Abstract
As one of the most promising fixators developed for anterior cruciate ligament (ACL) reconstruction, biodegradable magnesium (Mg)-based interference screws have gained increasing attention attributed to their appropriate modulus and favorable biological properties during degradation after surgical insertion. However, its fast degradation and insufficient mechanical strength have also been recognized as one of the major causes to limit their further application clinically. This review focused on the following four parts. Firstly, the advantages of Mg or its alloys over their counterparts as orthopaedic implants in the fixation of tendon grafts in ACL reconstruction were discussed. Subsequently, the underlying mechanisms behind the contributions of Mg ions to the tendon-bone healing were introduced. Thirdly, the technical challenges of Mg-based interference screws towards clinical trials were discussed, which was followed by the introduction of currently used modification methods for gaining improved corrosion resistance and mechanical properties. Finally, novel strategies including development of Mg/Titanium (Ti) hybrid fixators and Mg-based screws with innovative structure for achieving clinically customized therapies were proposed. Collectively, the advancements in the basic and translational research on the Mg-based interference screws may lay the foundation for exploring a new era in the treatment of the tendon-bone insertion (TBI) and related disorders.
Keywords: Magnesium, ACL reconstruction, Interference screw, Degradation, Mechanical properties
Graphical abstract
Highlights
-
•
The advantages of Mg or its alloys over their counterparts as orthopaedic implants in the fixation of tendon grafts in ACL reconstruction were discussed.
-
•
The underlying mechanisms behind the contributions of Mg ions to the tendon-bone healing were introduced.
-
•
The technical challenges and strategies of Mg-based interference screws towards clinical trials were discussed.
-
•
Promising directions for development of novel Mg-based fixators to achieve customized therapies in ACL reconstruction clinically were proposed.
1. Introduction
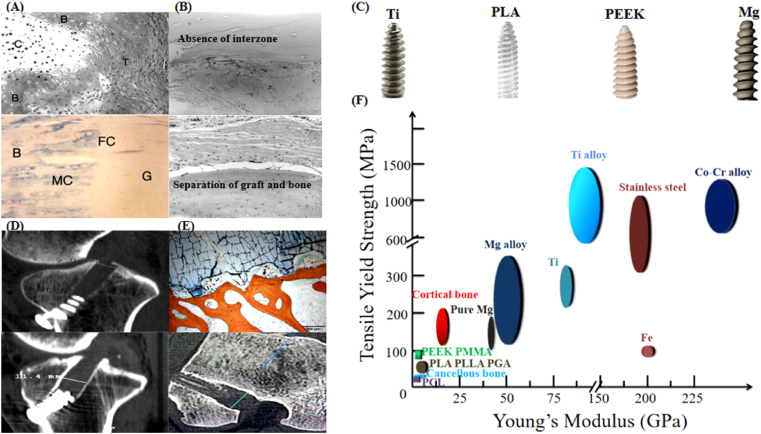
Anterior cruciate ligament (ACL) tear is one of the most common joint injuries, especially for those with high level of physical activities. ACL reconstruction can rapidly restore the knee function of the patients, thereby reducing the occurrence of osteoarthritis [1]. However, the estimated failure rate after ACL reconstruction is still higher than 10% [2]. The poor tendon-bone healing has been increasingly considered as one of the major causes for the unsatisfactory treatment outcomes [3]. In contrast with the interzone structure in the normal healed tendon-bone interface (Fig. 1A) [3], the failure of osteointegration of the tendon grafts into bone tunnels can gradually lead to the graft slippage (Fig. 1B), thus, ultimately impairing the knee stability. Traditionally, interference screws have been metallic referring to stainless steel (SS), particularly 316L and its variants, and titanium, which have maintained popularity in fixation devices because of a favorable combination of sufficient mechanical strength, corrosion resistance and cost effectiveness [4,5]. However, in recent years, an increasing number of studies have indicated that the drawbacks of these metallic interference screws have raised serious clinical concerns in ACL reconstruction [3]. For instance, the mismatch of modulus of elasticity, which may easily cause stress-shielding effects, and unsatisfactory osseointegration can deteriorate bone integration with tendon graft and thus cause graft slippage (Fig. 1C and D) [[6], [7], [8]]. Most recently, an innovative strategy was developed to modify rough surface and interconnecting porous structure of Ti interference screws through additive manufacturing (AM) technology [9]. Although the novel AM interference screws with favorable stiffness and porosity demonstrated improved tendon-bone-implant integration, the impaired mechanical performance may be a disadvantage preventing the adaptation of 3D printing into product development [10]. In addition, these metallic screws cause imaging artifacts, thus, precluding proper assessment by MRI [11]. Synthetic polymers have been widely accepted as the alternative to the traditional metallic counterparts in orthopaedics at low load-bearing skeletal sites [12,13]. Ultra-high molecular weight polyethylene (UHMWPE), polyurethanes (PU), poly(methylmethacrylate) (PMMA), and polyetheretherketone (PEEK) are the most common non-degradable polymers approved by United States Food and Drug Administration (US FDA) widely used in orthopaedic field [14], whereas poly(L- or D,l-lactic acid), poly(glycolic acid), and polycaprolactones (PCL) are widespread absorbable polymers as orthopaedic fixators with US FDA approval [12]. For example, a two-year clinical analysis of PEEK interference screws showed equivalent clinical performance to titanium counterparts [15]. Given an elastic modulus to the cortical bone, and the absence of metal artefact on MRI, these non-degradable polymers are superior to the metal devices as potential materials developed for interference screws in ACL reconstruction. However, the toxic residual monomers and wear debris from these non-degradable polymer implants may easily induce unexpected local risks for tissue healing [16]. The resorbable polymers demonstrate similar advantages as the non-degradable polymers. More importantly, the monomers released from the resorbable polymers during in vivo degradation exhibit high biocompatibility, thus, these polymers have been extensively used in orthopaedics, including ACL reconstruction as interference screws [16]. In addition, the mechanical properties of these degradable polymers can be remarkably improved through forming composites (e.g. polymer-Mg wire complex, etc.) for potential use as orthopaedic implants in the load-bearing skeletal sites [17]. However, the bulk erosion of the resorbable polymers leads to the accumulation of the acidic intermediate degradation products, thus, making it susceptible to induce aseptic inflammatory reactions. This subsequently results in the overexpression of the fibrous tissue at the peri-screw region, leading to impaired healing (Fig. 1E). Natural polymers, such as collagen and chitosan, demonstrate excellent biocompatibility, however, no orthopaedic implants have been developed and approved by US FDA as potential interference screws because of their low mechanical strength and possible inflammatory reactions caused by antigens and difficulties in processing [18].
Fig. 1.
The limitations and drawbacks of the existing orthopaedic implants in ACL reconstruction. (A) Representative histological images showing the normal tendon-bone healing with bony ingrowth towards the interface structure of the fibrous tissue or fibrocartilage after reconstruction. Figure adapted from Refs. [38,39]. (B) Representative histological images showing the healing failure between the tendon graft and bone after reconstruction. Figure adapted from Ref. [40]. (C) Representative images of Ti, PLA, PEEK, and Mg based interference screws. (D) Representative radiographic images showing the bone tunnel enlargement in patients after ACL reconstruction with currently used metallic interference screws. Figure adapted from Ref. [41]. (E) The degradation of PLA screws producing a significant amount of oligomers, resulting in aseptic inflammation around them, eventually leading to enlarged tendon-bone interface. Figure adapted from Ref. [42]. (F) Summary of the Young's modulus and tensile yield strength of natural bone, FDA-approved polymers and metals in orthopaedics, along with Mg or its alloys.
In the past few decades, bioceramics including hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP) or their mixtures with most similar composition to the inorganic component of bone, have been widely used in bone implants due to their advantages of biocompatibility and osteoinductive properties [19,20]. However, their poor mechanical properties (e.g. low fracture toughness, brittleness and extremely high stiffness, etc. [19]) have been considered as the major challenges for their use in load-bearing skeletal sites. Biodegradable metals (Zn, Fe, Mg) may be a good choice to address the aforementioned concerns. For instance, the recently developed Zn-based alloy systems showed outstanding mechanical strength and distinct osteopromotive property [21], exhibiting great potential as novel orthopaedic implants. However, the mechanical strength of pure Zn is low, which exceeds the minimum standards for load-bearing application. In addition, great attention should be paid to the Zn toxicity after degradation of Zn-based implants [22]. The approach of using iron (Fe) or its alloys has been also studied by increasing researchers in orthopaedic field because of their excellent biocompatibility and desirable mechanical properties [23]. As the magnetic nature of Fe and the production of iron oxide degradation by-products, there are still challenges for the further clinical use of Fe-based orthopaedic implants [24]. Taken together, biocompatibility, mechanical properties, and medical visualization are the major clinical concerns for either bioceramics or degradable metals as potential orthopaedic devices. In the last decades, the research and development (R&D) on the biodegradable magnesium (Mg)-based orthopaedics has attracted enormous attention from material engineers, preclinical scientists and clinicians [[25], [26], [27], [28], [29]], thus, igniting the hope of successfully addressing the flaws of the currently available commercial interference screws. Mg or its alloys exhibit good biocompatibility and appropriate Young's modulus close to the natural bone (Fig. 1F), indicating their suitability as promising orthopaedic implants [30]. More importantly, increasing evidences demonstrate that the Mg ions released from the Mg-based implants after surgical implantation in vivo can promote bone regeneration and accelerate healing of the bone diseases, showing great advantages over bio-inert PEEK counterparts as potential interference screws during the graft healing. In recent years, great progress has been achieved in the translational work of Mg-based orthopaedic implants. Germany is the first country to apply CE approved MAGNEZIX series compression screws made of MgYReZr alloy in clinical trials for hallux valgus surgery [31]. Then, a clinical study on the use of high-purity Mg (HPM) screws for fixation of autologous vascularized bony flaps to treat avascular necrosis of the femoral head was reported in China in 2013 [32]. After that, a multicenter clinical trial was approved by National Medical Products Administration (NMPA, China) to assess the safety and efficacy of HPM screws in 2019 [33]. In Korea, the K-MET screws made of MgCaZn alloy, developed by U&I company, was approved by Korea Food and Drug Administration in 2015 for repair of distal radius fractures [34]. Collectively, the favorable clinical outcomes further inspired the researchers and clinicians in sports medicine to develop the Mg-based interference screws for fixation of the tendon graft in ACL reconstruction [[35], [36], [37]]. Although the Mg-based interference screws can enhance the bony ingrowth towards the tendon-bone interface, insufficient mechanical strength and inappropriate degradation rate still pose major challenges for their further clinical applications. Therefore, this review introduces the in vivo degradation characteristics, clinical concerns associated with ACL reconstruction and potential repair mechanisms involved during tendon-bone healing, followed by summarizing the current modification methodologies to address the aforementioned drawbacks. Finally, novel strategies to develop the clinically available Mg/Ti hybrid fixators to support the tendon-bone healing after ACL reconstruction have been discussed.
2. Development of novel Mg-based interference screws
2.1. Appropriate Young's modulus
As shown in Table 1, Mg or its alloys exhibit the Young's modulus close to the natural bone, indicating the potential contributions of the Mg-based interference screws in attenuation of the peri-tunnel bone loss after ACL reconstruction [36].
Table 1.
Comparison of the material properties of natural bone, Mg and Ti.
| Material | Young's modulus (GPa) | Poisson's ratio (ν) |
|---|---|---|
| Trabecular bone | 1.0–1.4 | 0.3 |
| Pure Mg | 45 | 0.35 |
| Mg alloy | 35–69 | 0.27–0.35 |
| Commercial Ti | 114 | 0.3 |
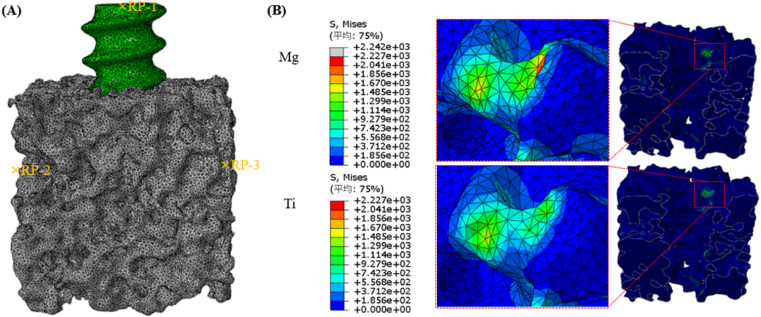
As shown in Fig. 2A, a 3D simplified solid model was designed to mimic femur or tibia implanted with commercial Ti or Mg-based interference screws. Finite element analysis (FEA) was subsequently conducted to compare stress distribution in the peri-screw bone tissue between the two groups (Fig. 2B). Compared to the Ti group, the Mg group demonstrated significantly higher maximum stress (nearly 50% increase) and larger stress distribution in the peri-screw bone tissue, indicating that the Mg-based interference screws are able to attenuate the stress-shielding induced bone loss in the peri-tunnel after ACL reconstruction.
Fig. 2.
Appropriate Young's modulus of the Mg-based orthopaedic implants. (A) FEA model of the trabecular bone with insertion of an interference screw under 1000 N loading condition from the horizontal direction. The bone model is fixed by restricting all 6° of freedom (DOF), while the interaction between the screw and bone is fixed using a binding constraint. (B) Compared to the Ti group in the FEA model, the Mg group exhibits higher von Mises stress distribution in the peri-screw bone tissue. Further, the maximum stress around the contacting bone along the force direction is 2227 MPa and 1670 MPa for the Mg and the Ti groups, respectively.
2.2. Enhancement in tendon-bone healing
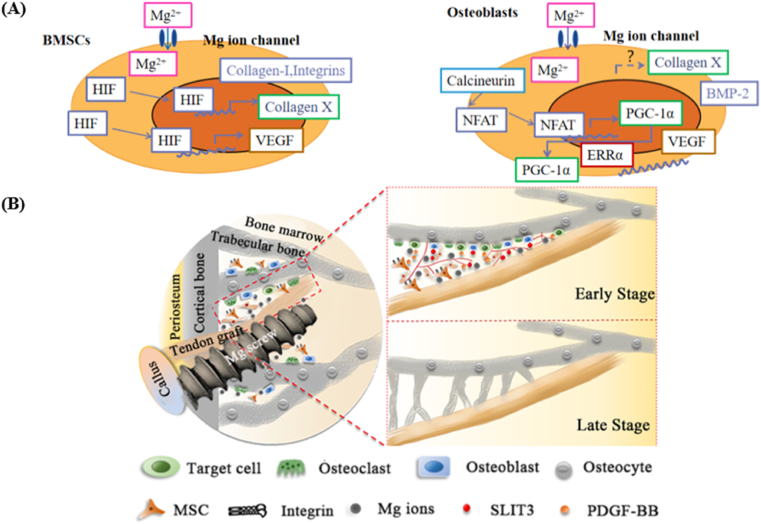
Mg or its alloys is classified as a type of “biodegradable metals” from the aspect of biofunctional materials because of their osteopromotive properties in orthopaedics [43]. Yoshizawa et al. reported that the addition of 10 mM Mg ions in the cell culture medium could significantly enhance the osteogenic differentiation capability of BMSCs and osteoblasts via upregulating collagen-X and vascular endothelial growth factor (VEGF), achieved through hypoxia-inducible factor 2a (HIF-2a) and peroxisome proliferator-activated receptor gamma coactivator (PGC) -1α, respectively (Fig. 3A) [44]. In addition, Wang et al. reported that the Mg ions at the same dosage significantly activated integrin α5β1 and focal adhesion kinase pathways in BMSCs [37]. Recently, Hung et al. observed that the canonical Wnt/β-catenin pathway could be activated in BMSCs treated with 10 mM Mg ions, confirmed by the increased LEF1 and Dkk1 [45]. The unique contribution of the Mg ions in bone regeneration underline the potential use of the Mg-based interference screws in tendon-bone interface healing. Therefore, Cheng et al. conducted ACL reconstruction in rabbits using high-purity Mg interference screws and found more fibrocartilaginous entheses regeneration owing to the significantly higher expression of BMP2 and VEGF under stimulation of the Mg ions [35]. In another study by Wang et al., the released Mg ions from the high-purity Mg interference screws favored the secretion of the transforming growth factor-β1(TGF-β1) and platelet-derived growth factor BB (PDGF-BB), which recruits more BMSCs for tendon-bone healing, thereby contributing to angiogenesis and bony ingrowth at the tendon-bone interface (Fig. 3B) [37].
Fig. 3.
Favorable osteopromotive functions of the Mg-based orthopaedic implants. (A) Higher extent of Mg ions can directly promote the osteogenic differentiation-related genes including BMP-2 and VEGF of BMSCs as well as osteoblasts via HIF-2α and PGC-1α-dependent mechanisms. Figure adapted from Ref. [44]. (B) The released Mg ions from the Mg-based screws enhance the recruitment, adhesion and osteogenic differentiation of BMSCs, along with the secretion of PDGF-BB and TGF-β1, thereby, contributing to angiogenesis and osteogenesis in the tendon-bone interface, ultimately leading to the improved bone ingrowth towards the tendon graft [37].
3. Challenges encountered by Mg-based interference screws for clinical application
3.1. Challenges in control of appropriate degradation behavior
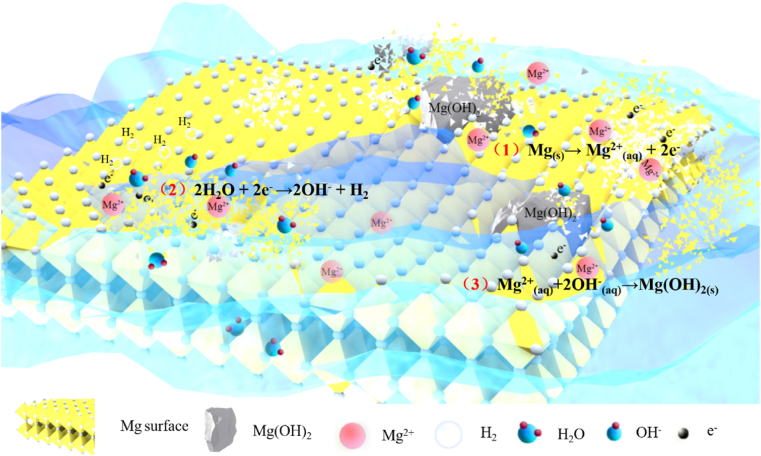
Mg metal has a lower standard electrode potential than hydrogen (−2.372 V vs. 0 V) and can be degraded into hydrogen gas and free Mg ions in an aqueous solution under standard condition (Fig. 4). The released hydrogen gas in the subcutaneous tissue escaped much faster than that trapped in the bone marrow cavity based on the measurement results by the electrochemical H2 sensor [46,47], which may lead to the formation of gas voids around the implants due to the gas accumulation. Therefore, the control of gas formation during the degradation of Mg-based implants is very critical, otherwise the tissue healing quality may be impaired because of the deteriorated cell migration, adhesion, and blood/bone ingrowth in the presence of gas voids at the interface between the implants and bone [48]. In addition to hydrogen gas, the release of the Mg ions in the alkaline conditions leads to the formation of the loose Mg(OH)2 layer [49]. The corresponding reactions are shown below:
| Anodic reaction: Mg(s)→ Mg2+(aq) + 2e− | (1) |
| Cathodic reaction: 2H2O + 2e− →2OH− + H2 | (2) |
| Overall reaction: Mg2+(aq)+2OH−(aq)→Mg(OH)2(s) | (3) |
Fig. 4.
Corrosion mechanism of Mg-based alloys in in vitro condition.
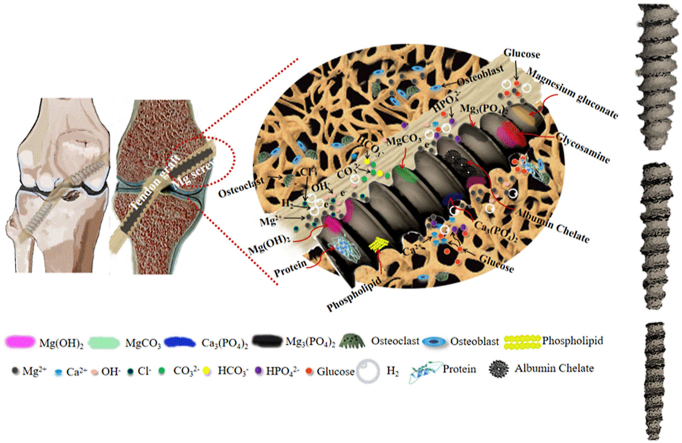
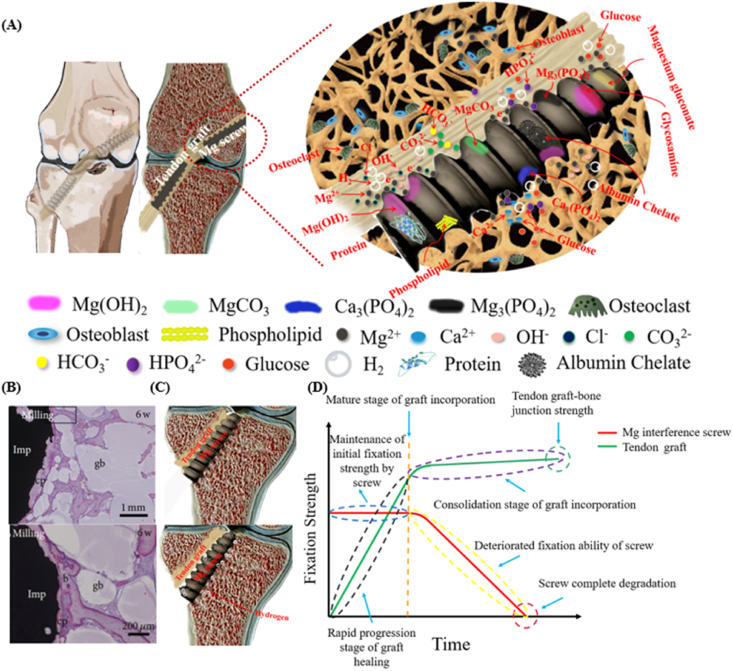
The degradation of the Mg-based interference screws is accompanied by the release of the Mg ions, the production of hydrogen gas, as well as alkaline substances, thereby leading to the alteration in the peri-screw micro-environment [50], ultimately affecting the tendon graft-bone healing quality. In human body, the inorganic ions (e.g. Ca2+, Cl−, OH−, HPO42−, H2PO4−, HCO3−, and CO32−) [51] and organic molecules (e.g. glucose, amino acids, and protein) [52,53] act as the major factors influencing the degradation of the Mg-based implants (Fig. 5A).
Fig. 5.
Challenges of Mg-based interference screws in clinical application. (A) The degradation behavior of the Mg-based interference screws in physiological condition involves the release of Mg ions, H2 evolution, and production of OH−, contributing to the formation of inorganic substances containing Mg(OH)2, MgCO3, Ca3 (PO4)2, and Mg3 (PO4)2 as well as organic products composed of magnesium gluconate, glycosamine, and albumin chelate. Importantly, the presence of amino acids, proteins, and lipids may alter the degradation rate of the Mg-based screws due to the deposition of the organic layers. (B) Representative histological images demonstrating the fast degradation of the Mg-based implants containing gas voids (b: bone, gb: gas voids, cp: corrosion product, square: area shown under high magnification in the below image). Figure adapted from Ref. [62]. (C) Fast degradation of the Mg-based interference screws in vivo leading to poor fixation. (D) Illustration of desirable Mg-based interference screws with favorable degradation behavior to maintain their mechanical integrity for sufficient fixation during the graft healing process in ACL reconstruction. Figure adapted from Ref. [63].
However, Mg(OH)2 is swiftly converted into soluble MgCl2 due to the Cl− attack at the OH− site in the body fluid [54]. The interaction of the free Mg ions with CO32− and HCO3− favors the passivation of the surface of the Mg-based implants via formation of the insoluble MgCO3 precipitates [55]. Besides, the presence of phosphate ions leads to precipitation of high-density amorphous magnesium phosphate (Mg3(PO4)2) and calcium phosphate (Ca3(PO4)2) to decelerate the Mg corrosion [51,56]. Apart from this, it is of note that the organic molecules in the human body are also involved in the degradation of the Mg-based implants. For instance, as one of the most common nutrients, glucose can rapidly transform into gluconic acid. On one hand, glucose at a low dose coordinates Ca2+ ions and subsequently increases the formation of Ca–P precipitates, thus, promoting the corrosion resistance of Mg [57]. On the other hand, low pH environment caused by the glucose acid accelerates Mg corrosion by the formation of magnesium gluconate ((CH2OH(CHOH)4COO)2Mg) [58]. Proteins/amino acids also participate in the in vivo degradation process of the Mg-based implants. The individual effect of proteins/amino acids on Mg degradation may be different due to the significantly different structures of various proteins/amino acids [59]. Herein, we take albumin for example to discuss its effect on Mg degradation. At a low concentration of albumin, the degradation rate of Mg is accelerated due to the metal chelating effect. Interestingly, for the albumin dose over a specific level, its effect on the degradation rate of Mg turns inhibitive due to the deposition of an integrated absorbed layer [53]. However, as the albumin level exceeds the maximal absorption amount on the surface of Mg, the further addition of albumin into the solution can impair their corrosion resistance as a result of protein aggregation [60,61]. In addition, the presence of glucose may influence the effect of proteins/amino acids on Mg degradation. The formation of glucosamine by glucose with proteins may decrease the degradation rate of Mg owing to rapid absorption of proteins/amino acids on the surface [60,61]. In summary, the current knowledge about the degradation products of the Mg-based implants in vivo is depicted in Fig. 5A.
In case the degradation rate of the Mg-based interference screws is above the expected window, the fixation strength impairment of the tendon graft after reconstruction may lead to surgical failure due to knee instability. Apart from the concerns associated with mechanical integrity, the reduction of gas cavities caused by the released hydrogen gas, closely related with the degradation rate of the Mg-based interference screws, is also considered as one of the main challenges for clinical trials [62](Fig. 5B and C). Therefore, the clinically available Mg-based interference screws must possess the desirable degradation behavior to match tissue healing without loss of mechanical integrity prior to complete healing (Fig. 4D).
3.2. Challenges in developing HPM interference screw with appreciated torque properties
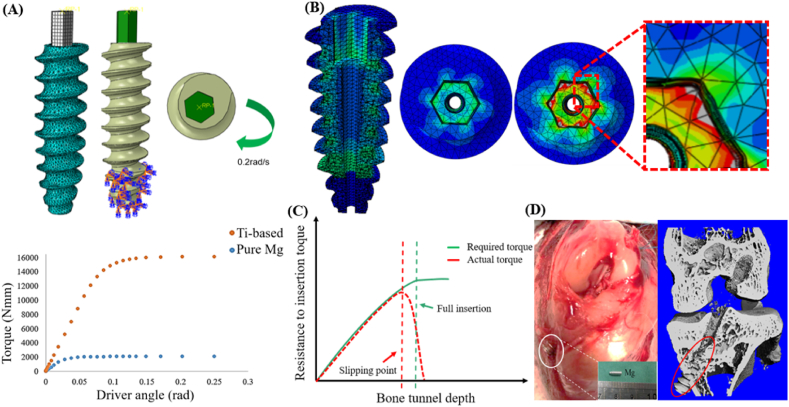
Although Mg-based alloys have better mechanical properties, the presence of second phases may impair their corrosion resistance due to internal galvanic corrosion. More importantly, the abundant synovial fluid in the joint cavity of knee can further accelerate the degradation rate of Mg-based interference screws if applied in sports medicine, so HPM, which possesses excellent corrosion resistance, has been increasingly considered as one of the most promising metals suitable for fabrication into interference screws. However, insufficient insertion torque is the major challenge for HMP interference screws prior to clinical use. A FEA model was set up to compare the maximum torque and stress distribution between the Ti and pure Mg interference screws (Fig. 6A), by employing the elastic constitutive model. Prior to simulation, the material properties of the Mg interference screw were defined as follows: yield strength σy = 24 MPa, Young's modulus E = 44.8 GPa, and Poisson ration n = 0.35, while for the Ti interference screw, these were defined as follows: σy = 910 MPa, E = 120 GPa and n = 0.342 [36,64]. Compared to the Ti interference screw, the Mg screw exhibited nearly 7-fold reduction in the maximum torque (Fig. 6A), indicating higher risks of surgical failure caused by slippage between screwdriver and screw head due to the deformation at the contacting sites (Fig. 6B). Therefore, the gap between the actual torque of screw and the required torque for full insertion (Fig. 6C) can easily damage screw head, leading to incomplete insertion of screws in bone tunnels (Fig. 6D) [60].
Fig. 6.
Insufficient torque for Mg-based interference screws. (A) A FEA model established to compare the maximal torque between Ti-based and pure Mg interference screws. (B) The model to illustrate the deformation in the internal hexagonal heads. (C) The distortion of screw head causes loss of screw torque, leading to insertion failure for fixation of the tendon graft. (D) Insufficient torque of pure Mg interference screws results in surgical failure due to the damage in the screw head.
4. Current strategies to address challenges of Mg or its alloys for potential use as orthopaedic implants
Tremendous efforts have been made in last decades to overcome the clinical concerns associated with inappropriate degradation behavior and insufficient mechanical properties of Mg or its alloys for orthopaedic applications (Table 2 and Table 3).
Table 2.
Alloying methods applied for improvement of mechanical or corrosion properties of Mg or its alloys.
| Phase | Type | Composition | Results (pre-treatment vs.post-treatment) | References |
|---|---|---|---|---|
| Crystalline | Pure Mg | Pure Mg (99.9 wt%) vs. high-purity Mg (99.99 wt%) | Five-fold increase in corrosion resistance due to the reduction of impurities | [108] |
| Binary | Mg–Zn | 58% higher than pure Mg in bending strength | [66] | |
| Mg–Ca | Dramatic increase in ultimate tensile strength | [67] | ||
| Mg–Ag | 100% increase in ultimate tensile strength | [68] | ||
| Ternary | Mg–Zn–Sr | Modulation of Mg–Zn alloys by addition of Sr | [36] | |
| Mg–2Sr–Ca | Improved corrosion resistance by addition of Ca or Zn into Mg–2Sr | [71] | ||
| Mg–2Sr–Zn | ||||
| Mg–Zn–Ag | Improvement in hardness while decrease in corrosion resistance by addition of Ag into Mg–Zn alloys | [72] | ||
| Mg–Zn–Ca | Improvement in corrosion resistance by addition of Ca into Mg–Zn alloys | [73] | ||
| Quaternary or above | Mg-Nd-Zn-Zr | Balanced mechanical strength and corrosion resistance by addition of Nd and Zr | [109] | |
| Mg-0.6Zr-0.5Sr-xSc | 25% increase in the ultimate tensile strength by addition of Sc in Mg–Zr–Sr alloys, | [79] | ||
| Mg–Li–Al-RE442 | Over 3.5-year degradation period vs. 12 weeks for ZX50 and approximate 24 weeks for WZ21 as intramedullary pins | [76] | ||
| Amorphous | Ternary | Mg–Zn–Ca alloy | Significant improvement in corrosion resistance compared to conventional crystalline Mg alloys | [81,82] |
| Amorphous & Crystalline dual-phase structure | Quaternary or above | Mg–Cu–Y alloy | SNDP-GC material increases the ultimate yield stress by nearly 8 times | [101] |
Table 3.
Surface modification methods applied for improvement of mechanical or corrosion properties of Mg or its alloys.
| Category | Sub-category | Surface composition | Method | Testing environment | Results (post-treatment vs. pre-treatment) | Shortcomings | References |
|---|---|---|---|---|---|---|---|
| Physical deposition | Polymer coatings | PCL | Deposition layer by layer by a custom designed spraying device | In vitro | 60%–80% decrease in corrosion rate while 50% increase in the remaining compressive strength after 60 days of immersion | Insufficient bonding strength between the substrate and coating layer | [97] |
| PLLA, PHB, PHBV, or PLGA | Spin coating | In vitro | Over a half reduction of weight loss for the coated samples after 4-week immersion test | [110] | |||
| Chitosan-based nanofibers with incorporated silver sulfadiazine and carbon nanotubes | Electrospinning technique | In vitro | Two times higher in charge transfer resistance | [111] | |||
| PLLA/AKT-DOXY | Electrospinning method | In vitro | Over 60% reduction in corrosion rate | [112] | |||
| Inorganic or hybrid (polymer + inorganic) coatings | HA | Deposition with Ca chelate compound in solution | In vivo | 25% decrease in corrosion rate | [113] | ||
| CeO2 | Sol-gel method on a fluorinated surface | In vitro | Approximate 90% reduction in corrosion current density | [114] | |||
| Fe-substituted tricalcium phosphate | Pulsed laser deposition | In vitro | 50% reduction in corrosion current density | [115] | |||
| Si-rich oxide and Si-rich layer | Ion implantation | In vitro | Over 90% reduction in corrosion current density | [116] | |||
| TiOx or AlOx film | Plasm vapor deposition | In vitro | Approximate 50% reduction in corrosion current density | [117] | |||
| PCL/bioglass nanoparticle composite | Spin coating | In vitro | Significant reduction in corrosion current density | [98] | |||
| Refinement in microstructure | Homogenous surface | Laser surface melting | In vitro | Over 60% reduction in corrosion current density after treatment and grinding | Residual stress exists after LSM treatment | [100] | |
| Nano-crystallization | Laser shock processing | In vitro | 86% increase in surface hardness | Impairment in corrosion resistance | [99] | ||
| Mesoporous silica | Selective laser melting | In vitro | 57% reduction in corrosion rate | Potential inhibition of bioactivity of Mg ions by the inert coating | [118] | ||
| Supra-nano-dual-phase alloy membrane | Mg–Cu–Y alloy | In vitro | Increase in the ultimate yield stress by nearly 8 times | Potential electrochemical reactions between membrane and Mg-based substrate | [101] | ||
| Chemical conversion coatings | Alkaline derived layers | Magnesium oxide layers | Alkaline-heat treatment | In vitro | Approximate 85% decrease in corrosion rate | Concerns in long-term corrosion performance caused by porosity and detachment of coatings | [88] |
| Acid derived layers | Mg3(PO4)2 | Phosphoric acid treatment | In vitro | Over 50% reduction in corrosion rate | [90] | ||
| Fluorinated layers | MgF2 | HF treatment | In vitro | 50%–70% reduction in corrosion current density | [89] | ||
| Electrochemical deposition | Ceramic coatings with dense micropores | Micro-arc oxidation | In vitro | Over 80% reduction in corrosion current density | [93] | ||
| Hydroxyapatite | Electrophoresis | In vitro | Slight increase in corrosion resistance depending on thickness | [92] | |||
| Others | DAHP/PEI | Chemical conversion and spin coating | In vitro | Reduction in corrosion current density by five orders of magnitude | [119] | ||
| Mechanical treatment | surface mechanical attrition treatment (SMAT) | Gradient nanostructures | Repeated multidirectional impact of flying balls on the surface | In vitro | Approximate 50% increase in tensile strength | Weaker corrosion resistance of alloy caused by high density of crystalline defects | [102] |
| Combination of SMAT and dual-phase metallic glass film | Hybrid nanostructures | Surface impact by ZrO2 balls and magnetron sputtering | In vitro | Improvement in both strength and ductility | Potential electrochemical reactions between the two layers for impairment of corrosion resistance | [120] |
4.1. Alloying
Alloying is one of the most effective methods to improve the mechanical properties of the Mg metals [65]. For instance, binary Mg-based alloys including Mg–Zn, Mg–Ca, and Mg–Ag showed dramatic increase in their mechanical strength [[66], [67], [68]]. However, the addition of alloying elements may normally impair corrosion resistance of the Mg matrix due to the presence of electrochemical reactions caused by the precipitation of intermetallic compounds along the grain boundaries [69]. As the corrosion performance of Mg alloys is also greatly influenced by the microstructure including the grain size, boundary and phase distribution [69], it is critical to carefully select alloying elements of Mg alloys with optimized composition design, e.g. the binary series alloys, the ternary series alloys, etc., to reduce the electrode potential differences between the matrix and second phase to retard corrosion. For example, the addition of appropriate Zn content into Mg–Ca alloys enhanced the corrosion resistance due to the changes in the corrosion potential in the second phase [70]. Chen et al. [71] reported that the addition of Ca or Zn in the Mg–2Sr alloys enhanced the in vitro and in vivo corrosion resistance of the binary alloys because of the refined microstructure. Silver has been commonly used to improve the mechanical properties of binary Mg-based alloys. For example, the addition of Ag into Mg–Zn alloys remarkably increase the hardness although the corrosion resistance was impaired [72]. Most recently, the micro-alloyed systems such as Mg-0.5Zn-(0.2X) alloys showed great potential in biomedical applications due to the reduction of precipitates [73]. Similarly, the compound of Mg12Nd greatly inhibited the galvanic corrosion of Mg-Nd-Zn-Zr alloys because of the reduction in the gap of the electrode potential between α matrix phase and Mg12Nd phase [74]. As these materials are implanted in the body without removal requirements, consideration must be taken to choose alloying elements that are non-toxic. Aluminum (Al) has been widely used as the alloying element for preparation of AZ series alloys in industrial applications. Some studies were tried to assess the use potential of Al containing Mg-based alloys in orthopaedics [75]. For instance, LAE442 is widely considered as a slow degrading Mg-based alloy. Angrisani et al. reported that LAE442 intramedullary pin maintained long-term degradation behavior up to 3.5 years without observation of gas formation and clinical problems in rabbits [76]. However, although a long-term implantation study (3.5 years) showed there was no accumulation of Al content in brain tissue samples for rabbits with LAE442 implants in their bone marrow cavities [76], more attention should be still paid by the introduction of Al into the Mg-based implants due to its link with neuro-toxicity [69,77]. Therefore, the nutrient elements (e.g. Ca, Zn, Sr, Mn, etc.) and rare earth elements have been increasingly explored as the alloying elements to fabricate the novel Mg-based alloys as potential orthopaedic implants to address the biosafety concerns [78]. Further, complexed alloys with quaternary or more phases were designed, aiming at gaining more satisfactory properties. Khurram Munir et al. [79] reported that the addition of a high concentration of Sc in the Sr-containing Mg alloys improved the corrosion resistance of Mg-0.6Zn-0.5Sr owing to the suppression of the intermetallic phases along the grain boundaries and formation of the chemically stable Sc oxide layer on the surface of the Mg alloys. In addition, another alloying technique using plasma ion implantation has also demonstrated the potential for modification of Mg or its alloys. For instance, implantation of pure Mg with Zn and Al ions as well as rare-earth WE43 Mg alloy with Nd ions exhibited improved corrosion resistance due to the formation of compact oxide film on the surface [80]. As the solubility of alloying elements in crystalline Mg is limited, the development of Mg-based bulk metallic glasses (BMGs) in the last ten years may be a promising direction to improve mechanical properties and corrosion resistance due to their single-phase, chemically homogeneous alloy system and the absence of second phases [81,82]. Although BMGs have great potential as future orthopaedic implants, there are still some technical challenges, e.g. the limitation of critical sizes, the lack of ductility, etc., prior to their further clinical applications [83].
Requirements for mechanical properties differ in Mg or its alloys when fabricated as nails, screws or plates. For instance, the biocompatibility and the degradation behavior of the LAE442 Mg-based intramedullary interlocked nailing system was evaluated in a sheep model without fractures during 24-week observation [84], laying a foundation for the design of clinical available fixation device from multiple aspects including safety, degradation, and mechanical properties. Generally, Mg-based orthopaedic implants are not suitable for consideration in heavy load-bearing skeletal sites. However, a recently developed Mg alloy containing 2% silver (Mg2Ag), which was cast and treated by a solidification cooling process, showed excellent mechanical properties to support long bone fractures as intramedullary pins with appropriate degradation rate [85].
4.2. Surface modification
In addition to alloying, surface modification represented another useful technology to improve the corrosion resistance of the Mg-based alloys. Generally, surface modification methods are categorized into chemical conversion coatings, physical deposition, and mechanical treatment according to the coating formation mechanisms [86]. If the new phase covered on the surface of Mg or its alloys is prepared by chemical or electrochemical treatment, it can be defined as chemical conversion coating [87]. Alkaline, acid, and fluoride treatments are widely used methods for surface modification to enhance the corrosion resistance of Mg-based alloys [[88], [89], [90]]. Hydrothermal method for preparation of hydroxide layers is also a promising strategy to improve the corrosion performance of Mg-based alloys [91]. In addition to the traditional chemical treatment, electrophoretic deposition (EPD) and micro-arc oxidation (MAO) have been increasingly recommended as effective surface modification methods to improve the corrosion resistance of the Mg-based alloys. For example, the deposition of bioceramics-like layers on the surface of the Mg-based alloys via EPD or MAO have been considered as a feasible technology in inhibition of corrosion [92,93]. In another study, Mehdi Razavi et al. [94] deposited nanostructured akermanite (Ca2MgSi2O7) coating on the AZ91 Mg alloys through EPD assisted MAO method and observed improved corrosion resistance and mechanical stability for the surface modified Mg alloys. As hydroxyapatite (HA) is one of the major components of the bone matrix, it has also been commonly used as the coating on Mg-based alloys for reducing the degradation rate of the metal matrix for effective orthopaedic use [95]. Most recently, the Mg alloy-based HA nanocomposites fabricated by Parande et al. by disintegrated melt deposition technology demonstrated a positive impact on the corrosion resistance and mechanical strength of the Mg–Zn–Si matrix [96]. However, most of the currently used surface modification methods mainly focus on reducing the degradation rate of Mg or its alloys, leading to minor improvement in their mechanical properties. Physical deposited coatings are defined as ex-situ coatings whose substrates do not involved in the formation of coatings. Biocompatible polymers such as PLA, PCL, and chitosan as well as inorganic substances have been widely studied as effective coatings to modify corrosion performance of Mg or its alloys [97,98]. In addition, laser surface melting and laser shock processing have been also tested in recent years for improvement of corrosion resistance or mechanical properties of Mg or its alloys as potential implants [99,100]. The development of supra-nano-dual-phase alloy membrane (SNDP) via magnetron sputtering is another advanced surface treatment pathway able to dramatically enhance the mechanical strength of the Mg alloys close to the theoretical ideal strength values, indicating the potential use in high weight-bearing skeletal sites [101].
In contrast with the other technologies, the surface mechanical attrition treatment (SMAT), with a gradient distribution (from several nanometers to a few micrometers) formed on the surface layer of the treated samples, is expected to dramatically improve the mechanical properties of the biodegradable Mg alloys [102] with deep treated depth. However, the corrosion resistance is observed to be impaired for the SMATed Mg-based alloys because of the increase of crystalline defects, such as grain boundaries and dislocations [103].
4.3. Other strategies
Apart from alloying and surface modifications, the preparation of Mg-based composites has shown great potential as orthopaedic implants with enhanced mechanical properties. For instance, Mg-nano-hydroxyapatite (Mg-nHA) composites showed 27% improvement in the compression strength after spark plasma sintering [104]. In addition to nHA, calcium polyphosphate (CPP) and Tri calcium phosphate (β-TCP) were also used as reinforcement in Mg composites [105]. Recently, the multiwall carbon nanotubes (MWCNs) was reported to reinforce Mg alloy composites, which showed 36% increase in the ultimate compressive strength [106]. In order to address the concerns on the low corrosion resistance of Mg alloys, a polymer matrix of PLLA has been reinforced with pure Mg particles, contributing to improved corrosion performance [107].
5. Novel strategies to develop Mg-containing interference screws
5.1. Optimizing the structure of new Mg-based interference screws
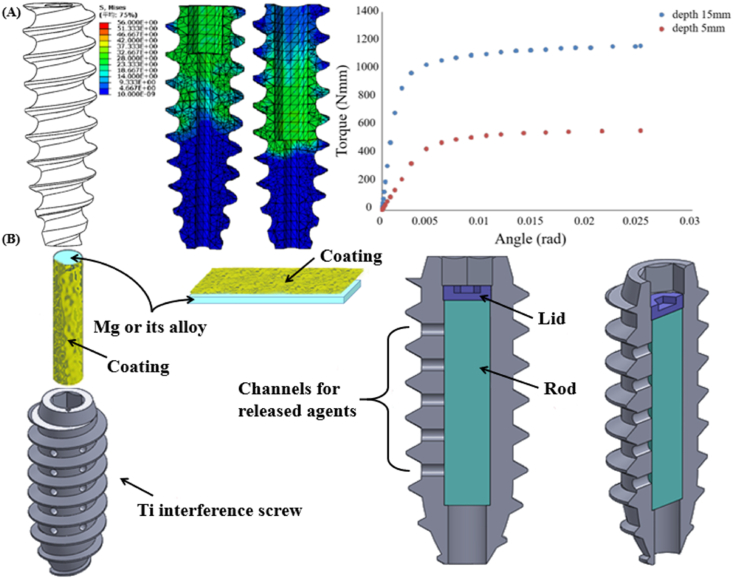
Although surface coatings are widely considered as a promising strategy to modify the properties of the Mg-based alloys, the fabrication of the satisfactory coatings without concerns of detachment from the surface of the Mg-based alloys is still challenging. The optimization of the structural design of the Mg-based orthopaedic implants represents a fundamental direction for achieving their widespread clinical use. For instance, alteration in the screw structure including thread geometry and screw head shape dramatically affect the mechanical behavior of the Mg-based screws [121,122], indicating that improving the design of the Mg-based interference screws is expected to increase the surgical success rates by decreasing the device failure at insertion. Based on our previous studies [123], one of the most challenging issues affecting the clinical use of the Mg-based interference screws is the lack of torque, leading to the screw head breakage during surgical insertion for ACL reconstruction. Therefore, with respect to the Mg-based interference screws, torque improvement becomes one of the prime targets prior to clinical trials. Our preliminary studies indicate that increasing the drive insertion depth can improve the maximal torque of the Mg-based screws (Fig. 7A), inspiring to focus on novel screw structure design using finite element analysis (FEA) models so as to attain improved mechanical properties.
Fig. 7.
R&D strategies for the novel Mg-containing interference screws feasible for clinical applications. (A) Optimization of the structural design of the Mg-based interference screw by altering the drill insertion depth and pitch distance for improved maximal torque. (B) Mg/Ti hybrid fixators composed of Mg rod and Ti-based interference screw with holes in the screw body to allow the release of the Mg ions in the surrounding bone tissue, exerting favorable biological effects while overcoming the concerns associated with insufficient mechanical strength.
5.2. Development of Mg-containing hybrid interference screws
In addition to the aforementioned technologies, the development of Mg-based hybrid fixators may be another promising strategy for pushing the Mg-containing orthopaedic implants into clinical trials [26,124]. Conceptually, the Mg-based implants, which favor bone regeneration, can be combined with the permanent metals to achieve improved mechanical properties. For example, the Mg-containing stainless steel nail with holes in the steel body, which allows the release of Mg ions into the surrounding tissue, could supported and significantly promoted bone fracture healing in rats with osteoporotic fractures conducted in their femora [125]. In addition, a novel hybrid device composed of Mg screws and Ti plate/screw, which has been recently developed to fix the fracture performed in the tibiae of rabbits, significantly enhanced bone callus formation [126]. Similarly, a cannulated Ti interference screw with the insertion of a coated Mg rod, which releases Mg ions through the holes in the Ti screw body, is proposed as a bio-functional fixator for the graft healing in ACL reconstruction (Fig. 7B). The coating on the surface of Mg-based alloys can prevent the direct contact between Mg and Ti, thereby inhibiting electrochemical reaction induced corrosion. Importantly, the released rate of Mg ions can be modulated to match the healing process by altering the coating composition and structure on the Mg surface.
5.3. R&D of porous Mg-based interference screws
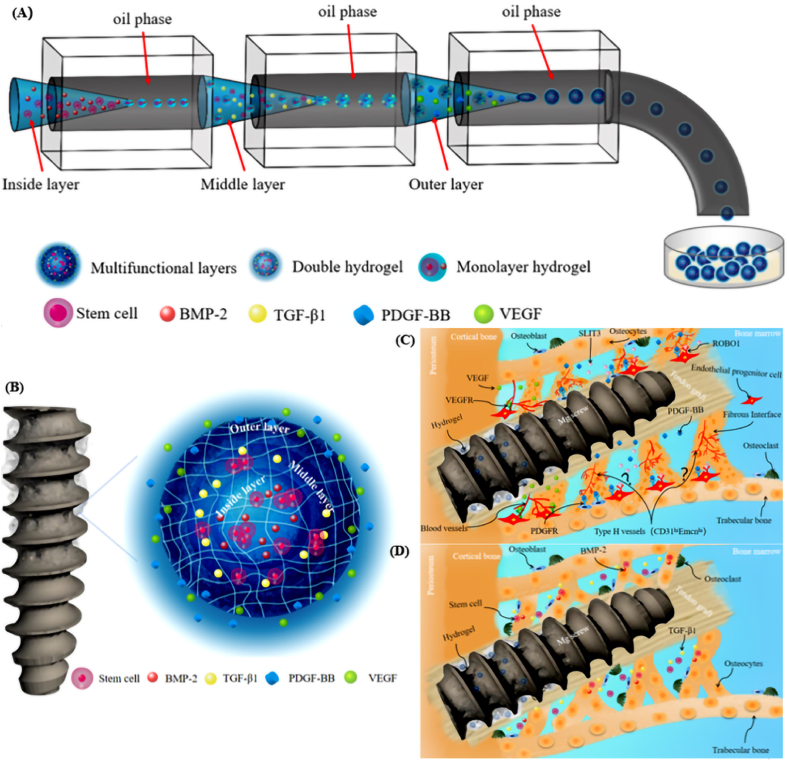
The clinical performance of an interference screw in ACL reconstruction is determined by the combination between the surgical technique, screw design, and potential incorporated biomaterials, which guarantees the integrity of the screw during insertion, the satisfactory graft healing, the bony ingrowth, and the stability of fixation [127]. Angiogenesis and osteogenesis are the key factors influencing the tendon-bone interface healing or integration quality, so efforts have been made to apply the exogenous osteoprogenitor cells (e.g. mesenchymal stem cells [128], synovial mesenchymal stem cells [129], etc.), growth factors (e.g. transforming growth factor-beta 1 (TGF-β1) [130], vascular endothelial growth factor (VEGF) [131], and bone morphogenetic proteins-2 (BMP-2) [132], etc.) and biomaterials (e.g. injectable tricalcium phosphate [133], Mg-based bone adhesive [134], etc.) for biological modulation, aiming to promote healing in ACL reconstruction [135]. To prolong the retention of the progenitor cells and growth factors in the local tissue to elicit cellular activity, biocompatible injectable in situ-forming hydrogels have been recently developed for encapsulation to achieving target delivery. Therefore, the Mg-based interference screws with holes in the screw body allowing the release of the injectable in situ-forming multifunctional hydrogel microparticles (HMP) loaded with progenitor cells or growth factors may be a promising option for improved osteogenesis and angiogenesis during the tendon-bone interface healing process (Fig. 8A and B). Accordingly, the development of the Mg-based interference screw with holes around the screw body to allow the release of drug-loading HMP after injection through the insertion tunnel of the screw may an option to favor the graft healing. For instance, injectable in situ-forming multi-layered hydrogel microparticles loaded with the growth factors and progenitor cells (PDGF-BB and VEGF in the outer layer, while TGF-β1 and BMP-2 in the middle and inner layers, respectively), which are distributed in the gap between the screw and bone tunnel surface, will favor angiogenesis, recruitment of endogenous progenitor cells for repair and osteogenesis at different healing stages, thereby contributing to enhanced coupling between angiogenesis and osteogenesis for improved osseous ingrowth at the tendon-bone interface (Fig. 8C and D). Regarding the thermosensitive hybrid HMP, the composition, the particle size and the degree of crosslinking must be adjusted to optimize the mechanical and rheological properties of the hydrogels for stable storage in the bone tunnel without clinical concerns [136].
Fig. 8.
R&D of the Mg-based interference screws with holes to release hydrogel microparticles with multifunctional layers for enhancing tendon-bone healing. (A) Schematic diagram of multifluidic technology for preparation of multi-layered hydrogel microparticles (W/O/W/O/W). (B) The structure diagram of multifunctional hydrogel particles showing PDGF-BB and VEGF in the outer layer, TGF-β1 in the middle layer and stem cells and BMP-2 in the inner layer. (C) The injected hydrogels in the Mg-based interference screws further improve angiogenesis including type H vessels in the presence of PDGF-BB and VEGF released from the outer layer during the early healing stages. (D) TGF-β1, stem cells and BMP-2 released from the middle and inner layers of the hydrogel increase the number of stem cells involved in tendon-bone healing, along with promoting their osteogenic differentiation capability for bony ingrowth towards the tendon-bone interface during the later healing stage (VEGF: vascular endothelial growth factor, PDGF-BB: platelet-derived growth factor-BB, TGF-β1: transforming growth factor-β1, and BMP-2: bone morphogenetic protein-2).
6. Conclusions
Innovative biodegradable Mg-based interference screws are considered as one of the most promising candidates to replace the Ti- or polymer-based counterparts in ACL reconstruction owing to their reduced stress shielding effects, osteopromotive contributions, and excellent biocompatibility. However, insufficient torque and corrosion resistance are still the major challenges of the Mg-based interference screws moving towards clinical trials. In addition to the traditional strategies including alloying and surface modification, optimization of screw structure, design of Mg/Ti hybrid fixators, and functionalized Mg-based interferences loaded with injectable in situ-forming hydrogel were discussed to address the challenges. The development of the novel Mg-containing interference screws is expected to open up a new era in the treatment of tendon-bone healing.
Credit author statement
Y.L. and J.W. wrote the manuscript. C.Z., J.W., F.F.L., K.C., L.Q. made contributions to language revisions and manuscript design. All authors commented on the manuscript.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Ref No. 81702165), Guangdong Natural Science Fund (Ref. No. 2019A1515011404), Key-Area Research and Development Program of Guangdong Province (Ref. No. 2020B090924004), and Theme-based Research Scheme (Ref No. T13-402/17-N).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ling Qin, Email: qin@ort.cuhk.edu.hk.
Jiali Wang, Email: wangjli8@mail.sysu.edu.cn.
References
- 1.Mather R.C., 3rd, Koenig L., Kocher M.S., Dall T.M., Gallo P., Scott D.J., Bach B.R., Jr., Spindler K.P., Group M.K. Societal and economic impact of anterior cruciate ligament tears. J. Bone Joint Surg. Am. 2013;95(19):1751–1759. doi: 10.2106/JBJS.L.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yabroudi M.A., Björnsson H., Lynch A.D., Muller B., Samuelsson K., Tarabichi M., Karlsson J., Fu F.H., Harner C.D., Irrgang J.J. Predictors of revision surgery after primary anterior cruciate ligament reconstruction. Orthop. J. Sports Med. 2016;4(9) doi: 10.1177/2325967116666039. 2325967116666039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajuied A., Wong F., Smith C., Norris M., Earnshaw P., Back D., Davies A. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am. J. Sports Med. 2014;42(9):2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 4.Disegi J.A., Eschbach L. Stainless steel in bone surgery. Injury. 2000;31(Suppl 4):2–6. doi: 10.1016/s0020-1383(00)80015-7. [DOI] [PubMed] [Google Scholar]
- 5.Myers P., Logan M., Stokes A., Boyd K., Watts M. Bioabsorbable versus titanium interference screws with hamstring autograft in anterior cruciate ligament reconstruction: a prospective randomized trial with 2-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2008;24(7):817–823. doi: 10.1016/j.arthro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Castellani C., Lindtner R.A., Hausbrandt P., Tschegg E., Stanzl-Tschegg S.E., Zanoni G., Beck S., Weinberg A.M. Bone-implant interface strength and osseointegration: biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011;7(1):432–440. doi: 10.1016/j.actbio.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Ciccone W.J., 2nd, Motz C., Bentley C., Tasto J.P. Bioabsorbable implants in orthopaedics: new developments and clinical applications. J. Am. Acad. Orthop. Surg. 2001;9(5):280–288. doi: 10.5435/00124635-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Karachalios T., Tsatsaronis C., Efraimis G., Papadelis P., Lyritis G., Diakoumopoulos G. The long-term clinical relevance of calcar atrophy caused by stress shielding in total hip arthroplasty: a 10-year, prospective, randomized study. J. Arthroplasty. 2004;19(4):469–475. doi: 10.1016/j.arth.2003.12.081. [DOI] [PubMed] [Google Scholar]
- 9.Tsai P.I., Chen C.Y., Huang S.W., Yang K.Y., Lin T.H., Chen S.Y., Sun J.S. Improvement of bone-tendon fixation by porous titanium interference screw: a rabbit animal model. J. Orthop. Res. 2018;36(10):2633–2640. doi: 10.1002/jor.24037. [DOI] [PubMed] [Google Scholar]
- 10.Picard M., Mohanty A.K., Misra M. Recent advances in additive manufacturing of engineering thermoplastics: challenges and opportunities. RSC Adv. 2020;10(59):36058–36089. doi: 10.1039/d0ra04857g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q.H., Cossey A., Tong J. Stress shielding in periprosthetic bone following a total knee replacement: effects of implant material, design and alignment. Med. Eng. Phys. 2016;38(12):1481–1488. doi: 10.1016/j.medengphy.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Ulery B.D., Nair L.S., Laurencin C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011;49(12):832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song R., Murphy M., Li C., Ting K., Soo C., Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018;12:3117–3145. doi: 10.2147/DDDT.S165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fda . 2019. Product Classification. [Google Scholar]
- 15.S S., E H., LJ S., JP R., JP L., M F., LA P. A randomized controlled trial of PEEK versus titanium interference screws for anterior cruciate ligament reconstruction with 2-year follow-up. Am. J. Sports Med. 2019;47(10):2386–2393. doi: 10.1177/0363546519861530. [DOI] [PubMed] [Google Scholar]
- 16.Navarro M., Michiardi A., Castaño O., Planell J.A. Biomaterials in orthopaedics. J. R. Soc. Interface. 2008;5(27):1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Chu C.L., Liu L., Liu X.K., Bai J., Guo C., Xue F., Lin P.H., Chu P.K. Biodegradable poly-lactic acid based-composite reinforced unidirectionally with high-strength magnesium alloy wires. Biomaterials. 2015;49:135–144. doi: 10.1016/j.biomaterials.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 18.Gohil S.V., Suhail S., Rose J., Vella T., Nair L.S. Polymers and composites for orthopedic applications. In: Bose S., Bandyopadhyay A., editors. Materials for Bone Disorders. Academic Press; 2017. pp. 349–403. [Google Scholar]
- 19.Wei S., Ma J.-X., Xu L., Gu X.-S., Ma X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020;7(1) doi: 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouler J.M., Pilet P., Gauthier O., Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: a review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 21.Yang H.T., Jia B., Zhang Z.C., Qu X.H., Li G.N., Lin W.J., Zhu D.H., Dai K.R., Zheng Y.F. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-019-14153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu X.H., Yang H.T., Yu Z.F., Jia B., Qiao H., Zheng Y.F., Dai K.R. Serum zinc levels and multiple health outcomes: implications for zinc-based biomaterials. Bioact. Mater. 2020;5(2):410–422. doi: 10.1016/j.bioactmat.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegener B., Sichler A., Milz S., Sprecher C., Pieper K., Hermanns W., Jansson V., Nies B., Kieback B., Muller P.E., Wegener V., Quadbeck P. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. (U.K.) 2020;10(1) doi: 10.1038/s41598-020-66289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad K., Bazaka O., Chua M., Rochford M., Fedrick L., Spoor J., Symes R., Tieppo M., Collins C., Cao A., Markwell D., Ostrikov K., Bazaka K. Metallic biomaterials: current challenges and opportunities. Materials. 2017;10(8) doi: 10.3390/ma10080884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2017;112:287–302. doi: 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J.Q., Chow D.H., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H.C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song B., Li W., Chen Z., Fu G., Li C., Liu W., Li Y., Qin L., Ding Y. Biomechanical comparison of pure magnesium interference screw and polylactic acid polymer interference screw in anterior cruciate ligament reconstruction-A cadaveric experimental study. J. Orthop. Transl. 2017;8:32–39. doi: 10.1016/j.jot.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marrella A., Lee T.Y., Lee D.H., Karuthedom S., Syla D., Chawla A., Khademhosseini A., Jang H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today. 2018;21(4):362–376. doi: 10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han H.S., Loffredo S., Jun I., Edwards J., Kim Y.C., Seok H.K., Witte F., Mantovani D., Glyn-Jones S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today. 2019;23:57–71. [Google Scholar]
- 30.Yun Y.H., Dong Z.Y., Lee N., Liu Y.J., Xue D.C., Guo X.F., Kuhlmann J., Doepke A., Halsall H.B., Heineman W., Sundaramurthy S., Schulz M.J., Yin Z.Z., Shanov V., Hurd D., Nagy P., Li W.F., Fox C. Revolutionizing biodegradable metals. Mater. Today. 2009;12(10):22–32. [Google Scholar]
- 31.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U., Schavan R., Stukenborg-Colsman C., Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online. 2013;12 doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao D.W., Huang S.B., Lu F.Q., Wang B.J., Yang L., Qin L., Yang K., Li Y.D., Li W.R., Wang W., Tian S.M., Zhang X.Z., Gao W.B., Wang Z.P., Zhang Y., Xie X.H., Wang J.L., Li J.L. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials. 2016;81:84–92. doi: 10.1016/j.biomaterials.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Wang J.L., Xu J.K., Hopkins C., Chow D.H., Qin L. Biodegradable magnesium-based implants in orthopedics-A general review and perspectives. Adv. Sci. 2020;7(8):1902443. doi: 10.1002/advs.201902443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.W., Han H.S., Han K.J., Park J., Jeon H., Ok M.R., Seok H.K., Ahn J.P., Lee K.E., Lee D.H., Yang S.J., Cho S.Y., Cha P.R., Kwon H., Nam T.H., Lo Han J.H., Rho H.J., Lee K.S., Kim Y.C., Mantovani D. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. U.S.A. 2016;113(3):716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng P., Han P., Zhao C., Zhang S., Wu H., Ni J., Hou P., Zhang Y., Liu J., Xu H., Liu S., Zhang X., Zheng Y., Chai Y. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Wu Y., Li H., Liu Y., Bai X., Chau W., Zheng Y., Qin L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials. 2018;157:86–97. doi: 10.1016/j.biomaterials.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Xu J., Song B., Chow D.H., Shu-Hang Yung P., Qin L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Eriksson K., Kindblom L.G., Wredmark T. Semitendinosus tendon graft ingrowth in tibial tunnel following ACL reconstruction: a histological study of 2 patients with different types of early graft failure. Acta Orthop. Scand. 2000;71(3):275–279. doi: 10.1080/000164700317411870. [DOI] [PubMed] [Google Scholar]
- 39.Ekdahl M., Wang J.H., Ronga M., Fu F.H. Graft healing in anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2008;16(10):935–947. doi: 10.1007/s00167-008-0584-0. [DOI] [PubMed] [Google Scholar]
- 40.Song E.K., Rowe S.M., Chung J.Y., Moon E.S., Lee K.B. Failure of osteointegration of hamstring tendon autograft after anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(4):424–428. doi: 10.1016/j.arthro.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Iorio R., Di Sanzo V., Vadalà A., Conteduca J., Mazza D., Redler A., Bolle G., Conteduca F., Ferretti A. ACL reconstruction with hamstrings: how different technique and fixation devices influence bone tunnel enlargement. Eur. Rev. Med. Pharmacol. Sci. 2013;17(21):2956–2961. [PubMed] [Google Scholar]
- 42.de Padua V.B.C., Vilela J.C.R., Espindola W.A., Godoy R.C.G. Bone tunnel enlargement with NON-metallic interference screws IN ACL reconstruction. Acta Ortopédica Bras. 2018;26(5):305–308. doi: 10.1590/1413-785220182605199995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. [Google Scholar]
- 44.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10(6):2834–2842. doi: 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Hung C.C., Chaya A., Liu K., Verdelis K., Sfeir C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019;98:246–255. doi: 10.1016/j.actbio.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhao D.L., Brown A., Wang T.T., Yoshizawa S., Sfeir C., Heineman W.R. In vivo quantification of hydrogen gas concentration in bone marrow surrounding magnesium fracture fixation hardware using an electrochemical hydrogen gas sensor. Acta Biomater. 2018;73:559–566. doi: 10.1016/j.actbio.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 47.Kuhlmann J., Bartsch I., Willbold E., Schuchardt S., Holz O., Hort N., Hoche D., Heineman W.R., Witte F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013;9(10):8714–8721. doi: 10.1016/j.actbio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Thormann U., Alt V., Heimann L., Gasquere C., Heiss C., Szalay G., Franke J., Schnettler R., Lips K.S. The biocompatibility of degradable magnesium interference screws: an experimental study with sheep. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/943603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esmaily M., Svensson J.E., Fajardo S., Birbilis N., Frankel G.S., Virtanen S., Arrabal R., Thomas S., Johansson L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017;89:92–193. [Google Scholar]
- 50.Gonzalez J., Hou R.Q., Nidadavolu E.P.S., Willumeit-Römer R., Feyerabend F. Magnesium degradation under physiological conditions - best practice. Bioact. Mater. 2018;3(2):174–185. doi: 10.1016/j.bioactmat.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng R.C., Hu Y., Guan S.K., Cui H.Z., Han E.H. Corrosion of magnesium alloy AZ31: the influence of bicarbonate, sulphate, hydrogen phosphate and dihydrogen phosphate ions in saline solution. Corrosion Sci. 2014;86:171–182. [Google Scholar]
- 52.Gray-Munro J.E., Strong M. A study on the interfacial chemistry of magnesium hydroxide surfaces in aqueous phosphate solutions: influence of Ca2+, Cl- and protein. J. Colloid Interface Sci. 2013;393:421–428. doi: 10.1016/j.jcis.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto A., Hiromoto S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2009;29(5):1559–1568. [Google Scholar]
- 54.Yan W., Lian Y.J., Zhang Z.Y., Zeng M.Q., Zhang Z.Q., Yin Z.Z., Cui L.Y., Zeng R.C. In vitro degradation of pure magnesium-the synergetic influences of glucose and albumin. Bioact. Mater. 2020;5(2):318–333. doi: 10.1016/j.bioactmat.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L., Shinohara T., Zhang B.P. Influence of chloride, sulfate and bicarbonate anions on the corrosion behavior of AZ31 magnesium alloy. J. Alloys Compd. 2010;496(1–2):500–507. [Google Scholar]
- 56.Jang Y., Collins B., Sankar J., Yun Y. Effect of biologically relevant ions on the corrosion products formed on alloy AZ31B: an improved understanding of magnesium corrosion. Acta Biomater. 2013;9(10):8761–8770. doi: 10.1016/j.actbio.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Zeng R.C., Li X.T., Li S.Q., Zhang F., Han E.H. In vitro degradation of pure Mg in response to glucose. Sci. Rep. 2015;5:13026. doi: 10.1038/srep13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui L.Y., Li X.T., Zeng R.C., Li S.Q., Han E.H., Song L. In vitro corrosion of Mg-Ca alloy - the influence of glucose content. Front. Mater. Sci. 2017;11(3):284–295. [Google Scholar]
- 59.Johnson I., Jiang W., Liu H. The effects of serum proteins on magnesium alloy degradation in vitro. Sci. Rep. 2017;7(1):14335. doi: 10.1038/s41598-017-14479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C.L., Wang Y.J., Zeng R.C., Zhang X.M., Huang W.J., Chu P.K. In vitro corrosion degradation behaviour of Mg–Ca alloy in the presence of albumin. Corrosion Sci. 2010;52(10):3341–3347. [Google Scholar]
- 61.Hou R.Q., Scharnagl N., Willumeit-Römer R., Feyerabend F. Different effects of single protein vs. protein mixtures on magnesium degradation under cell culture conditions. Acta Biomater. 2019;98:256–268. doi: 10.1016/j.actbio.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Thormann U., Alt V., Heimann L., Gasquere C., Heiss C., Szalay G., Franke J., Schnettler R., Lips K.S. The biocompatibility of degradable magnesium interference screws: an experimental study with sheep. BioMed Res. Int. 2015;2015:943603. doi: 10.1155/2015/943603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J.L., Xu J.K., Song B., Chow D.H., Yung P.S.H., Qin L. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Amendola A., Smith C.J., Goodall R., Auricchio F., Feo L., Benzoni G., Fraternali F. Experimental response of additively manufactured metallic pentamode materials confined between stiffening plates. Compos. Struct. 2016;142:254–262. [Google Scholar]
- 65.Chen J., Tan L., Yu X., Etim I.P., Ibrahim M., Yang K. Mechanical properties of magnesium alloys for medical application: a review. J. Mech. Behav. Biomed. Mater. 2018;87:68–79. doi: 10.1016/j.jmbbm.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Yu J.Y., Wang J.Z., Li Q., Shang J., Cao J.M., Sun X.D. Effect of Zn on microstructures and properties of Mg-Zn alloys prepared by powder metallurgy method. Rare Met. Mater. Eng. 2016;45(11):2757–2762. [Google Scholar]
- 67.Li Z.J., Gu X.N., Lou S.Q., Zheng Y.F. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29(10):1329–1344. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Tie D., Feyerabend F., Hort N., Hoeche D., Kainer K.U., Willumeit R., Mueller W.D. In vitro mechanical and corrosion properties of biodegradable Mg-Ag alloys. Mater. Corros. 2014;65(6):569–576. [Google Scholar]
- 69.Ding Y.F., Wen C.E., Hodgson P., Li Y.C. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: a review. J. Mater. Chem. B. 2014;2(14):1912–1933. doi: 10.1039/c3tb21746a. [DOI] [PubMed] [Google Scholar]
- 70.Zhang B.P., Hou Y.L., Wang X.D., Wang Y., Geng L. Mechanical properties, degradation performance and cytotoxicity of Mg-Zn-Ca biomedical alloys with different compositions. Mater. Sci. Eng. C. 2011;31(8):1667–1673. [Google Scholar]
- 71.Chen K., Xie X., Tang H., Sun H., Qin L., Zheng Y., Gu X., Fan Y. In vitro and in vivo degradation behavior of Mg-2Sr-Ca and Mg-2Sr-Zn alloys. Bioact. Mater. 2020;5(2):275–285. doi: 10.1016/j.bioactmat.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben-Hamu G., Eliezer D., Kaya A., Na Y.G., Shin K.S. Microstructure and corrosion behavior of Mg-Zn-Ag alloys. Mater. Sci. Eng. Struct. 2006;435:579–587. [Google Scholar]
- 73.Jin Y., Blawert C., Yang H., Wiese B., Feyerabend F., Bohlen J., Mei D., Deng M., Campos M.S., Scharnagl N., Strecker K., Bode J., Vogt C., Willumeit-Roemer R. Microstructure-corrosion behaviour relationship of micro-alloyed Mg-0.5Zn alloy with the addition of Ca, Sr, Ag, in and Cu. Mater. Des. 2020;195 [Google Scholar]
- 74.Chang J.W., Fu P.H., Guo X.W., Peng L.M., Ding W.J. The effects of heat treatment and zirconium on the corrosion behaviour of Mg-3Nd-0.2Zn-0.4Zr (wt.%) alloy. Corrosion Sci. 2007;49(6):2612–2627. [Google Scholar]
- 75.Witte F., Kaese V., Haferkamp H., Switzer E., Meyer-Lindenberg A., Wirth C.J., Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26(17):3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 76.Angrisani N., Reifenrath J., Zimmermann F., Eifler R., Meyer-Lindenberg A., Vano-Herrera K., Vogt C. Biocompatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5 years in a rabbit model. Acta Biomater. 2016;44:355–365. doi: 10.1016/j.actbio.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Bodelon O.G., Iglesias C., Garrido J., Clemente C., Garcia-Alonso M.C., Escudero M.L. Analysis of metallic traces from the biodegradation of endomedullary AZ31 alloy temporary implants in rat organs after long implantation times. Biomed. Mater. 2015;10(4) doi: 10.1088/1748-6041/10/4/045015. [DOI] [PubMed] [Google Scholar]
- 78.Guo K.W. A review of magnesium/magnesium alloys corrosion. Recent Pat. Corros. Sci. 2011;1(1):72–90. [Google Scholar]
- 79.Munir K., Lin J., Wen C., Wright P.F.A., Li Y. Mechanical, corrosion, and biocompatibility properties of Mg-Zr-Sr-Sc alloys for biodegradable implant applications. Acta Biomater. 2020;102:493–507. doi: 10.1016/j.actbio.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Jin W.H., Wu G.S., Feng H.Q., Wang W.H., Zhang X.M., Chu P.K. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corrosion Sci. 2015;94:142–155. [Google Scholar]
- 81.Gu X.N., Zheng Y.F., Zhong S.P., Xi T.F., Wang J.Q., Wang W.H. Corrosion of, and cellular responses to Mg-Zn-Ca bulk metallic glasses. Biomaterials. 2010;31(6):1093–1103. doi: 10.1016/j.biomaterials.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Zberg B., Uggowitzer P.J., Loffler J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009;8(11):887–891. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- 83.Li H.F., Zheng Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016;36:1–20. doi: 10.1016/j.actbio.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 84.Rossig C., Angrisani N., Helmecke P., Besdo S., Seitz J.M., Welke B., Fedchenko N., Kock H., Reifenrath J. In vivo evaluation of a magnesium-based degradable intramedullary nailing system in a sheep model. Acta Biomater. 2015;25:369–383. doi: 10.1016/j.actbio.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 85.Jahn K., Saito H., Taipaleenmaki H., Gasser A., Hort N., Feyerabend F., Schluter H., Rueger J.M., Lehmann W., Willumeit-Romer R., Hesse E. Intramedullary Mg2Ag nails augment callus formation during fracture healing in mice. Acta Biomater. 2016;36:350–360. doi: 10.1016/j.actbio.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 86.Li L.Y., Cui L.Y., Zeng R.C., Li S.Q., Chen X.B., Zheng Y., Kannan M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys - a review. Acta Biomater. 2018;79:23–36. doi: 10.1016/j.actbio.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 87.Wang J., Tang J., Zhang P., Li Y., Wang J., Lai Y., Qin L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100(6):1691–1701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 88.Gu X.N., Zheng W., Cheng Y., Zheng Y.F. A study on alkaline heat treated Mg-Ca alloy for the control of the biocorrosion rate. Acta Biomater. 2009;5(7):2790–2799. doi: 10.1016/j.actbio.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 89.Panemangalore D.B., Shabadi R., Gupta M., Ji G. Effect of fluoride coatings on the corrosion behavior of Mg-Zn-Er alloys. Surf. Interfaces. 2019;14:72–81. [Google Scholar]
- 90.Gray-Munro J.E., Seguin C., Strong M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. 2009;91a(1):221–230. doi: 10.1002/jbm.a.32205. [DOI] [PubMed] [Google Scholar]
- 91.Peng F., Li H., Wang D., Tian P., Tian Y., Yuan G., Xu D., Liu X. Enhanced corrosion resistance and biocompatibility of magnesium alloy by Mg-Al-layered double hydroxide. ACS Appl. Mater. Interfaces. 2016;8(51):35033–35044. doi: 10.1021/acsami.6b12974. [DOI] [PubMed] [Google Scholar]
- 92.Saadati A., Hesarikia H., Nourani M.R., Taheri R.A. Electrophoretic deposition of hydroxyapatite coating on biodegradable Mg-4Zn-4Sn-0.6Ca-0.5Mn alloy. Surf. Eng. 2020;36(9):908–918. [Google Scholar]
- 93.Zhang Y.F., Blawert C., Tang S.W., Hu J., Mohedano M., Zheludkevich M.L., Kainer K.U. Influence of surface pre-treatment on the deposition and corrosion properties of hydrophobic coatings on a magnesium alloy. Corrosion Sci. 2016;112:483–494. [Google Scholar]
- 94.Razavi M., Fathi M., Savabi O., Hashemi Beni B., Vashaee D., Tayebi L. Surface microstructure and in vitro analysis of nanostructured akermanite (Ca2MgSi2O7) coating on biodegradable magnesium alloy for biomedical applications. Colloids Surf. B Biointerfaces. 2014;117:432–440. doi: 10.1016/j.colsurfb.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Wang J.L., Tang J., Zhang P., Li Y.D., Wang J., Lai Y.X., Qin L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: a general review. J. Biomed. Mater. Res. B. 2012;100B(6):1691–1701. doi: 10.1002/jbm.b.32707. [DOI] [PubMed] [Google Scholar]
- 96.Parande G., Manakari V., Prasadh S., Chauhan D., Rahate S., Wong R., Gupta M. Strength retention, corrosion control and biocompatibility of Mg-Zn-Si/HA nanocomposites. J. Mech. Behav. Biomed. Mater. 2020;103:103584. doi: 10.1016/j.jmbbm.2019.103584. [DOI] [PubMed] [Google Scholar]
- 97.Wong H.M., Yeung K.W.K., Lam K.O., Tam V., Chu P.K., Luk K.D.K., Cheung K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31(8):2084–2096. doi: 10.1016/j.biomaterials.2009.11.111. [DOI] [PubMed] [Google Scholar]
- 98.Yang Y.Y., Michalczyk C., Singer F., Virtanen S., Boccaccini A.R. In vitro study of polycaprolactone/bioactive glass composite coatings on corrosion and bioactivity of pure Mg. Appl. Surf. Sci. 2015;355:832–841. [Google Scholar]
- 99.Ren X.D., Huang J.J., Zhou W.F., Xu S.D., Liu F.F. Surface nano-crystallization of AZ91D magnesium alloy induced by laser shock processing. Mater. Des. 2015;86:421–426. [Google Scholar]
- 100.Taltavull C., Torres B., Lopez A.J., Rodrigo P., Otero E., Atrens A., Rams J. Corrosion behaviour of laser surface melted magnesium alloy AZ91D. Mater. Des. 2014;57:40–50. [Google Scholar]
- 101.Wu G., Chan K.C., Zhu L.L., Sun L.G., Lu J. Dual-phase nanostructuring as a route to high-strength magnesium alloys. Nature. 2017;545(7652) doi: 10.1038/nature21691. 80-+ [DOI] [PubMed] [Google Scholar]
- 102.Meng X.C., Duan M., Luo L., Zhan D.C., Jin B., Jin Y.H., Rao X.X., Liu Y., Lu J. The deformation behavior of AZ31 Mg alloy with surface mechanical attrition treatment. Mater. Sci. Eng. Struct. 2017;707:636–646. [Google Scholar]
- 103.Li N., Li Y.D., Li Y.X., Wu Y.H., Zheng Y.F., Han Y. Effect of surface mechanical attrition treatment on biodegradable Mg-1Ca alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;35:314–321. doi: 10.1016/j.msec.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Khodaei M., Nejatidanesh F., Shirani M.J., Iyengar S., Sina H., Savabi O. Magnesium/Nano-hydroxyapatite composite for bone reconstruction: the effect of processing method. J. Bionic Eng. 2020;17(1):92–99. [Google Scholar]
- 105.Bommala V.K., Krishna M.G., Rao C.T. Magnesium matrix composites for biomedical applications: a review. J. Magnesium Alloys. 2019;7(1):72–79. [Google Scholar]
- 106.Li Q.Q., Viereckl A., Rottmair C.A., Singer R.F. Improved processing of carbon nanotube/magnesium alloy composites. Compos. Sci. Technol. 2009;69(7–8):1193–1199. [Google Scholar]
- 107.Cifuentes S.C., Frutos E., Gonzalez-Carrasco J.L., Munoz M., Multigner M., Chao J., Benavente R., Lieblich M. Novel PLLA/magnesium composite for orthopedic applications: a proof of concept. Mater. Lett. 2012;74:239–242. [Google Scholar]
- 108.J W., J X., B S., DH C., Yung P.S.-H., L Q. Magnesium (Mg) based interference screws developed for promoting tendon graft incorporation in bone tunnel in rabbits. Acta Biomater. 2017;63:393–410. doi: 10.1016/j.actbio.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Ding W.J. Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials. Regen. Biomater. 2016;3(2):79–86. doi: 10.1093/rb/rbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Witecka A., Yamamoto A., Idaszek J., Chlanda A., Swieszkowski W. Influence of biodegradable polymer coatings on corrosion, cytocompatibility and cell functionality of Mg-2.0Zn-0.98Mn magnesium alloy. Colloids Surf., B. 2016;144:284–292. doi: 10.1016/j.colsurfb.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 111.Bakhsheshi-Rad H.R., Chen X.B., Ismail A.F., Aziz M., Abdolahi E., Mahmoodiyan F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019;30(5):1333–1339. [Google Scholar]
- 112.Bakhsheshi-Rad H.R., Akbari M., Ismail A.F., Aziz M., Hadisi Z., Pagan E., Daroonparvar M., Chen X.B. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-akermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coating. Technol. 2019;377 [Google Scholar]
- 113.Kim S.M., Jo J.H., Lee S.M., Kang M.H., Kim H.E., Estrin Y., Lee J.H., Lee J.W., Koh Y.H. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. 2014;102(2):429–441. doi: 10.1002/jbm.a.34718. [DOI] [PubMed] [Google Scholar]
- 114.Zhong X.K., Li Q., Hu J.Y., Lu Y.H. Characterization and corrosion studies of ceria thin film based on fluorinated AZ91D magnesium alloy. Corrosion Sci. 2008;50(8):2304–2309. [Google Scholar]
- 115.Antoniac I.V., Filipescu M., Barbaro K., Bonciu A., Birjega R., Cotrut C.M., Galvano E., Fosca M., Fadeeva I.V., Vadala G., Dinescu M., Rau J.V. Iron ion-doped tricalcium phosphate coatings improve the properties of biodegradable magnesium alloys for biomedical implant application. Adv. Mater. Interfaces. 2020;7(16) [Google Scholar]
- 116.Jamesh M., Wu G.S., Zhao Y., Chu P.K. Effects of silicon plasma ion implantation on electrochemical corrosion behavior of biodegradable Mg-Y-RE Alloy. Corrosion Sci. 2013;69:158–163. [Google Scholar]
- 117.Wu G.S., Zeng X.Q., Ding W.B., Guo X.W., Yao S.S. Characterization of ceramic PVD thin films on AZ31 magnesium alloys. Appl. Surf. Sci. 2006;252(20):7422–7429. [Google Scholar]
- 118.Yang Y.W., Guo X.N., He C.X., Gao C.D., Shuai C.J. Regulating degradation behavior by incorporating mesoporous silica for Mg bone implants. ACS Biomater. Sci. Eng. 2018;4(3):1046–1054. doi: 10.1021/acsbiomaterials.8b00020. [DOI] [PubMed] [Google Scholar]
- 119.Yang Y.Y., Zhou J.C., Chen Q., Detsch R., Cui X.F., Jin G., Virtanen S., Boccaccini A.R. In vitro osteocompatibility and enhanced biocorrosion resistance of diammonium hydrogen phosphate-pretreated/poly(ether imide) coatings on magnesium for orthopedic application. ACS Appl. Mater. Interfaces. 2019;11(33):29667–29680. doi: 10.1021/acsami.9b11073. [DOI] [PubMed] [Google Scholar]
- 120.Liu C., Liu Y., Wang Q., Liu X.W., Bao Y., Wu G., Lu J. Nano-dual-phase metallic glass film enhances strength and ductility of a gradient nanograined magnesium alloy. Adv. Sci. 2020;7(19) doi: 10.1002/advs.202001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ezechieli M., Ettinger M., Konig C., Weizbauer A., Helmecke P., Schavan R., Lucas A., Windhagen H., Becher C. Biomechanical characteristics of bioabsorbable magnesium-based (MgYREZr-alloy) interference screws with different threads. Knee Surg. Sports Traumatol. Arthrosc. 2016;24(12):3976–3981. doi: 10.1007/s00167-014-3325-6. [DOI] [PubMed] [Google Scholar]
- 122.Mau J.R., Hawkins K.M., Woo S.L.Y., Kim K.E., McCullough M.B.A. Design of a new magnesium-based anterior cruciate ligament interference screw using finite element analysis. J. Orthop. Transl. 2020;20:25–30. doi: 10.1016/j.jot.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang J.L., Wu Y.H., Li H.F., Liu Y., Bai X.L., Chau W.H., Zheng Y.F., Qin L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials. 2018;157:86–97. doi: 10.1016/j.biomaterials.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 124.Tian L., Sheng Y., Huang L., Chow D.H., Chau W.H., Tang N., Ngai T., Wu C., Lu J., Qin L. An innovative Mg/Ti hybrid fixation system developed for fracture fixation and healing enhancement at load-bearing skeletal site. Biomaterials. 2018;180:173–183. doi: 10.1016/j.biomaterials.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 125.Tian L., Tang N., Ngai T., Wu C., Ruan Y.C., Huang L., Qin L. Hybrid fracture fixation systems developed for orthopaedic applications: a general review. J. Orthop. Transl. 2019;16:1–13. doi: 10.1016/j.jot.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J.L., Xu J.K., Hopkins C., Chow D.H.K., Qin L. Biodegradable magnesium-based implants in orthopedics-A general review and perspectives. Adv. Sci. 2020;7(8) doi: 10.1002/advs.201902443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Antoniac I., Laptoiu D., Popescu D., Cotrut C., Parpala R. Development of bioabsorbable interference screws: how biomaterials composition and clinical and retrieval studies influence the innovative screw design and manufacturing processes. In: Antoniac I., editor. Biologically Responsive Biomaterials for Tissue Engineering. Springer; 2012. pp. 107–136. [Google Scholar]
- 128.Jang K.M., Lim H.C., Bae J.H. Mesenchymal stem cells for enhancing biologic healing after anterior cruciate ligament injuries. Curr. Stem Cell Res. Ther. 2015;10(6):535–547. doi: 10.2174/1574888x10666150528153025. [DOI] [PubMed] [Google Scholar]
- 129.Ozeki N., Muneta T., Koga H., Nakagawa Y., Mizuno M., Tsuji K., Mabuchi Y., Akazawa C., Kobayashi E., Matsumoto K., Futamura K., Saito T., Sekiya I. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24(6):1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 130.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., Feng X., Van Hul W., Wan M., Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoshikawa T., Tohyama H., Enomoto H., Matsumoto H., Toyama Y., Yasuda K. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2006;14(9):804–810. doi: 10.1007/s00167-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 132.Crispim J.F., Fu S.C., Lee Y.W., Fernandes H.A.M., Jonkheijm P., Yung P.S.H., Saris D.B.F. Bioactive tape with BMP-2 binding peptides captures endogenous growth factors and accelerates healing after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2018;46(12):2905–2914. doi: 10.1177/0363546518787507. [DOI] [PubMed] [Google Scholar]
- 133.Huangfu X., Zhao J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy. 2007;23(5):455–462. doi: 10.1016/j.arthro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 134.Gulotta L.V., Kovacevic D., Ying L., Ehteshami J.R., Montgomery S., Rodeo S.A. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am. J. Sports Med. 2008;36(7):1290–1297. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 135.Fu S.C., Cheuk Y.C., Yung S.H., Rolf C.G., Chan K.M. Systematic review of biological modulation of healing in anterior cruciate ligament reconstruction. Orthop. J. Sports Med. 2014;2(3) doi: 10.1177/2325967114526687. 2325967114526687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimojo A.A.M., Pires A.M.B., Lichy R., Rodrigues A.A., Santana M.H.A. The crosslinking degree controls the mechanical, rheological, and swelling properties of hyaluronic acid microparticles. J. Biomed. Mater. Res. 2015;103(2):730–737. doi: 10.1002/jbm.a.35225. [DOI] [PubMed] [Google Scholar]