Abstract
Sporotrichosis in immunocompromised patients has a high morbidity and may cause deaths. Particularly, patients with acquired immunodeficiency syndrome (AIDS) with low T CD4 counts develop a chronic disease, with severe and widespread forms. Recently, the ability of Sporothrix brasiliensis, the main agent of zoonotic sporotrichosis, to increase its virulence in a diabetic patient without HIV infection was described. Since it was a unique finding, it is not known how often this occurs in patients with chronic and refractory sporotrichosis. The aim of this study is to compare sequential Sporothrix isolates obtained from patients with sporotrichosis and AIDS in order to detect changes in virulence-related phenotypes and acquisition of antifungal resistance during the evolution of the disease. Fungal growth in different substrates, antifungal susceptibility, thermotolerance, resistance to oxidative stress, and production of hydrolytic enzymes were evaluated. Correlations were assessed between clinical and phenotypic variables. Sixteen isolates, all identified as S. brasiliensis, obtained from five patients were studied. They grew well on glucose and N-acetyl-D-glucosamine, but poorly on lactate. Except from isolates collected from two patients, which were non-wild type for terbinafine, they were considered wild type for the antifungal drugs tested. Thermotolerance of the isolates was moderate to high. Except for phytase and phospholipase, isolates were able to produce virulence-related enzymes on different levels. Changes in all studied phenotypes were observed during the course of the disease in some patients. The results show that the HIV-driven immunosuppression is more relevant than fungal phenotypes on the unfavorable outcomes of disseminated sporotrichosis.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00297-y) contains supplementary material, which is available to authorized users.
Keywords: Sporotrichosis, Sporothrix brasiliensis, AIDS, Virulence
Introduction
Sporotrichosis is a worldwide mycosis of humans and other mammals caused by some species of the genus Sporothrix [1–3]. These species include Sporothrix schenckii, Sporothrix globosa, Sporothrix brasiliensis, Sporothrix luriei, Sporothrix pallida, Sporothrix mexicana, and Sporothrix chilensis [4, 5]. All Sporothrix species are saprobiotic fungi of living or decaying organic matter that occur mainly in tropical and subtropical areas. They are found abundantly in moss, hay, and rose thorns, among other substrates. These substrates are classically indicated as important transmission sources of these fungi [2, 6].
The history of sporotrichosis is constantly changing, from the transmission route to its geographical distribution. Environmental factors, urbanization, and the evolution in diagnosis methodologies partially explain the changes in disease profile [6]. In the late 1990s, sporotrichosis began to have relevance in Brazil, when domestic cats were identified as transmitters of this mycosis [6]. The zoonotic sporotrichosis in Brazil is caused mainly by S. brasiliensis and its occurrence is spreading across the country [7] and among neighboring countries [8].
Sporotrichosis has a wide range of clinical manifestations [5]. The fungal virulence and the host immunity are factors that can influence the occurrence of different clinical forms of the disease [9]. Virulence factors allow the fungus to survive and reproduce within the host. The mechanism by which human pathogenic species of Sporothrix acquire and maintain their virulence is not well understood, since they do not necessarily need an animal host in their life cycle. It is believed that their virulence may be related to environmental survival traits of the fungi in the intermicrobial interactions established in their habitat [10, 11]. In addition, interactions with host cells may also improve S. brasiliensis virulence [9].
Although sporotrichosis is a benign disease in most immunocompetent patients, it has high morbidity and may be fatal in immunocompromised individuals. In particular, patients with acquired immunodeficiency syndrome (AIDS) with a T CD4 cell count less than 200 cells/μl have a chronic mycosis course, characterized by severe, widespread forms and sometimes involvement of the central nervous system. HIV co-infection has a negative impact on the evolution of sporotrichosis [12]. Recently, the incidence of sporotrichosis among people living with HIV/AIDS has increased in Brazil, unlike other classic opportunistic mycoses, such as histoplasmosis and cryptococcosis [13]. The first case of sporotrichosis and HIV co-infection diagnosed at the Evandro Chagas National Institute of Infectious Diseases (INI), Oswaldo Cruz Foundation (Fiocruz), in 1999, coincides with the emergence of sporotrichosis as a public health issue in Brazil [14]. Since then, the increase in the number of patients with this co-infection has been approximately proportional to the overall increase in sporotrichosis cases over time [7].
It has been described that S. brasiliensis can increase its virulence in vivo, as demonstrated in a patient with disseminated sporotrichosis of 11 years of evolution. Fungal isolates from different sites, collected during the last 6 years of disease, presented no significant genetic variation. However, in in vivo assays, using the experimental infection model of Galleria mellonella larvae, a higher virulence of the last isolate was observed. The authors attributed this phenotypic differential to an ability to evade the immune system while maintaining infection throughout the years. This study is considered unique as it demonstrates the evolution of S. brasiliensis virulence in a host with disseminated and chronic sporotrichosis but without HIV co-infection [9]. Thus, considering that AIDS modifies the natural history of sporotrichosis and that S. brasiliensis is able to modify its virulence during infection, a similar scenario may occur in patients with chronic widespread sporotrichosis and AIDS. The study of clinical aspects and virulence-related phenotypes of the fungus will permit to clarify issues related to its biology and pathogenesis.
The aim of this study is to compare sequential isolates of S. brasiliensis from patients with sporotrichosis and AIDS in order to detect possible increases in virulence and acquisition of antifungal resistance during the chronic course of the disease. In addition, possible associations between virulence-related phenotypes and host immunity were assessed, to check whether the immune response affects S. brasiliensis phenotype expression and the significance of host immunity and fungal virulence on unfavorable outcomes of disseminated sporotrichosis associated with AIDS.
Material and methods
Patients
This study was approved by the Research Ethics Committee of INI/Fiocruz (CAAE: 03955818.8.0000.5262). A screening was performed in the database of the Clinical Research Laboratory in Infectious Dermatology, to collect data from patients followed up at INI from 1999 to 2018, diagnosed with HIV infection, and disseminated sporotrichosis. From this list, the electronic patient record system was used to verify if their records contained the following information: (i) mycological diagnosis of sporotrichosis, with at least three isolates of Sporothrix spp. obtained in culture in a minimum interval of 1 year; (ii) diagnosis of HIV infection, according to the recommendations of the Brazilian Ministry of Health; and (iii) chronic sporotrichosis, defined by the authors as a minimum of 1 year of specific treatment without cure. Patients that fulfilled these three criteria were included. Patients whose fungal strains were not viable at the time of the study were excluded.
Patient data
The variables of interest retrieved from the medical records of the patients included the following: (i) sociodemographic factors (age, gender, municipality of residence, and occupation), (ii) epidemiological factors (contact with cat, soil or plant, and history of trauma), (iii) clinical data (viral load, T CD4 and T CD8 cell counts, year of sporotrichosis diagnosis, year of HIV infection diagnosis, time of sporotrichosis evolution, clinical form of sporotrichosis, other comorbidities, type and duration of antifungal treatment), and (iv) mycological data (date and site of Sporothrix isolation as well as specific anti-Sporothrix IgG antibodies). Viral load, T CD4, and T CD8 cell counts were performed according to the guidelines of the Brazilian Ministry of Health [15]. Isolation of Sporothrix spp. in culture was performed by conventional diagnostic methodologies [5] and detection of specific anti-Sporothrix IgG antibodies was performed using an indirect ELISA against exoantigens from the mycelial form of S. brasiliensis [16].
Fungal reference strains
The strains S. brasiliensis IPEC 16490, S. schenckii IPEC 36275, and S. globosa IPEC 27135, previously characterized through partial calmodulin gene sequencing, were used as controls for molecular identification. The strains Candida albicans ATCC 18804 and Cryptococcus neoformans H99 were used as controls for tests of virulence-related phenotypes. For antifungal susceptibility tests, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, Aspergillus flavus ATCC 204305, and Aspergillus fumigatus ATCC 204304 strains were used as controls.
Fungal clinical strains
To carry out the below described methodologies, at least three clinical isolates from the patients who met the inclusion/exclusion criteria described above were used. In our institution, all Sporothrix spp. strains obtained from patients with sporotrichosis and HIV infection are maintained by freezing at − 20 °C in skim milk just after fungal isolation from the clinical material. Strains were recovered from freezing before the experiments and checked for viability on potato dextrose agar (Difco, Becton-Dickinson and Company, USA) at 25 °C and on brain heart infusion agar (Difco, Becton-Dickinson and Company, USA) at 35 °C. The mycelial and yeast-like forms of the fungus were maintained through subcultures on those media, every 7 days. No more than four subcultures were made with the strains, to preserve their virulence traits. If more subcultures were needed, a new vial from the − 20 °C freezer was used to recover the fungus.
Molecular identification
A polymerase chain reaction (PCR) targeting the calmodulin gene was performed for species identification of the clinical strains. This PCR uses specific primer pairs for the pathogenic species of the genus Sporothrix [17]. Specific primers for the three main etiological agents of sporotrichosis were used for identification: S. brasiliensis Sbra-F 5′-CCCCCGTTTGACGCTTGG-3′ and Sbra-R 5′-CCCGGATAACCGTGTGTCATAAT-3′; S. schenckii Ssch-F 5′-TTTCGAATGCGTTCGGCTGG-3′ and Ssch-R 5′-CTCCAGATCACCGTGTCA-3′; S. globosa Sglo-F 5′-CGCCTAGGCCAGATCACCACTAAG-3′ and Sglo-R 5′-CCAATGTCTACCCGTGCT-3′. PCR conditions were those described previously. For the visualization of the amplified DNA fragment, a 1% agarose gel electrophoresis was performed at 100 V for approximately 1 h. The DNA bands were stained with ethidium bromide (0.5 μg/ml) and visualized under 280 nm ultraviolet light. Band sizes were estimated according to the 1 kb plus molecular weight standard (Invitrogen, Thermo Fisher Scientific, Lithuania).
Growth curves
The growth of all clinical isolates in the presence of glucose, lactate, and N-acetyl-D-glucosamine was evaluated. An initial inoculum of 1 × 106 yeasts/ml was prepared on yeast nitrogen base medium (Difco, Becton-Dickinson and Company, USA) supplemented with 0.5% of each abovementioned carbon source (Difco, Becton-Dickinson and Company, USA). Aliquots of 100 μl were inoculated in triplicate on flat-bottom 96-well plates (Jet Biofil, Guangdong, China) and incubated at 35 °C for 7 days. The optical density of the wells at 530 nm was measured daily during the incubation period for the construction of growth curves.
Antifungal susceptibility
Amphotericin B, itraconazole, posaconazole, and terbinafine, all from Sigma-Aldrich (St. Louis, MO, USA), were tested. Fungal clinical isolates included in this study were submitted to the broth microdilution test following the protocols recommended by the Clinical and Laboratory Standard Institute (CLSI): M38-A2 [18] for the mycelial form of the fungi and M27-A3 [19] for the yeast form, with a few modifications. In order to obtain the inoculum of the isolates, cultures incubated at 35 °C for 7 days were made in potato dextrose agar (PDA) and brain heart infusion (BHI) media, to obtain the filamentous and yeast inoculum, respectively. Then, a cell suspension of each strain in their different morphologies was made at a McFarland scale standard 0.5, which yields a final concentration of 0.4–5.0 × 104 colony-forming units per milliliter (CFU/ml). The minimal inhibitory concentration (MIC) was determined visually. MICs from the mycelial forms were interpreted according to the epidemiological cutoff values (ECV) previously described [20]. At least two independent experiments were performed for each fungal morphology to confirm MIC determinations.
Thermotolerance determination
Clinical isolates were grown on PDA medium for 7 days at 25 °C. An inoculum of 1 × 106 conidia/ml of each isolate was prepared. Then, 5 μl aliquots of the suspensions were inoculated in triplicate into two Petri dishes (90 mm diameter) containing PDA medium. One culture was incubated at 25 °C and the other at 35 °C for a total of 7 days. At the end of the incubation period, the diameter of the colonies was measured in millimeters with the aid of a ruler and the growth inhibition percentage (% GI) was calculated as described [21]. Three replicates from three independent experiments served to calculate means and standard deviations of each isolate.
Resistance to nitrosative stress
Seven-day-old yeast cultures of each clinical isolate were washed three times on phosphate-buffered saline (PBS) solution. A suspension of 1 × 106 yeasts/ml was prepared on a nitrogen oxidant solution (0.5 mM NaNO2, 25 mM succinic acid, pH 4.0) as described [22]. Controls were prepared on PBS. After 3 h of incubation at 35 °C, the CFU/ml was determined for test and control conditions of each isolate. The percent survival of the isolates was determined as the ratio of the CFU/ml count on the nitrogen oxidant solution and the CFU/ml count of the control PBS solution. This experiment was performed three times and the results are expressed as mean and standard deviations of the replicates.
Resistance to oxidative stress
The sensitivities of the clinical isolates to hydrogen peroxide were measured by an agar plate diffusion assay, in which yeast cells are mixed with warm BHI medium to a final concentration of 1 × 106 yeasts/ml of culture medium. After the solidification of the agar in Petri dishes (150 mm diameter), four holes of approximately 5 mm diameter were punched in each plate, and 65 μl of hydrogen peroxide (Proquimios, Rio de Janeiro, RJ, Brazil) was added. Plates were incubated at 35 °C and inhibition zones of growth were measured 7 days after incubation. Four replicates from three independent experiments were used for the calculation of means and standard deviations.
Urease production
A volume of 200 μl of a 7-day-old fungal cell suspension (1 × 106 yeasts/ml) was diluted to a final volume of 2 ml Christensen urea broth (0.1% peptone, 0.5% NaCl, 0.2% KH2PO4, 2% urea, 0.1% glucose, 0.0016% phenol red). The yeast cell suspensions in Christensen urea broth were incubated at 35 °C. After 7 days, centrifugation was performed at 10,000g for 10 min and 100 μl of the supernatant was transferred in triplicate to a flat-bottom 96-well polystyrene plate. The absorbance of the samples was obtained by spectrophotometry at a wavelength of 559 nm (Epoch microplate spectrophotometer, BioTek Equipments, Winooski, VT, USA) as described [23]. Three replicates from two independent experiments were used to calculate means and standard deviations.
Aspartic protease production
The activity of this enzyme was evaluated on an albumin agar medium that is composed by yeast carbon base medium (Difco, Becton-Dickinson and Company, USA) supplemented with 0.2% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and 1.5% agar (Difco, Becton-Dickinson and Company, USA). Five microliters of a 1 × 106 yeasts/ml suspension was inoculated in triplicate on the surface of the culture medium in 90 mm diameter Petri dishes, which were incubated at 35 °C for 7 days. Positive samples present a halo around their colonies corresponding to the degradation of albumin. After the incubation time, both fungal colony and halo diameters were measured and the Pz value was calculated as described [24]. Experiments were repeated three times.
Hemolytic activity
Commercial Petri dishes containing sheep blood agar medium (PlastLabor, Rio de Janeiro, RJ, Brazil) were used to inoculate 5 μl of a 7-day-old cell suspension (1 × 106 yeasts/ml) in triplicate. Plates were incubated at 35 °C for 7 days. Positive samples present a translucent halo corresponding to the degradation of hematocytes around fungal colonies. After incubation, both fungal colonies and halo diameters were measured and the Pz value was calculated as described [24]. Experiments were repeated three times.
Phytase activity
The activity of myo-inositol hexakisphosphate phosphohydrolase, also known as phytase, was evaluated on a medium composed of the following ingredients: 1% glucose, 0.05% (NH4)2SO4, 0.02% KCl, 0.01% MgSO4·7 H2O, 0.0005% MnSO4, 0.0005% FeSO4, 0.2% calcium phytate (all from Sigma-Aldrich, St. Louis, USA), 0.05% yeast extract, and 1.5% agar (both from Difco, Becton-Dickinson and Company, USA), pH 7.0, as previously described [25]. The same inoculum and incubation conditions described above were used. Positive samples present a halo around the fungal colony corresponding to the solubilization of the organic phosphorus by the phytase activity. After incubation, both fungal colonies and halo diameters were measured and the Pz value was calculated as described [24]. Experiments were performed in triplicate.
Phospholipase activity
An egg yolk agar medium (2% glucose, 1% peptone, 0.5% yeast extract, 4% NaCl, 0.074% CaCl2, 8% organic egg yolk, 1.5% agar) was used in this set of experiments [24]. On the surface of the medium, 5 μl of a 7-day-old cell suspension (1 × 106 yeasts/ml) was inoculated in triplicate. Plates were incubated at 35 °C for 7 days. Positive samples present a dense white precipitation halo around the colonies corresponding to the formation of a complex between calcium and the fatty acids released after the fungal phospholipase action on phospholipids present in the egg yolk [26]. Both fungal colonies and halo diameters were measured after the incubation of the cultures and the Pz value was calculated as described [24]. Experiments were repeated three times.
Esterase activity
The activity of this enzyme was evaluated using the Tween agar medium (1% peptone, 0.5% yeast extract, 0.01% CaCl2, 0.1% Tween 80, 1.5% agar, pH 7.0) as described [27]. Inoculum and incubation conditions were the same as described in the previous experiments for enzymatic activity determination. Positive esterase fungi present a precipitation halo around the colonies correspondent to the binding between the calcium ions incorporated into the culture medium and the fatty acids released by the activity of the enzyme on the Tween. After the incubation of cultures at 35 °C for 7 days, both fungal colony and halo diameters were measured and the Pz value was determined as described [24]. Three Pz measurements were performed in two independent experiments.
Statistical analyses
The software GraphPad 7.0 was used for data analysis. Non-parametric tests such as Kruskal-Wallis, Mann-Whitney, and Wilcoxon tests were used to compare medians between different groups. Correlation between numeric variables was assessed using Spearman’s rank correlation test. The Spearman correlation coefficients of the different analyses were plotted in a heatmap format. A significance level of 0.05 was used in the statistical analyses.
Results
Patient information
Eighty-one patients with sporotrichosis and AIDS co-infection were found. Sixty-eight of them were excluded because of failure to fulfill all inclusion criteria. From the 13 remaining patients, eight were further excluded because their fungal samples were not viable at the time of the virulence-related phenotype studies. Therefore, five patients were included in this study. They had similar histories regarding antiretroviral and antifungal treatment abandonment, multiple hospitalizations, improvement of the general condition during hospitalization, and marked worsening after a few months of hospital discharge. All underwent therapeutic regimens involving amphotericin B, itraconazole, or terbinafine. All but one patient (patient 2) presented undetectable levels of anti-Sporothrix IgG antibodies in serum. Four patients died. Patient 1 died due to respiratory failure secondary to cryptococcosis, sporotrichosis, and advanced AIDS. Patient 3 became depressed, abandoned treatment for both sporotrichosis and AIDS, fractured the femur, and died after a bacterial sepsis. Patient 4 died during the course of disseminated sporotrichosis, also presented lung destruction due to a concomitant multi drug-resistant tuberculosis. Patient 5 died suddenly after neurological complications due to sequelae of meningoencephalitis caused by sporotrichosis. The clinical history of one of these patients is described in detail in a previous publication of our group [28]. Demographic and epidemiological characteristics of the included patients are described in Table 1. Clinical characteristics associated with the HIV infection are presented in Table 2, with the date of Sporothrix isolation.
Table 1.
Clinical and epidemiological characteristics of the five patients with chronic sporotrichosis and acquired immunodeficiency syndrome included in this study
| Patient | Sex | Age at sporotrichosis diagnosis | Municipality | Occupation | Year HIV diagnosis | Year sporotrichosis diagnosis | Sporotrichosis acquisition | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 45 | Nova Iguaçu | Bricklayer | 2011 | 2011 | Contact with cats | Deceased (2013) |
| 2 | Male | 37 | Rio de Janeiro | Hair stylist | 2011 | 2011 | Cat bite | Under treatment |
| 3 | Male | 38 | Rio de Janeiro | Mechanic | 2005 | 2016 | Contact with cats | Deceased (2018) |
| 4 | Male | 25 | Magé | Cleaning assistant | 2012 | 2012 | Not determined | Deceased (2015) |
| 5 | Female | 20 | Campos dos Goytacazes | Student | 2012 | 2012 | Contact with cats | Deceased (2014) |
Table 2.
Characteristics related to the HIV infection of the five patients with chronic sporotrichosis and acquired immunodeficiency syndrome included in this study at the time of Sporothrix isolation
| Patient | Sporothrix strain number | Fungal isolation date | Clinical specimen | T CD4 cell count | T CD8 cell count | Viral load |
|---|---|---|---|---|---|---|
| 1 | 41932 | 08/08/2011 | Skin lesion pus | 46 | 545 | 104,290 |
| 44886 | 10/15/2012 | Cerebrospinal fluid | 204 | 1967 | 194 | |
| 45308 | 12/21/2012 | Cerebrospinal fluid | 55 | 595 | < 50 | |
| 2 | 654H | 09/02/2011 | Blood | 66 | 292 | 423,153 |
| 42235 | 09/19/2011 | Oropharynx swab | 66 | 292 | 423,153 | |
| 45030 | 11/05/2012 | Skin tissue fragment | 127 | 506 | < 50 | |
| 3 | 49240 | 11/16/2016 | Skin tissue fragment | 53 | 1486 | 174,603 |
| 49263 | 12/24/2016 | Cerebrospinal fluid | 95 | 925 | < 50 | |
| 49413 | 01/17/2017 | Skin lesion pus | 95 | 925 | < 50 | |
| 50661 | 03/28/2018 | Skin lesion pus | 13 | 759 | 61,948 | |
| 4 | 43723 | 05/04/2012 | Bone marrow aspirate | 25 | 325 | 66,989 |
| 47418 | 06/11/2014 | Larynx fragment | 80 | 482 | < 50 | |
| 48294 | 12/10/2015 | Urine | 34 | 406 | < 50 | |
| 5 | 43987-1 | 06/14/2012 | Skin lesion pus | 111 | 605 | 82 |
| 44022 | 06/20/2012 | Sputum | 51 | 341 | < 50 | |
| 46670 | 07/23/2013 | Induced sputum | 304 | 1510 | < 50 |
Fungal characteristics
Sixteen clinical isolates from the five included patients were studied. Their macroscopic and microscopic characteristics were compatible with Sporothrix spp. (data not shown). All clinical strains were identified as S. brasiliensis since they presented a 469 bp amplified DNA band when the PCR was performed using the primer pair Sbra-F and Sbra-R. No bands were visualized when the other primer pairs (Ssch-F/Ssch-R and Sglo-F/Sglo-R) were used in the specific PCR. Reference control strains produced the expected amplicons.
Fungal growth on different substrates
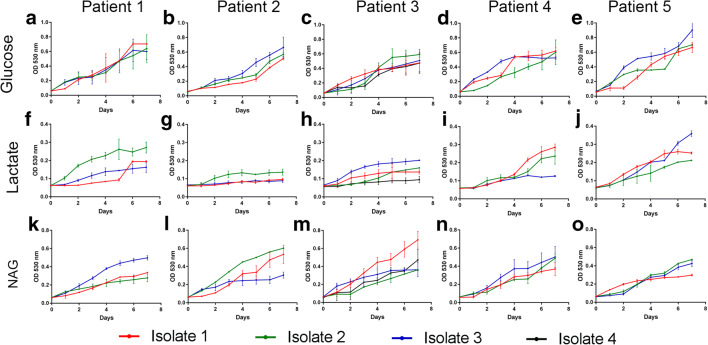
The growth profile of the isolates in the presence of glucose was similar among the five patients (Fig. 1a–e). In general, all isolates grew poorly in the presence of lactate (Fig. 1f–j). Two isolates from patient 2, for instance, consistently fail to grow in the presence of this carbon source. The growth of the Sporothrix clinical isolates herein studied was more abundant in the presence of N-acetyl-D-glucosamine than lactate (Fig. 1k–o). However, it was not possible to associate the growth of the isolates with the evolution time of neither the disease nor the clinical specimen that yielded the isolate. For instance, the third isolate from patient 1 (16 months of disease evolution, isolated from the cerebrospinal fluid) grew more abundantly in the presence of N-acetyl-D-glucosamine than the first two isolates. On the other hand, for patient 3, the first isolate (at diagnosis, from a skin tissue fragment) presented the best growth among the four studied Sporothrix isolates.
Fig. 1.
Growth of Sporothrix brasiliensis under different carbon sources. Glucose (a–e), lactate (f–j), and N-acetyl-D-glucosamine (k–o) served as carbon sources for the growth of clinical S. brasiliensis isolates obtained from patient 1 (a, f, k), patient 2 (b, g, l), patient 3 (c, h, m), patient 4 (d, i, n), and patient 5 (e, j, o), all with chronic sporotrichosis and acquired immunodeficiency syndrome. The optical density (530 nm) of the isolates grown in the different carbon sources was measured daily for 7 days. Results are expressed as the mean and standard deviation of the reads. NAG, N-acetyl-D-glucosamine; OD, optical density
Fungal response to the antifungal drugs used to treat the patients
Control strains used in the antifungal susceptibility tests yielded MIC values within the expected range (Online Resource 1). Table 3 depicts the overall antifungal susceptibility of the 16 clinical isolates, both on mycelial and yeast morphologies, against the four antifungal drugs tested in this study. According to the ECVs described for the mycelial form of S. brasiliensis, all studied clinical isolates were considered wild type for amphotericin B, itraconazole, and posaconazole. However, all isolates from patients 1 and 2 were classified as non-wild type for terbinafine. In order to check whether the fungal morphology of the fungus affects the clinical correlation of MIC and therapeutic response in sporotrichosis, MICs for the yeast form of S. brasiliensis were also determined. In general, MIC values for amphotericin B presented a maximum variation of one dilution. Seven isolates presented more than two dilution variations in itraconazole MIC values of mycelium and yeast forms of the fungus, two for posaconazole, and five for terbinafine. Except for isolates of patient 2, which yielded higher MICs for the S. brasiliensis yeast form, all other MICs were higher for the fungal mycelial form. It is interesting to note that isolates from patient 1, collected with more than 60 weeks of sporotrichosis evolution, developed a two-dilution increase in MIC values for amphotericin B, from 0.5 to 2.0 mg/l for the mycelial form and from 1.0 to 4.0 mg/l for the yeast form. The last isolate from patient 4 also presented a similar increase in the amphotericin B MIC value, from 1.0 to 4.0 mg/l, but only for the mycelial form of the fungus. All other variations observed on the MIC values from the mycelial form of studied strains were within one dilution range. On the other hand, patients 2 and 4 presented a two-dilution increase in itraconazole MIC values for the yeast form of the isolates, and patient 1 presented a similar variation for terbinafine yeast MICs.
Table 3.
Antifungal susceptibility of 16 Sporothrix brasiliensis isolates and antifungal drugs used by five patients with chronic sporotrichosis and acquired immunodeficiency syndrome
| Case | Sporothrix strain number | Disease evolution (weeks) | Previous antifungal treatments | MIC (mg/l) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B | Itraconazole | Posaconazole | Terbinafine | ||||||||
| Mycelium | Yeast | Mycelium | Yeast | Mycelium | Yeast | Mycelium | Yeast | ||||
| 1 | 41932 | 0 | None | 0.5 | 1.0 | 0.5 | 0.25 | 1.0 | 0.25 | 0.25a | 0.12 |
| 44886 | 60 | AMB (d)/ITC/FLC | 2.0 | 2.0 | 1.0 | 0.25b | 0.5 | 0.25 | 0.5 | 0.12 | |
| 45308 | 69 | AMB (d)/ITC/FLC | 2.0 | 4.0 | 0.5 | 1.0 | 0.5 | 0.25 | 0.5 | 0.25 | |
| 2 | 654H | 0 | None | 2.0 | 2.0 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 0.12 |
| 42235 | 2 | AMB (d) | 2.0 | 2.0 | 0.5 | 0.5 | 0.25 | 1.0 | 0.5 | 0.06 | |
| 45030 | 65 | AMB (d)/ITC | 4.0 | 4.0 | 0.25 | 1.0 | 0.5 | 1.0 | 0.5 | 0.12 | |
| 3 | 49240 | 0 | ITC | 4.0 | 2.0 | 1.0 | 1.0 | 0.5 | 0.25 | 0.03 | 0.03 |
| 49263 | 4 | AMB (d)/ITC | 2.0 | 2.0 | 2.0 | 0.25 | 0.5 | 0.25 | 0.03 | 0.03 | |
| 49413 | 9 | AMB (d) (l)/ITC | 2.0 | 1.0 | 2.0 | 0.5 | 0.5 | 0.5 | 0.06 | 0.015 | |
| 50661 | 66 | AMB (d) (l)/ITC | 2.0 | 2.0 | 1.0 | 0.5 | 0.5 | 0.25 | 0.03 | 0.015 | |
| 4 | 43723 | 0 | AMB (d)/ITC/TRB | 1.0 | 2.0 | 2.0 | 0.25 | 0.5 | 0.5 | 0.06 | 0.06 |
| 47418 | 105 | AMB (d) (l)/ITC | 1.0 | 1.0 | 2.0 | 0.25 | 0.5 | 0.25 | 0.06 | 0.03 | |
| 48294 | 187 | AMB (d) (l)/ITC/TRB/PSC | 4.0 | 2.0 | 1.0 | 1.0 | 0.5 | 0.25 | 0.06 | 0.06 | |
| 5 | 43987-1 | 0 | AMB (d) (l)/ITC | 4.0 | 2.0 | 2.0 | 1.0 | 0.5 | 0.5 | 0.03 | 0.06 |
| 44022 | 1 | AMB (d) (l)/ITC | 4.0 | 2.0 | 2.0 | 2.0 | 1.0 | 0.5 | 0.06 | 0.06 | |
| 46670 | 56 | AMB (d) (l)/ITC/TRB/PSC | 4.0 | 1.0 | 2.0 | 0.5 | 0.5 | 0.25 | 0.06 | 0.03 | |
aBold numbers indicate MICs of non-wild type isolates
bItalic numbers indicate MICs with a difference of two or more dilutions between the fungal mycelial and yeast forms
AMB (d), amphotericin B deoxycholate; (l), lipid complex; ITC, itraconazole; FLC, fluconazole; PSC, posaconazole
Fungal thermotolerance and its impact on the sporotrichosis outcome
Thermotolerance is inversely proportional to the % GI; that is, the lower the % GI, the more thermotolerant the fungus is. Therefore, except for patient 2, who is still under treatment for both sporotrichosis and HIV infection at our institution, fungal isolates obtained at late stages of S. brasiliensis infection presented slightly higher thermotolerances than the isolates obtained at the time of sporotrichosis diagnosis (Fig. 2). All these patients who had S. brasiliensis strains with higher thermotolerances presented an unfavorable outcome; that is, they died as a result of sporotrichosis and AIDS. The difference, however, was only significant for the last isolate of patient 3 (p = 0.0007).
Fig. 2.

Thermotolerance of clinical Sporothrix brasiliensis isolates from patients with chronic sporotrichosis and acquired immunodeficiency syndrome. Results are expressed as the mean and standard deviation of the percent growth inhibition of the isolates at 35 °C. The lower the percent growth inhibition, the higher the thermotolerance of the isolates. The asterisk symbol indicates a statistically significant difference between the thermotolerance of the isolate obtained at the time of sporotrichosis diagnosis and isolates obtained during disease evolution
Susceptibility of S. brasiliensis to nitrogen- and oxygen-derived oxidants
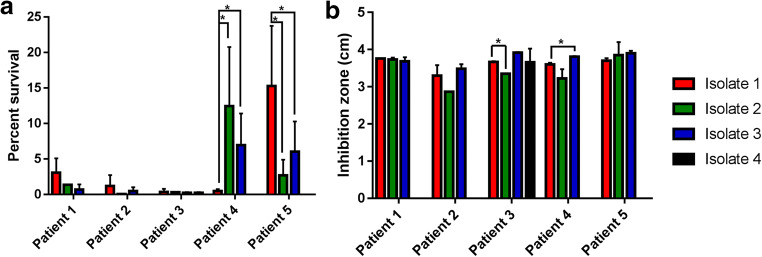
Figure 3 presents data about oxidative stress of the clinical S. brasiliensis isolates herein studied. The isolates from patient 4, obtained after more than 2 years of sporotrichosis evolution, were more resistant to nitrogen-derived oxidants than the isolate obtained at diagnosis (Fig. 3a), but the last isolate from this patient was more susceptible to hydrogen peroxide than the isolate obtained at sporotrichosis diagnosis (Fig. 3b). For all other patients, it was observed that resistance to nitrogen-derived oxidants decreased over time, although only patient 5 presented a statistically significant difference. The second isolate from patient 3, obtained from the cerebrospinal fluid, was more resistant to hydrogen peroxide (p = 0.0012), but for other isolates, all from skin lesions, the susceptibility profile to this oxidative stress was similar. The oxygen-derived oxidant susceptibility profile of isolates from other patients was similar over time.
Fig. 3.
Resistance of clinical Sporothrix brasiliensis isolates from patients with chronic sporotrichosis and acquired immunodeficiency syndrome from chemical oxidants. Results are expressed as the mean and standard deviation of a the percent survival of the yeast form of the isolates in a nitrogen-derived oxidant solution and b inhibition zone, in centimeters, of the yeast S. brasiliensis growth in the presence of hydrogen peroxide. The asterisk symbol indicates a statistically significant difference between the resistances of the isolate obtained at the time of sporotrichosis diagnosis and isolates obtained during disease evolution
Production of hydrolytic enzymes
The results obtained for the control strains were as expected (Online Resource 2). In general, phytase and phospholipase activities were not detected on the S. brasiliensis clinical isolates under the study conditions. Figure 4 depicts the overall profile of production of some hydrolytic enzymes associated with fungal virulence by the 16 S. brasiliensis isolates obtained from the five patients included in the present study. Some isolates were also not able to present activity for other studied enzymes: two did not present aspartic protease activity, five did not present hemolytic activity, other five did not present esterase activity, and one did not present urease activity. Significant variations on the production aspartic protease were observed in the second isolate of patient 2, which produced higher levels of the enzyme, and in the last isolate of patient 3. The first isolate from this patient did not produce aspartic protease, but the later isolates increased enzyme production. The last isolate from patient 1 was also not able to produce this enzyme. Regarding hemolysin production, isolates from patients 1 and 3 evolved from non-producers to low hemolysin producers, whereas the last isolate from patient 2 did not exhibit hemolysin activity, unlike previous isolates from the same patient. Hemolysin production profile of patient 4 was stable and patient 5 presented a significant decrease in hemolysin production in isolate 2 (p = 0.004). Only the second isolate from patient 1 and the last from patient 2 were able to produce esterase, in low and moderate levels, respectively. Variations on esterase production levels of isolates from patients 3 and 4 did not present statistical differences, despite the apparent increase on esterase production over time observed for patient 4. Esterase production by the third isolate from patient 5 was lower than that observed for the first isolate (p < 0.001). The urease production was the most stable phenotype among all isolates. Only the second isolate from patient 3 was not able to produce this enzyme.
Fig. 4.
Production of hydrolytic enzymes by clinical Sporothrix brasiliensis isolates from patients with chronic sporotrichosis and acquired immunodeficiency syndrome. Results are expressed as the mean and standard deviation of the a aspartic protease activity, b hemolytic activity, c esterase activity, and d urease activity. Aspartic protease, hemolytic, and esterase activities are expressed as the Pz of each enzyme in specific culture media to test the production of these enzymes by the yeast form of the fungus. Urease activity is expressed as the optical density (559 nm) of yeast S. brasiliensis cultures in Christensen’s urea broth. The asterisk symbol indicates a statistically significant difference between the production of the hydrolytic enzyme by the isolate obtained at the time of sporotrichosis diagnosis and isolates obtained during disease evolution. OD, optical density
Phenotypic differences according to the isolation site
To check whether isolates from the skin and disseminated sites have different phenotypic profiles, the 16 clinical isolates were grouped accordingly, and the phenotypic variables were evaluated using the Mann-Whitney test. As demonstrated in Fig. 5, isolates obtained from skin lesions produced more esterase than isolates from deep sites (Pz medians 0.6714 and 0.8742, respectively; p = 0.0387). All other studied phenotypes were similar among isolates from the skin and deep sites (p > 0.05). Although not significant (p = 0.1853), it is interesting to note that isolates with the lowest % GI, that is, with higher thermotolerance, were obtained from deep sites.
Fig. 5.
Differences between esterase production among clinical Sporothrix brasiliensis isolates obtained from the skin and deep sites of patients with chronic sporotrichosis and acquired immunodeficiency syndrome. Results are expressed as the mean value of six Pz measurements performed on two independent experiments. Pz values of isolates from different patients are represented in different colors
Fungal phenotypes and clinical aspects of patients
Correlations were assessed among clinical characteristics of the patients related to their immunological status (T CD4 cell count, T CD8 cell count, specific IgG response against S. brasiliensis), as well as to the virologic status of the HIV infection, that is, the viral load at the time of Sporothrix isolation in culture. As demonstrated in Fig. 6a, there were no significant correlations between the clinical data and the studied fungal phenotypes. We also evaluated possible correlations between different fungal phenotypes. Figure 6b presents Spearman’s correlation coefficient of all associations and the p values of significant correlations (p < 0.05). In brief, the following significant associations were found: (i) isolates with higher amphotericin B MIC values were more producers of esterase and urease; (ii) isolates with higher itraconazole MIC values presented lower terbinafine MIC values and had better growth with lactate as a carbon source; (iii) isolates with higher posaconazole MIC values were more susceptible to hydrogen peroxide and had better growth with glucose as a carbon source; (iv) isolates with increased resistance to nitrogen-derived oxidants presented lower thermotolerance but had better growth with glucose as a carbon source; (v) isolates with increased resistance to hydrogen peroxide grew less abundantly with glucose as a carbon source; and (vi) isolates that produced higher levels of esterase, also, produced high levels of urease.
Fig. 6.

Correlation analyses of clinical and mycological variables. Heatmaps representing Spearman’s correlation coefficients of a the associations between immunologic (T CD4 and CD8 cell counts, specific IgG response) and viral load of patients with the phenotypes of sequential Sporothrix brasiliensis isolates, and b the associations between different phenotypes of the studied clinical isolates. Green color represents negative associations, whereas the red color represents positive associations. Probability values of statistically significant correlations (p < 0.05) are displayed within the squares
Discussion
In order to survive the adverse conditions during parasitism, pathogenic fungi must adapt to the harsh microenvironments of the host and may display altered phenotypic characteristics due to the selection pressure, in a process known as microevolution, as demonstrated in the opportunistic fungi Aspergillus fumigatus, Cryptococcus neoformans, and Candida albicans [29–31]. The present study also shows evidence that S. brasiliensis, during opportunistic infections in HIV-infected patients, may also suffer microevolution, in a process that may be variable between different patients. This plasticity to adapt to a variety of stress conditions imposed during protracted infections on different patients was also observed previously for patients with cryptococcosis [32].
The clinical aspects of the patients included in this study were in accordance with other studies about sporotrichosis in people living with HIV/AIDS [14, 33]. The only patient who presented anti-Sporothrix IgG antibodies is the one who is still alive. The main immune response to Sporothrix infection is the cellular response, which is impaired in the HIV/AIDS patients. Our finding on the lack of serologic response among the four deceased patients brings the hypothesis of a possible role of the humoral response in these severe cases. Studies focusing on this poorly understood role and mechanisms may be helpful. Another hypothesis is that the uncontrolled HIV infection is responsible for the lack of antibody response and the main cause of death of the patients. In the present study, at least two patients (1 and 5) died due to consequences of the S. brasiliensis infection in hardly immunosuppressed patients with uncontrolled HIV infection. The treatment regimen is chosen according to the severity of each case. As for the possible drug interactions, our protocol involves a careful search, as well as a checkup for other factors that may impair the drug bioavailability. We could not measure the drug serum levels, because in Brazil, this test in unavailable in most laboratories. When patients did not respond to oral antifungal treatment, they also did not respond to HIV infection, since both infections are closely related. Sporotrichosis in people living with HIV presenting high CD4 levels and undetectable viral load has a clinical picture similar to that observed in immunocompetent patients [33].
As expected, S. brasiliensis was the species identified in all isolates from this study. This is the major sporotrichosis agent in Rio de Janeiro, Brazil, where the study was conducted [34]. It has been described that S. brasiliensis isolates that cause sporotrichosis in the Brazilian Southeast are clonal [35, 36], and therefore, genetic differences cannot explain the phenotypic plasticity observed by the isolates of this study. A similar scenario was observed for Cryptococcus spp. isolates from Botswana, which displayed a high phenotypic variety, not related to genetic diversity of the isolates [37].
The ability of microorganisms to grow under different nutritional conditions is known as metabolic plasticity and, for C. albicans, this trait is related to fungal virulence [38]. The S. brasiliensis isolates herein studied were able to grow in the presence of lactate, a compound largely abundant in most host niches, and more abundantly in the presence of the amino sugar N-acetyl-D-glucosamine, a constituent of host glycosylated proteins and a major component of the peptidoglycan found in the bacterial cell wall. This result, besides the aspartic protease production by most of isolates of this study, suggests that S. brasiliensis uses proteins from the host as molecules for growth. Recently, it was demonstrated that S. brasiliensis can exploit bacteria and use them as a sole source of nutrient for growth [11], which can further explain the more abundant growth of S. brasiliensis in the presence of N-acetyl-glucosamine, since the skin and other body sites have an associated bacterial microbiota that can also serve as nutrient for the fungus.
Sporothrix thermotolerance is historically associated with dissemination of the fungus through the host, both in humans [21] and cats [39]. In this study, all isolates presented less than 50% of growth inhibition at 35 °C, indicating that they present high thermotolerance, which is compatible with the disseminated sporotrichosis profile observed in the patients. Moreover, the last isolates from all deceased patients presented slightly higher thermotolerances than the initial isolate, ratifying the importance of thermotolerance in the pathogenesis of sporotrichosis.
All patients of this study used amphotericin B as treatment for sporotrichosis and two patients presented a two-dilution increase of MIC values for this antifungal drug. In a previous study, increases in MIC values were not observed for the isolates obtained during the sporotrichosis course, but only one patient treated with amphotericin B was included [40]. Hence, acquired or secondary resistance to amphotericin B may occur in patients during protracted treatments with this drug. Despite this increase, all isolates were classified as wild type for amphotericin B, which may be a result of the ECV being above the expected and achievable serum levels due to a natural resistance of S. brasiliensis to this antifungal drug [20]. Regarding the ECVs, only terbinafine non-wild type isolates were observed in this study. These isolates were obtained from patients 1 and 2, who were not treated with this antifungal drug. The existence of non-wild type S. brasiliensis isolates for terbinafine was previously demonstrated in Rio de Janeiro [41], suggesting that these patients may have become infected with non-wild type strains for this drug. MICs for itraconazole and terbinafine were found to be inversely associated in the isolates from this study. Both antifungals act on ergosterol biosynthesis, but on different enzymatic stages. Since the antifungal treatments applied to the patients do not explain this difference, this negative association may indicate that S. brasiliensis specializes in one specific resistance mechanism of ergosterol biosynthesis. Further experiments with a large number of wild type and non-wild type strains for both drugs are necessary to assess this issue.
The usual protocol for Sporothrix antifungal susceptibility uses the mycelial form of the fungus for MIC determinations [20]. Since yeasts are found in parasitism, we also checked whether the MIC determinations using the parasitic form of S. brasiliensis could offer a better indicator of the therapeutic response in sporotrichosis. In this study, most MICs were similar among different morphologies of the fungus, supporting that MIC determination of the mycelial form is sufficient to identify isolates refractory to the antifungal treatment. However, more studies with a large number of patients are necessary to confirm this hypothesis. Regarding the few variations between MICs from mycelial and yeast forms, all but one patient presented higher MICs for the mycelial form of the fungus, as described previously [42, 43]. Only isolates from patient 2 presented higher MICs for the yeast form, in comparison with the mycelium for itraconazole (third isolate) and posaconazole (second isolate). This patient was not treated with posaconazole, but the use of itraconazole during more than 1 year may have performed a selection pressure in the yeast parasitic form of the fungus. However, since it did not occur with the other patients that also were treated with this azole, it is unlikely that itraconazole has a high potential to induce in vivo resistance in yeast S. brasiliensis cells during infection.
It is important to note that the lack of clinical response in patients with sporotrichosis cannot be addressed solely on the antifungal susceptibility of the strains. Besides HIV, some immunosuppressor factors, such as uncontrolled diabetes, immunobiological therapy, and corticosteroid use, may affect the outcome of several mycoses. The five patients herein studied did not present any known immunosuppressive conditions other than the HIV infection. Another possible explanation for the lack of clinical response should be a higher virulence of the infective strain, as occurred in a previous patient treated by our group [9]. Therefore, we performed assays to address some virulence-related phenotypes of the strains isolated from the included patients.
The previous study from our group linked the increased resistance to nitrogen-derived oxidants with the in vivo evolution of S. brasiliensis virulence in a patient with chronic sporotrichosis [9]. In the present study, this occurred only in patient 4. Moreover, later isolates from patient 5 became more susceptible to this stress condition, reinforcing the virulence plasticity of S. brasiliensis. Interestingly, the last isolate from patient 4, which was more resistant to nitrogen-derived oxidants, was more susceptible to hydrogen peroxide. In other patients, except for patient 3, who presented increased resistance to hydrogen peroxide only for the second isolate, this phenotype was stable during infection, especially for patients 1 and 5, as occurred with our previous study in a patient without HIV infection. Resistances to nitrogen and oxygen oxidants were found to be differentially correlated with fungal growth in the presence of glucose. This indicates that fungal growth may be a mechanism that drives resistance to nitrogen oxidants and that, to resist oxygen oxidants, S. brasiliensis changes its metabolism, probably converging energy to cell rescue rather than cellular division.
This study described the production of aspartic protease, esterase, hemolysin, and urease by S. brasiliensis. Previous studies have demonstrated that around 20–25% of Brazilian Sporothrix strains have proteolytic activity in vitro [23, 44]. In this study, 87.5% of the isolates presented proteolytic activity, which supports previous observations that most isolates with this phenotype are isolated from patients infected with HIV [23]. The frequency of esterase-positive S. brasiliensis isolates in this study is similar to that observed in the study of Oliveira and collaborators [45]. In addition, isolates from the skin produced higher levels of this enzyme than isolates from deep sites, which may indicate a preferential use of short chain fatty acids during skin parasitism. To the best of our knowledge, this is the first description of hemolytic activity of S. brasiliensis isolates, which can be useful during hematogenic dissemination and for iron acquisition in host tissues. As described previously [23, 46], urease was observed in most of the studied isolates. The levels of these two last enzymes are statistically associated, indicating a joint action of them in the sporotrichosis pathogenesis. In addition, higher amphotericin B MIC values are associated with the productions of these two enzymes, suggesting a role for them in S. brasiliensis resistance to this drug. Phospholipase and phytase were not detected in any isolate from this study. A cytosolic phospholipase is important to the development of the yeast form of S. schenckii [47]. The incapability to detect phospholipase in this study may be related to the method herein applied or to differences between the yeast cell cycle of S. schenckii and S. brasiliensis. To our knowledge, phytase production by Sporothrix was not described so far.
We could not find statistically significant associations between the immunological and virologic status of the patients and the studied phenotypes. The lack of a particular phenotype of S. brasiliensis related to the immunity of the patients or with the disease outcome demonstrates that the fungal virulence has a low impact on the pathogenesis of the disseminated sporotrichosis associated with AIDS. In cryptococcosis, there is a relationship between host cellular immunity and some fungal phenotypes, such as cell and capsule size, which may play a role in fungal pathogenesis [37]. Sporothrix brasiliensis does not produce capsule and cell size was not evaluated in this study, which may explain this difference. Other factors that may explain the findings of the present study are the low number of included patients and the participation of other virulence-related phenotypes, not studied here, in the immune response. The low number of patients and isolates evaluated was indeed a limitation of the present study, particularly because of the inclusion criteria (patients with chronic refractory sporotrichosis with at least two clinical isolates 1 year apart).
The present study suggests that S. brasiliensis has a complex phenotype expression to adapt to the conditions found in the host, even in the absence of a proper immunologic response. In fact, the immunosuppression induced by the HIV infection is the most important factor related to unfavorable outcomes in disseminated sporotrichosis associated with AIDS. The study of other virulence-related phenotypes in a large cohort of patients may indicate some predictors of unfavorable outcomes and a better comprehension of sporotrichosis pathogenesis in people living with HIV/AIDS.
Electronic supplementary material
(PDF 15 kb)
(PDF 11 kb)
Acknowledgments
The authors would like to thank Fiocruz, for the financial support.
Funding information
RMZ-O was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 302796/2017-7) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ E-26/202.527/2019). RA-P was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 305487/2015-9).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, Feng P, Yang L, Chen M, Deng S, Li S, Liao W, Li R, Li F, Meis JF, Guarro J, Teixeira M, Al-Zahrani HS, Pires de Camargo Z, Zhang L, de Hoog GS. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14 000 human and animal case reports. Persoonia. 2015;35:1–20. doi: 10.3767/003158515X687416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues AM, Cruz Choappa R, Fernandes GF, de Hoog GS, de Camargo ZP. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016;120:246–264. doi: 10.1016/j.funbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Orofino-Costa R, de Macedo PM, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barros MBL, Almeida-Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcão EMM, de Lima Filho JB, Campos DP, Valle ACF, Bastos FI, Gutierrez-Galhardo MC, Freitas DFS. Hospitalizações e mortes relacionadas à esporotricose no Brasil (1992-2015) Cad Saude Publica. 2019;35:e00109218. doi: 10.1590/0102-311X00109218. [DOI] [PubMed] [Google Scholar]
- 8.Córdoba S, Isla G, Szusz W, Vivot W, Hevia A, Davel G, Canteros CE. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses. 2018;61:441–448. doi: 10.1111/myc.12760. [DOI] [PubMed] [Google Scholar]
- 9.Freitas DFS, Santos SS, Almeida-Paes R, Oliveira MME, Valle ACF, Gutierrez-Galhardo MC, Zancopé-Oliveira RM, Nosanchuk JD. Increase in virulence of Sporothrix brasiliensis over five years in a patient with chronic disseminated sporotrichosis. Virulence. 2015;6:112–120. doi: 10.1080/21505594.2015.1014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect Immun. 2004;72:3478–3488. doi: 10.1128/IAI.72.6.3478-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida-Paes R, Brito-Santos F, Oliveira MME, Bailão AM, Borges CL, Araújo GRS, Frases S, CMA S, Zancopé-Oliveira RM. Interaction with Pantoea agglomerans modulates growth and melanization of Sporothrix brasiliensis and Sporothrix schenckii. Mycopathologia. 2019;184:367–381. doi: 10.1007/s11046-019-00350-x. [DOI] [PubMed] [Google Scholar]
- 12.Moreira JAS, Freitas DFS, Lamas CC. The impact of sporotrichosis in HIV-infected patients: a systematic review. Infection. 2015;43:267–276. doi: 10.1007/s15010-015-0746-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-e-Silva M, Lima CMO, Schechtman RC, Trope BM, Carneiro S. Systemic mycoses in immunodepressed patients (AIDS) Clin Dermatol. 2012;30:616–627. doi: 10.1016/j.clindermatol.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Freitas DFS, do ACF V, da Silva MBT, Campos DP, Lyra MR, de Souza RV, Veloso VG, Zancopé-Oliveira RM, Bastos FI, Galhardo MCG. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:e3110. doi: 10.1371/journal.pntd.0003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brasil. Ministério da Saúde. Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais (2018). Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Brasília, DF
- 16.Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PCF, Peralta JM, Nosanchuk JD, Zancopé-Oliveira RM. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin Vaccine Immunol. 2007;14:244–249. doi: 10.1128/CVI.00430-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:e0004190. doi: 10.1371/journal.pntd.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CLSI - Clinical and Laboratory Standars Institute (2008). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard-second edition ed. Wayne, PA. USA: Clinical and Laboratory Standards Institute
- 19.CLSI - Clinical and Laboratory Standars Institute (2012) Reference method for both dilution antifungal susceptibility testing of yeasts. Approved standard-third edition. CLSI DOCUMENT M27-A3. Clinical and Laboratory Standards Institute. Wayne, PA: Clinical and Laboratory Standards Institute
- 20.Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, Govender NP, Guarro J, Johnson EM, Kidd SE, Pereira SA, Rodrigues AM, Rozental S, Szeszs MW, Ballesté Alaniz R, Bonifaz A, Bonfietti LX, Borba-Santos LP, Capilla J, Colombo AL, Dolande M, Isla MG, Melhem MSC, Mesa-Arango AC, Oliveira MME, Panizo MM, Pires de Camargo Z, Zancope-Oliveira RM, Meis JF, Turnidge J. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother. 2017;61:e01057–e01017. doi: 10.1128/AAC.01057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesa-Arango AC, Del Rocío R-MM, Pérez-Mejía A, Navarro-Barranco H, Souza V, Zúñiga G, Toriello C. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of sporotrichosis. J Clin Microbiol. 2002;40:3004–3011. doi: 10.1128/jcm.40.8.3004-3011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Casadevall A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect Immun. 1994;62:3004–3007. doi: 10.1128/IAI.62.7.3004-3007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida-Paes R, de Oliveira LC, Oliveira MME, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed Res Int. 2015;2015:212308. doi: 10.1155/2015/212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MF, Wilkinson ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982;20:7–14. doi: 10.1080/00362178285380031. [DOI] [PubMed] [Google Scholar]
- 25.Tsang PW-K. Differential phytate utilization in Candida species. Mycopathologia. 2011;172:473–479. doi: 10.1007/s11046-011-9453-3. [DOI] [PubMed] [Google Scholar]
- 26.Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13:122–143. doi: 10.1128/cmr.13.1.122-143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aktas E, Yigit N, Ayyildiz A. Esterase activity in various Candida species. J Int Med Res. 2002;30:322–324. doi: 10.1177/147323000203000315. [DOI] [PubMed] [Google Scholar]
- 28.Paixão AG, Galhardo MCG, Almeida-Paes R, Nunes EP, Gonçalves MLC, Chequer GL, Lamas CC. The difficult management of disseminated Sporothrix brasiliensis in a patient with advanced AIDS. AIDS Res Ther. 2015;12:16. doi: 10.1186/s12981-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballard E, Melchers WJG, Zoll J, Brown AJP, Verweij PE, Warris A. In-host microevolution of Aspergillus fumigatus: a phenotypic and genotypic analysis. Fungal Genet Biol. 2018;113:1–13. doi: 10.1016/j.fgb.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arras SDM, Ormerod KL, Erpf PE, Espinosa MI, Carpenter AC, Blundell RD, Stowasser SR, Schulz BL, Tanurdzic M, Fraser JA. Convergent microevolution of Cryptococcus neoformans hypervirulence in the laboratory and the clinic. Sci Rep. 2017;7:17918. doi: 10.1038/s41598-017-18106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S, Clancy CJ, Zhang Z, Hao B, Wang W, Iczkowski KA, Pfaller MA, Nguyen MH. Uncoupling of oxidative phosphorylation enables Candida albicans to resist killing by phagocytes and persist in tissue. Cell Microbiol. 2007;9:492–501. doi: 10.1111/j.1462-5822.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Farrer RA, Giamberardino C, Sakthikumar S, Jones A, Yang T, Tenor JL, Wagih O, Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP, Cuomo CA, Perfect JR. Microevolution of serial clinical isolates of Cryptococcus neoformans var. grubii and C. gattii. MBio. 2017;8:e00166–e00117. doi: 10.1128/mBio.00166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freitas DFS, Hoagland BS, Valle ACF, Fraga BB, Barros MBL, Schubach AO, Almeida-Paes R, Cuzzi T, Rosalino CMV, Zancopé-Oliveira RM, Gutierrez-Galhardo MC. Sporotrichosis in HIV-infected patients: report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med Mycol. 2012;50:170–178. doi: 10.3109/13693786.2011.596288. [DOI] [PubMed] [Google Scholar]
- 34.Almeida-Paes R, de Oliveira MME, Freitas DFS, Valle ACF, Zancopé-Oliveira RM, Gutierrez-Galhardo MC. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:e3094. doi: 10.1371/journal.pntd.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues AM, de Hoog GS, Zhang Y, de Camargo ZP. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg Microbes Infect. 2014;3:e32. doi: 10.1038/emi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira Mde M, Rodrigues AM, CKM T, de Almeida LGP, Van Diepeningen AD, van den Ende BG, Fernandes GF, Kano R, Hamelin RC, Lopes-Bezerra LM, ATR V, de Hoog S, de Camargo ZP, MSS F. Asexual propagation of a virulent clone complex in a human and feline outbreak of sporotrichosis. Eukaryot Cell. 2015;14:158–169. doi: 10.1128/EC.00153-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes KE, Brockway A, Haverkamp M, Cuomo CA, van Ogtrop F, Perfect JR, Carter DA. Phenotypic variability correlates with clinical outcome in Cryptococcus isolates obtained from Botswanan HIV/AIDS patients. MBio. 2018;9:e02016–e02018. doi: 10.1128/mBio.02016-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miramón P, Lorenz MC. A feast for Candida: metabolic plasticity confers an edge for virulence. PLoS Pathog. 2017;13:e1006144. doi: 10.1371/journal.ppat.1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boechat JS, Oliveira MME, Almeida-Paes R, Gremião IDF, Machado ACS, Oliveira RVC, Figueiredo ABF, Rabello VBS, Silva KBL, Zancopé-Oliveira RM, Schubach TMP, Pereira SA. Feline sporotrichosis: associations between clinical-epidemiological profiles and phenotypic-genotypic characteristics of the etiological agents in the Rio de Janeiro epizootic area. Mem Inst Oswaldo Cruz. 2018;113:185–196. doi: 10.1590/0074-02760170407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida-Paes R, Oliveira MME, Freitas DFS, Valle ACF, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Refractory sporotrichosis due to Sporothrix brasiliensis in humans appears to be unrelated to in vivo resistance. Med Mycol. 2017;55:507–517. doi: 10.1093/mmy/myw103. [DOI] [PubMed] [Google Scholar]
- 41.Almeida-Paes R, Brito-Santos F, Figueiredo-Carvalho MHG, Machado ACS, Oliveira MME, Pereira SA, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz. 2017;112:376–381. doi: 10.1590/0074-02760160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchotene KO, Brandolt TM, Klafke GB, Poester VR, Xavier MO. In vitro susceptibility of Sporothrix brasiliensis: comparison of yeast and mycelial phases. Med Mycol. 2017;55:869–876. doi: 10.1093/mmy/myw143. [DOI] [PubMed] [Google Scholar]
- 43.Trilles L, Fernández-Torres B, Lazéra MS, Wanke B, Schubach AO, Almeida-Paes R, Inza I, Guarro J. In vitro antifungal susceptibilities of Sporothrix schenckii in two growth phases. Antimicrob Agents Chemother. 2005;49:3952–3954. doi: 10.1128/AAC.49.9.3952-3954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandes GF, Do Amaral CC, Sasaki A, Godoy PM, De Camargo ZP. Heterogeneity of proteins expressed by Brazilian Sporothrix schenckii isolates. Med Mycol. 2009;47:855–861. doi: 10.3109/13693780802713216. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira DC, de Loreto ÉS, Mario DAN, Lopes PGM, Neves LV, da Rocha MP, Santurio JM, Alves SH. Sporothrix schenckii complex: susceptibilities to combined antifungal agents and characterization of enzymatic profiles. Rev Inst Med Trop Sao Paulo. 2015;57:289–294. doi: 10.1590/S0036-46652015000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendoza M, Alvarado P, Díaz de Torres E, Lucena L, de Albornoz MC. Physiological comportment and in vivo sensitivity of Sporothrix schenckii isolates maintained for 18 years by two preservation methods. Rev Iberoam Micol. 2005;22:151–156. doi: 10.1016/s1130-1406(05)70029-2. [DOI] [PubMed] [Google Scholar]
- 47.Valentín-Berríos S, González-Velázquez W, Pérez-Sánchez L, González-Méndez R, Rodríguez-Del Valle N. Cytosolic phospholipase A2: a member of the signalling pathway of a new G protein alpha subunit in Sporothrix schenckii. BMC Microbiol. 2009;9:100. doi: 10.1186/1471-2180-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 15 kb)
(PDF 11 kb)