Abstract
Objective
Vascular ultrasound (VUS) is a first‐line test for giant cell arteritis (GCA) in Europe but has been of limited use in the United States. We report clinical experience with a multidisciplinary model of VUS for the evaluation of GCA at a large US medical center.
Methods
Patients who underwent VUS for evaluation of GCA between 2013 and 2017 were reviewed. Trained vascular technologists followed a standardized protocol to visualize bilateral temporal, carotid, subclavian, and axillary arteries. Vascular medicine physicians interpreted VUS as no arteritis, hyperechoic wall thickening, or acute arteritis. Characteristics of patients with versus without acute arteritis (no arteritis or hyperechoic wall thickening) were compared. Among patients with suspected new‐onset GCA, the treating physician’s pretest and posttest suspicion for GCA were compared.
Results
Of 530 patients, 10.6% had prior‐onset GCA, 31.7% had polymyalgia rheumatica, and 57.6% were taking glucocorticoids. Most patients had no arteritis on VUS (84.3%); 10.6% had acute arteritis, and 5.1% had hyperechoic wall thickening. Typical GCA symptoms, such as jaw claudication and scalp tenderness, were significantly more frequent in patients with acute arteritis. For all 42 patients with suspected new‐onset GCA and acute arteritis, posttest suspicion was unchanged or increased. Of 415 patients with suspected new‐onset GCA and VUS without acute arteritis, suspicion decreased (76.4%) or was unchanged (20.2%).
Conclusion
We describe a multidisciplinary model for incorporating VUS into GCA care. When pretest suspicion was low and VUS did not reveal acute arteritis, posttest suspicion typically decreased, whereas when pretest suspicion was high and VUS revealed acute arteritis, posttest suspicion was reinforced.
Significance & Innovations.
A multidisciplinary model for performing and incorporating vascular ultrasound (VUS) into giant cell arteritis (GCA) clinical care impacted the treating physicians’ clinical impressions about the likelihood of GCA.
Typical GCA symptoms were more common among patients with versus without acute arteritis on VUS.
The treating physician’s clinical impression was generally reduced when pretest suspicion for GCA was low and VUS did not reveal acute arteritis, but it was reinforced when pretest suspicion for GCA was high and VUS revealed acute arteritis.
INTRODUCTION
Giant cell arteritis (GCA) has traditionally been diagnosed based on clinical evaluation and temporal artery biopsy. Proposed over two decades ago as a means for identifying large‐vessel vasculitis, vascular ultrasound (VUS) is increasingly used for this purpose (1). VUS demonstrates good sensitivity (77%) and excellent specificity (96%) for GCA when clinical diagnosis is the reference standard, does not involve ionizing radiation or invasive procedures, and is a relatively low‐cost test that can be readily performed in the ambulatory setting (2). Recognizing these favorable features, the European League Against Rheumatism recently recommended VUS as a first‐line diagnostic test for GCA (3).
VUS has been incorporated into routine practice for the evaluation of GCA in a number of European centers but has been slower to gain acceptance for such use in US medical centers (4, 5, 6, 7, 8). Since 2013, the Brigham and Women’s Hospital (BWH) has offered VUS as a tool for evaluating GCA. In the BWH model, vascular technologists perform VUS, vascular medicine physicians interpret VUS studies, and rheumatologists may provide clinical consultation with the patient but do not perform VUS themselves. We herein describe implementation, utilization, and impact of VUS on the evaluation of GCA at a large academic medical center.
PATIENTS AND METHODS
Background
BWH is a tertiary care academic medical center in Boston, Massachusetts, with approximately 1.7 million ambulatory visits annually and more than 1000 inpatient beds. VUS for the diagnosis of GCA is performed by a vascular technologist according to a standardized protocol; the study is then formally interpreted by a vascular medicine physician.
The BWH model for incorporating VUS into routine clinical care grew from a desire to emulate the success of European physicians, largely rheumatologists, in the use of this modality for the diagnosis of GCA and to broaden its availability and accessibility in a general medical center (1, 6, 7, 9, 10). Since 2013, more than 1200 VUS examinations for evaluation of arteritis have been performed at BWH.
General VUS examinations at BWH are performed in the Vascular Laboratory, which employs seven vascular technologists and performs more than 12, 000 vascular studies annually to evaluate a range of vascular diseases including carotid stenosis, deep vein thrombosis, and peripheral artery disease. The Vascular Laboratory is co‐directed by a radiologist and a vascular medicine physician. Owing to the Laboratory’s singular focus on imaging arterial and venous structures, the vascular technologists and vascular medicine physicians have a strong working knowledge of the sonographic characteristics of arteriosclerosis and other common vascular anomalies.
In 2013, a collaboration to implement VUS for the diagnosis of GCA was initiated among a member of the Division of Rheumatology (WPD), the Vascular Laboratory, and the Section of Vascular Medicine. All of the technologists that perform VUS to evaluate for GCA and the vascular medicine physicians that interpret these studies are credentialed registered vascular technologists (RVTs). This is a licensing examination that certifies expertise in VUS. The involved vascular technologists and vascular medicine physicians each have more than 5 years of vascular imaging experience. One of the vascular technologists had extensive interest in the application of VUS for the diagnosis of arteritis; training was augmented through the participation by two other vascular technologists and two physicians (WPD and PSS) in two separate European workshops.
In 2018, the Division of Rheumatology and Section of Vascular Medicine established a Fast Track Clinic for the Diagnosis of Giant Cell Arteritis to provide urgent evaluation for suspected GCA. Patients seen in the GCA Fast Track Clinic have a rheumatology clinic visit and VUS performed by the Vascular Laboratory; the VUS is generally performed immediately before or immediately following the rheumatology clinic visit so that it can inform real‐time clinical decision making.
Referral process
Any physician can refer a patient to the BWH Vascular Laboratory for VUS for the evaluation of arteritis. Although rheumatologists most frequently order the study, VUS referrals also are generated by neurologists, primary care doctors, ophthalmologists, inpatient general medicine physicians (hospitalists), emergency medicine physicians, and other physicians. Referrals are processed as an urgent priority; studies are frequently performed on the same day as an ambulatory visit with the referring provider, and nearly all referrals are completed within one business day. The protocolized VUS is performed by a vascular technologist and lasts about 45 minutes; it is then interpreted by a vascular medicine physician, and an official report as well as the VUS study itself are posted electronically in the patient’s medical record, typically on the day of the examination. The vascular technologist and vascular medicine physician are aware that the reason for VUS is to evaluate for GCA, though they are not routinely aware of the referring provider’s pretest clinical impression. Suspicious findings are promptly reported by the vascular technologist to the reading vascular medicine physician for review, and confirmed positive findings are relayed to the referring clinician by phone.
The order for VUS, if generated by a nonrheumatologist, is not routinely accompanied by a request for official rheumatology consultation, though cases of suspected GCA are often assessed by physicians in the Division of Rheumatology.
VUS protocol
Trained vascular technologists follow a standardized protocol to visualize 12 arteries: the right and left common superficial temporal arteries and their frontal and parietal branches, and the right and left common carotid, subclavian, and axillary arteries (11). Simultaneous color Doppler and duplex ultrasonography are performed using an 8‐18 MHz linear transducer (>15 MHz for temporal arteries, <15 MHz for large arteries) (LOGIQ S8 and E9 ultrasound systems; GE Healthcare). Gray scale is set to the highest available frequency, with dynamic range of 40 to 50 dB, and focus is set to approximately 5 mm below the skin surface. Color Doppler is set to the highest frequency with pulse repetition frequency (PRF) 2 KHz for temporal arteries, and lower frequency with PRF 3.5 KHz for large arteries. Frame rate is set as high as possible. Color PRF is 2.5 KHz Doppler frequency shift and is readjusted throughout the examination with velocity changes. Color gain is set such that color covers the lumen entirely, and color box angle correction is set to minimize the isonation angle. Power Doppler is used if occlusion is suspected. Pulse Doppler settings are 2 KHz for temporal arteries and 3 to 5 KHz for large arteries and are adjusted according to flow velocities. Doppler sample volume size is the same diameter as the arterial lumen (0.7 mm for temporal arteries; 1 mm for large arteries) and is positioned in the middle of the vessel with angle correction of 60° or less.
VUS interpretation
A vascular medicine physician provides an overall interpretation of the VUS study based on findings in individual arteries. Individual arteries are designated as having no arteritis‐related abnormality, halo sign, hyperechoic wall thickening without evidence of acute arteritis, stenosis, and/or occlusion.
A halo sign is defined as homogeneous, circumferential hypoechoic wall thickening visualized in longitudinal and transverse views (12, 13). Hyperechoic wall thickening is defined by circumferential wall thickening visualized in longitudinal and transverse views, hyperechogenicity compared to adjacent arterial segments within the same artery, and absence of arteriosclerosis.
Stenosis is defined as more than a two‐fold increased velocity in the artery segment distal to the affected artery, along with arterial waveform abnormality (14, 15). Occlusion is defined as complete absence of flow. For stenosis or occlusion to be considered attributable to arteritis, halo sign, or hyperechoic wall thickening is required in that artery.
In the carotid, subclavian, and axillary arteries, wall thickening is present when arterial wall thickness exceeds the thickness of the intima‐media complex with associated hypolucency or hyperlucency within the arterial wall without changes of arteriosclerosis (16). Because the intima‐media complex is not visible on VUS in a normal temporal artery, visualization of any thickening of the temporal artery wall with associated hypolucency or hyperlucency defines wall thickening in the temporal arteries. The 8‐18 MHz transducers used for VUS were not able to detect the intimal stripe in the temporal arteries (17).
The superficial temporal arteries, frontal, and parietal branches are considered abnormal if the halo sign or hyperechoic wall thickening is present. The common carotid and the subclavian and axillary arteries are considered abnormal if halo sign, hyperechoic wall thickening, stenosis, and/or occlusion are present. The presence of arteriosclerosis alone is not considered an arteritis‐related abnormality.
The overall interpretation for each VUS is one of three options: acute arteritis, no arteritis, or hyperechoic wall thickening without acute arteritis. Acute arteritis is characterized by presence of the halo sign in any of the visualized arteries; if halo sign is observed in one artery and hyperechoic wall thickening is observed in another artery, the overall interpretation is “consistent with acute arteritis.” Studies interpreted as “consistent with hyperechoic wall thickening without acute arteritis” do not show a halo sign in any artery and demonstrate hyperechoic wall thickening in at least one artery.
Study design and study population
We performed a retrospective cohort study of patients who underwent VUS for the evaluation of GCA in 2013 through 2017. The cohort includes patients with no prior diagnosis of GCA (ie, suspected new‐onset GCA) and patients previously diagnosed with GCA (ie, prior‐onset GCA). Among patients with suspected new‐onset GCA, a small subset had recent aortic surgery and were incidentally found to have giant cell aortitis, which then prompted evaluation with VUS. Among patients with prior‐onset GCA, some were actively treated for GCA at the time of VUS, whereas others had completed treatment. For all patients, VUS was performed as part of clinical practice either in the initial diagnostic evaluation (suspected new‐onset GCA) or to evaluate recurrent signs or symptoms (prior‐onset GCA). For patients with more than one VUS examination during the study period, only the first VUS was included to avoid correlated data. The Partners HealthCare Institutional Review Board approved this study.
Clinical, laboratory, and pathology data were extracted via electronic medical record review. Symptoms, physical examination findings, glucocorticoid use, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), and the treating physician’s clinical impression were assessed at the most recent visit before or on the VUS date. For patients with suspected new‐onset GCA, the treating physician’s clinical impression was characterized on a five‐point scale, ranging from “GCA is not the diagnosis” to “GCA is the diagnosis,” at two time points: the most recent visit before or on the VUS date and the visit after VUS. Overall VUS interpretation by the vascular medicine physician was categorized as acute arteritis, no arteritis, or hyperechoic wall thickening without acute arteritis. Temporal artery biopsy and/or aortic biopsy dates and histopathology before and/or after VUS were collected. For all patients, the treating provider’s final diagnosis (GCA or not GCA) was recorded.
Statistical analysis
Characteristics at the time of VUS were summarized among all patients and by overall VUS interpretation. Patients with acute arteritis on VUS were compared with those without acute arteritis on VUS (ie, either no arteritis or hyperechoic wall thickening without acute arteritis) using Kruskal‐Wallis tests and Fisher exact tests. Among patients with suspected new‐onset GCA, the treating physician’s pretest and posttest clinical suspicions for GCA were compared. Analyses were performed using SAS v9.4, and a two‐sided p < 0.05 was considered significant.
RESULTS
Clinical characteristics
Of the 530 patients that underwent VUS, 56 (10.6%) had prior‐onset GCA that was diagnosed a median of 19.2 months (interquartile range: 9.3‐63.3) prior to VUS (Table 1). The cohort was comprised of older adults (median age 70.6 years) that were predominantly White (87.6%) and female (69.1%). Nearly one‐third had a history of polymyalgia rheumatica. VUS was most often ordered by a rheumatologist (74.7%); specialties of other ordering providers were primary care (7.4%), inpatient general medicine/hospitalist (5.5%), ophthalmology (4.5%), cardiology (3.0%), neurology (2.6%), emergency medicine (0.4%), and other (1.9%). Headache was the most common symptom (50.2%). Symptoms had been present for 3 weeks or longer in the majority (63.0%). More than half of the cohort (57.6%) had been receiving treatment with glucocorticoids (GCs) at the time of VUS, and among these, the majority had been using GCs for 3 weeks or longer. Seventy‐five percent of VUS were performed in the ambulatory setting and the remaining 25% when patients were hospitalized. Two hundred six patients (38.9%) ever had a temporal artery biopsy performed (ie, either before or after VUS); 43 biopsies were interpreted as active arteritis.
Table 1.
Characteristics at the time of vascular ultrasound
|
All N = 530 |
Vascular ultrasound interpretation | |||
|---|---|---|---|---|
|
Acute arteritis n = 56 |
No acute arteritis a n = 474 |
p value | ||
| Age, y | 70.6 (62.9‐77.8) | 73.2 (64.8‐79.7) | 70.1 (62.7‐77.4) | 0.09 |
| Female | 69.1 | 66.1 | 69.4 | 0.65 |
| White b | 87.6 | 87.0 | 87.7 | 0.83 |
| Prior‐onset GCA | 10.6 | 23.2 | 9.1 | <0.01 |
| History of PMR | 31.7 | 30.4 | 31.9 | 0.88 |
| Recent biopsy‐proven aortitis | 3.2 | 1.8 | 3.4 | 0.99 |
| Elevated ESR and/or CRP | 83.0 | 94.6 | 81.7 | 0.01 |
| Fever | 13.8 | 14.3 | 13.7 | 0.84 |
| Weight loss | 13.8 | 23.2 | 12.7 | 0.04 |
| Fatigue | 37.2 | 42.9 | 36.5 | 0.38 |
| Headache | 50.2 | 51.8 | 50.0 | 0.89 |
| Jaw claudication | 11.5 | 30.4 | 9.3 | <0.01 |
| Scalp tenderness | 16.2 | 28.6 | 14.8 | 0.01 |
| Temporal artery tenderness or decreased pulsation | 14.2 | 25.0 | 12.9 | 0.02 |
| Vision loss (transient or permanent) | 10.4 | 16.1 | 9.7 | 0.16 |
| Symptom duration | ||||
| <1 week | 14.7 | 8.9 | 15.4 | |
| ≥1 to <3 weeks | 17.6 | 19.6 | 17.3 | 0.59 |
| ≥3 weeks | 63.0 | 66.1 | 62.7 | |
| Unclear | 4.7 | 5.4 | 4.6 | |
| Current GC use c | 57.6 | 73.2 | 55.7 | 0.01 |
| Prednisone equivalent d | ||||
| >0 to <15 mg/d | 33.8 | 31.7 | 34.1 | |
| ≥15 to <40 mg/d | 23.0 | 9.8 | 25.0 | 0.04 |
| ≥40 mg/d | 43.3 | 58.5 | 40.9 | |
| Duration of GC use d | ||||
| >0 to <1 week | 30.8 | 36.6 | 29.9 | |
| ≥1 to <3 weeks | 12.5 | 17.1 | 11.7 | 0.31 |
| ≥3 weeks | 56.7 | 46.3 | 58.3 | |
Reported as median (IQR) or %. P values from Kruskal‐Wallis test or Fisher exact test.
Abbreviation: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; GCA, giant cell arteritis; IQR, interquartile range; PMR, polymyalgia rheumatica.
No arteritis or hyperechoic wall thickening without acute arteritis.
Among 508 patients with data for race.
As treatment for GCA, PMR, or other indication for at least one day prior to ultrasound.
Among 305 patients using GCs.
Prior‐onset GCA was more common in patients with acute arteritis on VUS (23.2%) than patients without acute arteritis on VUS (9.1%) (p < 0.01) (Table 1). Current GC use—particularly high‐dose prednisone—was also more frequent among patients with acute arteritis on VUS than without. Elevated ESR and/or CRP was present in nearly all patients with acute arteritis on VUS (94.6%) and was also very common among patients without acute arteritis (81.7%). Jaw claudication, scalp tenderness, temporal artery tenderness or decreased pulsation, and weight loss were significantly more frequent among patients with acute arteritis than among patients without acute arteritis on VUS.
VUS interpretation and clinical suspicion for GCA
The overall VUS interpretation indicated no arteritis in the majority of patients (84.3%) (Table 2). Acute arteritis was observed in 10.6% of patients and hyperechoic wall thickening without acute arteritis in 5.1% of patients.
Table 2.
Vascular ultrasound interpretation among patients with and without prior‐onset GCA
| All (n = 530) | Prior‐onset GCA (n = 56) | Suspected new‐onset GCA a (n = 474) | |
|---|---|---|---|
| Acute arteritis | 10.6% | 23.2% | 9.1% |
| Hyperechoic wall thickening without acute arteritis | 5.1% | 10.7% | 4.4% |
| No arteritis | 84.3% | 66.1% | 86.5% |
Abbreviation: GCA, giant cell arteritis.
Includes n = 457 patients without biopsy‐proven aortitis and n = 17 patients with recent biopsy‐proven aortitis.
The cohort included 457 patients with no prior history of GCA and no recent aortic biopsy prior to VUS. Figure 1 depicts the treating physician’s clinical suspicion for GCA before and after the VUS for these patients, stratified by VUS result. For 42 patients with acute arteritis on VUS, the provider’s clinical suspicion for GCA was either unchanged or increased. Among 415 patients without acute arteritis on VUS, clinical suspicion for GCA was decreased (76.4%) or unchanged (20.2%).
Figure 1.
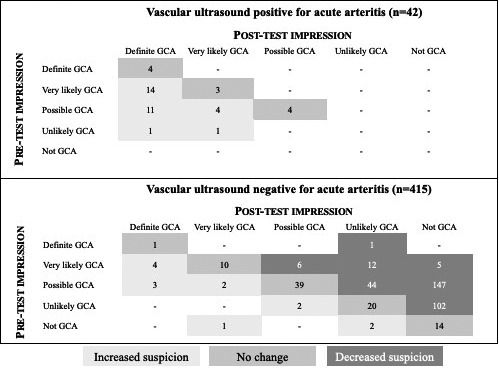
Treating physician’s clinical suspicion for giant cell arteritis (GCA) before and after vascular ultrasound among 457 patients without prior‐onset GCA or biopsy‐proven aortitis.
Temporal artery biopsies performed after VUS
One hundred twenty‐one patients had a temporal artery biopsy performed after VUS, and 16 were positive for active arteritis. Of these 16 patients, VUS showed acute arteritis in 5, hyperechoic wall thickening without acute arteritis in 2, and no arteritis in 9.
All 11 patients with positive temporal artery biopsy following a negative ultrasound (ie, either no arteritis or hyperechoic wall thickening without acute arteritis) had no prior history of GCA and had elevated ESR and/or CRP at the time of ultrasound. Clinical suspicion for GCA before the VUS was “very likely GCA” in 5 of 11, “possible GCA” in 5 of 11, and “unlikely GCA” in 1 of 11. Eight were taking GCs at the time of VUS (two low‐dose, one moderate‐dose, and five high‐dose GCs); four had been taking GCs for 3 weeks or longer. Six of eleven patients had a history of polymyalgia rheumatica, six presented with headache, and one had jaw claudication. Symptom duration varied: three had less than 1 week of symptoms, one had 1 to 3 weeks of symptoms, six had symptoms for three or more weeks, and one had an unclear duration.
Temporal artery biopsy was performed in 26 (13.6%) of 191 patients with a pretest impression of “possible GCA,” a negative VUS, and a posttest impression of “unlikely GCA” or “not GCA.”
Hyperechoic wall thickening without acute arteritis
Hyperechoic wall thickening without acute arteritis was observed in 27 patients (5.1%) (Figure 2). This interpretation was slightly more common among those with prior‐onset GCA (10.7%) than among those with suspected new‐onset GCA (4.4%). Six patients with hyperechoic wall thickening in the common superficial temporal artery and/or its branches had a temporal artery biopsy within 2 weeks after VUS. Three of these biopsies showed no evidence of active arteritis, and three showed evidence of active arteritis. Two of the biopsies with active arteritis had numerous eosinophils on histopathology; these patients were clinically diagnosed with GCA rather than eosinophilic forms of vasculitis.
Figure 2.
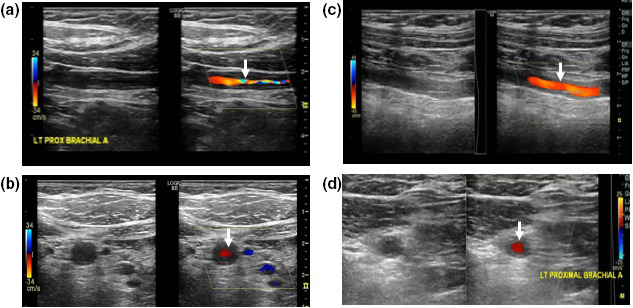
Comparison between the halo sign and hyperechoic wall thickening. Halo sign is characterized by homogeneous, circumferential hypoechoic wall thickening visualized in longitudinal (a) and transverse (b) views. Hyperechoic wall thickeningis defined by circumferential wall thickening visualized in longitudinal (c) and transverse (d) views, hyperechogenicity compared with adjacent arterial segments within the same artery, and absence of arteriosclerosis.
DISCUSSION
In the BWH model for the use of VUS in the evaluation of GCA, the VUS study is performed by vascular technologists and then interpreted by vascular medicine specialists.
Over the 4‐year period of this study, most VUS studies (84.3%) did not show active arteritis. Classic GCA symptoms, such as jaw claudication, scalp tenderness, and temporal artery tenderness or decreased pulsation, were significantly more common among patients with acute arteritis on VUS than among patients without acute arteritis on ultrasound. When VUS was interpreted as consistent with acute arteritis, the treating physician’s clinical suspicion for GCA increased or remained high; when VUS was interpreted as negative, the clinical suspicion for GCA decreased or remained low for nearly all patients.
Approximately one‐half of patients in this cohort were on GC therapy at the time of VUS, as GCA was either a suspected or known diagnosis. Concurrent GC use can affect sonographic findings, and thus, caution must be exercised in interpreting the prevalence of acute arteritis on VUS in this cohort (18, 19).
Hyperechoic wall thickening without acute arteritis was noted in a small percentage of the cohort (5.1%). Although the clinical importance of the halo sign for defining acute arteritis is well‐accepted, few studies have commented on the possible clinical significance of circumferential hyperechoic wall thickening without evidence for either acute arteritis or atherosclerosis.
Schmidt and colleagues described a patient with extracranial GCA in which hypoechoic wall thickening of the axillary, brachial, carotid, and subclavian arteries became hyperechoic 1 year after commencing treatment, and hypothesized that hyperechogenicity may represent fibrosis due to chronic disease (20). In a further report of follow‐up VUS in 40 patients with GCA with large‐vessel involvement, it was noted that “vasculitic wall swelling became brighter at follow‐up examinations” (21). In 6‐month follow‐up VUS of nine patients with initial halo signs of extracranial large arteries, Aschwanden et al described “marginally enhanced echogenicity of the vessel wall persisted” in the majority of examined segments (22). In follow‐up of six patients with hyperechoic swelling on initial VUS, we previously reported disappearance of this finding in three patients and emergence of a new halo sign in one (11). In the present study, hyperechogenicity without acute arteritis was seen in a small minority of patients with suspected new‐onset GCA or prior‐onset GCA. The finding of active arteritis on temporal artery biopsy in three patients is of interest and warrants further investigation. Because of small numbers, no certain conclusions can presently be drawn with regard to the clinical significance of hyperechoegneicity on VUS.
Temporal artery biopsy was performed in 38.9% of patients. Eleven patients with a VUS showing no acute arteritis and no prior history of GCA subsequently had a positive temporal artery biopsy. Assessment of the clinical characteristics of these patients in detail did not identify any common feature to all. Eight of 11 patients were receiving concurrent GC treatment. The sensitivity or specificity of VUS compared with temporal artery biopsy was not calculated owing to the small number of patients with temporal artery biopsy in our cohort. These 11 patients serve as a reminder that the diagnostic sensitivity of VUS is imperfect, whether temporal artery biopsy or clinical diagnosis is used as the reference standard (2). Data from our cohort revealed that providers sometimes sought further evaluation with a temporal artery biopsy following a negative VUS, even in a patient for whom the posttest clinical suspicion was low. The decision to pursue temporal artery biopsy in this circumstance is likely multifactorial, reflecting the physician’s confidence in the reliability of VUS as a diagnostic test for GCA and the patient’s desire or agreement to proceed with biopsy. Clinical judgment remains paramount in assessing GCA.
The increasing use of VUS led to the formal establishment of the BWH GCA Fast Track Clinic in January 2018, through which an evaluation routinely includes same‐day VUS and rheumatology clinic visit. The present study includes VUS only through the end of 2017; analysis of VUS data from 2018 onward is ongoing.
This retrospective study has a number of limitations. The cohort included patients with either suspected new‐onset GCA or prior‐onset GCA, which are clinically distinct groups. Patients with prior‐onset GCA were included because this subgroup reflects real‐world clinical care. We were unable to estimate sensitivity and specificity of VUS because of a lack of a reference standard: the clinical diagnosis (GCA or not) was informed by the ultrasound result and thus could not be used as a reference, and temporal artery biopsy was performed in less than half of the cohort. A major strength of this analysis is the high‐quality VUS data obtained through a standardized VUS protocol performed by expert vascular technologists and interpreted by vascular medicine physicians with extensive experience in VUS.
CONCLUSION
Our medical center offers a multidisciplinary model for GCA evaluation in which VUS examination is performed by vascular technologists and then interpreted and reported by vascular medicine physicians. The majority of the 530 studies performed during a four‐year period revealed no evidence of arteritis. Patients with acute arteritis on VUS often manifested classic GCA symptoms. VUS is a tool that can be usefully applied by clinicians in the diagnostic assessment of GCA.
AUTHOR CONTRIBUTIONS
Dr. Tedeschi drafted the manuscript. All authors performed critical revisions, and all authors approved the final version of the manuscript to be published.
Study conception and design
Tedeschi, Sobiesczyzk, Docken.
Acquisition of data
Ford, DiIorio.
Analysis and interpretation of data
Tedeschi, Docken.
Acknowledgments
The authors wish to acknowledge Magda Abdou, RPVI, RVT, RPhS, RDMS, Senior Vascular Technologist at BWH, for her expert and energetic contributions to our work on the use of vascular ultrasound for the diagnosis of arteritis.
This work was supported by the Brigham and Women’s Hospital Faculty Career Development Award, William P. Docken Inflammatory Autoimmune Disease Research Program, and the National Institutes of Health (K23 AR075070, L30 AR070514).
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica‐Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336–42. [DOI] [PubMed] [Google Scholar]
- 2. Duftner C, Dejaco C, Sepriano A, Falzon L, Schmidt WA, Ramiro S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta‐analysis informing the EULAR recommendations. RMD Open 2018;4:e000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 4. Monti S, Floris A, Ponte CB, et al. The proposed role of ultrasound in the management of giant cell arteritis in routine clinical practice. Rheumatology (Oxford) 2018;57:112–9. [DOI] [PubMed] [Google Scholar]
- 5. Laskou F, Coath F, Mackie SL, Banerjee S, Aung T, Dasgupta B. A probability score to aid the diagnosis of suspected giant cell arteritis. Clin Exp Rheumatol 2019;37 Suppl 117:104–8. [PubMed] [Google Scholar]
- 6. Patil P, Williams M, Maw WW, et al. Fast track pathway reduces sight loss in giant cell arteritis: results of a longitudinal observational cohort study. Clin Exp Rheumatol 2015;33 Suppl 89:S‐103–6. [PubMed] [Google Scholar]
- 7. Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast‐track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? [original research]. Rheumatology (Oxford) 2016;55:66–70. [DOI] [PubMed] [Google Scholar]
- 8. Schmidt WA. Ultrasound in the diagnosis and management of giant cell arteritis. Rheumatology (Oxford) 2018;57:ii22–31. [DOI] [PubMed] [Google Scholar]
- 9. Diamantopoulos AP, Haugeberg G, Hetland H, Soldal DM, Bie R, Myklebust G. Diagnostic value of color doppler ultrasonography of temporal arteries and large vessels in giant cell arteritis: a consecutive case series. Arthritis Care Res (Hoboken) 2014;66:113–9. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt WA, Seifert A, Gromnica‐Ihle E, Krause A, Natusch A. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large‐vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:96–101. [DOI] [PubMed] [Google Scholar]
- 11. Ford JA, DiIorio MA, Huang W, Sobiesczcyk P, Docken WP, Tedeschi SK. Follow‐up vascular ultrasounds in patients with giant cell arteritis. Clin Exp Rheumatol 2020;38 Suppl 124:107–11. [PMC free article] [PubMed] [Google Scholar]
- 12. Chrysidis S, Duftner C, Dejaco C, et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: a study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018;4:e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Czihal M, Zanker S, Rademacher A, et al. Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol 2012;41:231–6. [DOI] [PubMed] [Google Scholar]
- 14. Gerhard‐Herman M, Gardin JM, Jaff M, et al. Guidelines for noninvasive vascular laboratory testing: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vasc Med 2006;11:183–200. [DOI] [PubMed] [Google Scholar]
- 15. Yurdakul M, Tola M, Uslu OS. Color doppler ultrasonography in occlusive diseases of the brachiocephalic and proximal subclavian arteries. J Ultrasound Med 2008;27:1065–70. [DOI] [PubMed] [Google Scholar]
- 16. Schäfer VS, Juche A, Ramiro S, Krause A, Schmidt WA. Ultrasound cut‐off values for intima‐media thickness of temporal, facial and axillary arteries in giant cell arteritis. Rheumatology (Oxford) 2017;56:1479–83. [DOI] [PubMed] [Google Scholar]
- 17. Sundholm JK, Pettersson T, Paetau A, Albäck A, Sarkola T. Diagnostic performance and utility of very high‐resolution ultrasonography in diagnosing giant cell arteritis of the temporal artery. Rheumatol Adv Pract 2019;3:rkz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santoro L, D'Onofrio F, Bernardi S, Gremese E, Ferraccioli G, Santoliquido A. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology (Oxford) 2013;52:622. [DOI] [PubMed] [Google Scholar]
- 19. Hauenstein C, Reinhard M, Geiger J, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012;51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt WA, Kraft HE, Borkowski A, Gromnica‐Ihle EJ. Color duplex ultrasonography in large‐vessel giant cell arteritis. Scand J Rheumatol 1999;28:374–6. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt WA, Moll A, Seifert A, Schicke B, Gromnica‐Ihle E, Krause A. Prognosis of large‐vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:1406–8. [DOI] [PubMed] [Google Scholar]
- 22. Aschwanden M, Kesten F, Stern M, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis 2010;69:1356–9. [DOI] [PubMed] [Google Scholar]


