazole, ketoconazole, and potassium iodide [6, 43]. Due to the emergence of resistant species and the occurrence of therapeutic failures, in vitro antifungal susceptibility tests were developed. The determination of the minimal inhibitory concentration (MIC) is important for the treatment orientation and the evaluation of new antifungal agents [44]. There are studies of the antifungal susceptibility of Sporothrix isolates derived from human cases [45–48]. However, few studies on the antifungal susceptibility profiles of canine Sporothrix sp. isolates have been performed [8–34].
Only one report described the characterization of two isolates of Sporothrix from canine origin in Rio de Janeiro, Brazil [8], which is the region that has the highest number of animal sporotrichosis cases described in the world [12]. The aims of this study were to characterize at the species level the isolates of Sporothrix schenckii complex, obtained from canine cases of sporotrichosis in Rio de Janeiro, Brazil, to evaluate their antifungal susceptibility profile and to correlate them with the clinical and epidemiological characteristics.
Methodology
Isolates
Forty-seven isolates of Sporothrix spp. from dogs assisted at the Laboratory of Clinical Research on Dermatozoonoses in Domestic Animals—Evandro Chagas National Institute of Infectious Diseases (INI)/Fiocruz were included in this study. Sporothrix spp. were isolated from skin and mucosal lesions of dogs before they had received an antifungal treatment. The isolates were subcultured in potato dextrose agar (PDA) and then stored in 10% skimmed milk (Fluka Analytical, Sigma-Aldrich, Switzerland) at − 20 °C at the Mycology Laboratory—INI/Fiocruz. Clinical and epidemiological data were collected from the dog’s medical records.
Morphological and physiological studies
The polyphasic taxonomy was used to identify the fungal isolates at the species level, which included morphological, physiological, and molecular studies, with the filamentous form of the fungus used for all identification stages. For the phenotypic tests, the isolates were subcultured in PDA (Difco™; Becton, Dickinson and Company, Sparks, MD, USA) and visually examined for dihydroxynaphthalene melanin (DHN melanin) production [49]. To study conidiogenesis, these isolates were subcultured on corn meal agar (BBL™; BD, Franklin Lakes, NJ, USA) and incubated at 30 °C in a dark environment. After 10 days, the microscopic characteristics were evaluated [19].
The growth rate of colonies after 21 days of incubation at 30 °C and 37 °C in PDA medium was evaluated in triplicate at different times [19, 27]. Carbohydrate assimilation tests were performed in 96-well racks containing yeast nitrogen base (YNB) culture medium (Difco™; Becton, Dickinson and Company, Sparks, MD, USA) supplemented with sucrose or raffinose at a 0.5% concentration [19, 22, 32]. Cultures on YNB supplemented with glucose were used as positive controls for growth, and cultures on YNB without carbohydrates were used as negative controls. The results of morphological and physiological tests were interpreted based on a previously proposed taxonomic key [19, 20].
For the classification of the isolates regarding thermotolerance, the percent inhibition of growth was calculated as previously reported [50]. Isolates whose growth at 37 °C was reduced by 50% or more, and were classified as having low thermotolerance, and the isolates whose growth at 37 °C was reduced by less than 50% were classified as having high thermotolerance.
DNA extraction and analysis
For the identification through genotypic testing, the genomic DNA was extracted from the filamentous form using chloroform/isoamyl alcohol (24:1) [27]. For PCR, fingerprinting was used the universal T3B primer (5′-AGGTCGCGGGTTCGAATCC-3′) to distinguish between the species of the genus Sporothrix [32, 51]. Strains of species of the genus Sporothrix associated with cases found in humans and animals, S. brasiliensis (IPEC16490), S. globosa (IPEC27135), S. mexicana (MUM11.02), and S. schenckii (IPEC27722), were used as controls for molecular identification. Succinctly, we used the PCR reaction mix 10× buffer with KCl, dNTP mix 0.2 mM, MgCl2 50 mM, and platinum Taq DNA polymerase 1.0 U and 10 mM T3B primer [32, 51]. The T3B fingerprinting profiles obtained were analyzed with Bionumerics (version 5.1; Applied Maths BVBA, Sint-Martens-Latem, Belgium). Similarity coefficients were calculated using the Dice algorithm, and cluster analysis was performed by means of the unweighted paired group method using arithmetic averages (UPGMA).
Antifungal susceptibility testing
The antifungal susceptibility tests were performed in 11 isolates selected after polyphasic taxonomy. The inocula were prepared in sterile saline solution after incubation of each strain in PDA for 7 days at 37 °C. The antifungal susceptibility tests were conducted according to the M38-A2 protocol of the Clinical and Laboratory Standards Institute [52], using RPMI 1640 medium buffered to pH 7.0 with 0.165 mol/L morpholinepropanesulfonic acid (Sigma-Aldrich). The different working concentrations of antifungal drugs were distributed into wells of round-bottom 96-well microplates. Wells containing RPMI-1640 medium with DMSO and the fungal inoculum and without any antifungal agent were used as growth controls or, when only medium with DMSO was added, as sterility controls. Quality controls with the reference strains Aspergillus fumigatus ATCC 204305 and A. flavus ATCC 204304 were included. Tests were validated only if MIC values for these strains were within the range described in the M38-A2 reference document. MICs were determined by visual inspection after 48–72 h of incubation at 35 °C for better visualization of the fungal growth, as described [53, 54]. Amphotericin B (AMB), terbinafine (TRB), posaconazole (POS), ketoconazole (KTZ), and itraconazole (ITZ) (Sigma Chemical Corporation, St. Louis, MO, USA) were tested. All the tests were performed in triplicate for each isolate. According to the recent proposal of epidemiological cutoff values (ECV) for Sporothrix spp. [55], it is possible to classify the isolates of Sporothrix spp. as wild type (WT) and non-wild type (non-WT). Isolates with MIC values equal or less than the ECVs are defined as wild-type (WT) isolates, and isolates with MICs higher than the ECVs have elevated odds to present resistance mechanisms to the tested drug [53, 55]. According to the study, the MIC values proposed as ECV are 4 μg/ml for AMB, 2 μg/ml for ITZ, 2 μg/ml for POS, 2 μg/ml for KTZ, and 0.12 μg/ml for TRB [55]. It is possible to classify the isolates of S. brasiliensis and S. schenckii as wild type (WT) and non-wild type (non-WT); however, the study data were insufficient for the calculation of ECV of the S. schenckii isolates for KTZ and TRB.
Statistics
An exploratory analysis of the data was performed by applying the simple frequencies for each isolate to the species level of the genus Sporothrix as well as the clinical and epidemiological variables for the dogs, which were described in the evaluated individual medical records. For determining the association between related samples, the Wilcoxon test was used. p values < 0.05 indicated significant associations in the statistical tests. The obtained data were stored and analyzed in a database using the Statistical Package for Social Science (SPSS) software version 16.0.
Results
The clinical and epidemiological characteristics of the 47 dogs included in this study are reported in Table 1. Nineteen dogs presented only cutaneous lesions, 9 presented only mucosal lesions, and 19 presented mucosal and cutaneous lesions (Fig. 1).
Table 1.
Clinical and epidemiological aspects of 47 dogs with sporotrichosis assisted at INI/Fiocruz, Rio de Janeiro, Brazil, as well as phenotypic and molecular characterization of their isolates
| Clinical and epidemiological variables | Number (%) |
|---|---|
| Species determined by phenotypic tests | Sporothrix spp.—44 (93.62%) |
| S. schenckii—2 (4.26%) | |
| S. brasiliensis - 1 (2.12%) | |
| Species determined by molecular tests | S. brasiliensis—46 (97.88%) |
| S. schenckii—1 (2.12%) | |
| Gender | Male—30 (63.82%) |
| Female—17 (36.18%) | |
| Route of transmission | Unknown—19 (40.42%) |
| Scratch/bite of cats—18 (38.30%) | |
| Contact with cats—9 (19.15%) | |
| Contact with soil/plants—1 (2.13%) | |
| Clinical form | Fixed cutaneous—26 (55.32%) |
| Cutaneous disseminated—11 (23.40%) | |
| Mucosal—9 (19.15%) | |
| Lymphocutaneous—1 (2.13%) | |
| Respiratory signs | Yes—36 (76.60%) |
| No—11 (23.40%) | |
| Skin lesions | Yes—38 (80.85%) |
| No—9 (19.15%) | |
| Mucosal lesions | Yes—28 (59.57%) |
| No—19 (40.43%) | |
| Outcome | Clinical cure—22 (46.81%) |
| Treatment abandonment—13 (27.65%) | |
| Therapeutic failure—7 (14.90%) | |
| Death by another cause—3 (6.39%) | |
| Death by sporotrichosis—2 (4.25%) |
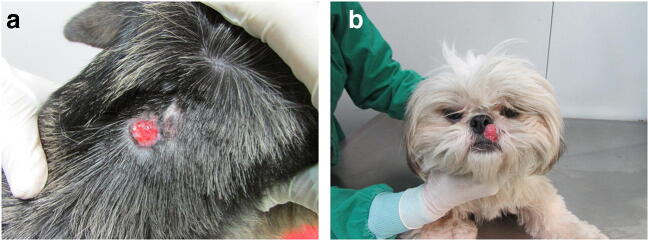
Fig. 1.
a Dog with ulcerated cutaneous lesion on the neck caused by S. schenckii (IPEC24C). b Dog with ulcerated mucocutaneous lesion on the nasal region caused by S. brasiliensis (IPEC17C)
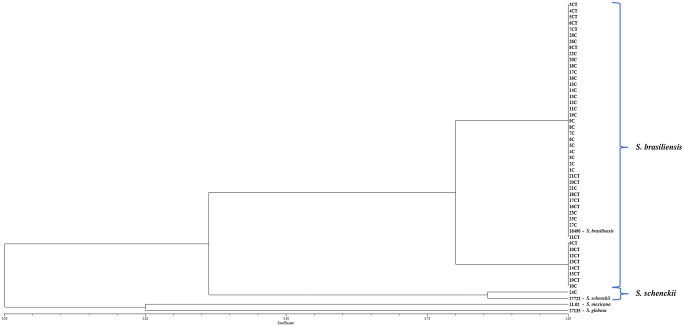
The results of phenotypic tests are reported in Online Resource 1. The median diameter of the colonies incubated at 30 °C after 21 days was 30 mm (range 13–54 mm), whereas the median for colonies at 37 °C was 10.5 mm (range 5–20 mm). It was possible to observe that the growth of colonies at 30 °C was better than at 37 °C (p < 0.05). In the carbohydrate assimilation tests, glucose was assimilated by all isolates, including the controls, after 10 days. All isolates from this study showed growth at 37 °C, and they were then classified as isolates with low and high thermotolerances, but only six isolates were classified as having high thermotolerance. With the results of the morphological and physiological tests, it was possible to compare the results of each isolate to a previously proposed taxonomic key [19]. Two isolates presented compatible phenotypic characteristics with S. schenckii and one with S. brasiliensis. However, the remaining 44 isolates did not presented compatible phenotypic characteristics that allowed to define to which species they belonged and were classified as Sporothrix spp. These isolates showed conclusive results at the species level using T3B PCR fingerprinting, 46 isolates were characterized as S. brasiliensis, and one isolate (IPEC24C) was characterized as S. schenckii (Fig. 2). In addition, eight isolates demonstrated a small intraspecific variability when compared with the S. brasiliensis type strain.
Fig. 2.
Phylogenetic tree showing species identification and the degree of similarity between T3B fingerprinting profiles among the Sporothrix isolates obtained of the 47 dogs at the Laboratory of Clinical Research on Dermatozoonoses in Domestic Animals (INI)/Fiocruz, Rio de Janeiro, Brazil, 2013 to 2018 using the UPGMA cluster method
Antifungal susceptibility tests were performed in the isolate characterized as S. schenckii and in isolates characterized as S. brasiliensis. The antifungal susceptibility profiles of the S. brasiliensis and S. schenckii isolates are reported in Table 2. AMB presented the highest MIC values for the canine isolates, ranging from 1.0 to 8.0 μg/ml. KTZ, ITZ, POS, and TRB showed good antifungal activity in the test (range 0.06–0.50 μg/ml, 0.12–0.50 μg/ml, 0.25–1.0 μg/ml, and 0.03–0.50 μg/ml, respectively) with no differences between species. For the antifungals that were tested, canine isolates of S. brasiliensis were all classified as WT for ITZ and POS, isolate IPEC13C was classified as non-WT for AMB, and IPEC22C was classified as non-WT for TRB. The ECV of the S. schenckii isolate (IPEC24C) was not evaluated for KTZ and TRB, but it was classified as WT for ITZ and POS as well as non-WT for AMB.
Table 2.
Antifungal treatment in 11 dogs with sporotrichosis assisted at INI/Fiocruz, Rio de Janeiro, Brazil, and the corresponding MIC values according to the antifungal susceptibility tests from their isolates
| Isolate code | Species determined by molecular tests | Initial therapeutic scheme | Change of therapeutic scheme | Treatment time to clinical cure (weeks) | MIC (μg/ml) | ||||
|---|---|---|---|---|---|---|---|---|---|
| AMB | KTZ | ITZ | TRB | POS | |||||
| 24C | S. schenckii | KTZ 200 mg/dog q24h | ITZ 250 mg/dog q24h | 24 | 8.00 | 0.50 | 0.50 | 0.06 | 0.50 |
| 10C | S. brasiliensis | ITZ 100 mg/dog q24h | – | 32 | 4.00 | 0.25 | 0.50 | 0.06 | 0.50 |
| 12C | S. brasiliensis | ITZ 200 mg/dog q24h | – | – | 2.00 | 0.50 | 0.50 | 0.03 | 0.50 |
| 13C | S. brasiliensis | ITZ 200 mg/dog q24h | ITZ 300 mg/dog q24h | 44 | 8.00 | 0.50 | 0.50 | 0.12 | 1.00 |
| 14C | S. brasiliensis | ITZ 300 mg/dog q24h | – | 32 | 2.00 | 0.06 | 0.25 | 0.06 | 0.25 |
| 16C | S. brasiliensis | ITZ 100 mg/dog q24h | – | 56 | 2.00 | 0.25 | 0.25 | 0.06 | 1.00 |
| 17C | S. brasiliensis | – | – | – | 2.00 | 0.50 | 0.25 | 0.12 | 0.50 |
| 18C | S. brasiliensis | ITZ 200 mg/dog q24h | – | 20 | 1.00 | 0.25 | 0.25 | 0.06 | 0.50 |
| 21C | S. brasiliensis | KTZ 100 mg/dog q24h | ITZ 150 mg/dog q24h | – | 2.00 | 0.50 | 0.25 | 0.06 | 0.50 |
| 22C | S. brasiliensis | ITZ 200 mg/dog q24h | ITZ 300 mg/dog q24h | – | 2.00 | 0.12 | 0.12 | 0.50 | 0.25 |
| 23C | S. brasiliensis | ITZ 150 mg/dog q24h | – | 76 | 2.00 | 0.50 | 0.50 | 0.06 | 0.50 |
MIC minimum inhibitory concentration, AMB amphotericin B, KTZ ketoconazole, ITZ itraconazole, POS posaconazole, TRB terbinafine, – not applicable
Discussion
After the description of new species from the genus Sporothrix, the identification of clinical isolates has been performed worldwide, especially in regions where a large number of sporotrichosis cases occurs [20, 22, 24, 28, 32, 37, 38, 56, 57].
Sporotrichosis in dogs is a poorly described disease compared to humans and cats; therefore, this study has an impact on the knowledge of different aspects of the disease in dogs, including the species of Sporothrix sp. that affect the canine population and their antifungal susceptibility profile. Few studies carried out in the southern and southeastern regions of Brazil have characterized canine isolates for which the predominant species was S. brasiliensis [8, 28, 40]. Sporothrix luriei has also been reported once in a study carried out in Rio Grande do Sul [34]. In our study, after molecular characterization using T3B PCR fingerprinting, the species S. brasiliensis was found in most of the isolates, which corroborates the findings of previous studies in animals from Brazil [8, 28, 32, 34, 37, 38, 58]. We documented here one isolate identified as S. schenckii. This study is the first report of this species affecting dogs in Brazil and the first report of another species besides S. brasiliensis affecting domestic animals from the metropolitan region of Rio de Janeiro, which is the major area of occurrence of animal sporotrichosis in the world. This finding highlights the possibility that S. schenckii may also circulate among cats in the same region as a causal agent since these animals are the main source of infection for dogs in this scenario [6]. To date, studies with isolates obtained from cats in Rio de Janeiro have only described S. brasiliensis [28, 32, 37, 38]. Other species, in addition to S. brasiliensis, have been identified in isolates from Rio de Janeiro that were obtained from human patients with sporotrichosis, such as S. schenckii and S. globosa [27, 56], which reinforces the hypothesis that other species may also circulate among animals.
The majority of dogs affected by sporotrichosis in this study acquired the infection through contact with cats that had sporotrichosis since they have an important role in the transmission of the fungus due the high fungal burden in their lesions [59]. Specifically, it was possible that the dog with sporotrichosis caused by S. schenckii (IPEC24C) was infected through contact with a stray cat that had sporotrichosis and frequented the backyard of the dog/owner’s home. This dog and its owner acquired the disease, but it was not possible to characterize the isolate from the owner. Also, it was not possible to isolate the fungus from the feline host. However, we cannot reject the possibility of S. schenckii transmission through the classical route. A previous study reported that a common route of infection for S. schenckii could occur in an environmental manner or in transmission through infected cats [25].
It was not possible to determine a taxonomic classification at the species level only with phenotypic characterization to classify the Sporothrix spp. in the majority of isolates tested. In most isolates, only glucose and sucrose were assimilated in the carbohydrate assimilation test, which suggested the species S. pallida or S. globosa. In fact, the isolates could not be classified as S. pallida because of the presence of dematiaceous conidia or as S. globosa because the isolates were thermotolerant at 37 °C [19], which did not allow the identification of the species by this method. One isolate (IPEC22C) had a hyaline colony without pigment production in PDA, but we observed the presence of dematiaceous and hyaline conidia in micromorphology that corroborates with the results of previous studies in humans and cats [22, 32]. However, most of the canine isolates had similar phenotypic profile regardless of the species identified by molecular technique, which differed from the results in previous studies of human and feline isolates [22, 32] which suggested a variability phenotypic in our isolates.
Unlike studies with feline and human isolates, it was not possible the identification at the species level only using a taxonomic key. Due to this diversity of results in the phenotypic tests, we suggest that mycologists should be cautious when identifying species of the genus Sporothrix in canine isolates through only morphological and physiological tests.
Despite the inconclusive results of the phenotypic tests in the most of studied isolates, the molecular technique has successfully identified all the isolates, and there was a disagreement between the results of phenotypic and molecular techniques, since only one isolate was correctly identified in both techniques as S. brasiliensis. The other two isolates characterized as S. schenckii in phenotypic tests were identified as S. brasiliensis in molecular identification. Other studies that performed the characterization of canine isolates using phenotypic and molecular techniques did not show such disagreement, which does not corroborate with our findings [28, 34]. The isolates used in other studies was from other regions of Brazil; in addition, the number used was lower compared with our study, which could justify the disagreement. However, our results were similar to others reported previously in studies with human isolates [22], as well as characterization of feline isolates [32, 60, 61].
There was a small intraspecific variability among our isolates classified as S. brasiliensis as observed in human and feline cases caused by S. brasiliensis described in a previous study [32, 51], and by correlating these findings with the clinical and epidemiological data of dogs, it was possible to observe that five of these animals presented therapeutic failure as an outcome. A larger number of phenotypic and genotypic studies in clinical isolates of Sporothrix sp. from dogs and cats are necessary, in areas where animal sporotrichosis occurs, for a more accurate evaluation of detected intraspecific variabilities and the possibility of association with the clinical and epidemiological characteristics of patients.
Few studies that evaluated antifungal susceptibility profile of canine isolates have been performed when compared with studies on human and feline isolates [8, 22, 45, 46, 48, 62, 63]. The identification of a new species in dogs may have implications in disease epidemiology, which emphasizes the importance of the study of the antifungal susceptibility profile of clinical isolates of these animals. From in vitro studies with clinical isolates, it is possible to choose a therapeutic alternative that is more effective and carries out detection of resistant strains [44].
In our study, the MIC values for AMB ranged from 1.0 to 8.0 μg/ml, which are values that have been previously observed in other studies with feline and canine isolates [8, 30, 48, 53]. Regarding AMB, only two isolates were classified as non-WT, according to classification proposed by Espinel-Ingroff and coworkers [55]; however, this drug was not used in any of the dogs in this study.
Of the 11 dogs that had the antifungal susceptibility from their isolates evaluated included the dog with sporotrichosis caused by S. schenckii and the other 10 dogs with sporotrichosis caused by S. brasiliensis, which were selected randomly. Of these dogs, six achieved the clinical cure outcome after ITZ treatment, including the dog with sporotrichosis caused by S. schenckii (IPEC24C). In this case series evaluated, the results of in vitro susceptibility tests for ITZ coincided, in most cases (6/11), with the favorable outcome of treatment with this drug. The MIC values for ITZ did not exceed 0.5 μg/ml, which indicates that these isolates were WT strains, and thus, there was a high probability of them being susceptible to ITZ. However, two dogs (IPEC21C and IPEC24C) started treatment with KTZ and needed to change to ITZ due to insufficient clinical response, despite the low MIC values for KTZ observed (≤ 1.0) and classification of isolates as WT strains. One dog obtained the clinical cure (IPEC24C), and in the other case, there was loss to follow-up. More importantly, other factors related to patients should be considered, such as their immune response or the use of adjuvants in the treatment might interfere with correlating in vitro and in vivo findings [55].
The MIC values found in our study corroborate the results previous described using POS [8, 45, 64]. The use of this drug in vivo was described in a murine model of sporotrichosis caused by S. brasiliensis, which showed a reduction of the fungal load after treatment [65]; however, it has a high commercial cost, which could limit the use of this drug as a therapeutic alternative.
Since the first studies of antifungal susceptibility, TRB has been a drug that constantly presents low MIC values against different species of Sporothrix [45, 48, 53, 62]. Recently, the clinical effectiveness of TRB was observed in two canine cases [8]. The results of antifungal susceptibility tests for TRB in our study remain promising, although we found a non-WT isolate (IPEC22C), with a MIC value of 0.50 μg/ml. Nevertheless, therapeutic studies with a larger number of animals are needed to define the role of this drug in the treatment of canine sporotrichosis.
Our study suggests that S. brasiliensis is the main etiologic agent associated with canine sporotrichosis in the metropolitan region of Rio de Janeiro. However, the genotypic characterization of S. schenckii from a dog that was infected through contact with a sick cat confirms the circulation of this pathogenic species in an animal population for the first time in the endemic region of Rio de Janeiro after the taxonomic changes made to the genus Sporothrix. Therefore, we believe that other species can also circulate among cats in this region, which play an important role in zoonotic transmission of sporotrichosis, which play an important role in zoonotic transmission of sporotrichosis. Characterization studies of canine and feline isolates of Sporothrix sp. (in endemic regions or where new cases are occurring) are strongly suggested to determine the circulating species among the animal population, which may present different modes of transmission, pathogenicity, and susceptibility to antifungal agents. This is the first report of S. schenckii in dog from Brazil and native of Rio de Janeiro. Nevertheless, no differences were observed in the clinical presentation of patients and antifungal susceptibility profiles between the S. brasiliensis and S. schenckii isolates.
The high prevalence of S. brasiliensis in the canine population and the identification of S. schenckii in the main endemic area of animal sporotrichosis in the world reaffirm the importance of studies on the identification of species and the antifungal susceptibility profile of clinical isolates from animals and their associations with clinical, epidemiological, and therapeutic aspects.
Electronic supplementary material
(DOCX 19 kb).
Acknowledgments
The authors are grateful for access to the Oswaldo Cruz Foundation’s sequencing platform (PDTIS/Fiocruz).
Funding information
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001 and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (E-26/200.839/2017). SAP was supported in part by Jovem Cientista do Nosso Estado 2016 (JCNE)—FAPERJ (grant no.: E-26/203.303/2016) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant no.: 309657/2016-4). RMZ-O was supported in part by CNPq (grant no.: 304976/2013-0) and FAPERJ (grant no.: E-26/103.157/2011). MMO was supported in part by FAPERJ (grant no.: INST E-26/010.001784/2016, JCNE E-26/203.301/2017).
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee on Animal Use of the Oswaldo Cruz Foundation (CEUA/Fiocruz; number: LW-17/17).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pereira SA, Gremiao ID, Kitada AA, Boechat JS, Viana PG, Schubach TM. The epidemiological scenario of feline sporotrichosis in Rio de Janeiro, State of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2014;47:392–393. doi: 10.1590/0037-8682-0092-2013. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues AM, Hoog GS, de Camargo ZP. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn Microbiol Infect Dis. 2014;78:383–387. doi: 10.1016/j.diagmicrobio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Pereira SA, Gremião IDF, Menezes RC. Sporotrichosis - new developments and future prospects. 1. Suíça: Springer; 2015. Sporotrichosis in animals: zoonotic transmission; pp. 83–102. [Google Scholar]
- 4.Cafarchia C, Sasaneli M, Lia RP, Caprariis D, Guillot J, Otranto D. Lymphocutaneous and nasal sporotrichosis in a dog from Southern Italy: case report. Mycopathologia. 2007;163:75–79. doi: 10.1007/s11046-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 5.Crothers SL, White SD, Ihrke PJ, Affolter VK. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007) Vet Dermatol. 2009;20:249–259. doi: 10.1111/j.1365-3164.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Schubach TM, Schubach A, Okamoto T, et al. Canine sporotrichosis in Rio de Janeiro, Brazil: clinical presentation, laboratory diagnosis and therapeutic response in 44 cases (1998-2003) Med Mycol. 2006;44:87–92. doi: 10.1080/13693780500148186. [DOI] [PubMed] [Google Scholar]
- 7.Mascarenhas MB, Lopes NL, Pinto TG, Costa TS, Peixoto AP, Ramadinha RR, Fernandes JI. Canine sporotrichosis: report of 15 advanced cases. Pesqui Vet Bras. 2018;38:477–481. [Google Scholar]
- 8.Viana PG, Figueiredo ABF, Gremião IDF, de Miranda LHM, da Silva Antonio IM, Boechat JS, de Sá Machado AC, de Oliveira MME, Pereira SA. Successful treatment of canine sporotrichosis with terbinafine: case reports and literature review. Mycopathologia. 2018;183:471–478. doi: 10.1007/s11046-017-0225-6. [DOI] [PubMed] [Google Scholar]
- 9.Barros MB, Almeida-Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva MB, Costa MM, Torres CC, et al. Esporotricose urbana: epidemia negligenciada no Rio de Janeiro, Brasil. Cad Saúde Pública. 2012;28:1867–1880. doi: 10.1590/s0102-311x2012001000006. [DOI] [PubMed] [Google Scholar]
- 11.Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:e3110. doi: 10.1371/journal.pntd.0003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gremião IDF, Miranda LHM, Reis EG, Rodrigues AM, Pereira SA. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog. 2017;13:e1006077. doi: 10.1371/journal.ppat.1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosser E, Dunstan R. Sporotrichosis. Infectious diseases of the dog and cat. 3. Philadelphia: Saunders Elsevier; 2006. [Google Scholar]
- 14.Barros MB, Schubach AO, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 15.Schubach A, Barros MB, Wanke B. Epidemic sporotrichosis. Curr Opin Infect Dis. 2008;21:129–133. doi: 10.1097/QCO.0b013e3282f44c52. [DOI] [PubMed] [Google Scholar]
- 16.Sykes JE, Torres SM, Armstrong PJ, Lindeman CJ. Itraconazole for treatment of sporotrichosis in a dog residing on a Christmas tree far. J Am Vet Med Assoc. 2001;218:1440–1443. doi: 10.2460/javma.2001.218.1440. [DOI] [PubMed] [Google Scholar]
- 17.Scott D, Miller W. Doenças fúngicas da pele. Dermatologia de pequenos animais. Rio de Janeiro: Interlivros Edições Ltda; 1996. pp. 301–369. [Google Scholar]
- 18.Marimon R, Gene J, Cano J, Trilles L, Lazera M, Guarro J. Molecular phylogeny of Sporothrix schenckii. J Clin Microbiol. 2006;44:3251–3256. doi: 10.1128/JCM.00081-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marimon R, Cano J, Gene J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marimon R, Gene J, Cano J, Guarro J. Sporothrix luriei: a rare fungus from clinical origin. Med Mycol. 2008;46:621–625. doi: 10.1080/13693780801992837. [DOI] [PubMed] [Google Scholar]
- 21.Romeo O, Scordino F, Criseo G. New insight into molecular phylogeny and epidemiology of Sporothrix schenckii species complex based on calmodulin-encoding gene analysis of Italian isolates. Mycopathologia. 2011;172:179–186. doi: 10.1007/s11046-011-9420-z. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira MM, Almeida-Paes R, Muniz MM, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia. 2011;172:257–267. doi: 10.1007/s11046-011-9437-3. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues AM, Choappa RC, Fernandes GF, De Hoog GS, Camargo ZP (2016) Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol 120:246–264 [DOI] [PubMed]
- 24.Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:e1005638. doi: 10.1371/journal.ppat.1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes-Bezerra LM, Mora-Montes HM, Zhang Y, Nino-Vega G, Rodrigues AM, de Camargo ZP, de Hoog S. Sporotrichosis between 1898 and 2017: the evolution of knowledge on a changeable disease and on emerging etiological agents. Med Mycol. 2018;56:S126–S143. doi: 10.1093/mmy/myx103. [DOI] [PubMed] [Google Scholar]
- 26.Moussa TAA, Kadasa NMS, Al Zahrani HS, et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J Med Microbiol. 2017;66:560–569. doi: 10.1099/jmm.0.000451. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira MM, Almeida-Paes R, Muniz MM, Barros MBL, Gutierrez-Galhardo MC, Zancope-Oliveira RM. Sporotrichosis caused by Sporothrix globosa in Rio de Janeiro, Brazil: case report. Mycopathologia. 2010;169:359–363. doi: 10.1007/s11046-010-9276-7. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues AM, Teixeira MM, Hoog GS, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kano R, Okubo M, Siew HH, Kamata H, Hasegawa A. Molecular typing of Sporothrix schenckii isolates from cats in Malaysia. Mycoses. 2015;58:220–224. doi: 10.1111/myc.12302. [DOI] [PubMed] [Google Scholar]
- 30.Han HS, Kano R, Chen C, Noli C. Comparison of two in vitro antifungal sensitivity tests and monitoring during therapy of Sporothrix schenckii sensu stricto in Malaysian cats. Vet Dermatol. 2017;28:156–e32. doi: 10.1111/vde.12417. [DOI] [PubMed] [Google Scholar]
- 31.Siew HH. The current status of feline sporotrichosis in Malaysia. Med Mycol J. 2017;58E:E107–E113. doi: 10.3314/mmj.17.014. [DOI] [PubMed] [Google Scholar]
- 32.Boechat JS, Oliveira MME, Almeida-Paes R, Gremião IDF, Machado ACS, Oliveira RVC, Figueiredo ABF, Rabello VBS, Silva KBL, Zancopé-Oliveira RM, Schubach TMP, Pereira SA. Feline sporotrichosis: associations between clinical-epidemiological profiles and phenotypic-genotypic characteristics of the etiological agents in the Rio de Janeiro epizootic area. Mem Inst Oswaldo Cruz. 2018;113:185–196. doi: 10.1590/0074-02760170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Marine M, Genis J, Cano J, Guarro J. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651–655. doi: 10.1111/j.1469-0691.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047–3049. doi: 10.1128/JCM.00255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Vergara ML, Camargo ZP, Silva PF, et al. Disseminated Sporothrix brasiliensis infection with endocardial and ocular involvement in an HIV-infected patient. Am J Trop Med Hyg. 2012;86:477–480. doi: 10.4269/ajtmh.2012.11-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Córdoba S, Isla G, Szusz W et al (2018) Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses 61:1–8 [DOI] [PubMed]
- 37.Macêdo-Sales PA, Souto SRL, Destefani CA, et al. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: a comparison between infected and non-infected populations. BMC Vet Res. 2018;14:1–10. doi: 10.1186/s12917-018-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza EW, Borba CM, Pereira SA, et al. Clinical features, fungal load, coinfections, histological skin changes, and itraconazole treatment response of cats with sporotrichosis caused by Sporothrix brasiliensis. Sci Rep. 2018;8:9074. doi: 10.1038/s41598-018-27447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etchecopaz AN, Lanza N, Toscanini MA, Devoto TB, Pola SJ, Daneri GL, Iovannitti CA, Cuestas ML. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J Mycol Med. 2019;30:100908. doi: 10.1016/j.mycmed.2019.100908. [DOI] [PubMed] [Google Scholar]
- 40.Waller SB, Hoffmann JF, Madrid IM, Picoli T, Cleff MB, Chaves FC, Zanette RA, de Mello JRB, de Faria RO, Meireles MCA. Polar Origanum vulgare (Lamiaceae) extracts with antifungal potential against Sporothrix brasiliensis. Med Mycol. 2018;56:225–233. doi: 10.1093/mmy/myx031. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Romero E, Reyes-Montes MR, Perez-Torres A, et al. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 2011;6:85–102. doi: 10.2217/fmb.10.157. [DOI] [PubMed] [Google Scholar]
- 42.Padhye AA, Kaufman L, Durry E, Banerjee CK, Jindal SK, Talwar P, Chakrabarti A. Fatal pulmonary sporotrichosis caused by Sporothrix schenckii var. luriei in India. J Clin Microbiol. 1992;30:2492–2494. doi: 10.1128/jcm.30.9.2492-2494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubach TM, Menezes RC, Wanke B. Sporotrichosis. Infectious diseases of the dog and cats. 4. Missouri: Elsevier; 2012. [Google Scholar]
- 44.Schreiber AZ. Antifungiograma: Quando solicitar e Como Interpretar. Prática Hospitalar. 2007;49:87–91. [Google Scholar]
- 45.Kohler LM, Monteiro PC, Hahn RC, Hamdan JS. In vitro susceptibilities of isolates of Sporothrix schenckii to itraconazole and terbinafine. J Clin Microbiol. 2004;42:4319–4320. doi: 10.1128/JCM.42.9.4319-4320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues AM, Hoog GS, Pires DC, et al. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14:219. doi: 10.1186/1471-2334-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stopiglia CDO, Magagnin CM, Castrillón MR, et al. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med Mycol. 2014;52:56–64. doi: 10.3109/13693786.2013.818726. [DOI] [PubMed] [Google Scholar]
- 48.Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, Nucci M, Lopes-Bezerra LM, Ishida K, Rozental S. Susceptibilidade of Sporothrix brasiliensis isolates to amphotericin B, azoles and terbinafine. Med Mycol. 2015;53:178–188. doi: 10.1093/mmy/myu056. [DOI] [PubMed] [Google Scholar]
- 49.Almeida-Paes R, Frases S, Monteiro PCF, Gutierrez-Galhardo MC, Zancopé-Oliveira RM, Nosanchuk JD. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes Infect. 2009;11:554–562. doi: 10.1016/j.micinf.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida-Paes R, Oliveira LC, Oliveira MME, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM (2015) Phenotypic characteristic associated with virulence of clinical isolates from Sporothrix complex. Biomed Res Int 2015:212308 [DOI] [PMC free article] [PubMed]
- 51.Oliveira MME, Franco-Duarte R, Romeo O, et al. Evaluation of T3B fingerprinting for identification of clinical and environmental Sporothrix species. FEMS Microbiol Lett. 2015;362:1–7. doi: 10.1093/femsle/fnv027. [DOI] [PubMed] [Google Scholar]
- 52.Clinical and Laboratory Standards Institute (CLSI) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 2. Wayne: Clincal and Laboratory Standards Institute; 2008. [Google Scholar]
- 53.Almeida-Paes R, Brito-Santos F, Figueiredo-Carvalho MHG, Machado ACS, Oliveira MME, Pereira SA, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz. 2017;112:376–381. doi: 10.1590/0074-02760160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brilhante RSM, Rodrigues AM, Sidrim JJC, et al. In vitro susceptibility of antifungal drugs against Sporothrix brasiliensis recovered from cats with sporotrichosis in Brazil. Med Mycol. 2016;54:275–279. doi: 10.1093/mmy/myv039. [DOI] [PubMed] [Google Scholar]
- 55.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, et al. Multicenter 1 and international study of MIC/MEC distributions for definition of epidemiological cutoff values (ECVs) for species of Sporothrix identified by molecular methods. Antimicrob Agents Chemother. 2017;61:1–8. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida-Paes R, Oliveira MME, Freitas DF, Francesconi AC, Zancopé-Oliveira RM, Gutierrez-Galhardo MC. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:e3094. doi: 10.1371/journal.pntd.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu T, Zhang K, Zhou X. Molecular identification of Sporothrix clinical isolates in China. J Zhejiang Univ Sci B. 2014;15:100–108. doi: 10.1631/jzus.B1300136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montenegro H, Rodrigues AM, Dias MAG, Silva EA, Bernardi F, Camargo ZP. Feline sporotrichosis due to Sporothrix brasiliensis: an emerging animal infection in São Paulo, Brazil. BMC Vet Res. 2014;10:269. doi: 10.1186/s12917-014-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miranda LHM, Silva JN, Gremião IDF, et al. Monitoring fungal burden and viability of Sporothrix spp. in skin lesions of cats for predicting antifungal treatment response. J Fungi (Basel) 2018;4:92. doi: 10.3390/jof4030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira MME, Maifrede SB, Ribeiro MA, Zancopé-Oliveira RM. Molecular identification of Sporothrix species involved in the first familial outbreak of sporotrichosis in the state of Espírito Santo, southeastern Brazil. Mem Inst Oswaldo Cruz. 2013;108:936–938. doi: 10.1590/0074-0276130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmoudi S, Zaini F, Kordbacheh P, Safara M, Heidari M. Sporothrix schenckii complex in Iran: molecular identification and antifungal susceptibility. Med Mycol. 2016;54:593–599. doi: 10.1093/mmy/myw006. [DOI] [PubMed] [Google Scholar]
- 62.Meinerz AR, Nascente OS, Schuch LFD, et al. In vitro susceptibility of isolates of Sporothrix schenckii to terbinafine and itraconazole. Rev Soc Bras Med Trop. 2007;40:60–62. doi: 10.1590/s0037-86822007000100012. [DOI] [PubMed] [Google Scholar]
- 63.Marimon R, Serena C, Gene J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomson J, Trott DJ, Malik R, Galgut B, McAllister MM, Nimmo J, Renton D, Kidd SE. An atypical cause of sporotrichosis in a cat. Med Mycol Case Rep. 2019;23:72–76. doi: 10.1016/j.mmcr.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Silva F, Capilla J, Mayayo E, Guarro J. Efficacy of posaconazole in murine experimental sporotrichosis. Antimicrob Agents Chemother. 2012;56:2273–2277. doi: 10.1128/AAC.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb).