Abstract
Objective
Forkhead box protein O1 (FOXO1) plays a key role in regulating hepatic glucose production, but investigations of FOXO1 inhibition as a potential therapeutic approach have been hampered by a lack of selective chemical inhibitors. By profiling structurally diverse FOXO1 inhibitors, the current study validates FOXO1 as a viable target for the treatment of diabetes.
Methods
Using reporter gene assays, hepatocyte gene expression studies, and in vivo studies in mice, we profiled our leading tool compound 10 and a previously characterized FOXO1 inhibitor, AS1842856 (AS).
Results
We show that AS has significant FOXO1-independent effects, as demonstrated by testing in FOXO1-deficient cell lines and animals, while compound 10 is highly selective for FOXO1 both in vitro and in vivo and fails to elicit any effect in genetic models of FOXO1 ablation. Chronic administration of compound 10 improved insulin sensitivity and glucose control in db/db mice without causing weight gain. Furthermore, chronic compound 10 treatment combined with FGF21 led to synergistic glucose lowering in lean, streptozotocin-induced diabetic mice.
Conclusions
We show that the widely used AS compound has substantial off-target activities and that compound 10 is a superior tool molecule for the investigation of FOXO1 function. In addition, we provide preclinical evidence that selective FOXO1 inhibition has potential therapeutic benefits for diabetes as a monotherapy or in combination with FGF21.
Keywords: FOXO1, Diabetes, Hepatic glucose production, FGF21, AS1842856, Compound 10
Highlights
-
•
Compound 10 is a highly-selective FOXO1 inhibitor and reduces hepatic glucose production in mice.
-
•
AS1842856 has substantial off-target, FOXO1-independent activities both in vitro and in vivo.
-
•
Selective pharmacological FOXO1 inhibition improves insulin sensitivity and normalized blood glucose in db/db mice.
-
•
FOXO1 inhibition in combination with FGF21 leads to synergistic glucose lowering in β-cell-ablated diabetic mice.
1. Introduction
Excessive hepatic glucose production (HGP) is a hallmark of both type 1 (T1D) and type 2 diabetes (T2D). In the liver, transcription factor Forkhead box protein O1 (FOXO1) promotes expression of gluconeogenic and glycogenolytic genes [1]. This activity is blocked by insulin via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, leading to phosphorylation and nuclear exclusion of FOXO1 followed by HGP suppression [2]. While FOXO3 and FOXO4 can also be phosphorylated by PI3K/Akt, only the former plays a modest role in HGP regulation [3]. In contrast, genetic ablation of Foxo1 alone in the liver is sufficient to prevent diabetes in mice [4], defining its predominant role in glucose control. Despite an intense interest in FOXO1 as a therapeutic target, investigations of direct pharmacological FOXO1 inhibition have been largely limited to a single compound, AS1842856 (AS), discovered more than a decade ago [5,6]. This molecule remains the only commercially available small molecule FOXO1 inhibitor. Even though AS has been studied in numerous papers and patent applications, its selectivity profile and effects on glucose metabolism after chronic treatment have not been reported. More recently, we described the discovery of a panel of novel FOXO1 chemical inhibitors that are structurally unrelated to AS [7]. However, whether these compounds can lead to FOXO1 inhibition in vivo and provide substantial therapeutic benefits in diabetes remains to be investigated.
Fibroblast growth factor 21 (FGF21) analogs are emerging as novel therapies that address multiple metabolic abnormalities and comorbidities associated with T2D, including obesity, dyslipidemia, and non-alcoholic steatohepatitis (NASH), but their glucose-lowering effects are less pronounced, especially in T1D [8,9]. Intriguingly, this novel metabolic regulator is increased in mice with hepatic FOXO1 ablation [10], suggestive of an integrated metabolic pathway whose significance in HGP regulation is yet unexplored. To note, in combination with another diabetes therapy, the glucose lowering efficacy of FGF21 is robustly potentiated [11].
In this study, we sought to determine the effects of selective pharmacological inhibition of FOXO1 on in vivo glucose metabolism in mouse models of diabetes and investigated the efficacy of FOXO1 inhibition when co-administered with FGF21.
2. Materials and methods
2.1. Chemicals
Compound 10 (N-[3-(1H-1,3-benzodiazol-2-yl)-1H-pyrazol-5-yl]-4-(4-methylpiperazin-1-yl)benzamide) was synthesized by IntelliSyn Pharma, characterized by mass spectrometry and 1H NMR spectroscopy, and determined to be ≥ 98% pure. AS1842856 was purchased from MedChemExpress (>98% purity).
2.2. Mouse PK
In vivo PK study was performed in male ICR mice (N = 3 per route). Compound 10 was formulated in Solutol HS-15:Saline (5:95 v/v). Mice were dosed i.v. (1 mg/kg) or p.o. (10 mg/kg), and blood was collected at 0.083, 0.25, 0.5, 1, 2, 4, 8, and 24 h and processed to obtain plasma. Compound concentration in plasma was determined by LC-MS/MS and the results were used to calculate PK parameters.
2.3. Reporter gene assays
HEK293 cells (ATCC, Cat# CRL-1573) were seeded at 7,500 cells/well in EMEM supplemented with 1% fetal bovine serum (FBS) and 1x penicillin-streptomycin onto 384-well plates (Perkin Elmer, Cat# 6007680) and incubated at 37 °C/5% CO2 overnight. Cells were transfected with pGL4.26-4xIRE-luc2 (FFluc), pRL-CMV (Promega), and pcDNA3.1 vector containing RFP (as negative control), FOXO1-AAA (Addgene, #9023), FOXA2 (GenScript, #OHu31644), FOXO3 (GenScript, #OHu23372), or FOXO4 (GenScript, #OHu23105) using Lipofectamine3000 (Invitrogen). For the FOXO1-WT (Addgene, #13507) assay, cells were transfected with pGL4.26-4xIRE-NanoLuc and pGK-FFluc (Promega). Compounds were added in 10-point half-log dilution in duplicate wells using a Mosquito dispenser immediately after transfection mixtures were added, with a final dimethyl sulfoxide (DMSO) concentration of 0.5%. Plates were sealed with Breathe Easy tape (Research Products International Corp). After 24 h of incubation, reporter gene activities in cell lysates were measured by Dual-Glo Luciferase Assay System or Nano-Glo Dual-Luciferase Assay System (Promega) according to the manufacturer's protocol using an EnVision 2105 plate reader (Perkin Elmer). Medium removed from the cell plate was used to determine lactate dehydrogenase activity using LDH-Glo Cytotoxicity Assay (Promega). Z′ was calculated as previously described [12] to be > 0.5 for all assays. At least two independent experiments were performed for each compound for each assay. A four-parameter logarithmic curve fit was used to determine relative IC50.
2.4. Primary hepatocytes
All animal procedures were approved by the Columbia University Institutional Animal Care and Use Committee. Hepatocytes were isolated from 8- to 10-week-old male C57/BL6 mice as previously described [7], resuspended in M199 medium containing 10% FBS, 1x penicillin-streptomycin, and 50 μg/ml of G418 (ThermoFisher), and seeded onto collagen-coated 24-well plates at 200,000 cells/well. After 4–5 h of recovery, cells were washed twice with phosphate-buffered saline (PBS) and incubated for 15–16 h in M199 medium containing 1% FBS, 1x penicillin-streptomycin, and 50 μg/ml of G418. Cells were then treated with freshly prepared 100 μM of cAMP (Sigma–Aldrich), 1 μM of dexamethasone (Sigma–Aldrich), and 100 nM of insulin (Sigma–Aldrich), or compounds at various concentrations. The final DMSO concentration was 0.1% for all wells. After 6 h of incubation, RNA was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. One hundred nanograms of RNA were reverse-transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Q-PCR was performed on a CFX Connect Real-Time PCR system (Bio-Rad) using iTaq Universal SYBR Green SuperMix. G6pc, Pck1, Foxo1, and Ppia primer sequences were described previously [13]. AS was tested in 2 independent experiments. Compound 10 was tested in 10 independent experiments.
2.5. Pyruvate tolerance test (PTT)
Normal C57, liver-specific Foxo1 knockout mice (LKO) [4], or their control littermates were randomized by body weight and fed blood glucose levels. Compound 10 was formulated in Optiform Select #8 (Catalent) and dosed at 16 mg/kg. AS was formulated in 5% DMSO/95% PEG-400 and dosed at 30 or 100 mg/kg. Mice were p.o. dosed at 10 ml/kg twice daily for a total of 3 doses (8 am, 5 pm, 8 am). Mice were either fasted for 4 h (from 6 am to 10 am) or 16 h (6 pm–10 am) before 2 g/kg (10 ml/kg) of sodium pyruvate (Sigma) dissolved in saline was injected intraperitoneally (i.p.). Tail blood glucose was measured by Contour glucose monitor (Bayer) at 0, 15, 30, 60, and 120 min. Both compounds were tested once in LKO and control littermates. PTT in normal C57 mice was performed at least twice for each compound for each fasting condition.
2.6. Chronic studies in db/db mice
Six-to 7-week-old male db/db (C57BL/6J background) mice were purchased from Jackson Labs, acclimated for two weeks, and randomized according to body weight and blood glucose. Mice were p.o. dosed with vehicle, compound 10 (16 mg/kg, twice daily) or rosiglitazone (10 mg/kg in 0.5% methylcellulose, twice daily for days 1–4 and once daily for days 5–10). Ad libitum blood glucose levels and body weight were monitored daily in the morning before the first dose. Food intake was measured from two mice receiving the same treatment co-housed in a single cage, and average daily food intake was calculated per animal. Tail blood glucose after 6 h of fasting was measured on days 0, 5, and 10. Plasma insulin was measured by enzyme-linked immunosorbent assay (ELISA, Mercodia). Plasma triglycerides (ThermoFisher), total cholesterol (Wako Pure Chemicals), alanine aminotransferase (ALT, TECO Diagnostics), and aspartate aminotransferase (AST, ThermoFisher) were measured by colorimetric assays according to the manufacturers’ protocols. Insulin tolerance test (ITT) by i.p. injection (0.75U/kg Novolin) was performed on day 9 in 7-h-fasted mice, and blood glucose was monitored for the next 120 min. On day 11, mice were sacrificed by CO2 inhalation followed by cervical dislocation. Livers were collected, fixed, and embedded in paraffin. Sections were stained with hematoxylin and eosin. Images were acquired on a Keyence BZ-X800 microscope. Compound 10 treatment in db/db mice was performed twice.
2.7. Chronic studies in streptozotocin (STZ) mice
Six-to 7-week-old male C57/BL6J mice were purchased from Jackson Labs, acclimated for a week, and i.p. injected with 50 mg/kg of STZ (Sigma–Aldrich) freshly prepared in sodium citrate buffer (pH4.5) for 5 consecutive days. Blood glucose was monitored twice weekly until mice became hyperglycemic (300–450 mg/dL). Mice were randomized by blood glucose and body weight. Human recombinant FGF21 (Sino Biological) was formulated in saline and s.c. injected at 0.45 mg/kg once daily. For mice receiving combination treatment, FGF21 was injected daily from day 1, and compound 10 was administered twice daily from day 4. Compound 10 was formulated in Solutol HS-15:Saline (5:95 v/v) and p.o. dosed twice daily for 7 days at 32 mg/kg/dose. Blood and plasma parameters were measured as described above. For oral glucose tolerance test, mice were fasted for 6 h, p.o., dosed with 2 g/kg glucose, and tail blood glucose levels were measured at 0, 30, 60, and 120 min after the glucose challenge. On day 11, mice were sacrificed and both sides of perigonadal fat pads were dissected and weighed. Liver triglyceride (TG) content was measured as previously described [7] and normalized by tissue weight. Compound 10/FGF21 combination treatment in STZ mice was performed once.
3. Results
3.1. Activities of FOXO1 inhibitors compound 10 and AS1842856 in cell-based assays
We profiled a panel of previously reported FOXO1 inhibitors [7] for their aqueous solubility, intrinsic clearance, and cellular permeability. Compound 10 (N-[3-(1H-1,3-benzodiazol-2-yl)-1H-pyrazol-5-yl]-4-(4-methylpiperazin-1-yl)benzamide) emerged as the molecule with the best overall profile. In vivo pharmacokinetic study for compound 10 in mice showed significant plasma exposure and oral bioavailability despite relatively short half-life (Table S1), suggesting that this compound is suitable for in vivo studies by oral dosing.
To compare compound 10 to AS [5], the FOXO1 inhibitor widely used in the literature, we tested both compounds in a series of cell-based activity and selectivity assays (Table 1). We used HEK293 cells for transcriptional reporter assays as this cell line has minimal endogenous expression of FOXO proteins and low basal activity of a transfected insulin response element (IRE) [14]-luciferase reporter (data not shown). Transfection of FOXO1 or other forkhead transcription factors into HEK293 cells leads to >100-fold increase in IRE-luciferase activity. A separate plasmid containing a divergent luciferase enzyme under the control of a constitutive promoter is co-transfected as an internal control for transfection efficiency. Notably, compound 10 displayed similar inhibitory activities in the IRE-reporter assay against both wild-type (WT) FOXO1 and a constitutively active form of FOXO1, which has three serine/threonine residues mutated to alanine (AAA) and thus cannot be phosphorylated and inactivated by Akt [15]. These findings show that compound 10 acts distal to PI3K/Akt. In contrast, AS potently inhibited FOXO1-WT but was much less active against FOXO1-AAA, consistent with the previous report that the compound's interaction with FOXO1 depends on these key serine/threonine residues [5], and also with the possibility of off-target effects on the PI3K/Akt pathway.
Table 1.
Activities of FOXO1 inhibitors in HEK293 cell-based assays.
| HEK293 cell-based assays | Compound 10, IC50 (μM) | AS1842856, IC50 (μM) | |
|---|---|---|---|
| IRE-reporter assays | FOXO1-WT | 0.076 × /÷ 1.7∗ | 0.131 × /÷ 3.3 |
| FOXO1-AAA | 0.046 × /÷ 1.3 | 9.03 × /÷ 1.1 | |
| FOXO3 | 15.6 × /÷ 1.1 | 2.21 × /÷ 1.7 | |
| FOXO4 | 18.0 × /÷ 1.1 | 1.16 × /÷ 1.8 | |
| FOXA2 | 22.4 × /÷ 2.0 | EC50 = 2.35 × /÷ 2.5 | |
| Non-specific activities | pGK-FFluc | >10∗ | 1.08 × /÷ 1.5 |
| pCMV-Rluc | >24 | 0.647 × /÷ 1.9 | |
| Cell viability (LC50) | >24 | 19.1 × /÷ 1.5 | |
IRE: insulin response element. FFluc: firefly luciferase. Rluc: Renilla luciferase. Data are geometric mean × /÷ standard deviation. ∗Highest concentration tested = 10 μM. All other assays were tested up to 50 μM.
We examined the compounds’ activities against FOXO3, FOXO4, as well as the more distantly related FOXA2. Compound 10 showed minimal activity for these three forkhead transcription factors, with >200-fold selectivity for FOXO1 (Table 1). Furthermore, compound 10 showed no significant inhibition of firefly (FFluc) or Renilla luciferase (Rluc) reporters driven by constitutive promoters (pGK and pCMV, respectively), and no significant cellular toxicity (measured by lactate dehydrogenase release into supernatant). In contrast, although AS did not cause significant cell death, it paradoxically activated FOXA2-dependent reporter activity, inhibited FOXO3- and FOXO4-dependent reporter gene expression and inhibited the activities of constitutively expressed FFluc and Rluc, all in the sub-to low-micromolar range, with 5- to 18-fold selectivity for FOXO1. These data indicate that AS has significant FOXO1-independent effects in HEK293 cells.
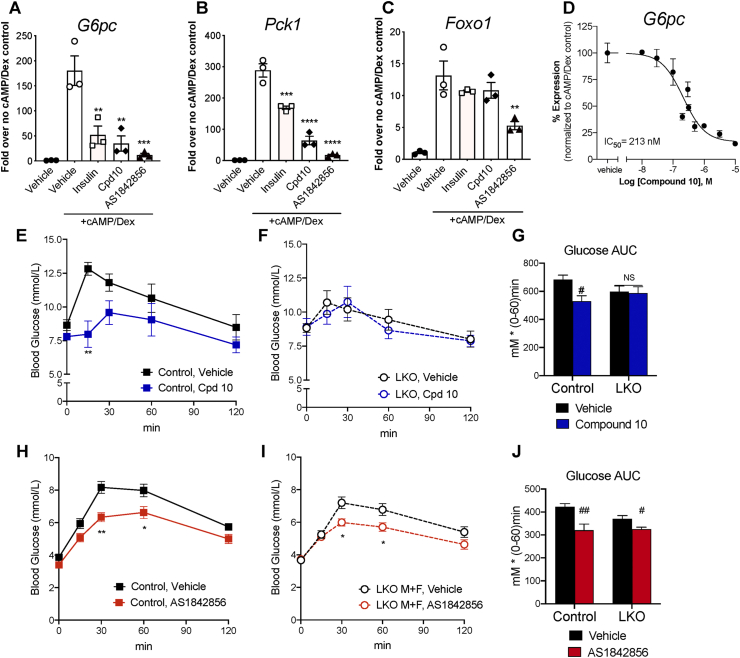
We sought to confirm that the compounds can inhibit endogenous FOXO1 function, using an orthogonal assay format that avoids potential artefacts due to overexpression. The hepatocyte is a model in which hormone-dependent transcriptional regulation of gluconeogenic genes, such as glucose-6 phosphatase (G6pc) and phosphoenolpyruvate carboxykinase 1 (Pck1) by endogenously expressed FOXO1 is well established [1]. In primary hepatocytes isolated from normal mice, compound 10, AS, as well as insulin (the physiological inhibitor of FOXO1 in this context) significantly suppressed cyclic AMP (cAMP)/dexamethasone (Dex)-induced G6pc and Pck1 mRNA expression (Figure 1A–B). Noteworthy, AS suppressed cAMP/Dex-stimulated Foxo1 expression by 60%, while insulin and compound 10 did not affect Foxo1 levels (Figure 1C). Dose titration studies in hepatocytes showed that compound 10 suppressed G6pc expression in a dose-dependent manner with an estimated IC50 of 213 nM (Figure 1D). Taken together, the data indicate that compound 10 and AS inhibit endogenous FOXO1 target gene expression in hepatocytes; and the effect of AS (but not that of compound 10) is at least in part mediated by a reduction in Foxo1 mRNA expression rather than inhibition of FOXO1 protein function.
Figure 1.
In vitro and in vivo effects of FOXO1 inhibitors. (A–C) mRNA expression of G6pc, Pck1, and Foxo1 in primary mouse hepatocytes treated by vehicle, cAMP (100 μM)+Dex (1 μM), cAMP + Dex + insulin (100 nM), or cAMP + Dex + FOXO1 inhibitors (10 μM) was quantified by q-RT-PCR and normalized against Ppia. Expression level of each gene in control hepatocytes (without cAMP + Dex) was set to 1. Values of 3 replicate wells and mean ± SEM are shown. Data are representative of at least 2 independent experiments. ∗∗, ∗∗∗, ∗∗∗∗: p < 0.01, 0.001, 0.0001 vs. cAMP + Dex by one-way ANOVA. (D) G6pc mRNA expression in mouse hepatocytes treated by compound 10 at concentrations ranging from 10 nM to 10 μM in the presence of cAMP + Dex. G6pc expression in control hepatocytes (without cAMP + Dex) was set to 0%, and that in hepatocytes treated by cAMP + Dex was set to 100%. Mean ± SEM of 3–6 replicate wells per concentration are shown. Aggregated data from 4 independent experiments was analyzed by 4-parameter curve fit in GraphPad Prism. (E–J) Blood glucose levels and area-under-the-curve (AUC) during intraperitoneal PTT in control mice (Foxo1 flox/flox) and liver-specific Foxo1 knockouts (LKO; Albumin-Cre, Foxo1 flox/flox) after oral treatment of compound 10 at 16 mg/kg/dose (E–G) or AS at 30 mg/kg/dose (H–J). N = 7–15 mice per group; both male and female animals are included. ∗, ∗∗: p < 0.05, 0.01 vs. vehicle by two-way ANOVA. #, ##: p < 0.05, 0.01 vs. vehicle by one-way ANOVA. NS: not significant.
3.2. Effects of compound 10 and AS1842856 in normoglycemic mice
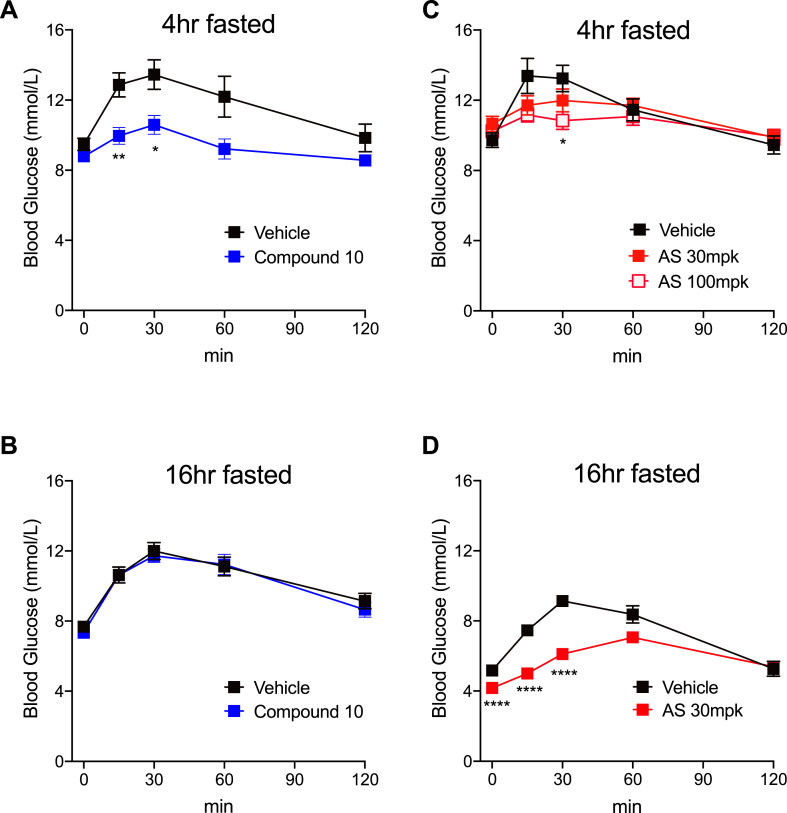
FOXO1 inhibition has been shown to suppress HGP in vivo [4,5], leading to reduced glucose excursions when mice receive a bolus of gluconeogenetic precursors. Indeed, after a 4-h fast, normal C57 mice (Fig. S1A) or control mice (Foxo1 flox/flox, Figure 1E) that received three oral doses (on a b.i.d. schedule) of compound 10 followed by an intraperitoneal injection of pyruvate showed significantly lower glucose excursion compared to mice receiving vehicle treatment. Furthermore, compound 10 failed to reduce glucose levels during pyruvate tolerance test (PTT) in liver-specific Foxo1 knockout (LKO) mice (Figure 1F–G), confirming the compound's effect requires intact FOXO1. Of note, compound 10 at the same dose level was ineffective at lowering glucose in normal C57 mice that were fasted overnight (Fig. S1B). This is consistent with the notion that the primary target of FOXO1 is G6pc, which regulates glycogenolysis in the early time points after fasting, whereas gluconeogenesis, which is partially FOXO1-independent, predominates after prolonged fasting [16]. AS was able to reduce glucose levels during PTT in overnight-fasted C57 mice (Fig. S1D) and control mice (Figure 1H) at 30 mg/kg, the previously reported minimum efficacious dose [5]. In 4-h-fasted mice, AS also significantly lowered glucose excursion during PTT, albeit at a higher dose level (Fig. S1C). However, AS also reduced glucose levels in liver Foxo1 knockouts (Figure 1I–J), indicating that its glucose-lowering effect in vivo is partly independent of FOXO1.
3.3. Effects of compound 10 in insulin-resistant diabetic mice
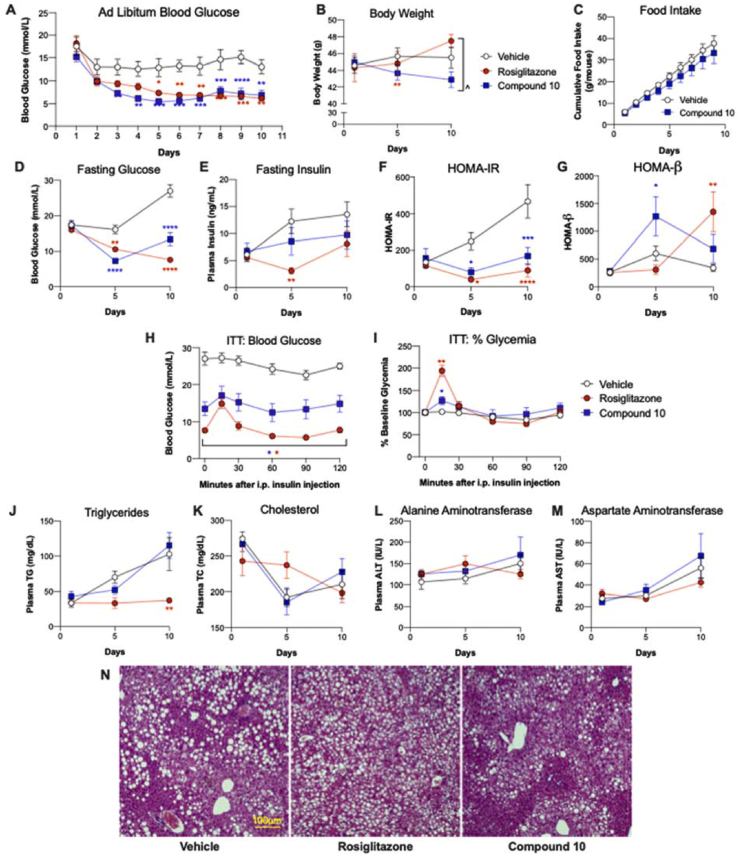
We observed poor tolerability (moribund appearance and unanticipated death) of AS after repeated dosing in diabetic mice, which may partly account for the lack of prior reports on this compound's chronic efficacy for diabetes. In light of the superior in vitro and in vivo selectivity of compound 10 compared to AS, we further examined the in vivo effects of compound 10 in diabetic mice. In db/db mice, a model with severe obesity and insulin resistance, compound 10 treatment for ten days reduced blood glucose to an extent similar to the PPARγ agonist rosiglitazone (Figure 2A, D), although it did not significantly affect insulin levels (Figur 2E). The calculated HOMA-IR index showed a substantial worsening of insulin resistance in control animals over the course of the study that was staunched by both compound 10 and rosiglitazone (Figure 2F). HOMA-β, an indicator of β-cell function, tended to be improved by both compound 10 and rosiglitazone (Figure 2G). ITT showed significantly reduced glucose levels in both compound 10 and rosiglitazone treated mice at all time points (Figure 2H–I), while data interpretation regarding insulin sensitivity was confounded by major differences in baseline glycemia and acute response to handling stress across different groups. Importantly, there was a trend towards weight loss (5%) in db/db mice treated by compound 10, in contrast to the weight gain induced by Rosiglitazone (Figure 2B). In a follow-up study, we found that db/db mice treated by compound 10 showed a trend towards reduced food intake (12%) compared to those receiving vehicle treatment (Figure 2C), which likely contributed to the weight loss. Compound 10 had no significant effects on plasma TG and total cholesterol (TC) (Figure 2J–K), while rosiglitazone reduced plasma TG. ALT and AST were not affected by either compound (Figure 2L–M). In addition, compound 10 had no apparent effect on hepatic histology, while rosiglitazone exacerbated hepatic steatosis (Figure 2N). In summary, FOXO1 inhibition normalized blood glucose in db/db mice, associated with improvements in insulin sensitivity and β-cell function (as evidenced by HOMA-IR and HOMA-β), which are unlikely to be explained solely by the modest weight loss observed. Furthermore, the FOXO1 inhibitor's effect on body weight differentiate it from PPARγ agonist rosiglitazone, indicative of a distinct mechanism and a potentially superior therapeutic profile. Finally, compound 10 did not increase liver enzymes, confirming its in vivo safety.
Figure 2.
Effects of FOXO1 inhibitor compound 10 in db/db mice. Male db/db mice were treated by vehicle, compound 10 (32 mg/kg/dose), or Rosiglitazone (10 mg/kg/dose) for 10 days. (A) Ad libitum blood glucose levels measured daily approximately 1 h after dosing. (B) Body weight, (C) daily food intake, (D) blood glucose, and (E) plasma insulin after a 6 h fast on the indicated days are shown. Fasting glucose and insulin levels were used to calculate HOMA-IR (F) and HOMA-β (G). (H–I) Intraperitoneal insulin tolerance test was performed on day 9; and (H) blood glucose levels and (I) % glycemia normalized to time 0 are shown. (J–M) 6-h-fasted plasma levels of (J) TG, (K) TC, (L) ALT, and (M) AST are shown. (N) Representative H&E staining of liver tissues collected after 10 days of treatment. N = 6–8 per group. Data are Mean ± SEM. ∗, ∗∗, ∗∗∗, ∗∗∗∗: p < 0.05, 0.01, 0.001, 0.0001 vs. vehicle by two-way ANOVA. ˆ: p < 0.05 between indicated groups by two-way ANOVA.
3.4. Effects of compound 10 and FGF21 in β-cell-ablated diabetic mice
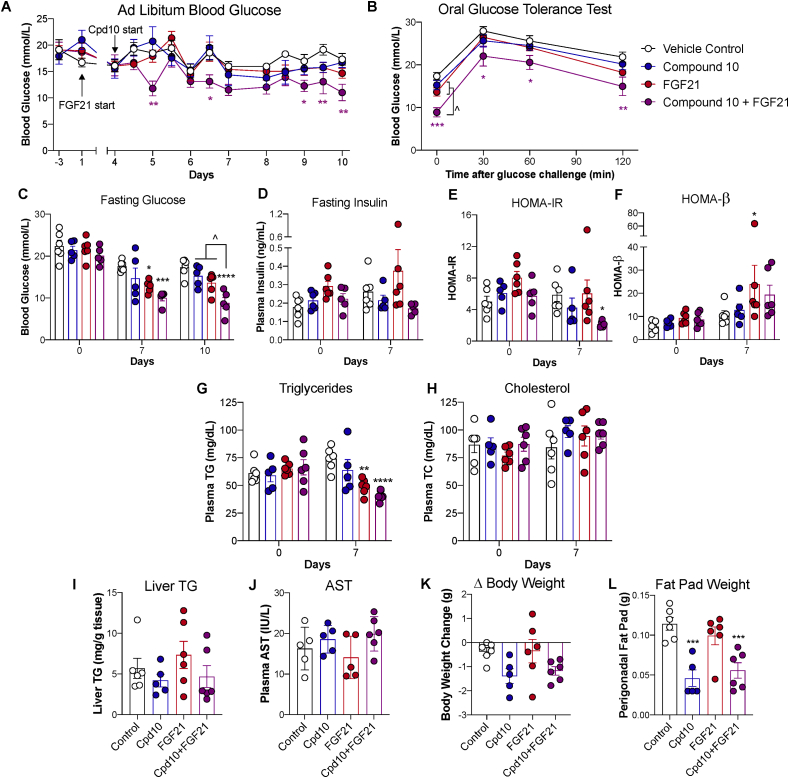
To assess the efficacy of FOXO1 inhibition in an insulin-deficient model similar to type 1 diabetes, we went on to examine the effect of compound 10 in lean mice rendered diabetic by STZ, a model of β-cell ablation with normal insulin sensitivity. Compound 10 treatment alone did not significantly reduce blood glucose levels in this model (Figure 3A–B, blue line). We hypothesized that if insulin sensitivity is further enhanced, the effect of FOXO1 inhibition may become more pronounced. FGF21 has been shown to enhance insulin action even in mice that have normal body weight and insulin sensitivity [17,18], although it did not significantly lower glucose in lean STZ diabetic mice as a monotherapy (Figure 3A–B, red line). STZ-induced diabetic mice receiving FGF21 treatment combined with compound 10 showed lower glucose levels and reduced glucose excursion during an oral glucose tolerance test (Figure 3A–C, purple line). Insulin levels were not significantly different among groups (Figure 3D). HOMA-IR and HOMA-β indices showed improvements in insulin sensitivity and β-cell function only in animals receiving the FGF21/compound 10 combination treatment (Figure 3E–F) and not in animals receiving either treatment alone. Plasma TG, a well-established biomarker for FGF21 [19], were significantly reduced in animals receiving FGF21 monotherapy and combination treatment (Figure 3G), confirming FGF21 target engagement. Plasma TC and AST levels and liver TG content were not significantly different among groups (Figure 3H–J). Animals receiving compound 10 monotherapy and combination treatment showed a trend toward reduced body weight (Figure 3K) and >50% reduction in perigonadal fat pad weight at the end of the study (Figure 3L). Collectively, these data show FOXO1 inhibition and FGF21 have synergistic glucose-lowering effects in insulin-deficient diabetes.
Figure 3.
Effects of FOXO1 inhibitor compound 10 in STZ lean mice. STZ-induced diabetic male mice were treated by vehicle, compound 10 (32 mg/kg/dose), FGF21 (0.45 mg/kg), or both compound 10 and FGF21. (A) Ad libitum blood glucose levels were measured 1 h after dosing. (B) Blood glucose levels were measured during an OGTT performed on day 11. (C–H) Blood glucose (C) and plasma levels of insulin (D), triglycerides (G), and total cholesterol (H) were measured after a 6-h fast on indicated days. Fasting glucose and insulin levels were used to calculate HOMA-IR (E) and HOMA-β (F). (I) Liver TG content was measured on day 11. (J) AST level was measured in ad libitum plasma on day 9. (K) Change in body weight on day 10 compared to day 0 and (L) perigonadal fat pad weight on day 11 were recorded. N = 5–6 per group. Data are Mean ± SEM. ∗, ∗∗, ∗∗∗, ∗∗∗∗: p < 0.05, 0.01, 0.001, 0.0001 vs. vehicle control by two-way ANOVA. ˆ: p < 0.05 between indicated groups by two-way ANOVA.
4. Discussion
As the nexus of HGP regulation in normal hormonal response and dysregulation in insulin resistance, there has been long-standing interest in FOXO1 as a potential therapeutic target for the treatment of diabetes. However, investigations to date into FOXO1 pharmacology have been handicapped by the dearth of selective and well-tolerated chemical inhibitors. A key advance of the current report is the demonstration that the widely used inhibitor AS has FOXO1-independent effects in vitro and in vivo, including widespread effects on transcription in HEK293 cells, suppression of Foxo1 mRNA expression in hepatocytes, and the ability to reduce glucose levels in liver Foxo1 knockout mice. The current data suggest that conclusions drawn from studies using this molecule should be re-examined.
We show that the recently reported inhibitor compound 10 is highly selective for FOXO1, has significant oral exposure, is well tolerated in vivo, and lowers glucose in mice in a FOXO1-dependent manner. Therefore, it is a superior tool molecule to probe FOXO1 pharmacology. Chronic FOXO1 inhibition by compound 10 leads to insulin sensitization and improves glucose control in diabetic mice. These salutary effects are more pronounced in the insulin-resistant and hyperinsulinemic db/db mouse model than in the β-cell-ablated and insulin-sensitive STZ lean mouse model, consistent with FOXO1 inhibition having a predominant effect on insulin sensitization. The increase in HOMA-β associated with FOXO1 inhibitor treatment may be secondary to improvements in glycemia, and should be investigated in future studies. Interestingly, FOXO1 inhibition tended to reduce body weight and adiposity, in contrast to the weight gain associated with PPARγ agonists as well as insulin [20,21]. Furthermore, notwithstanding the theoretical liability that FOXO1 inhibition may activate lipogenesis and worsen dyslipidemia [10], compound 10 was neutral on hepatic steatosis and did not increase circulating triglycerides or cholesterol. While the mechanism underlying compound 10's body weight- and lipid-sparing effects deserves further investigation, the current report delineates a path to a new class of insulin sensitizers that can potentially normalize glucose control in diabetes without exacerbating comorbidities, such as obesity, atherosclerosis, and NASH.
Combination therapy adopted early in the course of disease has become a mainstay for the treatment of diabetes [22]. FGF21 analogs have shown promise for the treatment of hypertriglyceridemia and NASH. Although their glycemic efficacy as monotherapy are limited, clinical studies of native FGF21, several FGF21 analogs and FGFR1/KLB agonistic antibodies have shown improvements in insulin sensitivity, glucose levels and/or HbA1c [23]. Thus, the beneficial effect of this therapeutic class is fully translatable in humans. The current study is the first to report a synergistic glucose-lowering effect of FOXO1 inhibition and FGF21 in diabetic mice. Remarkably, the combination treatment further improves insulin sensitivity in STZ lean mice, which do not have underlying insulin resistance. Whether FOXO1 regulates FGF21 sensitivity has not been studied and will be subject to future investigations. This study outlines a potential combination approach that enhances the glycemic efficacy of the FGF21 therapeutic class to address multiple comorbidities of diabetes.
Acknowledgments
This work was funded by Forkhead BioTherapeutics, Inc. and a grant awarded by the National Institute of Diabetes and Digestive and Kidney Diseases (R43DK120177). We thank Carmen Lam, Kasia Dover, Xiaoming Xu, Junjie Yu, Q. Lina Xu, Meiyan Liu, and Mingfei Zeng for expert technical assistance.
Conflict of interest
None declared.
Appendix.
Supplementary Table 1.
PK properties of compound 10.
| Compound 10 mouse PK | ||
|---|---|---|
| IV (1 mg/kg) | T1/2 | 1.23 h |
| AUCinf | 1.86 μM∗h | |
| Vss | 1.71 L/kg | |
| CL | 22.7 mL/kg∗min | |
| PO (10 mg/kg) | Tmax | 0.67 h |
| Cmax | 1.62 μM | |
| AUCinf | 4.66 μM∗h | |
| F% | 25.5% | |
PK: pharmacokinetics. IV: intravenous. PO: per os. CL: clearance. Vss: steady state volume of distribution. F%: oral bioavailability.
Figure S1.
Effects of compound 10 and AS on pyruvate tolerance test in normal mice. Six-to 8-week-old male C57 mice were treated by oral gavage of compound 10 at 16 mg/kg/dose (A-B) or AS at 30 or 100 mg/kg/dose (C-D). Pyruvate was injected after 4hr (A, C) or 16 h (B, D) fasting. N = 10 mice per group. ∗, ∗∗, ∗∗∗∗: p < 0.05, 0.01, 0.0001 vs. vehicle by two-way ANOVA.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nakae J., Kitamura T., Silver D.L., Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. Journal of Clinical Investigation. 2001;108(9):1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakae J., Park B.C., Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. Journal of Biological Chemistry. 1999;274(23):15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 3.Haeusler R.A., Kaestner K.H., Accili D. FoxOs function synergistically to promote glucose production. Journal of Biological Chemistry. 2010;285(46):35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto M., Pocai A., Rossetti L., Depinho R.A., Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metabolism. 2007;6(3):208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Nagashima T., Shigematsu N., Maruki R., Urano Y., Tanaka H., Shimaya A. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Molecular Pharmacology. 2010;78(5):961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 6.Calissi G., Lam E.W., Link W. Therapeutic strategies targeting FOXO transcription factors. Nature Reviews Drug Discovery. 2020 doi: 10.1038/s41573-020-0088-2. [DOI] [PubMed] [Google Scholar]
- 7.Langlet F., Haeusler R.A., Linden D., Ericson E., Norris T., Johansson A. Selective inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171(4):824–835. doi: 10.1016/j.cell.2017.09.045. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharitonenkov A., DiMarchi R. Fibroblast growth factor 21 night watch: advances and uncertainties in the field. Journal of Internal Medicine. 2017;281(3):233–246. doi: 10.1111/joim.12580. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.H., Bae K.H., Choi Y.K., Go Y., Choe M., Jeon Y.H. Fibroblast growth factor 21 analogue LY2405319 lowers blood glucose in streptozotocin-induced insulin-deficient diabetic mice by restoring brown adipose tissue function. Diabetes, Obesity and Metabolism. 2015;17(2):161–169. doi: 10.1111/dom.12408. [DOI] [PubMed] [Google Scholar]
- 10.Haeusler R.A., Han S., Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. Journal of Biological Chemistry. 2010;285(35):26861–26868. doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilroy C.A., Capozzi M.E., Varanko A.K., Tong J., D'Alessio D.A., Campbell J.E. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Advancement of Science. 2020;6(35):eaaz9890. doi: 10.1126/sciadv.aaz9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iversen P.W., Beck B., Chen Y.F., Dere W., Devanarayan V., Eastwood B.J. HTS assay validation. In: Markossian S., editor. Assay guidance manual [Internet] Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD: 2012. [Google Scholar]
- 13.Wang L., Liu Q., Kitamoto T., Hou J., Qin J., Accili D. Identification of insulin-responsive transcription factors that regulate glucose production by hepatocytes. Diabetes. 2019;68(6):1156–1167. doi: 10.2337/db18-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durham S.K., Suwanichkul A., Scheimann A.O., Yee D., Jackson J.G., Barr F.G. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140(7):3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- 15.Tang E.D., Nunez G., Barr F.G., Guan K.L. Negative regulation of the forkhead transcription factor FKHR by Akt. Journal of Biological Chemistry. 1999;274(24):16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 16.Kuo T., McQueen A., Chen T.C., Wang J.C. Regulation of glucose homeostasis by glucocorticoids. Advances in Experimental Medicine & Biology. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wente W., Efanov A.M., Brenner M., Kharitonenkov A., Koster A., Sandusky G.E. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55(9):2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 18.Andersen B., Omar B.A., Rakipovski G., Raun K., Ahren B. Fibroblast growth factor 21 prevents glycemic deterioration in insulin deficient mouse models of diabetes. European Journal of Pharmacology. 2015;764:189–194. doi: 10.1016/j.ejphar.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal G., Thanikachalam P.V., Maurya R.K., Chawla P., Ramamurthy S. An overview on medicinal perspective of thiazolidine-2,4-dione: a remarkable scaffold in the treatment of type 2 diabetes. Journal of Advanced Research. 2020;23:163–205. doi: 10.1016/j.jare.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larger E. Weight gain and insulin treatment. Diabetes & Metabolism. 2005;31(4 Pt 2):4S51–4S56. doi: 10.1016/s1262-3636(05)88268-0. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Addendum. 9. Pharmacologic approaches to glycemic treatment: Diabetes Care. 2020;43(8):1979. doi: 10.2337/dc20-ad08a. [DOI] [PubMed] [Google Scholar]
- 23.Talukdar S., Kharitonenkov A. NASH we trust. Mol Metab; 2020. FGF19 and FGF21; p. 101152. [DOI] [PMC free article] [PubMed] [Google Scholar]