Abstract
Cystic fibrosis (CF) causes a variety of symptoms in different organs, but the majority of the morbidity and mortality of CF is related with pulmonary conditions. Primary infections are usually bacterial, and when treated with antibiotics, yeast infections appear or become more evident. Studies show that different microorganisms can co-inhabit the same environment and the interactions could be synergistic or antagonistic. Using techniques including viable and non-viable cell-to-cell interactions, mixed culture in liquid, and solid media sharing or not the supernatant, this study has evaluated interactions between the fungal species Scedosporium apiospermum and Scedosporium boydii with the bacterial species Staphylococcus aureus, Pseudomonas aeruginosa, and Burkholderia cepacia. Cell-to-cell interactions in liquid medium showed that P. aeruginosa and B. cepacia were able to reduce fungal viability but only in the presence of alive bacteria. Interactions without cell contact using a semi-permeable membrane showed that all bacteria were able to inhibit both fungal growths/viabilities. Cell-free supernatants from bacterial growth reduced fungal viability in planktonic fungal cells as well as in some conditions for preformed fungal biomass. According to the chemical analysis of the bacterial supernatants, the predominant component is protein. In this work, we verified that bacterial cells and their metabolites, present in the supernatants, can play anti-S. apiospermum and anti-S. boydii roles on fungal growth and viability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-020-00415-w.
Keywords: Cystic fibrosis, Scedosporium, Bacteria, Growth, Viability
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease as a result of a mutation in the CFTR gene. Patients presenting this genetic alteration produce thicker mucus in different sites, such as pancreas and lungs, which causes problems in digestive absorbance and higher susceptibility to chronic pulmonary infections [1]. The high morbidity and mortality present by children and adults are due to severe lung infections caused by bacterial and fungal pathogens. Staphylococcus aureus, Pseudomonas aeruginosa, and the complex Burkholderia cepacia are some of the most common bacteria found in CF lungs [2, 3]. The last one is associated with worse prognosis due to its high patient-to-patient transmission and antibiotic resistance [4, 5]. Bacterial colonization in CF lungs varies along the patient’s life. For instance, S. aureus is the most prevalent species in patients between 5 and 20 years old, whereas P. aeruginosa tends to become more frequent in older patients. This data indicates that the airway microbial colonization is not static and varies along the time [6].
Fungal pathogens are also isolated from CF patients’ airways, especially Aspergillus, Penicillium, and Scedosporium species [7]. Among Scedosporium species, S. apiospermum is the most frequent (28.6%), followed by S. boydii (19.3%), S. aurantiacum (10.0%), and L. prolificans (3.6%) [7]. Regarding the airway colonization by Scedosporium species, this group of fungi has been considered by several studies as the second most frequent in CF lungs (after Aspergillus) [8–10]. In this context, Scedosporium pathogens are late colonizers, because it was seen that the mean age of patients presenting Scedosporium colonization was higher than other pathogens [9]. It suggests that previous lesions on pulmonary epithelial cells are necessary for the establishment of Scedosporium colonization and that its long-lasting presence contributes to the chronic inflammation of CF lungs [8]. In addition, treatment of Scedosporium infections is not easy due to their resistance to many antifungals available in clinical settings, such as polienes, echinocandins, and the first-generation azoles [8, 11–13]. Best results are found using voriconazole [13] and posaconazole [12, 14, 15]. All these reasons highlight the importance of studying Scedosporium airway colonization and its interaction with other pathogens in the context of CF.
It is well established that CF patients’ airways are colonized by polymicrobial colonization during their lives, so a variety of studies have been done to understand especially the relationship between bacteria and fungi [16]. For instance, Mowat and colleagues observed that the co-culture of P. aeruginosa and Aspergillus fumigatus resulted in fungal growth inhibition [17]. It is also known that bacteria produce molecules able to inhibit fungal growth, even in CF context. Some P. aeruginosa metabolites, such as phenazine, pyoverdine, dirhamnolipids, and quorum-sensing molecules, have already been described to potentially inhibit Aspergillus fumigatus, Candida albicans, and Scedosporium species [18–21]. Burkholderia species, such as B. cenocepacia, have already been shown to display antifungal activity against A. fumigatus and C. albicans [22, 23], but the majority of studies regarding Burkholderia species is related to its interaction with fungal plant pathogens [24–26]. Thus, more studies are needed in order to clarify the mechanisms by which bacteria and fungi interact and influence each other, which could help to improve the knowledge regarding the prognosis of CF.
Since B. cepacia, P. aeruginosa, and S. aureus inhibited fungal growth even with no cell-cell contact, it is reasonable to believe that these bacteria secrete substances in the growth media that play an antifungal role.
A variety of extracellular P. aeruginosa metabolites have already been described playing antifungal roles, especially against A. fumigatus [27]. Interestingly, P. aeruginosa isolates derived from CF patients present a more prominent inhibitory effect compared to those originated from non-CF patients, suggesting that this effect is phenotype dependent [28]. It is known that P. aeruginosa supernatants present some antifungal molecules, such as phenazine, suggesting that secreted molecules might play a role in bacteria-fungi competition [18, 21]. Other extracellular products have already been described playing crucial roles in its relation with A. fumigatus, such as pyoverdine, a bacterial siderophore, which has been considered the major antifungal molecule produced by Pseudomonas spp., promoting iron starvation to A. fumigatus [29]. The quorum-sensing molecule PQS (Pseudomonas quinolone signal; 2-heptyl-3,4-dihydroxyquinoline) also displays antifungal properties affecting iron acquisition [30, 31]. Dirhamnolipids have also been identified in P. aeruginosa playing anti-A. fumigatus activity by inhibiting β-1,3 glucan synthase activity [32].
Burkholderia species have already been described to produce extracellular molecules that display antifungal properties, such as occidiofungin from B. pyrrocinia [26], but the majority of the studies are related to fungal plant pathogens [24, 25]. In the clinical field, Burkholderia signaling molecule cis-2-dodecenoic acid (BDSF) has been shown to display inhibitory effect against C. albicans and also presented synergism with itraconazole and fluconazole [23]. In the context of CF, B cenocepacia possesses antifungal activity against A. fumigatus by producing phenylacetic acid, but more studies are needed to clarify its inhibitory effect [22].
Despite the data found in the literature, little is known about how bacteria inhibit Scedosporium species, and it is not clear yet the mechanisms by which bacteria and fungi, especially Scedosporium species, interact with each other. In addition, although some reports are found in the literature regarding P. aeruginosa and Burkholderia spp. molecules with antifungal activity, very little is observed for other CF relevant bacteria, such as S. aureus, which could also interfere in Scedosporium growth. Considering that polymicrobial respiratory infections are frequent in CF patients, the understanding of these microbial interactions in CF lungs could help to understand the dynamic of airway colonization and also to manage the disease.
Aiming to increase the knowledge regarding polymicrobial cultures containing Scedosporium species, the present study analyzed the interaction between the bacteria P. aeruginosa, S. aureus, and B. cepacia with S. apiospermum and S. boydii and their influence on fungal growth. The study was performed using co-culture techniques, in order to evaluate the effect of bacterial cells on S. apiospermum and S. boydii growth/viability. Moreover, bacterial supernatants were analyzed to check whether they could contain molecules able to inhibit S. apiospermum and S. boydii growth and biofilm formation.
Material and methods
Microrganisms and growth conditions
Staphylococcus aureus strain ATCC 25923, Pseudomonas aeruginosa strain ATCC 27853, and Burkholderia cepacia strain ATCC 25416 were maintained in nutrient broth (Difco) for storage. To obtain bacterial suspensions, cells were inoculated in nutrient broth and incubated at 37 °C for 24 h. Bacterial quantification was determined according to the McFarland turbidity scale (0.5 McFarland turbidity standard).
Scedosporium boydii strain HLPB/Clade 4 (isolated from eumycotic mycetoma) and Scedosporium apiospermum strain RK107-0417/Clade 5 were maintained in Potato Dextrose Broth (PDB; Acumedia) at room temperature with shaking. The fungal strains were supplied by Dr. Bodo Wanke, from the Instituto de Pesquisa Evandro Chagas, Fundação Oswaldo Cruz. To obtain conidial suspension, fungal cells were spread onto potato dextrose agar (PDA; Acumedia) and incubated at room temperature for 7 days. Further, phosphate buffered saline (PBS) 0.01 M pH 7.2 was added onto fungal growth, and conidia were harvested. After centrifugation (3.5 × 100 r.p.m. for 10 min), the pellet was washed with PBS 0.01 pH 7.2 twice, and conidia were counted with a hemocytometer.
Bacterial supernatant
To obtain cell-free supernatant from bacterial growth, cells were incubated as described above for 7 days. After that time, the content was filtered with a 0.22-μm membrane (DISMIC® - 13cp), dialyzed for 3 days in distilled water and lyophilized. Cell-free supernatants from bacterial growth were then analyzed for their contents of sugar and protein, using the phenol-sulfuric acid and Lowry methodologies, respectively [33, 34].
To evaluate the influence of different concentrations of protein present in the supernatants, 105 conidia were incubated in 96-well plates for 4 and 24 h at 37 °C with 25, 50, and 100 μg of protein from bacterial supernatant. Dried nutrient broth was used as a control to exclude any effect due to the high concentration of salts.
Cell-cell contact in liquid media
Conidia (105) and 100 μl of bacterial suspension (about 105 cells) were added in a 24-well plate containing nutrient broth. Positive fungal control was performed by adding 100 μl nutrient broth instead of bacterial suspension. The plate was incubated for 24 h at 37 °C. After incubation, each well was scraped with a cell scraper. The well content was homogenized and diluted (1:10) in PBS 0.01 M pH 7.2. The sample was treated with 1% of streptomycin-penicillin for 2 h at room temperature to kill bacteria (adapted from [21]). Fungal cell viability was determined by XTT reduction assay. Optical density was measured in a spectrophotometer at 490 nm [35, 36].
Cell-cell contact with non-viable bacteria
Bacterial pellet was treated with methanol for 2 h at room temperature. After methanol treatment, the pellet was washed with PBS 0.01 M pH 7.2 and added (about 105 bacterial cells) to a 24-well plate containing 500 μl nutrient broth and 105 conidia. Positive fungal control was performed by adding nutrient broth instead of bacterial suspension. The plate was incubated for 24 h at 37 °C [17].
After incubation, each well was scraped with a cell scraper. The well content was homogenized and diluted (1:10) in PBS 0.01 M pH 7.2. Fungal cell viability was determined by XTT reduction assay. Optical density was measured in a spectrophotometer at 490 nm [35, 36].
Cell-cell interaction through 0.22-μm membrane
A tube with semi-permeable membrane (Corning® Costar® Spin-X® centrifuge tube filters, Sigma-Aldrich) was used to avoid cell contact and allow only nutrient broth to pass through the membrane. Using this system, bacteria were grown at the bottom of the tube, whereas fungi were grown on top of the tube. Each empty basket was weighted before starting the experiment. At the bottom part of the tube, 250 μl bacterial suspension in nutrient broth (about 105 bacterial cell) or only nutrient broth as positive control (no bacteria) was added. The basket with semi-permeable membrane was introduced at the microtube, and 250 μl PDB containing 105 conidia was added. Then the tubes were incubated for 24 h at 37 °C. After incubation, the basket was dried in a 50 °C stove and then weighted again to determine the fungal dry weight (adapted from [20]).
Effect of bacterial supernatant on fungal viability
In a 96-well plate, equal volumes of PDB and bacterial supernatant were added in each well with 105 conidia, in a total volume of 200 μl. Nutrient broth was used as positive control instead of bacterial supernatant. The plate was incubated for 48 h at 37 °C. After incubation, the wells were washed with PBS 0.01 M pH 7.2 to remove non-adherent cells [37]. Fungal cell viability was determined by XTT reduction assay. Optical density was measured in a spectrophotometer at 490 nm [35, 36].
Effect of bacterial supernatant on preformed fungal biomass
Before exposure to bacterial supernatant, 105 fungal cells were incubated in a 96-well plate with PDB for 48 h at 37 °C. After 48 h (time to allow fungal cells to grow and adhere on the bottom of the wells), the plate was washed to remove non-adherent fungal cells, and then equal volumes of PDB and bacterial supernatant were added. Nutrient broth (no bacteria cells) was used as positive control instead of bacterial supernatant. The plate was again incubated for 48 h at 37 °C to allow the interaction between fungal biomass with the bacterial cell-free supernatants. After incubation, the wells were washed with PBS 0.01 M pH 7.2 [37]. Viability of the remaining fungal cells was determined by XTT reduction assay. Optical density was measured in a spectrophotometer at 490 nm [35, 36].
Statistics
All the experiments were performed, at least 3 times (biological replicates) with 3 technical replicates each time. Statistical analyses were done using GraphPad Prism version 8.00 for Mac OS X (GraphPad Software, San Diego, CA, USA). One-way analysis of variance using a Kruskal-Wallis nonparametric test was used to compare the differences between groups, and individual comparisons of groups were performed using a Bonferroni post-test. The 90–95% confidence interval was determined in all experiments.
Results
Fungal inhibition effect by bacteria species in liquid co-cultures
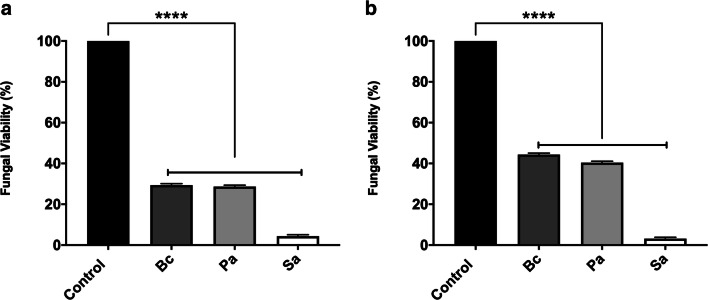
In order to evaluate fungal viability in a co-culture model, bacterial and fungal isolates were co-cultured in a 1:1 ratio in nutrient broth for 24 h at 37 °C. Then, the mixed bacterial–fungal suspension was treated with penicillin-streptomycin 1% to avoid any bacterial viability reading when XTT reduction assay was performed. All three bacteria species statistically (p < 0.0001) decreased both S. apiospermum (Fig. 1a) and S. boydii (Fig. 1b) viability at more than 50% compared to the control in which fungi were grown alone.
Fig. 1.
Fungal inhibition effect in the co-culture of S. apiospermum and S. boydii with B. cepacia, P. aeruginosa, and S. aureus. Bacterial and fungal isolates were co-cultured in a 1:1 ratio in nutrient broth for 24 h at 37 °C. After this time, the mixed co-cultures were treated with penicillin-streptomycin 1%, and fungal viability was measured by XTT, as showed for S. apiospermum (a) and S. boydii (b). Control means only the fungal growth, without bacteria cells. Bc, B. cepacia; Pa, P. aeruginosa; Sa, S. aureus. ****p < 0.0001
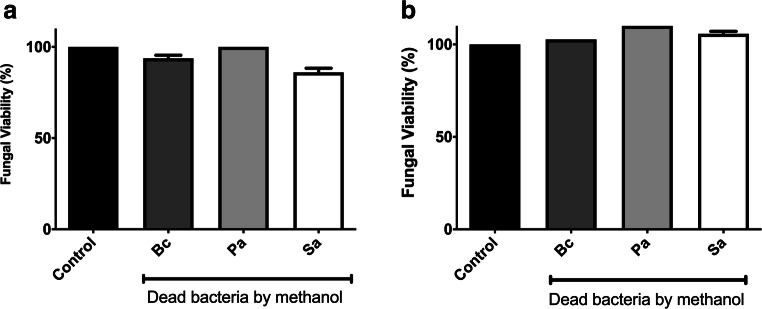
To analyze whether the effect on fungal viability was dependent on bacteria viability, B. cepacia, P. aeruginosa, and S. aureus were killed by methanol prior to the co-culture incubation with the fungal species. Fungi were grown alone as a positive control, which was considered 100% of metabolic activity. Methanol-killed bacteria did not decrease S. apiospermum (Fig. 2a) and S. boydii (Fig. 2b) viability, suggesting that fungal inhibition in co-culture conditions is dependent on the presence of viable bacterial cells.
Fig. 2.
S. apiospermum and S. boydii inhibitions are dependent on B. cepacia, P. aeruginosa, and S. aureus viabilities. B. cepacia, P. aeruginosa, and S. aureus were killed by methanol for 2 h at room temperature prior to the co-culture incubation with the fungal species. Fungal viability was measured after 24 h at 37 °C incubated with dead bacteria species by XTT, as showed for S. apiospermum (a) and S. boydii (b). Control means only the fungal growth, without bacteria cells. Bc, B. cepacia; Pa, P. aeruginosa; Sa, S. aureus
Indirect interactions between S. apiospermum and S. boydii with B. cepacia, P. aeruginosa, and S. aureus
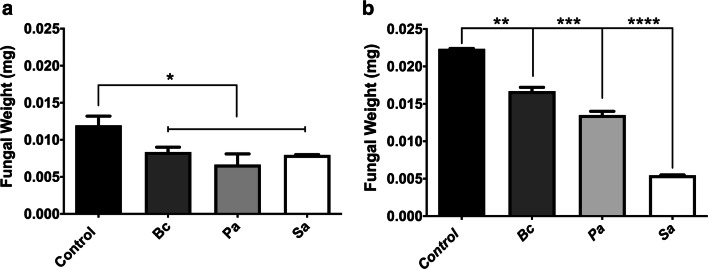
To investigate whether physical contact between bacteria and fungi is important to trigger fungal growth inhibition, co-cultures were performed in tubes with polycarbonate (semi-permeable) membranes to prevent direct contact between fungal and bacterial cells while allowing free exchange of nutrients and extracellular molecules between the organisms. S. apiospermum growth was inhibited when co-cultured with all three bacteria species (Fig. 3a), and the inhibition was more evident in S. boydii co-cultured with all three bacteria, specially S. aureus, according to the substantial reduction of fungal biomass (Fig. 3b) when compared to the control (with no co-culture with bacteria).
Fig. 3.
Non-physical interaction between S. apiospermum and S. boydii with B. cepacia, P. aeruginosa, and S. aureus separated by a filter semi-permeable membrane. Co-cultures were performed in tubes with polycarbonate membrane, and it was observed that direct contact between fungal and bacterial species during 24 h at 37 °C is not essential for fungal growth inhibition, as showed for S. apiospermum (a) and S. boydii (b). Control means only the fungal growth, without bacteria cells. Bc, B. cepacia; Pa, P. aeruginosa; Sa, S. aureus. p = 0.0332(*), 0.0021(**), 0.0002(***), < 0.0001(****)
Protein and sugar composition of the bacterial supernatants
In order to evaluate the chemical composition of the bacterial supernatants, protein and sugar contents were analyzed. It was observed that there is an increase of protein in all the bacterial supernatants in comparison to the control (only nutrient broth/no bacterial growth). Protein and sugar measurements showed that supernatants present higher protein content (60–80%) compared to sugar (approximately 10%). The sugar content was similar in all the conditions (Fig. 4).
Fig. 4.
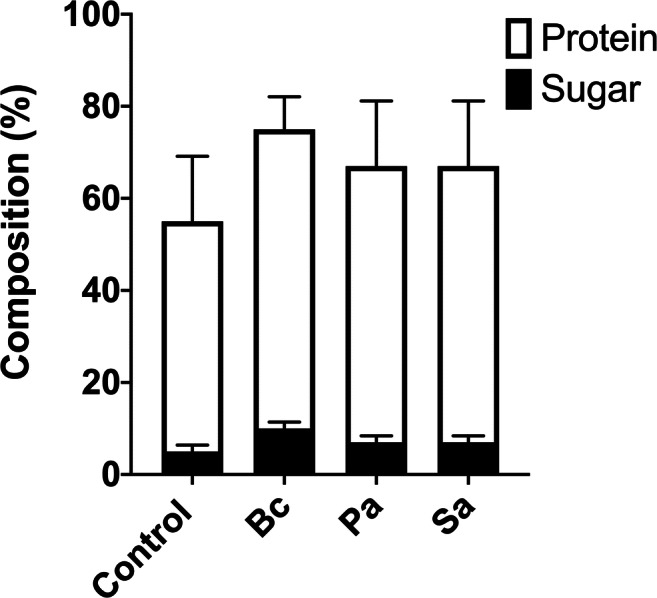
B. cepacia, P. aeruginosa, and S. aureus supernatants present a higher content of protein in comparison to sugar. Chemical characterization of the cell-free supernatants from bacterial growth were analyzed for their contents of sugar and protein (after the concentration and dialysis of each supernatant), showing an increase of protein in all the bacterial supernatants in comparison to the control, as well as a higher protein content compared to sugar. Control means only proteins from nutrient broth, without bacteria cells. Bc, B. cepacia; Pa, P. aeruginosa; Sa, S. aureus
Inhibition of S. apiospermum and S. boydii metabolic activity by bacterial supernatants
To understand the mechanism by which bacteria inhibit fungal viability, the effect of B. cepacia, P. aeruginosa, and S. aureus supernatants on S. apiospermum and S. boydii cells was screened. Supernatants were prepared as outlined in the Materials and Methods, and dried bacterial supernatants containing 25, 50, and 100 μg of protein were added to nutrient broth containing conidia of S. apiospermum or S. boydii.
All concentrations of P. aeruginosa proteins supernatants reduced both S. apiospermum and S. boydii viability after 4 and 24 h of interaction (Online Resource 5a-d). S. aureus supernatants decreased S. apiospermum viability in both times of interaction (Online Resource 5a-b), but similar results were only observed for S. boydii at the highest concentration tested after 4 h of interaction (Online Resource 5c-d). No inhibition on S. apiospermum and S. boydii by B. cepacia supernatants was observed at 4 h of interaction (Online Resource 5a and 5c), but some inhibitory effect could be observed at 100 μg of protein for S. apiospermum and 50 μg of protein for S. boydii at 24 h of interaction (Online Resource 5b and 5d).
Interference on the preformed fungal biomass by bacterial supernatants
Since the previous results showed a significant metabolic reduction of S. apiospermum and S. boydii planktonic conidia by the B. cepacia, P. aeruginosa, and S. aureus supernatants, it was evaluated whether this effect could happen when fungi are associated as adherent cells. Preformed biomass forming S. apiospermum displayed higher inhibition in the presence of all three bacterial supernatants (Online Resource 6a) when compared to S. boydii, which only a significant reduction was observed in the presence of S. aureus supernatant (Online Resource 6b).
Discussion
Patients who carry the CFTR mutation and present CF symptoms were expected to live only few months some decades ago. Now, due to the development of diagnostic and therapeutic approaches, they can reach 40 years old in some countries [38]. Although advances have been observed in the management of the disease, several studies have been developed aiming to increase life quality of CF patients. In this context, considering that pulmonary infections are one of the most important causes of CF mortality, researches have been focusing on the lung microbiome and the relations among species that compose the polymicrobial lung colonization [39].
Several studies have already evaluated the relationship between bacteria and fungi in the context of CF respiratory tract. One of the best characterized bacteria-fungi relation is that of P. aeruginosa and Aspergillus, Candida, and Scedosporium species, in which P. aeruginosa inhibit these fungi through the production of phenazine and other secreted molecules [19–21]. Considering that Scedosporium species are known to be the third more frequent fungi isolated from lungs of CF patients [7], the present study aimed to analyzed how S. apiospermum and S. boydii viability is influenced by three of the most frequent bacteria found in these patients, B. cepacia, P. aeruginosa, and S. aureus.
Our study found that B. cepacia, P. aeruginosa, and S. aureus decreased S. apiospermum and S. boydii growth/viability when a co-culture was performed in liquid media. Similar results were observed by Chen and colleagues, who demonstrated that P. aeruginosa reduced L. prolificans and S. aurantiacum growth when it was cultivated together with both fungi [21]. In addition, fluorescence microscopy using FUN-1 staining showed that L. prolificans and S. aurantiacum lose viability in the presence of P. aeruginosa [21]. Similar inhibitory results were also demonstrated in A. fumigatus when it is co-cultured with P. aeruginosa in liquid media [17], suggesting that this bacterium presents an antifungal effect against different fungi.
Since live bacteria led to fungal viability reduction in a co-culture system, we evaluated whether this effect is due to metabolic active bacterial cells or simply due to some cell structure component, such as protein and carbohydrate. When methanol-killed bacteria, which did not display active metabolism, were added to fungal culture, no effect was observed, because S. apiospermum and S. boydii grew at similar levels in controls and in the presence of killed bacteria, suggesting that inhibitory effect is only observed when bacteria is growing actively. P. aeruginosa also did not decrease S. boydii, S. apiospermum, and A. fumigatus when it was killed by methanol prior to co-culture [17, 21], indicating that bacterial cells need to be metabolically active to possess antifungal effect.
It is known that microorganisms secrete many molecules that play a variety of roles, including in competition with other species. Considering that B. cepacia, P. aeruginosa, and S. aureus reduced S. apiospermum and S. boydii growth only when their metabolism is active, we decided to check whether the cell-cell contact is essential for the inhibitory effect. When bacteria and fungi were both growing in the same media, but separated by a 0.22-μm membrane to avoid cell-cell contact, fungal mass decreased in the presence of all bacteria compared to the control, indicating that bacteria-fungi contact is not necessary for the antifungal effect displayed by B. cepacia, P. aeruginosa, and S. aureus. A. fumigatus and S. aurantiacum have been shown to be inhibited by P. aeruginosa when they are grown in a Transwell® system which impairs cell contact, corroborating with the results found in the present study [17, 20]. Similar results have been observed with Cryptococcus neoformans, which presented a 50% reduced CFU when grown in a U-shape tube in the presence of P. aeruginosa separated by a 0.22-μm membrane [40].
In the present work, we analyzed the bacterial supernatant content in order to check which types of molecules are found in bacterial supernatants as secondary metabolites after 7 days of incubation. Protein and sugar measurements showed that supernatants present higher protein content (60–80%) compared to sugar (approximately 10%). In this context, protein content was quantified in the cell-free supernatant, and it was used to check its antifungal activity in early (4 h) and longer time points (24 h). Although B. cepacia supernatant did not influence fungal viability at 4 h of incubation, all three bacterial supernatants reduced S. apiospermum and S. boydii viability after 24 h of incubation. In addition, supernatant from P. aeruginosa culture was the one presenting the highest antifungal effect, corroborating with the studies that have already demonstrated the presence of secreted secondary metabolites with antifungal properties in its culture supernatants. Interestingly, although cell-cell interaction showed that bacteria inhibit fungal viability after 24 h of incubation, bacterial supernatants continued presenting antifungal activity after longer time, as observed with supernatants obtained after 7 days of bacterial viability. However, as a limitation of our study, more studies are needed in order to characterize which molecules are found in these supernatants that are involved with the antifungal effect observed in our results.
Fungal biofilm inhibition by bacterial molecules has already been described [29, 41, 42]. It is interesting due to the fact that fungal biofilms are an important way to develop a more resistant infection in human. Aspergillus and Scedosporium species have already been described to form biofilm in abiotic and biotic surfaces [43–45]. In the present work, S. apiospermum adherent cells were decreased by supernatants from all three bacteria, but S. boydii adherent cells were only affected by S. aureus supernatants. However, more studies are needed to clarify the effect of B. cepacia, P. aeruginosa, and S. aureus supernatants on S. apiospermum and S. boydii biofilms. Ferreira and colleagues have already demonstrated that culture filtrates of P. aeruginosa, especially CF isolates, can inhibit A. fumigatus biofilms, and its inhibitory effect is enhanced when P. aeruginosa is grown as biofilm [28]. Granillo and colleagues have shown that when S. aureus and A. fumigatus form a mixed biofilm, S. aureus lead to scarce production of fungal biofilm and disorganized fungal structures, suggesting that it displays anti-biofilm activity against A. fumigatus [46].
The present study described that S. aureus, P. aeruginosa, and B. cepacia, three of the most isolated bacteria in CF patients’ lung, secrete metabolites found in the supernatant that play anti-S. apiospermum and anti-S. boydii roles. Considering the relevance of these bacteria and fungi for respiratory infections in CF patients, more studies are needed to increase the knowledge about the interaction between bacteria and Scedosporium species.
Supplementary information
S. apiospermum and S. boydii viability can be inhibited for different protein concentrations of B. cepacia, P. aeruginosa and S. aureus supernatants. Effect of different concentration of proteins (25, 50 and 100 ⌠g) in the B. cepacia, P. aeruginosa and S. aureus supernatants was analyzed in the viability of S. apiospermum (A-B) and S. boydii (C-D) at 37 °C during 4 h (A and C) and 24 h (B and D), respectively. Fungal growth was accessed according to the fungal viability by XTT. Control means only the fungal growth, without bacterial supernatants. Bc: B. cepacia, Pa: P. aeruginosa, Sa: S. aureus. p= 0.0332(*), 0.0021(**), 0.0002(***) (TIFF 1035 kb).
B. cepacia, P. aeruginosa and S. aureus supernatants are able to inhibit S. apiospermum preformed biomass. S. apiospermum and S. boydii conidia were incubated for 48 h at 37 °C to allow the growth and adherence of fungal cells. After this time, B. cepacia, P. aeruginosa and S. aureus supernatants were added on the top of the fungal biomass and incubated for 48 h at 37 °C, to allow the interaction between fungal biomass with the bacterial cell-free supernatants. All the three bacterial supernatants were able to inhibit the preformed biomass of S. apiosmermum (A), but only S. aureus supernatant was able to significantly inhibit preformed biomass of S. boydii (B). Control means only the fungal growth, without bacterial supernatants. Bc: B. cepacia, Pa: P. aeruginosa, Sa: S. aureus. p<0.0001(****) (TIFF 183 kb).
Acknowledgments
The authors would like to thank the support of CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnologico), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Authors’ contributions
Experimental procedures were performed by AJM, RRP, and MIDSX. Data analysis was done by AJM, RRP, MIDSX, ALSS, EBB, and LCLL. Manuscript writing was prepared by AJM, RRP, and LCLL. Manuscript revision was performed by ALSS, EBB, and LCLL.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent of participation
All authors consent to participate in this publication.
Consent for publication
All authors consent to publish the manuscript.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Andressa de Jesus Marques and Rodrigo Rollin-Pinheiro contributed equally to this work.
References
- 1.King J, Brunel SF, Warris A. Aspergillus infections in cystic fibrosis. J Inf Secur. 2016;72(Suppl):S50–S55. doi: 10.1016/j.jinf.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Ciofu O, Hansen CR, Hoiby N. Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med. 2013;19(3):251–258. doi: 10.1097/MCP.0b013e32835f1afc. [DOI] [PubMed] [Google Scholar]
- 3.Vandeplassche E, Tavernier S, Coenye T, Crabbe A. Influence of the lung microbiome on antibiotic susceptibility of cystic fibrosis pathogens. Eur Respir Rev. 2019;28(152):190041. doi: 10.1183/16000617.0041-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenna DTD, Lilley D, Coward A, Martin K, Perry C, Pike R, Hill R, Turton JF. Prevalence of Burkholderia species, including members of Burkholderia cepacia complex, among UK cystic and non-cystic fibrosis patients. J Med Microbiol. 2017;66(4):490–501. doi: 10.1099/jmm.0.000458. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard AC, Waters VJ. Microbiology of cystic fibrosis airway disease. Semin Respir Crit Care Med. 2019;40(6):727–736. doi: 10.1055/s-0039-1698464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Brazilian Patient Registry Cystic Fibrosis (2017) REBRAFC
- 7.Engel TGP, Slabbers L, de Jong C, Melchers WJG, Hagen F, Verweij PE, Merkus P, Meis JF, Dutch Cystic Fibrosis Fungal Collection C Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients - a Dutch, multicentre study. J Cyst Fibros. 2019;18(2):221–226. doi: 10.1016/j.jcf.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Bouchara JP, Le Govic Y, Kabbara S, Cimon B, Zouhair R, Hamze M, Papon N, Nevez G. Advances in understanding and managing Scedosporium respiratory infections in patients with cystic fibrosis. Expert Rev Respir Med. 2020;14(3):259–273. doi: 10.1080/17476348.2020.1705787. [DOI] [PubMed] [Google Scholar]
- 9.Cimon B, Carrere J, Vinatier JF, Chazalette JP, Chabasse D, Bouchara JP. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2000;19(1):53–56. doi: 10.1007/s100960050011. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz C, Brandt C, Melichar V, Runge C, Heuer E, Sahly H, Schebek M, Koster H, Bouchara JP, Biedermann T, Meissner P, Grosse-Onnebrink J, Skopnik H, Hartl D, Sedlacek L, Tintelnot K. Combined antifungal therapy is superior to monotherapy in pulmonary scedosporiosis in cystic fibrosis. J Cyst Fibros. 2019;18(2):227–232. doi: 10.1016/j.jcf.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Lackner M, de Hoog GS, Verweij PE, Najafzadeh MJ, Curfs-Breuker I, Klaassen CH, Meis JF. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother. 2012;56(5):2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo AJ, Guarro J. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob Agents Chemother. 2001;45(7):2151–2153. doi: 10.1128/AAC.45.7.2151-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuenca-Estrella M, Ruiz-Diez B, Martinez-Suarez JV, Monzon A, Rodriguez-Tudela JL. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999;43(1):149–151. doi: 10.1093/jac/43.1.149. [DOI] [PubMed] [Google Scholar]
- 14.Lelievre B, Legras P, Godon C, Franconi F, Saint-Andre JP, Bouchara JP, Diquet B. Experimental models of disseminated scedosporiosis with cerebral involvement. J Pharmacol Exp Ther. 2013;345(2):198–205. doi: 10.1124/jpet.112.201541. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez MM, Pastor FJ, Salas V, Calvo E, Mayayo E, Guarro J. Experimental murine scedosporiosis: histopathology and azole treatment. Antimicrob Agents Chemother. 2010;54(9):3980–3984. doi: 10.1128/AAC.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delhaes L, Monchy S, Frealle E, Hubans C, Salleron J, Leroy S, Prevotat A, Wallet F, Wallaert B, Dei-Cas E, Sime-Ngando T, Chabe M, Viscogliosi E. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community--implications for therapeutic management. PLoS One. 2012;7(4):e36313. doi: 10.1371/journal.pone.0036313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mowat E, Rajendran R, Williams C, McCulloch E, Jones B, Lang S, Ramage G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol Lett. 2010;313(2):96–102. doi: 10.1111/j.1574-6968.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 18.Gibson J, Sood A, Hogan DA. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl Environ Microbiol. 2009;75(2):504–513. doi: 10.1128/AEM.01037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee P, Sass G, Swietnicki W, Stevens DA (2020) Review of potential Pseudomonas weaponry, relevant to the Pseudomonas-Aspergillus interplay, for the mycology community. J Fungi (Basel) 6(2). 10.3390/jof6020081 [DOI] [PMC free article] [PubMed]
- 20.Kaur J, Pethani BP, Kumar S, Kim M, Sunna A, Kautto L, Penesyan A, Paulsen IT, Nevalainen H. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front Microbiol. 2015;6:866. doi: 10.3389/fmicb.2015.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen SC, Patel S, Meyer W, Chapman B, Yu H, Byth K, Middleton PG, Nevalainen H, Sorrell TC. Pseudomonas aeruginosa inhibits the growth of Scedosporium and Lomentospora in vitro. Mycopathologia. 2018;183(1):251–261. doi: 10.1007/s11046-017-0140-x. [DOI] [PubMed] [Google Scholar]
- 22.Lightly TJ, Phung RR, Sorensen JL, Cardona ST. Synthetic cystic fibrosis sputum medium diminishes Burkholderia cenocepacia antifungal activity against Aspergillus fumigatus independently of phenylacetic acid production. Can J Microbiol. 2017;63(5):427–438. doi: 10.1139/cjm-2016-0705. [DOI] [PubMed] [Google Scholar]
- 23.Yang DL, Hu YL, Yin ZX, Zeng GS, Li D, Zhang YQ, Xu ZH, Guan XM, Weng LX, Wang LH. Cis-2-dodecenoic acid mediates its synergistic effect with Triazoles by interfering with efflux pumps in fluconazole-resistant Candida albicans. Biomed Environ Sci. 2019;32(3):199–209. doi: 10.3967/bes2019.027. [DOI] [PubMed] [Google Scholar]
- 24.Mao S, Lee SJ, Hwangbo H, Kim YW, Park KH, Cha GS, Park RD, Kim KY. Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr Microbiol. 2006;53(5):358–364. doi: 10.1007/s00284-005-0333-2. [DOI] [PubMed] [Google Scholar]
- 25.Kilani-Feki O, Zouari I, Culioli G, Ortalo-Magne A, Zouari N, Blache Y, Jaoua S. Correlation between synthesis variation of 2-alkylquinolones and the antifungal activity of a Burkholderia cepacia strain collection. World J Microbiol Biotechnol. 2012;28(1):275–281. doi: 10.1007/s11274-011-0817-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang XQ, Liu AX, Guerrero A, Liu J, Yu XQ, Deng P, Ma L, Baird SM, Smith L, Li XD, Lu SE. Occidiofungin is an important component responsible for the antifungal activity of Burkholderia pyrrocinia strain Lyc2. J Appl Microbiol. 2016;120(3):607–618. doi: 10.1111/jam.13036. [DOI] [PubMed] [Google Scholar]
- 27.Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV, Groleau MC, Dietl AM, Visca P, Haas H, Deziel E, Stevens DA. Aspergillus-Pseudomonas interaction, relevant to competition in airways. Med Mycol. 2019;57(Supplement_2):S228–S232. doi: 10.1093/mmy/myy087. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira JA, Penner JC, Moss RB, Haagensen JA, Clemons KV, Spormann AM, Nazik H, Cohen K, Banaei N, Carolino E, Stevens DA. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS One. 2015;10(8):e0134692. doi: 10.1371/journal.pone.0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sass G, Nazik H, Penner J, Shah H, Ansari SR, Clemons KV, Groleau MC, Dietl AM, Visca P, Haas H, Deziel E, Stevens DA (2018) Studies of Pseudomonas aeruginosa mutants indicate Pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J Bacteriol 200(1). 10.1128/JB.00345-17 [DOI] [PMC free article] [PubMed]
- 30.Nazik H, Sass G, Ansari SR, Ertekin R, Haas H, Deziel E, Stevens DA. Novel intermicrobial molecular interaction: Pseudomonas aeruginosa Quinolone Signal (PQS) modulates Aspergillus fumigatus response to iron. Microbiology (Reading) 2020;166(1):44–55. doi: 10.1099/mic.0.000858. [DOI] [PubMed] [Google Scholar]
- 31.Bredenbruch F, Geffers R, Nimtz M, Buer J, Haussler S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ Microbiol. 2006;8(8):1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- 32.Briard B, Rasoldier V, Bomme P, ElAouad N, Guerreiro C, Chassagne P, Muszkieta L, Latge JP, Mulard L, Beauvais A. Dirhamnolipids secreted from Pseudomonas aeruginosa modify anjpegungal susceptibility of Aspergillus fumigatus by inhibiting beta1,3 glucan synthase activity. ISME J. 2017;11(7):1578–1591. doi: 10.1038/ismej.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168(4265):167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 35.Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly JP, Verweij PE, Network E. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol. 2001;39(9):3402–3408. doi: 10.1128/jcm.39.9.3402-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loures FV, Levitz SM (2015) XTT assay of antifungal activity. Bio Protoc 5(15). 10.21769/bioprotoc.1543 [DOI] [PMC free article] [PubMed]
- 37.Homa M, Sandor A, Toth E, Szebenyi C, Nagy G, Vagvolgyi C, Papp T. In vitro interactions of Pseudomonas aeruginosa with Scedosporium species frequently associated with cystic fibrosis. Front Microbiol. 2019;10:441. doi: 10.3389/fmicb.2019.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 39.Rabin HR, Surette MG. The cystic fibrosis airway microbiome. Curr Opin Pulm Med. 2012;18(6):622–627. doi: 10.1097/MCP.0b013e328358d49a. [DOI] [PubMed] [Google Scholar]
- 40.Rella A, Yang MW, Gruber J, Montagna MT, Luberto C, Zhang YM, Del Poeta M. Pseudomonas aeruginosa inhibits the growth of Cryptococcus species. Mycopathologia. 2012;173(5–6):451–461. doi: 10.1007/s11046-011-9494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nazik H, Penner JC, Ferreira JA, Haagensen JA, Cohen K, Spormann AM, Martinez M, Chen V, Hsu JL, Clemons KV, Stevens DA. Erratum for Nazik et al., effects of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob Agents Chemother. 2015;59(11):7160. doi: 10.1128/AAC.02353-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazik H, Penner JC, Ferreira JA, Haagensen JA, Cohen K, Spormann AM, Martinez M, Chen V, Hsu JL, Clemons KV, Stevens DA. Effects of iron chelators on the formation and development of Aspergillus fumigatus biofilm. Antimicrob Agents Chemother. 2015;59(10):6514–6520. doi: 10.1128/AAC.01684-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Ramirez AI, Ramirez-Granillo A, Medina-Canales MG, Rodriguez-Tovar AV, Martinez-Rivera MA. Analysis and description of the stages of Aspergillus fumigatus biofilm formation using scanning electron microscopy. BMC Microbiol. 2016;16(1):243. doi: 10.1186/s12866-016-0859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mello TP, Aor AC, Goncalves DS, Seabra SH, Branquinha MH, Santos AL. Assessment of biofilm formation by Scedosporium apiospermum, S. aurantiacum, S. minutisporum and Lomentospora prolificans. Biofouling. 2016;32(7):737–749. doi: 10.1080/08927014.2016.1192610. [DOI] [PubMed] [Google Scholar]
- 45.Rollin-Pinheiro R, de Meirelles JV, Vila TVM, Fonseca BB, Alves V, Frases S, Rozental S, Barreto-Bergter E. Biofilm formation by Pseudallescheria/Scedosporium species: a comparative study. Front Microbiol. 2017;8:1568. doi: 10.3389/fmicb.2017.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez Granillo A, Canales MG, Espindola ME, Martinez Rivera MA, de Lucio VM, Tovar AV. Antibiosis interaction of Staphylococcus aureus on Aspergillus fumigatus assessed in vitro by mixed biofilm formation. BMC Microbiol. 2015;15:33. doi: 10.1186/s12866-015-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. apiospermum and S. boydii viability can be inhibited for different protein concentrations of B. cepacia, P. aeruginosa and S. aureus supernatants. Effect of different concentration of proteins (25, 50 and 100 ⌠g) in the B. cepacia, P. aeruginosa and S. aureus supernatants was analyzed in the viability of S. apiospermum (A-B) and S. boydii (C-D) at 37 °C during 4 h (A and C) and 24 h (B and D), respectively. Fungal growth was accessed according to the fungal viability by XTT. Control means only the fungal growth, without bacterial supernatants. Bc: B. cepacia, Pa: P. aeruginosa, Sa: S. aureus. p= 0.0332(*), 0.0021(**), 0.0002(***) (TIFF 1035 kb).
B. cepacia, P. aeruginosa and S. aureus supernatants are able to inhibit S. apiospermum preformed biomass. S. apiospermum and S. boydii conidia were incubated for 48 h at 37 °C to allow the growth and adherence of fungal cells. After this time, B. cepacia, P. aeruginosa and S. aureus supernatants were added on the top of the fungal biomass and incubated for 48 h at 37 °C, to allow the interaction between fungal biomass with the bacterial cell-free supernatants. All the three bacterial supernatants were able to inhibit the preformed biomass of S. apiosmermum (A), but only S. aureus supernatant was able to significantly inhibit preformed biomass of S. boydii (B). Control means only the fungal growth, without bacterial supernatants. Bc: B. cepacia, Pa: P. aeruginosa, Sa: S. aureus. p<0.0001(****) (TIFF 183 kb).
Data Availability Statement
Not applicable.