Abstract
Itraconazole is the first drug of choice for the treatment of sporotrichosis and it is available at different concentrations for veterinary patients. However, therapeutic failure has been reported, limiting clinical treatment. This study evaluated the in vitro efficacy of brand-name and compounded itraconazole formulations against Sporothrix brasiliensis and estimated the itraconazole content in each tested formulation. Oral capsules were acquired from two brand-name products for human (H-IND) and veterinary (V-IND) uses, and three from compounding pharmacies in Pelotas, RS, for human (H-COMP1/H-COMP2) and veterinary (V-COMP) uses. Capsule purity was analyzed by liquid chromatography–electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF-MS). Antifungal activity was determined against 29 Sporothrix brasiliensis by the M38-A2 guideline of CLSI. H-IND/H-COMP1/H-COMP2 had high efficacy against S. brasiliensis (approximately 70% of total isolated susceptible), V-COMP showed moderate efficacy (51.7%), and V-IND was the least effective formulation (37.9%). Thirty-four percent of the total isolates were resistant to all formulations. Furthermore, itraconazole content did not match the concentration indicated by the manufacturers, ranging from 387.70 to 7.81 μg/mg (H-COMP2 > V-COMP > H-IND > H-COMP1 > V-IND). Therefore, it is possible that the formulations showed different in vitro efficacy due to the difference in their itraconazole contents. Given the emergence of antifungal resistance for all formulations, the choice product to be used must follow susceptibility testing. Stringent quality control measures are recommended for product manufactures to assure drug content uniformity.
Keywords: Sporotrichosis, Sporothrix brasiliensis, Antifungal, Itraconazole, Compounding drug, Antifungal resistance
Introduction
Sporotrichosis is a subcutaneous mycosis in humans and animals and caused by Sporothrix schenckii complex species [1]. Infections caused by S. brasiliensis are highly prevalent in feline and canine outbreaks in Brazil [2, 3]. For the treatment, itraconazole is the drug of choice due to its good pharmacological response and safety [4] and should be administered daily from least 3 to 6 months with a therapeutic extension of 1 month after the remission of the clinical signs [5, 6]. Considering the long duration of the treatment, the therapeutic management in cats and dogs is often challenging to owners in vulnerable socio-economic conditions due to the high cost of the treatment [7].
This scenario is common in Southern Brazil; for example, in the cities Pelotas and Rio Grande, most cases of feline and canine sporotrichosis occur among animal patients from low-income households [2, 8]. Owners often choose the compounded formulations which are comparatively cheaper than the brand-name formulations [8, 9].
Compounded drugs are prepared in a pharmaceutical establishment following medical prescription containing the details of pharmacological composition, dosage form, and posology, according to Resolution 67 of the Brazilian National Health Surveillance Agency [10]. However, there have been reports of compounded formulations not being effective, missing the active ingredient, and having quality control issues [11, 12].
Given that little is known about the efficacy of compounded itraconazole formulations in the treatment of sporotrichosis, this study evaluated their in vitro activity against Sporothrix brasiliensis isolates and determined the respective purity of the antifungal compound.
Material and methods
Itraconazole
Five different itraconazole containing products were purchased as oral capsules and used in the in vitro tests. Of these, two were brand-name products registered by the pharmaceutical industries and marketed in human (H-IND, 100 mg) and veterinary (V-IND, 25 mg) pharmacies, and three were purchased from compounding pharmacies located in Pelotas, RS, Brazil, two marketed for human (H-COMP1, 64 mg; H-COMP2, 65 mg) and one for veterinary use (V-COMP, 64 mg).
Stock solutions were individually prepared from each capsule according to the M38-A2 guidelines of the Clinical and Laboratory Standards Institute [13]. No physical alterations in coloration, odor, and texture were noted in the capsules and the respective contents.
For the preparation of the stock solutions for both antifungal assays and chromatographic analysis, one capsule of each product was opened in Class II biological safety cabinet, and the content was individually deposited in a sterile porcelain mortar, followed by the addition of 10 mL of dimethyl sulfoxide. The content was dissolved with the aid of a pestle until complete dissolution of the antifungal formulation in the solvent, resulting in a final concentration of 10 mg/mL (H-IND), 2.5 mg/mL (V-IND), 6.4 mg/mL (H-COMP1 and V-COMP), and 6.5 mg/mL (H-COMP2). These solutions were kept at − 80 °C. The characteristics of the itraconazole formulations used in this study are described in Table 1.
Table 1.
Description of the itraconazole formulations acquired as oral capsules according to the manufacturers
| IDa | Origin | Use | Concentrationc | Origin of the pharmaceutical company |
|---|---|---|---|---|
| H-INDb | Brand name | Human | 10 mg/mL | Beerse, Belgium |
| V-IND | Brand name | Veterinary | 2.5 mg/mL | São Paulo, SP, Brazil |
| H-COMP1 | Compounded | Human | 6.4 mg/mL | Pelotas, RS, Brazil |
| H-COMP2 | Compounded | Human | 6.5 mg/mL | Pelotas, RS, Brazil |
| V-COMP | Compounded | Veterinary | 6.4 mg/mL | Pelotas, RS, Brazil |
aID identification
bReference drug
cConcentration after dissolution, according to the M38-A2 guidelines [12]
Antifungal susceptibility assay
For the antifungal susceptibility assay, the broth microdilution technique was performed according to M38-A2 guidelines [13] against 28 Sporothrix brasiliensis isolates from cats (n = 13) and dogs (n = 15) from cases occurring in Rio Grande do Sul state (Southern Brazil). The clinical isolates were stored in the mycology collection of Centro de Diagnóstico e Pesquisa em Micologia Veterinária (Federal University of Pelotas, Brazil), in addition to a standard strain of human case from Rio de Janeiro, RJ (IPEC 16969, FIOCRUZ, Brazil), totaling 29 S. brasiliensis isolates. The fungal identification was performed by macro- and micromorphological analyses of the colonies, with evidence of fungal dimorphism. Molecular identification was performed by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP), using restriction endonuclease digestion [14].
Each fungal inoculum was individually prepared from the respective subculture in potato dextrose agar (Acumedia, Lansing, MI, USA) at 27 °C for 7 days for promoting conidia formation. Each inoculum was suspended in sterile saline solution and the concentration was adjusted using a spectrophotometer (Spectrum Instruments Co., Shanghai, China) at an optical density of 80–82% and 530 nm of transmittance. Adjusted fungal suspensions were diluted in RPMI-1640 (Roswell Park Memorial Institute medium, Sigma, Steinheim, Germany) containing MOPS (3-(N-morpholino) propane-sulfonic acid) and supplementation with glucose at a pH of 7.0, to obtain a final inoculum of 104 CFU/mL (colony forming units).
In 96-well microplates, 100 μL of RPMI-1640 with MOPS was added in each well, whereas 100 μL of an itraconazole solution prepared at the concentration of 32 μg/mL from the stock solutions was added in the well correspondent to the higher concentration of the product. Subsequently, a serial dilution was performed to obtain the final concentration, which ranged from 16 to 0.03 μg/mL. Fungal conidia suspensions (100 μL) were then added to each well-containing itraconazole.
Positive (100 μL of conidia suspension and 100 μL of RPMI-1640) and negative (200 μL RPMI-1640) controls were utilized to confirm normal fungal growth and the sterility of the culture medium, respectively. Plates were incubated at 27 °C for 72 h and the minimal inhibitory concentration (MIC) was determined visually by comparison with growth-free well (negative control). MIC was designed as the lowest concentration that produced no visible fungal growth.
For the sensitivity criteria, although there is no breakpoint for Sporothrix species, we followed the recommendations of M38-A2 guideline [13], which states that MIC values < 4 μg/mL and ≥ 4 μg/mL may be considered sensitive and resistant for itraconazole, respectively. The criteria for establishing antifungal efficacy are listed in Table 2.
Table 2.
Criteria for categorizing the efficacy of brand-name and compounded itraconazole formulations against Sporothrix brasiliensis, based on percentage of fungal inhibition in the in vitro antifungal susceptibility assay
| Antifungal efficacy | Fungal inhibition (%)a |
|---|---|
| Very high | 81–100 |
| High | 61–80 |
| Moderate | 41–60 |
| Low | 21–40 |
| Very low | 0–20 |
aConsidering the total number of Sporothrix brasiliensis isolates tested (n = 29) as 100%
LC-ESI-QTOF-MS analysis
The content of the active ingredient in each capsule was analyzed by liquid chromatography–electrospray ionization quadrupole time-of-flight (LC-ESI-QTOF) mass spectrometry (MS), which was performed according to Da Luz et al. [15]. All chemicals were of analytical grade. Formic acid was from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile was of HPLC grade (JT Baker, Phillipsburg, NJ, USA) and ultrapure water was obtained using MegaPurity purification system. The commercial standard of itraconazole was purchased from Abcam (Cambridge, MA, USA).
Briefly, samples were analyzed using high-performance liquid chromatography (UFLC) system (Shimadzu, Japan) coupled to a QTOF mass spectrometer (Impact HD, Bruker Daltonics, Bremen, Germany). The analysis was performed on a Shim-pack XR-ODS C18 (2.0 × 75 mm, 2.2 μm) column using 0.1% formic acid in water as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B, and following gradient program: the run started at 10% B and maintained for 2 min; from 2 to 7 min phase B increased linearly to 50%; from 7 to 22 min phase B was increased to 100% and maintained for 3 min (from 22 to 25 min). Before each run, the column was re-equilibrated for 2 min using the initial solvent composition. The flow rate was set constant at 0.2 mL/min and the column temperature was maintained at 40 °C for all separations. Ten microliters of each sample solution was injected and analyzed in triplicate.
MS operation parameters were the following: capillary voltage, 4500 V; nebulizer pressure, 40 psi; drying gas flow rate, 9 L/min; gas temperature, 200 °C. MS accurate mass spectra were recorded across the range of m/z 50–1000 in positive ionization mode. External calibration of the MS was carried out using sodium formate solution (10 mM). For quantification of itraconazole, external calibration curve with commercial standard was prepared at concentrations from 20 to 2500 ng/mL (equation: Y = 966.03x + 7177.6, R2 = 0.9969). The MS data were processed through Data Analysis 4.0 and compass quant analysis from Bruker Daltonics (Bremen, Germany). The results were expressed as an average of three replicates ± standard deviation. Limit of detection (LOD) and limit of quantification (LOQ) were estimated from the linearity of the calibration curve for itraconazole. They were determined based on the slope and standard deviation (σ) of the linear coefficient of the analytical curve:
Statistical analysis
Antifungal susceptibility data were evaluated by Wilcoxon’s test at p ≤ 0.05 using the statistical software BioEstat® version 5.3.
Results
All itraconazole formulations were standardized at the same concentration based on manufacturer information and ranged from 16 to 0.03 μg/mL, according to the M38-A2 guideline [13]. Sensitivity and resistance of Sporothrix brasiliensis isolates were observed for all formulations (Fig. 1). MIC ranged from 0.25 to > 16 μg/mL for the brand-name (H-IND and V-IND) and compounded (V-COMP) products, and of 0.5 to > 16 μg/mL for compounded (H-COMP1 and H-COMP2) product (Table 3). These findings demonstrated the emergence of itraconazole-resistant S. brasiliensis isolates from feline (06/29) and canine (04/29) cases to all formulations.
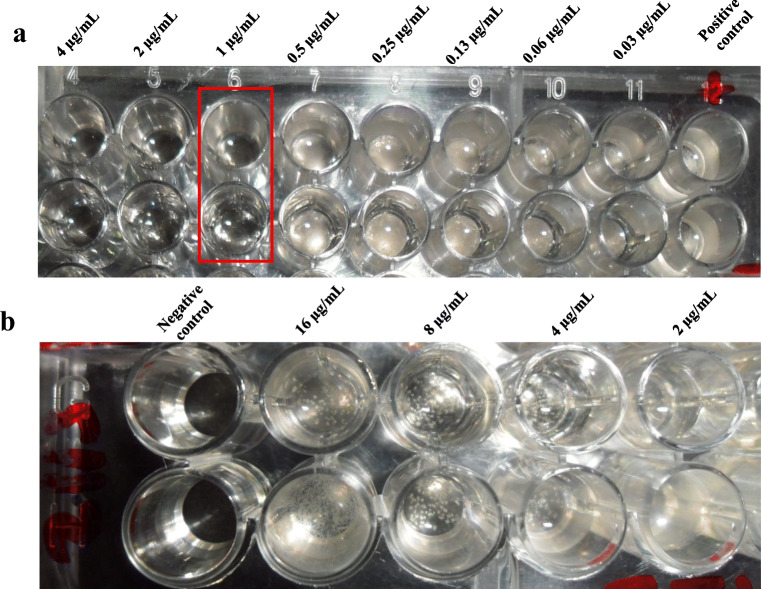
Fig. 1.
In vitro sensitivity and resistance of Sporothrix brasiliensis isolates from feline cases in Pelotas/RS, Southern Brazil, to different itraconazole formulations. In 96-well microplates, the visualization of fungal growth is noted into wells containing the itraconazole concentrations ranging from 16 to 0.03 μg/mL, in comparison with the positive and negative controls, by the antifungal susceptibility assay (M38-A2 of the Clinical Laboratory Standard Institute). The fungistatic activity of a compounded formulation for human use, identified as H-COMP2 was observed in both duplicate test lines against a feline isolate, showing a MIC of 1 μg/mL (a). No fungistatic activity was observed in a brand-name formulation for veterinary use, identified as V-IND, which MIC was greater than 16 μg/mL (b) against another feline isolate
Table 3.
Minimal inhibitory concentration (MIC) of brand-name and compounded itraconazole formulations against Sporothrix brasiliensis isolates from feline and canine cases occurring in Southern Brazil
| MIC (μg/mL)a | Sporothrix brasiliensis | Criteriac | |||||
|---|---|---|---|---|---|---|---|
| Cats (n = 13) | Dogs (n = 15) | Humanb (n = 1) | Overall (n = 29) | Sensitivity % (n) | Resistance % (n) | ||
| H-IND | Range | 0.25–> 16 | 0.25–> 16 | 1 | 0.25–>16 | 72.4 (21/29) | 27.6 (08/29) |
| GM | 4.32 | 2.11 | – | 4 | |||
| V-IND | Range | 0.25–> 16 | 0.25–> 16 | > 16 | 0.25–> 16 | 37.9 (11/29) | 62.1 (18/29) |
| GM | 7.13 | 5.22 | – | 6.29 | |||
| H-COMP1 | Range | 0.5–> 16 | 0.5–> 16 | 0.5 | 0.5–> 16 | 69 (20/29) | 31 (09/29) |
| GM | 4 | 2.35 | – | 3.05 | |||
| H-COMP2 | Range | 0.5–> 16 | 0.5–> 16 | 4 | 0.5–> 16 | 69 (20/29) | 31 (09/29) |
| GM | 3.76 | 2.1 | – | 2.75 | |||
| V-COMP | Range | 0.25–> 16 | 0.5–> 16 | 8 | 0.25–> 16 | 51.7 (15/29) | 48.3 (14/29) |
| GM | 6.96 | 4.26 | – | 5.48 | |||
aH-IND (brand name for human use); V-IND (brand name for veterinary use); H-COMP1 and H-COMP2 (compounded for human use); V-COMP (compounded for veterinary use); GM geometric mean
bHuman sporotrichosis from Rio de Janeiro (RJ/Brazil)—IPEC 16969 strain code (Instituto de Pesquisa Evandro Chagas/FIOCRUZ, RJ, Brazil)
cCriteria of in vitro antifungal susceptibility according to the M38-A2 guideline [13], which MIC values < 4 μg/mL were considered sensitive, and MIC ≥ 4 μg/mL were considered resistant to itraconazole
Considering the criteria of antifungal efficacy (Table 2) and the findings of itraconazole-susceptible and itraconazole-resistant S. brasiliensis isolates (Table 3), we found that compounded formulations showed fungistatic and fungicidal activity against S. brasiliensis from feline and canine cases. Compounded drugs showed high efficacy of fungal inhibition (69%—20/29, for both H-COMP1 and H-COMP2), similarly to the brand-name itraconazole for human use (H-IND, 72.4%—21/29), with no significant difference among themselves. Although the compounded drug for veterinary use (V-COMP) has shown moderate efficacy (51.7%—15/29), it did not differ statistically to formulations categorized with high anti-Sporothrix brasiliensis activity (H-IND, H-COMP1, and H-COMP2). A low efficacy was noted in the brand-name formulation for veterinary use (V-IND, 37.9%—11/29), which differed statistically from H-IND, H-COMP1, and H-COMP2 (p < 0.05).
Feline isolates were less susceptible than canine isolate to the itraconazole formulations for the formulations H-COMP1 and H-COMP2 (p < 0.05). MIC values were higher for feline isolates (H-COMP1Geometric Mean (GM) = 4 μg/mL; H-COMP2GM = 3.76 μg/mL) than for canine isolates (H-COMP1GM = 2.35 μg/mL; H-COMP2GM = 2.1 μg/mL).
Regarding the purity of the capsules measured by HPLC-MS, the linearity of the analytical method was assessed by the coefficient of determination (R2) obtained with the calibration curve by the least-squares linear regression. Good linear calibration (R2 = 0.9969) was obtained in the studied range (20 to 2500 ng/mL for itraconazole). The LOD and LOQ were 8.48 ng/mL and 25.71 ng/mL, respectively. Itraconazole was found present in all tested formulations at different amounts. The concents of the active ingredient did not correspond to those described in the respective packaging. Itraconazole was present at a significantly lower concentration than expected, corresponding to only 38.8% (H-COMP2), 33.9% (V-COMP), 11% (H-IND), 4.7% (H-COMP1), and 0.78% (V-IND) of the concentration indicated by the manufacturer (Table 4). The degree of antifungal efficacy did not directly correlate with the observed concentration.
Table 4.
Measured itraconazole content (μg/mg) in the different products of brand-name and compounded formulations for human and veterinary use
| Itraconazole formulationsa | Manufacturer information | Measured (mean ± SD)b |
|---|---|---|
| H-IND | 100 mg | 11 mg (110.01 ± 0.29) |
| V-IND | 25 mg | 0.195 mg (7.81 ± 0.12) |
| H-COMP1 | 64 mg | 3 mg (47.00 ± 0.07) |
| H-COMP2 | 65 mg | 25.2 mg (387.70 ± 2.64) |
| V-COMP | 64 mg | 21.7 mg (338.44 ± 2.52) |
aH-IND (brand name for human use); V-IND (brand name for veterinary use); H-COMP1 (compounded for human use); H-COMP2 (compounded for human use); V-COMP (compounded for veterinary use)
bAverage of three replicates ± standard deviation
Discussion
Among the formulations for human use, the brand-name formulation H-IND was attributed to possessing high efficacy, corroborating the studies performed on humans [16–19] and feline [20, 21] isolates of S. brasiliensis. Most of the in vitro studies with itraconazole evaluating the efficacy of Sporothrix species were performed with brand-name products for human [19–22] and veterinary use [23] or with a product known to be of high purity [16, 17].
Meanwhile, in vitro studies with compounded itraconazole in Sporothrix species are scarce [24]. Yet, these products have been successfully employed in the treatment of human sporotrichosis [25], as well as for the treatment of cats and dogs [8]. Although in vivo studies are needed to assure the efficacy of these products against animal sporotrichosis, the low in vitro efficacy of some of the formulations may result in a lower dose than necessary if administered to an animal.
In the antifungal assays, each formulation was adjusted according to the M38-A2 guidelines [13], and the final concentrations ranged from 16 to 0.03 μg/mL for all tested itraconazole. The MIC values for H-IND, H-COMP1, and H-COMP2 were two to six times higher than the MIC values reported in the literature [16, 19–21], whereas for V-COMP and V-IND were until 15 times higher than these cited studies. Our study showed a variation in the degree of antifungal efficacy and that high MIC values were required for the tested fungal isolates, independent of the pharmaceutical origin of the formulations. This finding could indicate a greater difficulty to control this mycosis in dogs and cats from Southern Brazil.
Therapeutic failures after high doses of itraconazole in human cases [26] reflect the current worrying scenario for sporotrichosis control. It is known that the antifungal resistance has been of concern for the control of mycoses nowadays [27, 28] and that isolates of Sporothrix brasiliensis from feline [20, 22–24] and canine [22, 23] cases have been described as in vitro itraconazole-resistant isolates. Our study showed that 34.5% (10/29) of S. brasiliensis isolates were considered resistant to all tested formulations, which were derived from cats and dogs living in Pelotas (09/10), except for a feline from São Lourenço do Sul (01/10). This finding highlighted the emergence of itraconazole-resistant isolates from animal cases in Southern Brazil.
Antifungal resistance may arise when isolates can overexpress efflux pumps, like ATP-binding cassette (ABC) proteins, causing an efflux of the drug by exocytosis [27]. The ability of S. brasiliensis to grow even when high concentrations of itraconazole are applied may be a paradoxical effect, this is, an effect contrary to the desired. This phenomenon was reported in Candida spp. isolates treated with elevated concentrations of antifungals [29], including itraconazole [30], in which the adaptive responses were associated with the capacity to accumulate of chitin [28]. The resistance mechanism of Sporothrix species remains unknown, and further studies should be undertaken to evaluate the mechanism that leads to this ability.
Considering the temporal analysis, all animal isolates came from cases between 2005 and 2013 in Southern Brazil. Interestingly, all those considered as itraconazole-resistant isolates were from cases occurred between 2012 and 2013. This finding corroborates with Borba-Santos et al. [17], which also reported that isolates from recent epidemic cases in Rio de Janeiro (2011–2012) had higher MIC values compared with isolates from cases before that period.
According to a phylogenetic study performed by Rodrigues et al. [14], the strains of S. brasiliensis from the Rio Grande do Sul shared a distinct clone of strains of S. brasiliensis from São Paulo, Minas Gerais, Paraná, and Rio de Janeiro. This genotypic distinction in the extreme south of Brazil [14] may indicate a spread of S. brasiliensis clones with itraconazole-resistant genes among the animal isolates used in our study. This hypothesis could justify the findings of in vitro resistance to all itraconazole formulations and should be investigated in more detail.
Thus, it is suggested to carry out in vitro antifungal susceptibility tests with the chosen itraconazole, either brand-name or compounded, to verify its efficacy, mainly in cases of feline and canine sporotrichosis in Southern Brazil. Regarding itraconazole from compounding pharmacies, it is known that the quality of the formulations is regulated through Resolution 67 of the National Health Surveillance Agency [10]. However, the matrix pH and particle size [31], as well as the different ways to prepare a compounded formulation [32], are some factors that may influence the final product, giving rise to different degrees of quality. Furthermore, failures during the process can lead to the absence of the active ingredient in the compounded product [11].
A study revealed that the compounded capsules identified as fluconazole from an establishment did not show retention time similar to standard fluconazole by high-performance liquid chromatography [11], showing that the content in the capsule did not correspond to the product described. In our study, all formulations presented the active ingredient, showing the presence of itraconazole in its pure form (C35H38Cl2N8O4) by the HPLC-MS method.
However, all formulations presented itraconazole in a concentration lower than that described by the manufacturer. This finding could indicate degradation of the active ingredient [33]; however, no degradation products were identified in our LC-MS analyzes. It is believed that non-pure forms of itraconazole have been used to prepare them, such as hydrochloride itraconazole [34] or ditosylate salt of itraconazole [35], among others. Although extremely low concentrations of the pure form could justify a low antifungal efficacy, as observed in V-IND, the same was not noted for all formulations. This is because the brand-name (H-IND) and compound (H-COMP1 and H-COMP2) formulations for human use showed high antifungal efficacy. This finding highlighted that the concentrations of the pure form did not seem to directly influence the antifungal efficacy. Although it was not possible to determine the factors that influenced the anti-Sporothrix brasiliensis activity, comparative studies of intrinsic and extrinsic factors related to pharmaceutical formulation should be performed in brand-name and compounded itraconazole.
In this study, we demonstrated a variation in the degree of antifungal efficacy among the formulations on S. brasiliensis, highlighting H-IND, H-COMP1 and H-COMP2 with high efficacy; V-COMP with moderate efficacy and V-IND with low efficacy. Furthermore, the emergence of itraconazole-resistant S. brasiliensis isolates from feline and canine from Southern Brazil were shown for all formulations, regardless of the pharmaceutical origin. All formulations presented the pure form of itraconazole; however, the concentrations did not match the concentration indicated by the manufacturers. Antifungal efficacy was not directly correlated to the observed itraconazole concentration, suggesting that other components present in the matrix may be influencing either the antifungal activity or even the compound measurement. It is recommended to perform in vitro antifungal assays with the chosen itraconazole as a strategy for therapeutic purposes, regardless of pharmaceutical origin. Furthermore, the variable itraconazole contents found among the analyzed formulations points to the need for requiring a stringent quality control to assure the itraconazole concentration.
Acknowledgments
We thank Dr. Zoilo Pires de Camargo (Universidade Federal de São Paulo, Brazil) for the biomolecular analysis; and Dr. João Luiz Zani (Universidade Federal de Pelotas, Brazil) for providing one of the itraconazole used in this study. The authors are grateful for the scholarships provided by CAPES, FAPERGS, and CNPq.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
This is not applicable. This paper does not contain any studies with experimental animals.
Informed consent
This is not applicable. This manuscript does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rodrigues AM, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP (2020) The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia.:1–30. 10.1007/s11046-020-00425-0 [DOI] [PubMed]
- 2.Poester VR, Mattei AS, Madrid IM, Pereira JTB, Klafke GB, Sanchotene KO, Brandolt TM, Xavier MO. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health. 2018;65:815–821. doi: 10.1111/zph.12504. [DOI] [PubMed] [Google Scholar]
- 3.Sanchotene KO, Madrid IM, Klafke GB, Bergamashi M, Terra PPD, Rodrigues AM, de Camargo ZP, Xavier MO. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses. 2015;58:652–658. doi: 10.1111/myc.12414. [DOI] [PubMed] [Google Scholar]
- 4.Pereira SA, Passos SRL, Silva JN, Gremiao IDF, Figueiredo FB, Teixeira JL, Monteiro PCF, Schubach TMP. Response to azolic antifungal agents for treating feline sporotrichosis. Vet Rec. 2010;166:290–294. doi: 10.1136/vr.b4752. [DOI] [PubMed] [Google Scholar]
- 5.Reis ÉG, Schubach TM, Pereira SA, Silva JN, Carvalho BW, Quintana MS, Gremião ID. Association of itraconazole and potassium iodide in the treatment of feline sporotrichosis: a prospective study. Med Mycol. 2016;54:684–690. doi: 10.1093/mmy/myw027. [DOI] [PubMed] [Google Scholar]
- 6.Madrid IM, Mattei A, Martins A, Nobre M, Meireles M. Feline sporotrichosis in the southern region of Rio Grande do Sul, Brazil: clinical, zoonotic and therapeutic aspects. Zoonoses Public Health. 2010;57:151–154. doi: 10.1111/j.1863-2378.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 7.Barros MBDL, Schubach TMP, Coll JO, Gremião ID, Wanke B, Schubach AO. Esporotricose: A evolução e os desafios de uma epidemia. Rev Panam Salud Publica. 2010;27:455–460. [PubMed] [Google Scholar]
- 8.Nakasu CCT (2018) Esporotricose: aspectos clínicos e terapêuticos, correlação com retroviroses e susceptibilidade a compostos químicos. [Master’s dissertation]. Pelotas: Universidade Federal de Pelotas, Faculdade de Veterinária, UFPEL
- 9.Renschler J, Albers A, Sinclair-Mackling H, Wheat LJ. Comparison of compounded, generic, and innovator-formulated itraconazole in dogs and cats. J Am Anim Hosp Assoc. 2018;54(4):195–200. doi: 10.5326/JAAHA-MS-6591. [DOI] [PubMed] [Google Scholar]
- 10.Agência Nacional de Vigilância Sanitária (Brasil). Resolução n° 67, de 8 de outubro de 2007. Boas Práticas de Manipulação de Preparações Magistrais e Oficinais para Uso Humano em Farmácias. Diário Oficial da União, 8 out 2007; Seção 1
- 11.Markman BEO, Koschtschak MRW, Uessugui O, Magnelli RF, Wu EM (2009) Identificação de antifúngicos azólicos em produtos manipulados por CLAE-UV. Bol Epidemiol Paul 6(63):15–19
- 12.Mota TF, Soares AF. Análise Físico Química de Cápsulas Manipuladas de Fluconazol 150 mg. Rev Cient Faminas. 2012;8(3):1–13. [Google Scholar]
- 13.Clinical and Laboratory Standard Institute . Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved M38-A. 2. CLSI: Wayne; 2008. p. 52. [Google Scholar]
- 14.Rodrigues AM, de Melo TM, de Hoog GS, et al. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Luz SR, Pazdiora PC, Dallagnol LJ, Dors GC, Chaves FC. Mycotoxin and fungicide residues in wheat grains from fungicide-treated plants measured by a validated LC-MS method. Food Chem. 2017;220:510–516. doi: 10.1016/j.foodchem.2016.09.180. [DOI] [PubMed] [Google Scholar]
- 16.Almeida-Paes R, Brito-Santos F, Figueiredo-Carvalho MHG, Machado ACS, Oliveira MME, Pereira SA, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz. 2017;112:376–381. doi: 10.1590/0074-02760160527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borba-Santos LP, Rodrigues MA, Gagini TB, et al. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol. 2015;53:178–188. doi: 10.1093/mmy/myu056. [DOI] [PubMed] [Google Scholar]
- 18.Stopiglia CDO, Magagnin CM, Castrillón MR, Mendes SD, Heidrich D, Valente P, Scroferneker ML. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med Mycol. 2014;52:56–64. doi: 10.3109/13693786.2013.818726. [DOI] [PubMed] [Google Scholar]
- 19.Marimon R, Serena C, Gene J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52:732–734. doi: 10.1128/aac.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchotene KO, Brandolt TM, Klafke GB, Poester VR, Xavier MO. In vitro susceptibility of Sporothrix brasiliensis: comparison of yeast and mycelial phases. Med Mycol. 2017;55:869–876. doi: 10.1093/mmy/myw143. [DOI] [PubMed] [Google Scholar]
- 21.Brilhante RS, Rodrigues AM, Sidrim JJ, et al. In vitro susceptibility of antifungal drugs against Sporothrix brasiliensis recovered from cats with sporotrichosis in Brazil. Med Mycol. 2016;54:275–279. doi: 10.1093/mmy/myv039. [DOI] [PubMed] [Google Scholar]
- 22.Waller SB, Peter CM, Hoffmann JF, Picoli T, Osório LG, Chaves F, Zani JL, de Faria RO, de Mello JRB, Meireles MCA. Chemical and cytotoxic analyses of brown Brazilian propolis (Apis mellifera) and its in vitro activity against itraconazole-resistant Sporothrix brasiliensis. Microb Pathog. 2017;105:117–121. doi: 10.1016/j.micpath.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Waller SB, Hoffmann JF, Madrid IM, Picoli T, Cleff MB, Chaves FC, Zanette RA, de Mello JRB, de Faria RO, Meireles MCA. Polar Origanum vulgare (Lamiaceae) extracts with antifungal potential against Sporothrix brasiliensis. Med Mycol. 2018;56:225–233. doi: 10.1093/mmy/myx031. [DOI] [PubMed] [Google Scholar]
- 24.Waller SB, Serra EF, Silva AL, et al. Eficácia in vitro de itraconazóis de referência e de uso veterinário frente ao Sporothrix brasiliensis de origem felina. Sci Anim Health. 2017;5:34–35. doi: 10.15210/sah.v5i4. [DOI] [Google Scholar]
- 25.Soto PA, Cavallera E, Zerpa O. Esporotricosis Cutánea Fija: Reporte de un caso en un lactante mayor. Dermatol Venez. 2010;48(1–2):32–34. [Google Scholar]
- 26.Crestani L, Souza BCE, Kakizaki P, Valente NYS. Therapeutic failure with itraconazole in sporotrichosis due to bariatric surgery. An Bras Dermatol. 2020;S0365-0596(20):30033–30037. doi: 10.1016/j.abd.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha MFG, Bandeira SP, de Alencar LP, Melo LM, Sales JA, Paiva MAN, Teixeira CEC, Castelo-Branco DSCM, Pereira-Neto WA, Cordeiro RA, Sidrim JJC, Brilhante RSN. Azole resistance in Candida albicans from animals: highlights on efflux pump activity and gene overexpression. Mycoses. 2017;60:462–468. doi: 10.1111/myc.12611. [DOI] [PubMed] [Google Scholar]
- 28.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell. 2008;7:747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcos-Zambrano LJ, Escribano P, Sánchez-Carrillo C, Bouza E, Guinea J. Frequency of the paradoxical effect measured using the EUCAST procedure with micafungin, anidulafungin, and caspofungin against Candida species isolates causing candidemia. Antimicrob Agents Chemother. 2016;61:e01584–e01516. doi: 10.1128/AAC.01584-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arikan S, Sancak B, Hascelik G. In vitro activity of caspofungin compared to amphotericin B, fluconazole, and itraconazole against Candida strains isolated in a Turkish University hospital. Med Mycol. 2005;43:171–178. doi: 10.1080/13693780410001731565. [DOI] [PubMed] [Google Scholar]
- 31.Boothe DM. Veterinary compounding in small animals: a clinical pharmacologist’s perspective. Vet Clin Am Small Anim Pract. 2006;36:1129–1173. doi: 10.1016/j.cvsm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Okuyama SSK (2010) Proposta de Padronização para o Preparo de Medicamentos nas Formas Farmacêuticas Semi- Sólidas e Líquidas. [Master’s dissertation]. Curitiba: Pontifícia Universidade Católica do Paraná, Centro de Ciência Biológica e da Saúde, UCP
- 33.Parikh SK, Dave JB, Patel CN, Ramalingan B. Stability-indicating high-performance thin-layer chromatographic method for analysis of itraconazole in bulk drug and in pharmaceutical dosage form. Pharm Methods. 2011;2(2):88–94. doi: 10.4103/2229-4708.84442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kushwaha A, Jacob M, Shiva Kumar HN, Hiremath S, Aradhya S, Repka MA, Murthy SN. Trans-ungual delivery of itraconazole hydrochloride by iontophoresis. Drug Dev Ind Pharm. 2015;41(7):1089–1094. doi: 10.3109/03639045.2014.927481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar N, Shishu BG, Kumar S, Jana AK. Ditosylate salt of itraconazole and dissolution enhancement using cyclodextrins. AAPS PharmSciTech. 2012;13(3):863–874. doi: 10.1208/s12249-012-9804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]