Abstract
Objective
The aim of this systemic review is to compare different treatments for patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) to inform evidence‐based recommendations for the American College of Rheumatology (ACR)/Vasculitis Foundation (VF) Vasculitis Management Guidelines.
Methods
A systemic review was conducted by searching articles in English using OVID Medline, PubMed, Embase, and the Cochrane Library. Articles were screened for suitability in addressing PICO questions, with studies presenting the highest level of evidence given preference.
Results
A total of 729 full‐text articles addressing GPA and MPA PICO questions were reviewed. For remission induction, rituximab was shown to be noninferior to cyclophosphamide (CYC) (odds ratio [OR]: 1.55, moderate certainty of evidence). The addition of plasma exchange to induction therapy in severe disease did not improve the composite end point of death or end stage renal disease (hazard ratio [HR]: 0.86 [95% confidence interval CI: 0.65, 1.13], moderate certainty of evidence). In nonsevere disease, methotrexate was noninferior to CYC for induction of remission (remission at 6 months of 90% vs. 94%). For maintenance of remission, methotrexate and azathioprine showed no difference in the risk of relapse over a mean follow‐up of 29 months (HR: 0.92, [95% CI: 0.52, 1.65]low certainty of evidence). As maintenance therapy, rituximab was superior to a tapering azathioprine strategy in major relapse‐free survival at 28 months (HR: 6.61, [95% CI: 1.56, 27.96], moderate certainty of evidence). In two randomized trials, longer‐term azathioprine maintenance therapy (>24 months) is associated with fewer relapses without an increase in adverse events.
Conclusion
This comprehensive systematic review synthesizes and evaluates the benefits and toxicities of different treatment options for GPA and MPA.
INTRODUCTION
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are forms of small‐medium vessel vasculitis, more specifically, antineutrophil cytoplasmic antibody (ANCA)‐associated vasculitis (AAV). GPA and MPA are rare diseases with a prevalence of 24 to 160 per million and 39 to 94 per million, respectively (1). Although no validated diagnostic criteria exist, the 1990 American College of Rheumatology (ACR) classification criteria (for GPA) and the 2012 Chapel Hill Consensus Conference nomenclature help define these diseases for the purposes of clinical trials (2, 3). Both GPA and MPA commonly cause a pulmonary‐renal syndrome, with GPA frequently affecting the upper airway as well. Because of their clinical similarities, GPA and MPA are frequently studied together in clinical trials.
Prior to modern therapies, prognosis was poor, with a mean survival of 5 months for patients with GPA. In 1971, Fauci and colleagues published the first report of their experience with the use of cyclophosphamide (CYC) for the treatment of GPA (4). For the first time, most patients could achieve a lasting remission (5). However, the toxicity of CYC and long‐term glucocorticoids (GCs) have led to treatment strategies to limit or reduce CYC and/or GC use. Treatment paradigms have evolved to initially treating aggressively with induction regimens to achieving remission, generally defined as no disease activity. The choice of induction therapy is typically determined by whether patients have severe manifestations, defined as life‐ or organ‐threatening disease. After remission is achieved, less toxic maintenance regimens are utilized to prevent relapses while minimizing toxicities (6).
The aim of this systematic review is to search and compare the benefits and toxicities of different treatments for patients with GPA and MPA. It includes randomized controlled trials and nonrandomized studies and presents the evidence and an assessment of its certainty for important outcomes. These reviews were used to inform the evidence‐based recommendations for GPA and MPA presented in the 2020 ACR/Vasculitis Foundation (VF) Guideline for the Management of ANCA‐associated Vasculitis.
MATERIALS AND METHODS
Search strategy and data sources
An information specialist conducted systematic searches of the published English‐language literature, including OVID Medline, PubMed, Embase, and the Cochrane Library (including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, and Health Technology Assessments) from the inception of each database through August 2018 to obtain direct evidence in patient populations with vasculitis relating to vasculitis questions (Supplementary Appendix 1). The information specialist updated the searches conducted on August 2019. Of note, we conducted a targeted update search on July 16, 2020, for the questions addressing steroids and Plasma exchange (PLEX). The methods team used DistillerSR software (Evidence Partners) to identify duplicate records (https://distillercer.com/products/distillersr‐systematic‐reviewsoftware/). The search was specific to address interventions specified in each PICO question for each vasculitis type. The ACR/VF Vasculitis Guideline Core Team developed 47 PICO questions for GPA/MPA that addressed relevant or commonly encountered diagnostic, treatment, and management scenarios (Supplementary Appendix 2).
The systemic review was performed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta‐Analysis guidelines. For additional information on study selection, screening, data extraction, assessment of bias, and data analysis see Supplementary Appendix 3.
RESULTS
Description of studies
The initial search retrieved 13,800 nonduplicate studies, of which 2596 were included for full‐text review. Following full‐text review, we found 1156 articles to be potentially eligible for data abstraction and inclusion in the systematic reviews of the different vasculitis types. For this review, we considered 729 articles for data abstraction for GPA/MPA. We conducted an updated search and captured 352 nonduplicate studies, 18 of which included full texts; 2 were considered for data abstraction. Additionally, our targeted updated search in 2020 led to the inclusion of one trial (7). Reasons for exclusion for full‐text review included ineligible study design, study population, intervention, sample size less than 10 patients, and unacceptable reference standard or index test (Figure 1) (Supplementary Appendix 4). The single‐arm data can be found in Supplementary Appendix 5.
Figure 1.
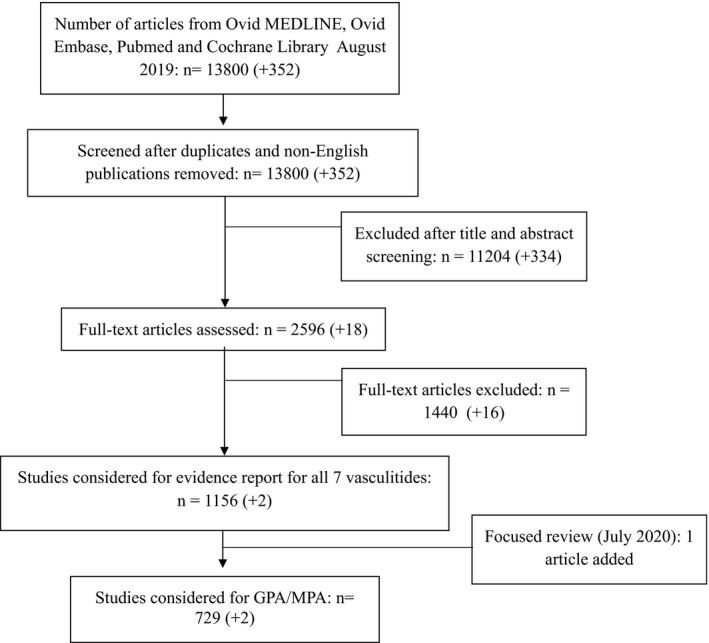
PRISMA flow diagram for included studies.
Treatment: remission induction
Pulse intravenous GCs vesus no‐pulse intravenous GCs in severe disease
Two retrospective, observational studies evaluated the outcomes of intravenous (IV) pulse GCs compared with high‐dose oral GCs in AAV with severe disease, not excluding eosinophilic granulomatosis with polyangiitis. In a Chinese cohort of patients with severe renal insufficiency (estimated glomerular filtration rate [eGFR] ≤10 ml/min/1.73 m), IV pulse GC (500 mg methylprednisolone daily for 3 days) was associated with fewer deaths (odds ratio [OR]: 0.41, [95% confidence interval (CI): 0.17, 0.96], low certainty of evidence) and improved dialysis‐free survival (OR: 7.29, [95% CI: 2.30, 23.07], low certainty of evidence) (8). However, a variety of induction strategies were used (oral GC with or without IV CYC or mycophenolate mofetil [MMF]). In a European and North American cohort, there was no clear benefit in survival, renal recovery, or relapse at 12 months with IV GC (median 1‐3 g total). However, IV pulse GC was associated with more serious infections (OR: 2.40, [95% CI: 1.03, 5.59], low certainty of evidence) and new‐onset diabetes at 12 months (OR: 5.34, [95% CI: 1.63, 17.46], moderate certainty of evidence). The IV pulse GC group had significantly more PLEX, less CYC, and less oral prednisolone (9).
Rituximab versus CYC in severe disease
One randomized, double‐blind, noninferiority trial directly compared a single course of rituximab (RTX) (375 mg/m2 given weekly for four doses) with oral CYC (2 mg/kg/d) followed by azathioprine (AZA) (2 mg/kg/d) (10). The mean doses of both methylprednisolone and prednisone were similar prior to enrollment. RTX was demonstrated to be noninferior to CYC in terms of the primary end point of sustained remission at 6 months without GC (OR: 1.55, [95% CI: 0.88, 2.74], moderate certainty of evidence), sustained remission for 12 months without GC (OR: 1.43, [95% CI: 0.81, 2.51], moderate certainty of evidence), and sustained remission at 18 months (OR: 1.34, [95% CI: 0.75, 2.40], moderate certainty of evidence ). In a post hoc analysis, patients who were positive for proteinase 3–ANCA treated with RTX had a higher rate of sustained remission off GCs at 6 months (OR: 2.11, [95% CI: 1.04, 4.30], moderate certainty of evidence). At 18 months, severe leukopenia was less common with RTX (OR: 0.17, [95% CI: 0.06, 0.48], moderate certainty of evidence), but there was no difference in serious adverse events (SAEs) (OR: 1.21, [95% CI: 0.69, 2.15], moderate certainty of evidence) or infections (OR: 1.09, [95% CI: 0.46, 2.61], moderate certainty of evidence). RTX may be superior at sustaining remission in patients presenting with relapsing disease at 6 months (OR: 2.76, [95% CI: 1.23, 6.20], moderate certainty of evidence), with superiority sustained at 12 months (OR: 3.04, [95% CI: 1.30, 7.12], moderate certainty of evidence) (10–13).
Comparison of different RTX induction regiments
One small (n = 58) multicenter retrospective trial compared RTX induction using two different regimens (375 mg/m2 given weekly for four doses vs. 1000 mg for two infusions, 2 weeks apart). Remission rates were similar between regimens (81% and 75%, respectively), with no difference in time to first relapse, over a median of 20 months. No direct comparisons in SAE were made between groups. (14)
IV CYC versus oral CYC in severe disease
Four randomized trials compared the efficacy of IV pulse CYC to daily oral CYC for remission induction in severe disease. With IV pulse CYC, there was a higher rate of patients with relapses (OR: 2.04, [95% CI: 1.11, 3.75], low certainty of evidence), but fewer patients developed leukopenia (OR: 0.37, [95% CI: 0.20, 0.69], low certainty of evidence). There was a trend toward fewer deaths with IV pulse CYC (OR: 0.56, [95% CI: 0.29, 1.07], low certainty of evidence). There were no differences in the overall number of participants with at least one adverse event, severe infections, or complete remission at 3 to 5 years (15–18) (Figure 2).
Figure 2.
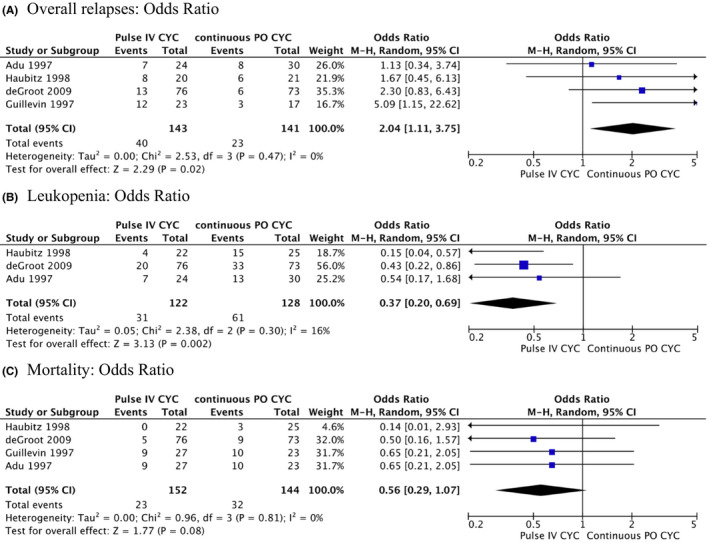
Meta‐analysis on comparative trials comparing intravenous CYC with oral CYC: overall relapses: odds ratio (A), leukopenia: odds ratio (B), and mortality: odds ratio (C). CYC = cyclophosphamide.
C5a inhibition versus prednisone in severe disease
A phase II randomized, double‐blind, placebo‐controlled trial compared an oral C5a receptor inhibitor, avacopan (CCX168) 30 mg twice daily, with or without reduced‐dose prednisone (initial dose 20 mg daily) to oral high‐dose prednisone alone (initial dose 60 mg daily) for remission induction. The analysis to address the PICO question focused on the avacopan monotherapy compared with prednisone monotherapy groups. All patients received either RTX or CYC for induction therapy. Almost all patients had renal involvement and ANCA positivity at baseline. There was no difference in the primary end point (≥50% reduction in Birmingham Vasculitis Activity Score (BVAS) by week 12 with no worsening in any organ system) in the avacopan monotherapy group compared with the high‐dose prednisone group (OR: 1.82, [95% CI: 0.42, 7.76], low certainty of evidence), which met criteria for noninferiority. There were also no differences in complete remission at 12 weeks (OR: 0.75, [95% CI: 0.21, 2.68], moderate certainty of evidence), renal response at 12 weeks (OR: 0.75, [95% CI: 0.20, 2.83], moderate certainty of evidence), participants with SAE (OR: 2.71, [95% CI: 0.68, 10.84], low certainty of evidence), or infections (OR: 1.05, [95% CI: 0.06, 17.85], low certainty of evidence). The avacopan group had significantly more participants who had a sustained remission from week 4 through 12 of the study (OR: 7.60, [95% CI: 0.82, 70.16], low certainty of evidence) (19).
PLEX versus placebo or IV pulse GCs in severe disease
Seven randomized controlled trials evaluated the use of PLEX for the treatment of glomerulonephritis in AAV (19, 20, 21, 22, 23, 24, 25), six compared to placebo and one compared to IV pulse GCs (22). Combined data from two of the largest trials suggested a potential protective effect of PLEX against end stage renal disease (ESRD) in patients with glomerulonephritis (hazard ratio [HR]: 0.72, [95% CI: 0.53, 0.98], moderate certainty of evidence) using a fixed‐effects model. The benefit was most pronounced in patients at the highest risk of ESRD with 142 fewer per 1000 cases (95% CI: 296, 38, low certainty of evidence). However, no difference in mortality was demonstrated (relative risk [RR]: 1.15, [95% CI: 0.78, 1.70], low certainty of evidence) (Figure 3). Two trials evaluated the use of PLEX in patients presenting with alveolar hemorrhage. There were no differences in mortality (RR: 0.95, [95% CI: 0.70, 1.30], low certainty of evidence) or remission rates (RR: 1.09, [95% CI: 0.92, 1.31], low certainty of evidence). Among four trials, there were no statistically significant differences in severe infections (RR: 1.19, [95% CI: 0.99, 1.42], moderate certainty of evidence).
Figure 3.
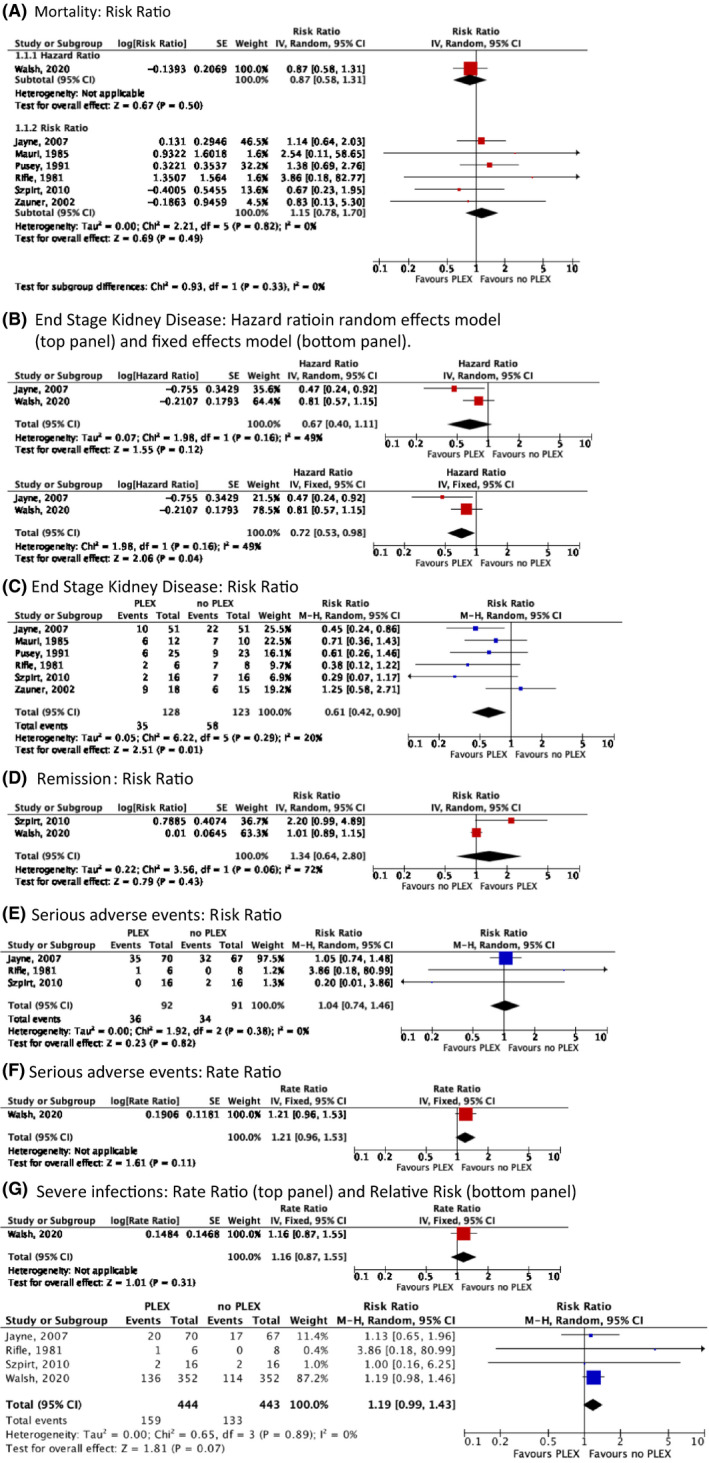
Meta‐analysis on comparative trials comparing the addition of plasma exchange (PLEX) for induction therapy in patients with glomerulonephritis: mortality: risk ratio (A); end stage kidney disease: hazard ratio in random effects model (top panel) and fixed‐effects model (bottom panel) (B); end stage kidney disease: risk ratio (C); remission: risk ratio (D); serious adverse events: risk ratio (E); serious adverse events: rate ratio (F); and severe infections: rate ratio (top panel) and relative risk (bottom panel) (G).
High‐dose versus reduced‐dose GCs in severe disease
One randomized controlled trial compared a standard (high‐dose) prednisone taper to a reduced‐dose GC taper (19). All participants received either RTX or CYC for induction therapy. The use of PLEX was equally distributed among groups. Each group received the same GC dosage for the first week; however, by the second week, the reduced‐dose group was reduced by approximately 50%. By 6 months, the cummulative GC exposure was 60% less in the reduced‐dosage group. The reduced‐dose group was found to be noninferior for the primary composite end point of death or end stage kidney disease (HR: 1.04, [95% CI: 0.81, 1.33], low certainty of evidence). Similarly, there were no differences in the secondary end points of sustained remission (RR: 1.04, [95% CI: 0.92, 1.19], low certainty of evidence) and SAEs (incidence rate ratio: 0.95, [95% CI: 0.75, 1.20], moderate certainty of evidence). However, there were fewer serious infections at 1 year in the reduced‐dose group (incidence rate ratio: 0.69, [95% CI: 0.52, 0.93], moderate certainty of evidence).
Methotrexate versus CYC in nonsevere disease
One randomized, unblinded, multicenter controlled trial compared methotrexate (MTX) (20‐25 mg/wk oral) to CYC (2 mg/kg/d oral) in participants with nonsevere disease (26, 27). The remission rate was noninferior with MTX compared with CYC at 6 months (90% vs. 94%). At 18 months, the relapse rate was higher with MTX (70% vs. 47%, p = 0.023). At a median follow‐up of 6 years, cumulative relapse‐free survival tended to be lower in the MTX arm (p = 0.056). The cumulative duration of GC was higher in the MTX group at 18 months (median: 15 months [interquartile range (IQR): 12‐18] vs 12 months [IQR: 12‐15], p = 0.005), which persisted between months 18 to 60 (median: 36 months [IQR: 18‐42] vs. 18 months [IQR: 0‐36], p = 0.004).
MMF versus IV CYC for severe disease
Two prospective trials, one randomized and one nonrandomized, have evaluated the efficacy of MMF for induction of remission at doses of 1000 to 1500 mg twice daily (28, 29). The overall remission rate at 6 months was 69% (60 of 87). Among patients achieving remission, 32% (24 of 76) relapsed at 18 months. Major relapses occurred in 6% (4 of 63) of participants. The overall mortality rate at 18 months was 6% (5 of 87). At 18 months, SAEs were seen in 40% (35 of 87), including serious infections (26%; 18 of 70) and malignancy (1%; 1 of 70). MMF was noninferior to CYC in remission rates at 6 months (67% vs. 61%; risk difference: 5.7%, 90% CI: −7.5%, 19%, low certainty of evidence). Among 17 participants, there was significant improvement in eGFR (average baseline of 46‐52 ml/min/m2, p < 0.05) and proteinuria (889 mg/24 hours to 149 mg/24 hours, p < 0.001).
Addition of IV immunoglobulins to standard of care for refractory disease
One randomized, placebo‐controlled trial evaluated the addition of IV immunoglobulins (IVIGs) (single dose of 2 mg/kg) in addition to standard induction therapy for participants with GPA and MPA with refractory disease in which there was an intention to escalate therapy. The baseline cumulatative prednisolone dose was similar (13.9 g [standard deviation (SD) 9.6] in the IVIG group vs. 8.4 g [SD 4.8] in placebo). Patients were followed for 12 months. Participants receiving IVIGs were more likely to have a therapeutic response (OR: 8.56, [95% CI: 1.74, 42.17], low certainty of evidence) as well as improved BVAS scores at 1 month (mean difference: 2.33 moderate certainty of evidence) and 3 months (mean difference: 1.8, 95% CI: 0.35, 3.25], moderate certainty of evidence). However, the number of patients with adverse events was higher when they were treated with IVIG (OR: 7.80, [95% CI: 1.69, 36.06], moderate certainty of evidence) (30).
Treatment: remission maintenance
MTX versus AZA
One open‐label, randomized trial compared remission maintenance with oral MTX (titrated to 25 mg/wk) to AZA (2 mg/kg/d) for 12 months following remission induction with IV CYC. Low‐dose prednisone (5 mg/d) was continued up to 24 months after the start of induction therapy. When comparing participants who received MTX with participants who received AZA, there was no difference in rates of relapse (HR: 0.92 low certainty of evidence), SAEs (OR: 2.45, [95% CI: 0.80, 7.53], very low certainty of evidence), severe infections (OR: 5.34, [95% CI: 0.61, 47.13], very low certainty of evidence), cancer (OR: 0.49 very low certainty of evidence), or death (OR: 3.05, [95% CI: 0.12, 76.26], very low certainty of evidence) after a mean follow‐up of 29 months. Most relapses (73%) occurred after discontinuation of the study drug (31).
RTX versus AZA and comparison of RTX maintenance regiments
One randomized trial compared RTX (500 mg IV on days 0 and 14, then on months 6, 12, and 18 after remission) with AZA (2 mg/kg/d for 12 months, 1.5 mg/kg/d for 6 months, then 1 mg/kg/d for 4 months) after induction of remission with IV CYC. Participants were kept on low‐dose prednisone (5 mg/d) for up to 18 months and then continued at the discretion of the treating physician. Compared with RTX, the AZA maintenance group had more major relapses at 28 months (HR: 6.61, [95% CI: 1.56, 27.96], moderate certainty of evidence) and 60 months (HR: 2.51, [95% CI: 1.35, 4.69], moderate certainty of evidence). There were no significant differences in the number of participants with SAE at 60 months (HR: 1.02, [95% CI: 0.63, 1.62], low certainty of evidence). Overall survival at 60 months was better in the RTX group (100% vs. 95%; p = 0.045). At 60 months, the time spent free of relapse or toxicity was significantly higher in the RTX group (difference of 12.6 months; p < 0.001). At 24 months, SF‐36 physical component scores tended to be better in the RTX group (mean difference: 3.95, [95% CI: 0.28, 8.18], very low certainty of evidence), whereas SF‐36 mental component scores were significantly better in the AZA group (mean difference: 4.23, [95% CI: 0.17, 8.29], low certainty of evidence). Blinding of participants and personnel was not performed (32, 33, 34).
One randomized controlled trial compared a tailored maintenance regimen of RTX based on biomarkers with the fixed RTX regimen (500 mg every 6 months). In the tailored regimen, patients received RTX 500 mg at the start of remission maintenance therapy followed by monitoring of the B‐cell counts and ANCA (both by indirect immunofluorescence [IIF] and enzyme‐linked immunosorbent assay [ELISA]) every 3 months. Repeat infusions of RTX were given if there was at least one of the following: 1) detectable B‐cells, 2) a change in ANCA serologies from negative to positive, 3) a twofold dilutional increase in ANCA by IIF, or 4) doubling of ANCA by ELISA. Induction of remission was achieved with CYC (62%), RTX (38%), or MTX (0.6%). Low‐dose prednisone (5 mg/d) was continued at the discretion of the site investigator; however, there was no significant difference in GC dose or duration in either group. The tailored group received fewer RTX infusions with a median of 3 (IQR 2‐4) in the tailored group versus 5 (IQR 5‐5) in the fixed schedule group over 28 months. A nonsignificant trend toward more relapses was seen in tailored group at 28 months (OR: 1.91, [95% CI: 0.75, 4.83], very low certainty of evidence). However, the study had fewer events (relapses) than anticipated and may have been underpowered to detect a difference between groups. There was no difference in the Vasculitis Damage Index (VDI) between groups at 28 months (mean difference: 0.1, [95% CI: ‐0.65, 0.45], very low certainty of evidence), occurrence of SAEs (OR: 0.76 low certainty of evidence), or mortality (OR: 0.33, [95% CI: 0.03, 3.19], very low certainty of evidence) between groups (35).
There is limited prospective experience regarding maintenance therapy after RTX induction therapy. A large randomized controlled trial comparing RTX 1000 mg every 4 months with AZA (2 mg/kg/d) after RTX induction therapy will be completed soon (36). There have been no direct comparisons of different RTX maintenance therapies (eg, 1000 mg every 6 months vs. 500 mg every 6 months vs. 1000 mg every 4 months).
MMF versus AZA
One open‐label, randomized controlled trial compared MMF (2000 mg/d at onset, 1500 mg/d at 12 months, then 1000 mg/d at 18 months) with AZA (2 mg/kg/d started, 1.5 mg/kg/d at 12 months, then 1 mg/kg/d at 18 months) after remission induction with IV or oral CYC. GCs were slowly withdrawn throughout the trial (prednisolone 15 mg/d at remission, decreased to 5 mg/d at 12 months, then stopped at 24 months). At a median follow‐up of 39 months (IQR: 0.66‐53.6 months), the MMF group had a higher rate of relapse (unadjusted HR: 1.69, [95% CI: 1.06, 2.70], low certainty of evidence) and a trend toward higher rate of severe relapses (HR: 2.14, [95% CI: 0.99, 4.64], low certainty of evidence). There were no differences in SAE (HR: 0.53, [95% CI: 0.23, 1.18], low certainty of evidence, severe infections (HR: 0.52, [95% CI: 0.11, 2.36], low certainty of evidence), leukopenia (HR: 0.57 low certainty of evidence), malignancies (HR: 0.25, [95% CI: 0.02, 2.62], low certainty of evidence), or drug intolerance leading to discontinuation (HR: 2.59, [95% CI: 0.55, 12.08], low certainty of evidence) between MMF and AZA groups. One death occurred in each group during follow‐up (37).
Leflunomide versus MTX
One multicenter, prospective randomized control trial compared oral leflunomide (LEF) (titrated to 30 mg/d) with oral MTX (titrated to 20 mg/wk) after induction of remission with oral or IV CYC. At the start of maintenance therapy, participants were receiving prednisolone 15 mg daily, tapered to 5 mg/d after 12 months, and withdrawn at 24 months. The study was prematurely terminated because of an unacceptably high rate of relapses in the MTX group; however, the dose of MTX in this group was titrated up very slowly (8‐week titration). The LEF group had a lower number of both relapses (OR: 0.35, [95% CI: 0.11, 1.12], very low certainty of evidence) and major relapses (OR: 0.12, [95% CI: 0.01, 1.06], very low certainty of evidence) after a median follow‐up of 21 months. Drug withdrawal due to intolerance was more common in the LEF group compared with the MTX group (OR: 6.43, [95% CI: 0.70, 59.28], very low certainty of evidence) (38).
Trimethoprim/sulfamethoxazole versus MTX or placebo in GPA
A retrospective, comparative trial compared trimethoprim/sulfamethoxazole (T/S) (160/800 mg twice daily) with MTX (IV 0.3 mg/kg/wk) with and without low‐dose prednisone (median 10 and 3 mg/d, respectively) in participants with generalized GPA (ie, not limited to disease in the upper and lower airways) for maintenance therapy after induction of remission with oral or IV CYC. Patients from each arm of the study were treated at different time periods (T/S from 1986 to 1993; MTX from 1992 to 1995). In the participants off maintenance prednisone, the MTX‐treated group had higher rates of partial or sustained remission (OR: 4.52, [95% CI: 1.05, 19.54], very low certainty of evidence) and fewer relapses (OR: 0.22, [95% CI: 0.05, 0.96],very low certainty of evidence) for a median duration of 16 and 22 months, respectively (MTX vs. T/S). Adverse events were twice as common in the MTX group (including in patients treated with additional low‐dose prednisone) (OR: 2.48, [95% CI: 0.79, 7.71], very low certainty of evidence) (39).
Two prospective randomized controlled trials evaluated T/S compared with placebo as maintenance therapy. Stegeman and colleagues (40) randomized participants in remission to T/S 800 mg/160 mg twice daily or placebo for 24 months with or without maintenance CYC and/or prednisolone therapy. Zycinska and colleagues (41) compared T/S 800 mg/160 mg three times weekly compared with placebo for 18 months therapy after prior induction of remission with CYC and prednisolone. Both studies included patients without significant baseline renal impairment. At 18 to 24 months, the T/S group had significantly more people in remission (OR: 2.38, 95% CI: 1.06, 5.32], low certainty of evidence). Specifically, the T/S group had fewer relapses of the upper airway. Fewer infections, both respiratory tract infections (p = 0.005) and nonrespiratory tract infections (p = 0.05), were seen in the T/S group (40). Both studies saw a 17% to 21% rise in creatinine with T/S, which returned to baseline after the study.
Short‐term (up to 24 months) versus long‐term continuation of maintenance therapy
Two randomized controlled trials evaluated AZA maintenance therapy (1.5 mg/kg/d) for 18 to 24 months, a duration used in many prospective trials, versus 48 months after induction of remission with CYC. Karras and colleagues included patients with GPA, MPA, and renal limited vasculitis and continued low‐dose prednisolone (5 mg/d) for the duration of maintenance therapy (42). Sanders and colleagues only included patients with newly diagnosed disease who were postiive for PR3‐ANCA, discontinuing GCs 24 to 34 weeks after diagnosis (43). In both studies, there were more relapses seen in the shorter‐term maintenance groups (OR: 4.70, [95% CI: 2.31, 9.55], moderate certainty of evidence). There were no differences in the number of patients with adverse events (OR: 0.82, [95% CI: 0.38, 1.75], low certainty of evidence), mortality (OR: 0.44, [95% CI: 0.08, 2.38], moderate certainty of evidence), or VDI (mean difference: 0, [95% CI: 0.07, 0.07] moderate certainty of evidence) (42, 43).
Additional comparative studies results and the risk of bias can be found in Supplementary Appendixes 6 and 7, respectively.
DISCUSSION
This review presents pooled estimates of patient important outcomes (relapse, deaths, SAEs, etc) for commonly available treatments for GPA/MPA.
GCs are currently part of standard induction regimens for both GPA and MPA. For severe disease, there are conflicting results regarding the utility of IV (pulse) GCs, which may be related to different induction and IV GC regimens used in these retrospective trials. PLEX is sometimes used as a supplement to GC for rapid control of disease activity. From a randomized controlled trial, the use of PLEX in those patients presenting with glomerulonephritis with a high risk of ESRD may be protective against dialysis within the first year. Of note, among the largest trials evaluating PLEX, there were discordant results (19, 22, 44), which may be related to improvements in therapies for these diseases (eg, the addition of RTX as an induction agent) and may have limited the additional benefits seen from PLEX seen in later trials. In addition, earlier studies may have been confounded by small number of events or systemic errors from bias resulting from unclear assignment methods. Also of note, one of the studies compared PLEX directly with IV pulse GCs rather than placebo (22). Small studies suggest C5a inhibitors can potentially replace GCs for induction therapy, but larger phase III randomized control trials are not yet available.
Current induction strategies include the combination of GCs and another immunosuppressive agent. Although CYC has been proven as an effective induction agent for severe GPA/MPA, there are subtle differences based on the route. IV pulse CYC is associated with a higher rate of relapse but fewer episodes of leukopenia. However, the incidence of severe leukopenia with oral CYC may be diminished with more frequent monitoring. The decision about the route of CYC administration should be made on an individual basis. RTX was demonstrated to be noninferior to CYC for the induction in severe disease in a randomized controlled trial and may be superior in relapsing disease. A concurrently published randomized trial by Jones and colleagues also supported RTX as an induction agent in severe disease, but the study was excluded from this review because RTX was combined with CYC and did not directly address the PICO question (45). Patients included in this trial had more severe disease. In a small retrospective trial, two RTX regimens (1000 mg for two infusions and 375 mg/m2 weekly for four infusions) appear to have similar rates of inducing remission. In randomized trials, MTX was effective as induction therapy in those without severe manifestations. Limited evidence supports MMF as an induction agent; however, future large randomized trials are warranted. IVIG can be considered for induction therapy in patients with refractory disease or contraindications to conventional induction agents (eg, severe infections).
Once remission is achieved, transition to a remission maintenance agent is recommended to prevent disease relapse, especially those with PR3‐ANCA positivity and/or GPA phenotypes. In the majority of trials, CYC or MTX was given as induction therapy. Within this population, a randomized trial demonstrated MTX and AZA to be equivalent in terms of prevention of relapses. In a large randomized trial, RTX maintenance dosing (500 mg every 6 months) was associated with fewer relapses compared with AZA; however, blinding of participants was not addressed and AZA was tapered over the 24‐month trial period. It is unclear whether there is an advantage of basing the dosage of RTX on B‐cell counts and/or ANCA serologies. The one study available was likely underpowered, and information about allocation, concealment, or blinding of participants/personnel was not available, raising the risk of selection and performance bias, respectively. LEF may be considered for maintenance therapy based on unblinded comparisons with MTX in a small randomized trial; however, it has been associated with a higher rate of drug intolerance that is likely due to a higher dosage (30 mg/d). A large randomized trial has demonstrated a higher relapse rate with MMF compared with AZA; however, the study was open‐label, thus raising the risk of detection bias. MMF can be considered in patients refractory to or with contraindications to conventional therapy. There is limited evidence to suggest that T/S may prevent sinonasal flares of GPA, and it was not as effective at relapse prevention when compared with methotrexate. T/S should be utilized for pneumocystis pneumonia prevention when appropriate and can be considered as adjuvant therapy. Most trials continued maintenance therapy for a period of 18 to 24 months. Combined data from two randomized trial have demonstrated that continuing AZA for 48 months (2 additional years) prevents relapse without a higher risk for adverse events. Currently, there is not enough evidence to evaluate the most effective maintenance regimen after RTX induction. The publication of a large randomized trial is expected.
This review has several strengths. The comprehensive and systematic approach for identifying studies makes it unlikely that relevant studies were missed. Additionally, we assessed the certainty of evidence in this area and identified sources of bias. We note a few limitations in this comprehensive systematic review. We limited our review by English language. Additionally, the outcome data were combined on studies that have heterogeneous designs. For the majority of the studies, confidence intervals remained wide, which was generally a function of the low numbers of patients included, reflecting the difficulty in conducting trials in rare diseases.
This comprehensive systematic review synthesizes and evaluates the benefits and toxicities of different treatment options of GPA/MPA. Estimates of benefits and toxicities as well as sensitivity and specificity from this review were used to model diagnostic and management strategies and to inform evidence‐based recommendations for the ACR/VF Vasculitis Management Guideline.
AUTHOR CONTRIBUTIONS
Drs. Kalot, Husainat, and Mustafa contributed to study selection. Drs. Springer, Kalot, Husainat, and Mustafa contributed to drafting the report. Drs. Springer, Byram, Dua, James, Lin, and Villa‐Forte critically revised the report. Drs. Langford, Abril, Maz, and Chung provided feedback on the included studies and contributed to the critical revision of the report. All authors approved the final version of the manuscript.
Study conception and design
Kalot, Husainat, Mustafa
Acquisition of data
Springer, Kalot, Husainat, Byram, Dua, James, Lin, Villa‐Forte, Mustafa
Analysis and interpretation of data
Springer, Kalot, Husainat, Byram, Dua, James, Lin, Villa‐Forte, Abril, Langford, Maz, Chung, Mustafa.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The systematic review team would like to acknowledge Amy Turner, Regina Parker, and Robin Lane for their assistance with administrative support, data management, and coordination of the project. The review team would also like to acknowledge the panel members of the American College of Rheumatology Vasculitis Practice Clinical Guidelines 2020 for their review of the evidence and input during the guideline development process.
This systematic review was conducted to support the development of the American College of Rheumatology 2020 Guidelines for Diagnosis and Management of Vasculitis. The entire guideline development process was funded by the American College of Rheumatology. Through the Outcomes and Implementation Research Unit at Kansas University Medical Center, Mohamad Kalot and Nedaa Husainat received salary support. Reem Mustafa received grant support, others volunteered their time.
Drs. Springer and Kalot contributed equally to this work.
Dr. Springer has served as site investigator for ChemoCentryx and InfaRx. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Ntatsaki E, Watts RA, Scott DG. Epidemiology of ANCA‐associated vasculitis. Rheum Dis Clin North Am 2010;36:447–61. [DOI] [PubMed] [Google Scholar]
- 2. Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum 1990;33:1101–7. [DOI] [PubMed] [Google Scholar]
- 3. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 4. Fauci AS, Wolff SM, Johnson JS. Effect of cyclophosphamide upon the immune response in Wegener's granulomatosis. N Engl J Med 1971;285:1493–6. [DOI] [PubMed] [Google Scholar]
- 5. Fauci AS, Wolff SM. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine (Baltimore) 1973;52:535–61. [DOI] [PubMed] [Google Scholar]
- 6. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349:36–44. [DOI] [PubMed] [Google Scholar]
- 7. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma exchange and glucocorticoids in severe ANCA‐associated vasculitis. N Engl J Med 2020;382:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Y, Han F, Chen L, Wang H, Han H, Yu B, et al. The impact of intravenous methylprednisolone pulses on renal survival in anti‐neutrophil cytoplasmic antibody associated vasculitis with severe renal injury patients: a retrospective study. BMC Nephrol 2017;18:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chanouzas D, McGregor JA, Nightingale P, Salama AD, Szpirt WM, Basu N, et al. Intravenous pulse methylprednisolone for induction of remission in severe ANCA associated vasculitis: a multi‐center retrospective cohort study. BMC Nephrol 2019;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unizony S, Villarreal M, Miloslavsky EM, Lu N, Merkel PA, Spiera R, et al. Clinical outcomes of treatment of anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis based on ANCA type. Ann Rheum Dis 2016;75:1166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geetha D, Specks U, Stone JH, Merkel PA, Seo P, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis with renal involvement. J Am Soc Nephrol 2015;26:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission‐induction regimens for ANCA‐associated vasculitis. N Engl J Med 2013;369:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jones RB, Ferraro AJ, Chaudhry AN, Brogan P, Salama AD, Smith KG, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Rheum 2009;60:2156–68. [DOI] [PubMed] [Google Scholar]
- 15. Adu D, Pall A, Luqmani RA, Richards NT, Howie AJ, Emery P, et al. Controlled trial of pulse versus continuous prednisolone and cyclophosphamide in the treatment of systemic vasculitis. QJM 1997;90:401–9. [DOI] [PubMed] [Google Scholar]
- 16. Haubitz M, Schellong S, Göbel U, Schurek HJ, Schaumann D, Koch KM, et al. Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody‐associated vasculitis and renal involvement: a prospective, randomized study. Arthritis Rheum 1998;41:1835–44. [DOI] [PubMed] [Google Scholar]
- 17. de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody‐associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. [DOI] [PubMed] [Google Scholar]
- 18. Guillevin L, Cordier JF, Lhote F, Cohen P, Jarrousse B, Royer I, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis Rheum 1997;40:2187–98. [DOI] [PubMed] [Google Scholar]
- 19. Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA‐associated vasculitis. J Am Soc Nephrol 2017;28:2756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pusey CD, Rees AJ, Evans DJ, Peters DK, Lockwood CM. Plasma exchange in focal necrotizing glomerulonephritis without anti‐GBM antibodies. Kidney Int 1991;40:757–63. [DOI] [PubMed] [Google Scholar]
- 21. Mauri JM, Gonzalez MT, Poveda R, Seron D, Torras J, Andujar J, et al. Therapeutic plasma‐exchange in the treatment of rapidly progressive glomerulonephritis. Plasma Ther Transfus 1985;6:587–91. [Google Scholar]
- 22. Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high‐dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007;18:2180–8. [DOI] [PubMed] [Google Scholar]
- 23. Rifle G, Chalopin JM, Zech P, Deteix P, Ducret F, Vialtel P, et al. Treatment of idiopathic acute crescentic glomerulonephritis by immunodepression and plasma‐exchanges. A prospective randomised study. Proc Eur Dial Transplant Assoc 1981;18:493–502. [PubMed] [Google Scholar]
- 24. Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener's granulomatosis–a clinical randomized controlled trial. Nephrol Dial Transplant 2011;26:206–13. [DOI] [PubMed] [Google Scholar]
- 25. Zäuner I, Bach D, Braun N, Krämer BK, Fünfstück R, Helmchen U, et al. Predictive value of initial histology and effect of plasmapheresis on long‐term prognosis of rapidly progressive glomerulonephritis. Am J Kidney Dis 2002;39:28–35. [DOI] [PubMed] [Google Scholar]
- 26. De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Rheum 2005;52:2461–9. [DOI] [PubMed] [Google Scholar]
- 27. Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Höglund P, et al, and the European Vasculitis Study Group . Brief Report: long‐term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Rheum 2012;64:3472–7. [DOI] [PubMed] [Google Scholar]
- 28. Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA‐associated vasculitis: a randomised, non‐inferiority trial. Ann Rheum Dis 2019;78:399–405. [DOI] [PubMed] [Google Scholar]
- 29. Silva F, Specks U, Kalra S, Hogan MC, Leung N, Sethi S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement–a prospective, open‐label pilot trial. Clin J Am Soc Nephrol 2010;5:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jayne DR, Chapel H, Adu D, Misbah S, O'Donoghue D, Scott D, et al. Intravenous immunoglobulin for ANCA‐associated systemic vasculitis with persistent disease activity. QJM 2000;93:433–9. [DOI] [PubMed] [Google Scholar]
- 31. Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, et al. Azathioprine or methotrexate maintenance for ANCA‐associated vasculitis. N Engl J Med 2008;359:2790–803. [DOI] [PubMed] [Google Scholar]
- 32. Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA‐associated vasculitis. N Engl J Med 2014;371:1771–80. [DOI] [PubMed] [Google Scholar]
- 33. Terrier B, Pagnoux C, Perrodeau É, Karras A, Khouatra C, Aumaître O, et al. Long‐term efficacy of remission‐maintenance regimens for ANCA‐associated vasculitides. Ann Rheum Dis 2018;77:1150–6. [DOI] [PubMed] [Google Scholar]
- 34. Pugnet G, Pagnoux C, Terrier B, Perrodeau E, Puéchal X, Karras A, et al. Rituximab versus azathioprine for ANCA‐associated vasculitis maintenance therapy: impact on global disability and health‐related quality of life. Clin Exp Rheumatol 2016;34 Suppl 97:S54–9. [PubMed] [Google Scholar]
- 35. Charles P, Terrier B, Perrodeau É, Cohen P, Faguer S, Huart A, et al. Comparison of individually tailored versus fixed‐schedule rituximab regimen to maintain ANCA‐associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann Rheum Dis 2018;77:1143–9. [DOI] [PubMed] [Google Scholar]
- 36. Gopaluni S, Smith RM, Lewin M, McAlear CA, Mynard K, Jones RB, et al. Rituximab versus azathioprine as therapy for maintenance of remission for anti‐neutrophil cytoplasm antibody‐associated vasculitis (RITAZAREM): study protocol for a randomized controlled trial. Trials 2017;18:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody‐associated vasculitis: a randomized controlled trial. JAMA 2010;304:2381–8. [DOI] [PubMed] [Google Scholar]
- 38. Metzler C, Miehle N, Manger K, Iking‐Konert C, de Groot K, Hellmich B, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener's granulomatosis. Rheumatology (Oxford) 2007;46:1087–91. [DOI] [PubMed] [Google Scholar]
- 39. de Groot K, Reinhold‐Keller E, Tatsis E, Paulsen J, Heller M, Nölle B, et al. Therapy for the maintenance of remission in sixty‐five patients with generalized Wegener's granulomatosis. Methotrexate versus trimethoprim/sulfamethoxazole. Arthritis Rheum 1996;39:2052–61. [DOI] [PubMed] [Google Scholar]
- 40. Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG, and the Dutch Co‐Trimoxazole Wegener Study Group . Trimethoprim‐sulfamethoxazole (co‐trimoxazole) for the prevention of relapses of Wegener's granulomatosis. N Engl J Med 1996;335:16–20. [DOI] [PubMed] [Google Scholar]
- 41. Zycinska K, Wardyn KA, Zielonka TM, Krupa R, Lukas W. Co‐trimoxazole and prevention of relapses of PR3‐ANCA positive vasculitis with pulmonary involvement. Eur J Med Res 2009;14 Suppl 4:265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karras A, Pagnoux C, Haubitz M, de Groot K, Puechal X, Tervaert JW, et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA‐associated vasculitis. Ann Rheum Dis 2017;76:1662–8. [DOI] [PubMed] [Google Scholar]
- 43. Sanders JS, de Joode AA, DeSevaux RG, Broekroelofs J, Voskuyl AE, van Paassen P, et al. Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase‐3 anti‐neutrophil cytoplasmic antibody‐associated vasculitis patients who remain cytoplasmic anti‐neutrophil cytoplasmic antibody‐positive after induction of remission: a randomized clinical trial. Nephrol Dial Transplant 2016;31:1453–9. [DOI] [PubMed] [Google Scholar]
- 44. Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta‐analysis. Am J Kidney Dis 2011;57:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA‐associated renal vasculitis. N Engl J Med 2010;363:211–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


