Abstract
Purpose of Review
The goal of this review is to summarize the current knowledge of the epidemiology, clinical manifestations, diagnosis, and treatment of cutaneous, mucosal, and visceral leishmaniasis. We will describe the most recent findings and suggest areas of further research in the leishmaniasis field.
Recent Findings
This article reviews newer leishmaniasis tests (including rapid diagnostic tests using rK39 antibodies), vaccine candidates, and updated treatment recommendations.
Summary
While leishmaniasis is a complex disease, learning the prominent clinical manifestations and major parasite species can guide the recommendations for diagnosis and treatment.
Keywords: Leishmaniasis, Cutaneous leishmaniasis, Mucocutaneous leishmaniasis, Visceral leishmaniasis, Leishmania vaccine
Introduction
Leishmania is a vector-borne, obligate intracellular, protozoan parasite (family Trypanosomatidae) causing cutaneous, mucocutaneous, and visceral disease in the Old and New World. Leishmaniasis is a complex disease caused by multiple subspecies with diverse clinical manifestations, leading to confusion even among experts. The purpose of this review is to summarize the current knowledge of this important zoonotic and vector-borne disease and to guide future directions in the field of leishmaniasis.
Epidemiology
Leishmaniasis is a neglected tropical disease infecting the world’s poorest populations in over 90 countries throughout Asia, Africa, the Middle East, and Central and South America. While likely underreported, current estimates of cutaneous leishmaniasis (CL) incidence range from 700,000 to 1.2 million cases per year [1] with approximately 95% of cases occurring in the Americas, the Mediterranean basin, the Middle East, and Central Asia [2]. Estimates of annual visceral leishmaniasis (VL) are currently less than 100,000, which is a significant decrease from prior estimates of 400,000 [1], with more than 95% of case reported to the World Health Organization (WHO) from Brazil, China, Ethiopia, India, Kenya, Nepal, Somalia, and Sudan [2]. Risk factors for leishmaniasis include poverty, population migration, malnutrition, poor hygiene, and an immunocompromised state [2].
Over 20 species of the Leishmania parasite have been characterized and are transmitted from approximately 70 different types of phlebotomine sand flies (of the Diptera Family Psychodidae genera subdivided into Phlebotomus in the Old World and Lutzomyia in the New World) [3]. Given the large variety of species, leishmaniasis has been divided geographically into the Old World and New World. The Old World, referring to the Eastern Hemisphere, includes Asia, the Middle East, Africa, and Southern Europe. Conversely, the New World refers to the Western Hemisphere specifically Mexico, Central America, South America, and the USA.
The sandflies are found across the globe and tropical species can complete the lifecycle throughout the year [4•]. In subtropical areas, however, species can only complete their life cycles during warmer months [4•]. Most active at night, the sandflies fly silently and are often not heard or seen by their prey [4•].
The majority of CL cases in the USA are attributed to travel or immigration [1]; however, the WHO classified the USA as endemic for leishmaniasis in 2015 [5]. Furthermore, in a recent study, of 69 novel cases of leishmaniasis identified in Texas, 41 (59%) were autochthonous CL with no history of travel outside the USA [6]. With climate change, the incidence and geographical distribution of leishmaniasis is expected to increase [7].
There are 22 species belonging to the genus Leishmania that has been further subdivided into the subgenera Leishmania and Viannia based on development of the organism in the digestive tract of the sandfly. Each parasite species has specific geographical predilections, host factors, and symptom characteristics. For example, L. donovoni presents as VL in South Asia (Bangladesh, India, Nepal) and East Africa (Sudan, Somalia, Ethiopia, and Kenya) and often predominates among younger individuals while sparing older adults due to acquired immunity [8]. L. infantum (same species as L. chagasi in Latin America), on the other hand, also can present as VL, but is usually found in the Mediterranean, Middle East, Pakistan, Iran, and Brazil. In Texas, all endemic cases of leishmaniasis were found to be caused by L. mexicana CL [6].
Life Cycle
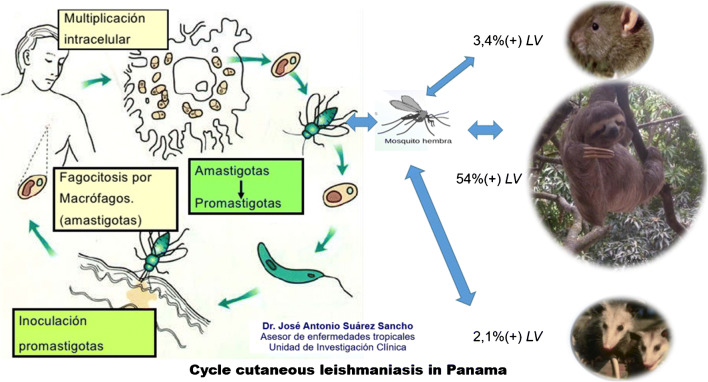
The female phlebotomine sand fly, mostly active in the nighttime hours (“from dusk till dawn”), transmits the leishmaniasis parasite to humans or other animal reservoirs. Leishmania sp. has two distinct phases in its life cycle: promastigote and amastigote [4•, 9]. The promastigote form has a flagellum allowing mobility in the sand fly’s intestines [4•, 9]. When the sand fly takes a blood meal from the mammalian host, the promastigote form is injected into the skin and is phagocytosed by mononuclear cells of the mammalian host, transforming it into the amastigote form, also known as Leishman-Donovan body (Fig. 1) [4•, 9]. The amastigotes multiply and develop within the reticulo-endothelial system of the host, causing either the asymptomatic or symptomatic forms of the disease based on underlying host and parasite species’ factors [4•]. The amastigotes can travel hematogenously and lymphatically to cause mucosal and visceral disease [4•]. Recently, researchers have discovered that the Leishmania RNA Virus (LRV1) coinfects L. Viannia (V.) guyanensis and L.V. braziliensis species, eliciting a hyperimmune response through toll-like receptors causing mucosal damage and metastatic infection [10]. LRV outside of the Viannia subspecies was discovered in L. major (categorized as LRV2), but no association between clinical phenotype and severity has been elicited [11].
Fig. 1.
Leishmaniasis life cycle. By Dr. José Antonio Suárez Sancho
The transmission of Leishmania sp. is predominantly driven by symptomatic infection and post-kala-azar dermal leishmaniasis (PKDL), as asymptomatic cases are thought to not infect sandflies [12, 13]. In some areas, humans are required to maintain the lifecycle (anthroponotic transmission), which is characteristic of L. tropica (responsible for CL in the New World) and L. donovoni (responsible for VL in India) [4•, 14•, 15]. Animals, however, can maintain the lifecycle and may or may not exhibit signs or symptoms of disease. Dogs, rodents, marsupials, monkeys, and edentates are among the susceptible hosts. In particular, dogs are the most important animal reservoir for L. infantum [14•, 16]. Less commonly, leishmaniasis can also be transmitted through organ transplantation, blood transfusion, intravenous drug use, or congenitally.
Pathogenesis
Leishmaniasis is classically thought of as an imbalance of TH1 versus TH2 CD4+ helper cells [17, 18]. Those with a primary TH1 response have excellent parasite control with low levels of parasitemia; however, they are primed toward mucocutaneous disease as a result of overactive cellular immunity and cellular destruction [18, 19]. Those with a TH2 response have increased parasite load as antibody neutralization is ineffective against the intracellular parasite [18]. TH2 responders are more likely to develop disseminated disease, which leads to visceral disease and, in the New World, results in disseminated cutaneous leishmaniasis (DCL) [18]. DCL is clinically represented by innumerable lesions throughout the body.
Clinical Symptoms
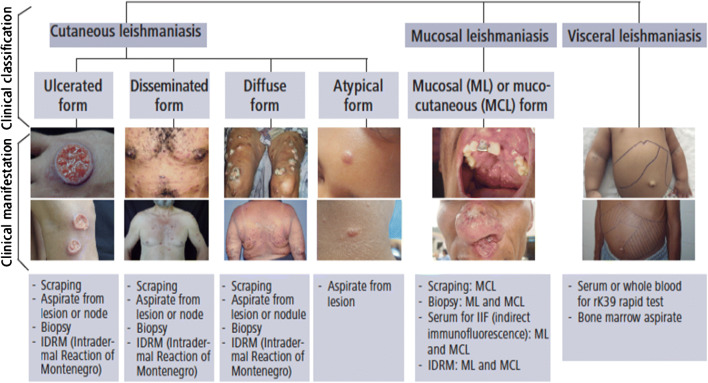
While leishmaniasis can present with a variety of different clinical manifestations, the main three phenotypic categories of disease are cutaneous (CL), mucosal (ML), and visceral leishmaniasis (VL). These categories can be subdivided further to encompass the spectrum of clinical disease to include ML of the Americas, VL, CL of the New World, CL of the Old World, post-kala-azar CL, Leishmania recidivante, diffuse CL, and disseminated CL (Table 1) [19]. Moreover, the appearance of cutaneous disease can be variable (Figs. 2, 3, 4, and 5). In some individuals, infection can remain asymptomatic or subclinical, but it can also present as acute, subacute, or as a chronic disease.
Table 1.
Clinical syndrome and species
| Disease pattern | Old World species | New World species | Symptoms, exam, lab findings |
|---|---|---|---|
| Visceral leishmaniasis | L. donovoni, L. infantum, L. tropica | L. chagasi (identical species to L. infantum but in New World), L. amazonensis | Fever, weight loss, fatigue, hepatosplenomegaly, pancytopenia, hypergammaglobulinemia |
| Post-kala azar dermal leishmaniasis | L. donovoni, L. infantum | L. chagasi (identical species to L. infantum) | Skin lesions (always starts on face) 6 months following VL |
| Cutaneous leishmaniasis | L. tropica, L. major, L. aethiopica, L. infantum, L. donovani | L. mexicana species complex, L. mexicana, L. amazonensis, L. venezuelensis, L. Vianna (V) subgenus, L. (V) braziliensis, L. (V) panamensis, L. (V) guyanesis, L. (V) peruviana, L. major like organisms, L. chagasi | Skin lesions on extremities and face:Painless papules which progress to nodules then ulcers |
| Leishmaniasis recidivans | L. tropica, L. major | N/A | Satellite lesions around prior ulcer site difficult to treat and may relapse |
| Diffuse cutaneous leishmaniasis | L. aethiopica | L. mexicana, L. amazonensis, L. panamensis (rarely) | Diffuse, anergic skin lesions with non-ulcerative nodules and plaques progressing from primary lesion. Rare but more common in immunocompromised individuals |
| Disseminated leishmaniasis | N/A | L. Vianna subgenus, L. (V) braziliensis (most common), L. amazonensis | Noncontiguous pleomorphic lesions in immunocompetent hosts. Difficult to treat |
| Mucosal leishmaniasis | L. tropica, L. major, L. donovani, L. infantum | L. Vianna (V) subgenus, L. (V) brazilensis, L. (V) panamensis, L. (V) guyanesis, L. (V) peruviana, L. amazonensis | Nasal secretions, nasal obstruction, pain, epistaxis. Destructive lesions in nose, oropharynx. Initially involves nose and mouth, can progress to include pharynx and larynx |
*Adapted from Bennet et al.
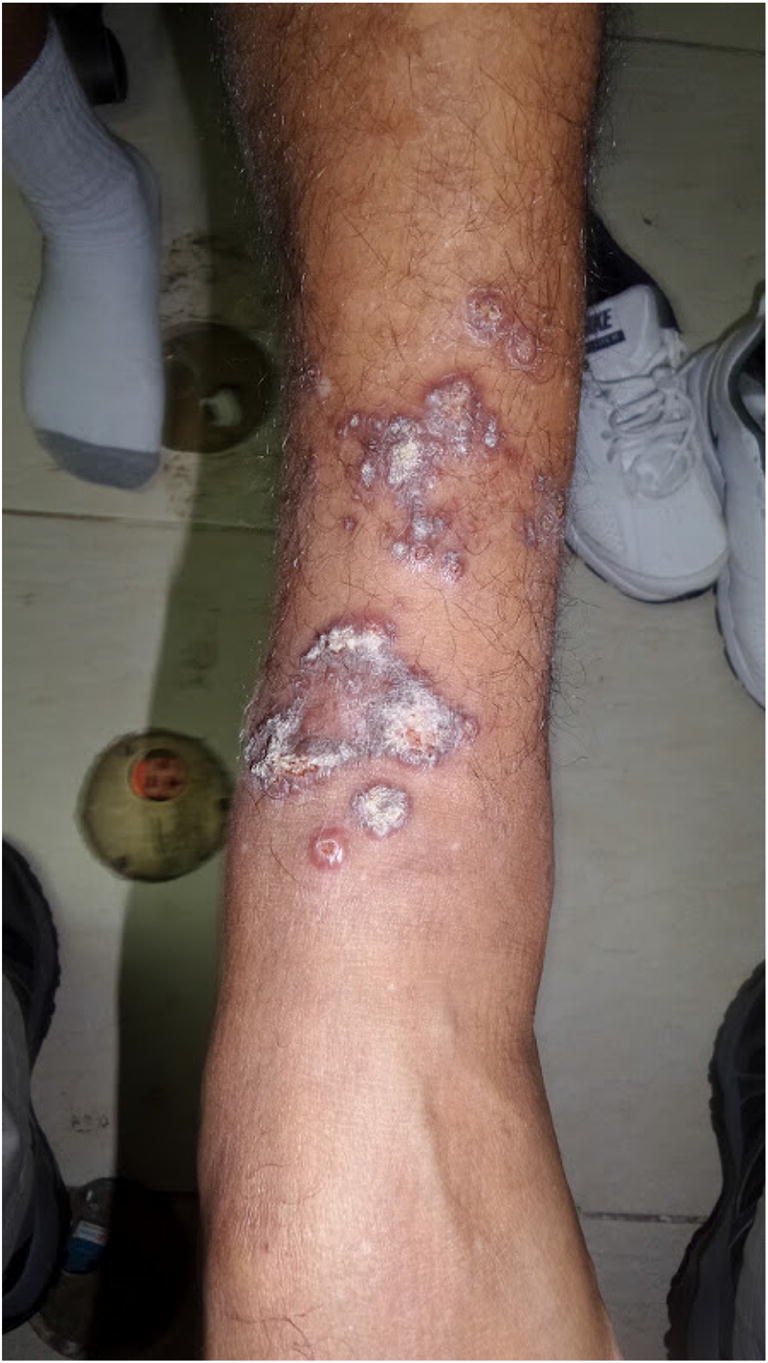
Fig. 2.

Cutaneous leishmaniasis: arm ulcer with typical appearance in a patient with L. panamensis
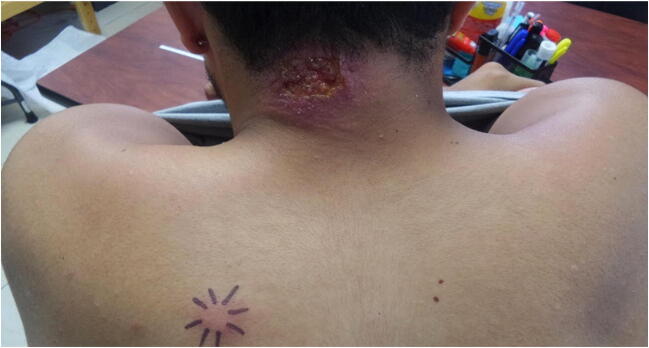
Fig. 3.
Cutaneous leishmaniasis: L arm revealing diffuse CL disease
Fig. 4.

Cutaneous leishmaniasis: sporotrichoid appearance of left arm
Fig. 5.
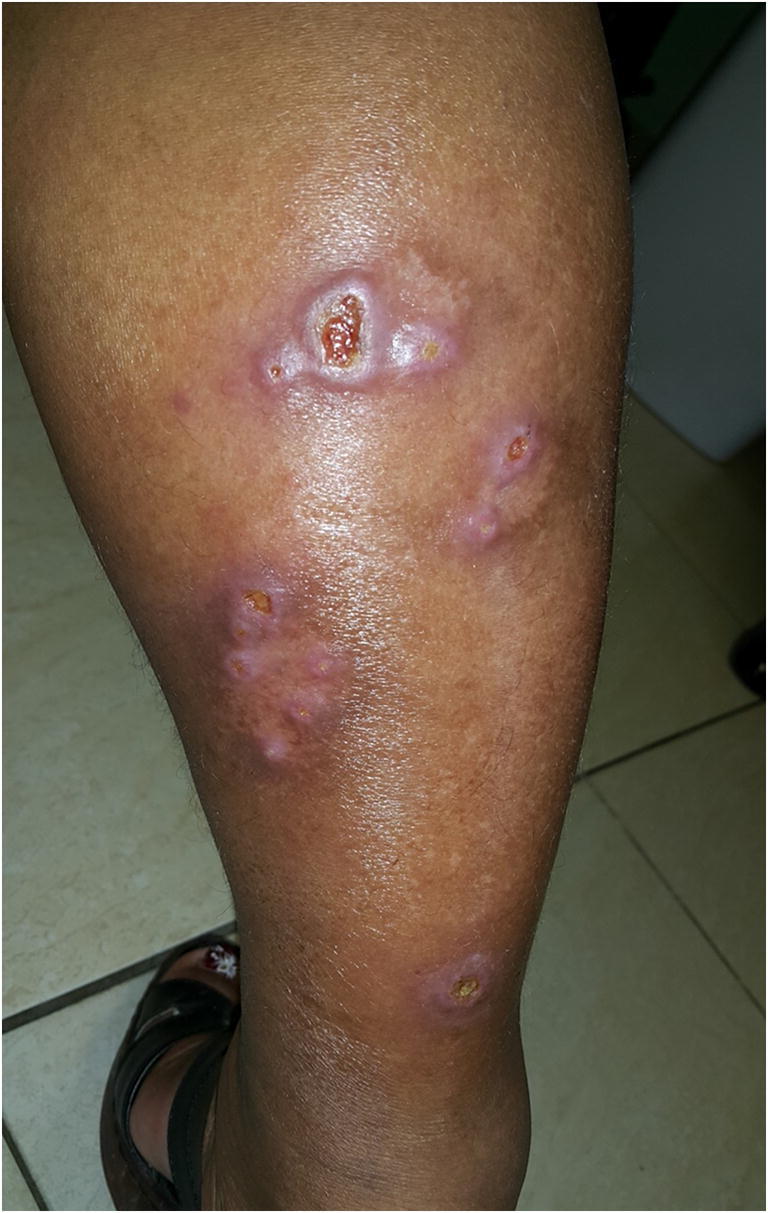
Cutaneous leishmaniasis: small satellite lesions outside of the plaque/ulcer on right leg
CL lesions typically occur at the sand fly bite site (commonly on well-exposed areas of the face and extremities) as a solitary non-suppurative papule, although multiple lesions can occur. CL occurs in the Old World forms (L. tropica, L major, L. aethiopica and less commonly L. infantum and L. donovani) as well as the New World forms (L. mexicana, L. amazonesis, L. venezuelensis, and L. viannia subgenus including L. V. braziliensis, panamensis, guyanesis) [19, 20]. Over weeks to months, the papules progress to painless ulcers with heaped-up borders, which can spontaneously heal over months to years, or cause scars and disfigurement (Fig. 2) [21]. There are a variety of atypical cutaneous manifestations, however, including nodular, sporotrichoid, disseminated, psoriasiform, verrucous, zosteriform, eczematous, and/or erysipeloid (Figs. 3 and 4) [4•, 22]. Other atypical presentations include small satellite lesions outside of the plaque/ulcer (nodular lymphangitis) (Fig. 5). All patients with cutaneous leishmaniasis should be evaluated for evidence of mucosal lesions through a naso-oropharyngeal exam [23•]. Leishmaniasis recidivins (associated most commonly with L. tropica) occurs as satellite lesions surrounding old scars and is often confused for cutaneous tuberculosis [14•].
The most disfiguring form of disease, ML (also known as espundia in Latin America), leads to facial deformities typically years after the initial cutaneous symptoms have resolved (Fig. 6). Frequently caused by the L Viannia subgenus, the parasite disseminates from amastigotes on the skin to the nasopharyngeal mucosa through the hematogenous and lymphatic system [20]. Although less typical, mucosal lesions can also co-occur with dermal manifestations. Patients often report chronic nasal symptoms including secretions, epistaxis, and pain; evidence of ulceration, bleeding, and inflammation is often found on physical exam [24]. Initially, there is mucosal involvement of the nose and mouth with oropharyngeal and laryngeal involvement later in the course of the disease. Unlike cutaneous disease, ML does not heal spontaneously [21, 25]. The cartilaginous septum inside the anterior nares is commonly involved and perforation of the nasal septum can occur. Other complications include collapse of the anterior nose and destruction in the nose and mouth [26, 27].
Fig. 6.
Mucocutaneous leishmaniasis in a patient with L. panamensis
The most lethal form of leishmaniasis, VL (also known as kala-azar, which is Hindi for black fever), can cause systemic infection affecting the liver, spleen, hematogenous and lymphatic system. The species that are typically associated with visceral disease include L. donovani, L. infantum (in the old World), and L. chagasi (same as L. infantum but found in the New World) [20]. Other species that have also been associated with visceral disease include L. tropica (Old World), which typically causes dermatologic manifestations, but was described among seven servicemen experiencing visceral symptoms in the Persian Gulf conflict in 1990s [28]. L. amazonensis has also been the suspected cause of cases of visceral disease [20]. A report among patients in Sudan describes some of the severe manifestations of the disease including cachexia, hepatosplenomegaly, fever, pancytopenia, and hypergammaglobulinemia [29]. Subjective symptoms often described in the history of patients with visceral leishmaniasis include fatigue, abdominal pain, and un-intentional weight loss. Among patients with HIV, visceral leishmaniasis is considered an opportunistic infection, contributing to 25–70% of HIV co-infections reported in Europe [30]. Post-kala-azar dermal leishmaniasis, occurring after the initial visceral leishmaniasis syndrome, leads to cutaneous papules and nodules, commonly found on the face, or macular hypopigmentation (Table 1) [31].
Diagnosis
The diagnosis of leishmaniasis is often challenging but can be made clinically with direct (parasitic) and indirect (immunologic) confirmation. Confirmation of diagnosis is often not practical in low-resource countries. The history, epidemiology, clinical symptoms, and signs on physical examination should alert the clinician of the possible diagnosis of leishmaniasis. In particular, a painless, non-purulent skin ulcer on the face or extremities in a person with recent travel to or migration from an endemic area should prompt concern for cutaneous leishmaniasis. Alternatively, prolonged fevers, fatigue, weight loss, anemia, leucopenia, and hepatosplenomegaly in a patient from an endemic region such as Africa may suggest visceral leishmaniasis (in addition to HIV, TB, and malignancy). Given that treatment can be toxic and differs substantially based on species identification, clinical diagnosis of leishmaniasis is not sufficient and should ideally be confirmed by evidence of amastigotes in tissue and/or molecular testing such as polymerase chain reaction (PCR) (Table 2, Fig. 7) [23•, 32•].
Table 2.
Diagnostic methods
| Disease pattern | Direct vs indirect | Diagnostic method | Comments |
|---|---|---|---|
| Cutaneous leishmaniasis | Direct | Biopsy, scraping, aspirate | Sensitivity dependent on expertise of pathologist and quality of specimen. Obtain from edge of ulcer and base |
| Microscopy | Giemsa stained microscopy | ||
| Culture | Amastigote typically 2–4 μm in diameter, round to oval in shape with nucleus and kinetoplast | ||
| Histology | Special media, as organism is fastidious it can take weeks to become positive. | ||
| PCR | Most sensitive, identifies species which is helpful in excluding ML associated species. PCR is also helpful in cases with low parasite burden. | ||
| Indirect | CL Detect | Immunochromatographic assay for the rapid detection of Leishmania species antigen in ulcerative skin lesions | |
| Sensitivity 96%, specificity 90% | |||
| Serologic tests (see below) | Not recommended for diagnosis of CL | ||
| Visceral leishmaniasis | Direct | Splenic aspirate (parasite isolation, culture, histology, and PCR per above) | Most sensitive (93–99%) compared to bone marrow and lymph node aspirate for diagnosing VL but risk of splenic hemorrhage |
| Bone marrow aspirate | Bone marrow sensitivity (52–85%) sensitivity. | ||
| Safer to perform than splenic aspirate | |||
| LN Aspirate | Lymph node aspirate sensitivity (52%–58%) | ||
| Peripheral blood | Assess blood for buffy coat, in vitro culture, and molecular analyses. Helpful in diagnosis for immunocompromised and HIV | ||
| Indirect | Serological tests: | Cannot distinguish active from prior infection. Not helpful for CL. Often non-reactive in immunocompromised hosts. | |
| Rapid Diagnostic Test (rK-39)* | Detect specific antibody against antigen present in L. donovani, chagasi-infantum | ||
| Results available in 20–25 min | |||
| Easy to perform, quick and cheap- particularly helpful in underserved areas | |||
| Sensitivity varies depending on region and parasite species | |||
| Can cross react with other infections—for example Chagas disease | |||
| Direct Agglutination Test (DAT)* | Uses whole organisms to look for antibody. | ||
| Gives antibody tires ranging from 1:100 up to 1: 151200. | |||
| Sensitive (>95%) and specific (>85%) test when performed correctly | |||
| Needs well trained technician to perform over 2-3 days | |||
| Not available in North America | |||
| Other antibody test, ELISA, Indirect immunofluorescence, indirect agglutination test, antigen test | Serologic antigen and urine antigen available | ||
| Sensitivity and specificity varies based on test | |||
| False positive results in persons with Chagas, leprosy, tuberculosis, malaria | |||
| Mucosal leishmaniasis | Direct | Biopsy, scraping, aspirate of mucosal lesion/LN (culture, histology, and PCR per above) | Direct diagnosis is preferred. |
| Indirect | Serological tests per above | Not as helpful for ML as for VL. Direct diagnosis is preferred | |
| Leishmanin Test | Delayed type hypersensitivity response | ||
| Also known as Montenegro test, works similarly to tuberculin skin test | |||
| Most useful in diagnosis of ML | |||
| Negative in diffuse CL, active VL | |||
| False positives with other skin disease | |||
| Not available in North America |
CL, cutaneous leishmaniasis; ML, mucosal leishmaniasis; VL, visceral leishmaniasis; LN, lymph node
*Most common serological tests
Adapted from Aronson et al. CID, PAHO, Burza et al., Berman et al.
Fig. 7.
Clinical classification and sample collection for leishmaniasis diagnosis in the Americas. Source: PAHO/WHO, 2018
As direct evidence is preferred, microscopy, culture, and PCR through correct specimen collection are critical [23•, 32•]. While PCR-based methods are more sensitive than culture or microscopy, these are often not standardized and their availability is limited to large hospitals or clinics. Tissue can be collected through scrapings, biopsies, or aspirates. For CL, the cleansed base of an ulceration should be collected for PCR and culture, while the less disruptive border of the ulceration should be biopsied for histology [32•]. For VL, sensitivity varies based on the tissue collection site with splenic biopsy yielding the highest rate of positive specimens (> 90% sensitivity), though this procedure carries a high risk of life-threatening bleeding. Therefore, bone marrow is the preferred first source despite lower sensitivity (50–80%) whereas other potential sources of tissue specimens include the liver, enlarged lymph nodes, and whole blood, especially in immunocompromised hosts [14•, 33]. Blood specimens (i.e., buffy coat preparation) are not typically diagnostic, except in HIV or immunocompromised patients who may have higher levels of parasitemia [14•]. Patients with erythematous and nodular PKDL have large number of parasites in skin. In general, to improve the sensitivity of leishmaniasis diagnostics, various diagnostic methods should be used and multiple tissue specimens should be collected from a variety of sites especially in the setting of chronic skin lesions and mucosal disease, where fewer parasites are present [14•].
Microscopic detection of amastigote stages within or near macrophages using Giemsa stained tissue confirms the diagnosis of leishmaniasis, though species level identification is not possible based on morphology [34]. Found intra- or extra-cellularly, the amastigote is typically 2–4 μm in diameter, round to oval in shape, and with a large nucleus and characteristic rod-shaped kinetoplast (mitochondrial structure containing extranuclear DNA) [32•]. On Giemsa stain, the cytoplasm stains blue and the nucleus and kinetoplast stain red to purple in color [20]. An expert should always evaluate the slide to exclude other causes of “dot” like organisms (i.e., Histoplasma capsulatum or Sporothrix sp.) [20]. Unlike amastigotes, intracellular fungal pathogens will not have a kinetoplast and will stain positive with mucicarmine, silver or periodic acid Schiff stains [35]. Of note, the amastigotes of Leishmania spp. are indistinguishable from those of Trypanosoma cruzi [36]. Although much less sensitive, histopathology can also be utilized to confirm leishmaniasis diagnosis. Findings vary from necrotizing and non-necrotizing granulomas to an inflammatory infiltration, necrotic ulceration, parakeratosis, and intraepithelial abscesses [37].
Other methods for direct diagnosis include culture of infected tissues. Culture media such as Novy, MacNeal, and Nicolle’s medium and Schneider’s Drosophila medium supplemented with 30% fetal bovine serum are incubated at 25 °C for up to 4 weeks given the fastidious nature of the parasite. Promastigote stages are identified microscopically in wet mounts from positive cultures [35]. Species level identification of culture isolates may be pursued using DNA-based assays or isoenzyme analysis in reference laboratories.
Molecular amplification assay using PCR is particularly important in the diagnosis of pauciparasitic syndromes such as ML. Leishmania species confirmation is currently recommended to guide management decisions [23•]. Moreover, knowledge of the Leishmania species (particularly L. Viannia) provides information about the risk of mucocutaneous disease and, thus, directs treatment recommendations [32•]. For example, L. panamensis often presents as cutaneous disease, but can lead to a mucosal syndrome which requires systemic treatment. By providing species identification, PCR can inform the clinician of L panamensis, prompting systemic therapy to prevent mucosal disease. Furthermore, as PCR is one of the more sensitive diagnostic methods, it is particularly helpful for diagnosis in scenarios with low parasitological burden [14•].
As direct parasitological diagnosis through microscopy, culture, and molecular testing are not always confirmatory or available, indirect immunologic tests may also be obtained. Evaluation for antibodies, usually IgG through direct agglutination, ELISA, immunofluorescence, and Western Blot, can be used as adjunctive tools in serologic diagnosis. These serologic tests are typically not helpful in cases of CL but can be of benefit in diagnosis of ML and VL [14•]. The Infectious Disease Society of America (IDSA) recommends serological tests in persons with suspicion for VL when definitive diagnostic parasitic testing (parasite identification, culture, histopathology, or parasite DNA) is negative or not available [23•]. A rapid diagnostic test, called rK-39 antibody tests (through ELISA and immunochromotography) sold under the manufacturing names Kalazar Detect, Diamed-IT Leish, and Onsite Leishmania Ab Rapid Test, involves the detection of antibodies against the K39 protein antigen found in Leishmania. These tests report having over a 90% sensitivity and specificity for diagnosing VL from certain regions [38–41]. Leishmania antibody can also be rapidly evaluated through the direct agglutination test (DAT) with a sensitivity of greater than 95% and a specificity of 85% when performed correctly [42]. While antibody tests represent an advancement in the serologic diagnosis of VL, they can cross react with other infections such as tuberculosis and malaria [43]. They also have decreased sensitivities in specific geographic regions [44, 45] and should not be used in patients who have had a history of a prior leishmania infection (as antibodies can persist in patients after treatment) or who are asymptomatic (as antibodies can be found in asymptomatic individuals from endemic areas) [21]. In addition, the potential for false negative serology results in patients with VL who are immunocompromised (HIV or other reasons) may limit its diagnostic utility in this setting.
In addition to antibody tests, antigen tests are also available to support the diagnosis. CL Detect, an immunochromatographic assay for rapid detection of Leishmania species antigen, is a novel immunologic test that can be used on ulcerative skin lesions to diagnose CL [23•]. An antigen detection method using latex agglutination called KAtex has a reported 93% specificity but a lower 64% sensitivity [14•]. ELISA urine antigen tests have also been developed with a sensitivity of over 90% [46, 47].
Lastly, intradermal skin testing, the Montenegro or Leishmanin Test, is a delayed type hypersensitivity reaction similar to the Mantoux tuberculin skin test (Fig. 8). The test is not widely available, and may be useful for epidemiological surveys to identify high-risk populations. Positive test results may be seen in patients with ML and CL [4•, 14•]; however, those with active VL and diffuse CL typically test negative [48, 49]. Also, the test cannot differentiate current infections from prior (Table 2) [4•].
Fig. 8.
Montenegro skin reaction in patient with cutaneous leishmaniasis
Treatment
Similar to diagnosis, treatment of leishmaniasis can be quite challenging (Table 3). Overall, leishmaniasis treatment should be tailored individually to the patient and the type of leishmaniasis, as well as to the parasite subspecies. PCR testing for species identification is particularly helpful in guiding therapy for patients with cutaneous disease, who may be at risk of ML.
Table 3.
Summary of systemic therapy
| Drug | Route of administration | Laboratory monitoring | Adverse effects | Comments |
|---|---|---|---|---|
| Parental | ||||
| Pentavalent Antimonials- sodium stibogluconate and meglumine antimoniate | IV, IM | CBC, CMP, Lipase, amylase, EKG | Myalgia, headaches, fatigue, nausea are common, elevated LFT, lipase, amylase (usually reversible), QT Prolongation, ST-T wave changes, cytopenias | Interrupt therapy if clinical pancreatitis, arrythmias, or worsening EKG changes. AIDS and immunosuppressed at risk of life threatening pancreatitis and cardiotoxicity. In the USA, must obtain from CDC. |
| Amphotericin- deoxycholate and liposomal amphotericin B | IV | CBC, K, Mg, creatinine | Infusion related reactions (fevers, rigors, nausea, vomiting, hypotension), malaise, nephrotoxicity, electrolyte abnormalities (K, Mg) | Liposomal amphotericin is better tolerated. Consider premedicating and slowing infusion to decrease risk of infusion reaction |
| Pentamidine | IV, IM | CBC, CMP, EKG, electrolytes | Nausea, vomiting, dysgeusia, headache, hypoglycemia, Insulin dependent DM, pancreatitis, hypotension, qt prolongation, nephrotoxicity, hepatotoxicity, cytopenias, electrolyte abnormalities, IM injection pain, rhabdomyolysis | Infuse drug slowly to decrease risk of hypotension. Avoid other nephrotoxic medications |
| Oral | ||||
| Miltefosine | Oral | CBC, kidney, and liver function tests in setting of VL. Pregnancy test prior to starting treatment | Nausea/vomiting, teratogenic. Rare risk of hepatic, renal impairment. If > 45 kg, need to increase dose | Administer with high fat meal to decrease nausea. Medication is costly |
| Azoles-Fluconazole, Ketoconazole | Oral | Hepatic function test, EKG, CBC | GI symptoms, hepatotoxicity, QTc prolongation, hair loss, agranulocytosis, decreased secretion of adrenal corticosteroids | Avoid use with other heptotoxic medications, qtc prolongation. Not recommended for treatment for ML or VL |
Adapted from IDSA and ASTMH guidelines Aronson et al. CID
Cutaneous leishmaniasis treatment is based on the clinical presentation, host, and species type. In an immunocompetent individual, with no evidence of mucosal disease, CL caused by L mexicana and L major does not require treatment, as it usually spontaneously resolves [21]. However, local treatment is recommended for simple lesions that do not self-resolve, while systemic treatment is recommended for complex CL (Table 3). Complex CL is defined as >five lesions; an area greater than 5 cm; lesions on face, fingers, toes, or genitalia; regional adenopathy; duration greater than 6 months; diffuse CL; immunocompromised host; prior clinical failure; or infection with a species associated with mucosal disease [23•]. Treatment in these cases can prevent morbidity, relapse, and progression to mucosal disease. Multiple systemic therapies (see Table 3) are available including pentavalent antimonials (meglumine antimoniate and sodium stibogluconate), amphotericin (deoxycholate and liposomal formulation), pentamidine and oral therapies (including fluconazole and miltefosine). Pentavalent antimonials are considered standard of care for systemic treatment of CL in Latin America [50].
For clinically simple lesions not associated with the risk of development of ML, photo or laser therapy, thermotherapy, topical paromomycin, and intralesional injections of antimonials are recommended [23•]. Thermotherapy is increasingly being utilized in South America and is associated with decreased lesion size and clinical cure [50]. While there is no ideal treatment regimen, experts recommend individualized therapy for the patient based on the extent and location of lesion involvement, immune status, co-morbid conditions, pregnancy plans, adverse effects of the medications, and published reports on the efficacy of treatment based on species and geographic data (see Table 3 for more details). Collagenase ointment has been used to decrease fibrous scarring and promote the final stages of healing [51].
Unlike CL, mucosal leishmaniasis does not spontaneously resolve and may lead to progressive destruction, disfigurement, and even death (due to aspiration pneumonia or respiratory obstruction). As a result, after a thorough naso-oropharyngeal exam, early systemic therapy is warranted. The IDSA recommends inpatient monitoring and prophylactic steroids in patients at risk for respiratory obstruction, which can be worsened by initiation of anti-leishmaniasis therapy [23•]. Similar to persons with complex CL, persons with ML should receive individualized systemic therapy (with the systemic agents previously described for CL).
As visceral leishmaniasis is the most serious manifestation of leishmaniasis, any person with signs and symptoms and a confirmed diagnosis of VL warrants early systemic therapy. Pentavalent antimonials were previously considered standard of care, but there is concern for developing resistance among Leishmania species. The IDSA recommends liposomal amphotericin B for treatment of VL in immunocompetent patients with the consideration for miltefosine in non-pregnant, non-breastfeeding individuals [23•]. Pentavalent antimonials can be used as an alternative for persons with leishmaniasis who cannot tolerate liposomal amphotericin B or miltefosine in areas of known low drug resistance patterns [23•]. Immunocompromised patients (i.e., HIV or transplant patients) should ideally receive liposomal amphotericin B; however, combination therapy with miltefosine can also be considered [23•]. HIV patients should receive antiretroviral therapy (ART) and secondary prophylaxis targeting the specific parasite, while these are not recommended for transplant patients [23•].
Patients who receive successful treatment for all clinical forms of leishmaniasis should be monitored for relapse. In patients who have recurrence, repeat treatment is advised (usually with liposomal amphotericin B) and longer duration treatment regimens should be considered [23•]. Drug resistance testing is, unfortunately, not commercially available and repeat biopsies and serological testing are generally not recommended.
Future Research
Multiple areas in the prevention, diagnosis, and treatment of leishmaniasis could be significantly advanced. The current limited diagnostic methods and the absence of clinician familiarity with endemic leishmaniasis are both significant obstacles to timely and accurate detection of this disease. Leishmaniasis is cumbersome to diagnose; thus, advances in serological tests could potentially lead to earlier detection and treatment. Furthermore, as WHO considers the USA to be a Leishmania endemic region, leishmaniasis should not be characterized as a uniquely tropical neglected disease. This mischaracterization likely leads to clinicians overlooking the diagnosis and delaying testing and treatment for leishmaniasis. Furthermore, when the diagnosis is made, reporting to both local, national, and international health agencies should be required. Texas, for example, is the only US state where leishmaniasis diagnosis requires a state health department report; however, federal health department notification is not necessary. If all states and nations required health agency reporting, a better understanding of the geographic distribution, risk factors, host species characteristics, and climate change contribution for leishmaniasis will be available.
Therapeutically, drug toxicities, cost, and emerging drug resistance limit the antiparasitic treatment armamentarium. Unfortunately, there are no molecular markers of drug resistance for Leishmania and testing for drug resistance is limited. Pentavalent antimonial was considered the historic standard of care, but inappropriate dosing and treatment regimens have likely contributed to increasing drug resistance with this agent [52]. While miltefosine is one of the newest drugs available, there are increasing rates of resistance to this agent, and the cost of the drug can be prohibitive [53]. In addition to drug development campaigns, directly observed therapy (DOT) of newer treatment regimens may be useful in supporting treatment success and combating resistance. Moreover, free leishmaniasis treatment through organized drug assistance programs (and restriction of over the counter medication) would encourage appropriate prescriptive use and possibly prevent emerging drug resistance.
Given the limitations in cost, toxicity, and availability of treatment regimens, vaccination is the best hope for controlling leishmaniasis. The goal would be a safe, effective, affordable, and widely available vaccine. Unfortunately, despite years of investigations and trials, there is currently no human vaccine available for this disease [54, 55]. Leishmune, CaniLeish (using fractionated leishmania antigens), and Leish-Tec (a second-generation vaccine using recombinant leishmania proteins) have shown efficacy in dogs (one of the primary parasite reservoirs) and could help control and prevent transmission of leishmaniasis [56•]. Infectious Disease Research Institute (IDRI, Washington, Seattle) has developed a vaccine candidate (LEISH-F3 +GLA SE) using recombinant Leishmania proteins from L. donovani and L. infantum and is currently testing subjects in phase I clinical trials [57]. The Sabin Vaccine Institute Product Development Partnership (Sabin PDP), similarly, is also working on a vaccine using recombinant L. donovani protein with sand fly salivary antigens [55]. Most recently, a third-generation leishmaniasis vaccine, ChAd63-KH, was developed to induce leishmania specific CD8+ T cells and has shown promise in a phase 1 clinical trial [58]. The European Multivalent Vaccine for Human Visceral Leishmaniasis (MeLeVaClin) is also working on recombinant protein–based vaccine strategies in pre-clinical models, and funding from the Bill & Melinda Gates Foundation, Carlos Slim Foundation, European Commission, German Federal Ministry of Education and Research NIH and Wellcome Trust is being directed toward vaccine development [55]. Hopefully, this research will provide an effective, prophylactic vaccine that could be used for individuals in endemic areas and also for animals to reduce their risk as Leishmania parasite carriers.
Conclusions
Leishmaniasis is a complex clinical syndrome that is difficult to diagnose and treat. Advances in vaccine development, diagnosis, reporting, and treatment could prevent substantial morbidity and mortality from this disease.
Abbreviations
- HIV
Human immunodeficiency virus
- TB
Tuberculosis
- PCR
Polymerase chain reaction
- DNA
Deoxyribonucleic acid
- ELISA
Enzyme-linked immunosorbent assay
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Skin and Soft Tissue Infections in Returning Travelers from the Tropics
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.CDC. Epidemiology and risk factors. [Available from: https://www.cdc.gov/parasites/leishmaniasis/epi.html].
- 2.WHO. Leishmaniasis [Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis].
- 3.Boelaert M, Sundar S. 47 - Leishmaniasis. In: Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White NJ, editors. Manson’s Tropical Infectious Diseases (Twenty-third Edition). London: W.B. Saunders; 2014. p. 631-51.e4.
- 4.•Organization PAHO. Manual of procedures for leishmaniases surveillance and control in the Americas 2019. [Available from: https://iris.paho.org/handle/10665.2/51838. This article gives a thorough explanation of the clinical symptoms, diagnosis, and treatment of leishmaniasis.
- 5.WHO. Status of endemicity of cutaneous leishmaniasis data by country [Available from: https://apps.who.int/gho/data/node.main.NTDLEISHCEND?lang=en].
- 6.McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatology. 2018;154(9):1032–1039. doi: 10.1001/jamadermatol.2018.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S. Climate change and risk of leishmaniasis in north america: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4(1):e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bern C, Amann J, Haque R, Chowdhury R, Ali M, Kurkjian KM, et al. Loss of leishmanin skin test antigen sensitivity and potency in a longitudinal study of visceral leishmaniasis in Bangladesh. Am J Trop Med Hyg. 2006;75(4):744–748. doi: 10.4269/ajtmh.2006.75.744. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Leishmaniasis: Biology. [Available from: https://www.cdc.gov/parasites/leishmaniasis/biology.html].
- 10.Hartley M-A, Ronet C, Zangger H, Beverley SM, Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol. 2012;2:99. doi: 10.3389/fcimb.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheffter SM, Ro YT, Chung IK, Patterson JL. The complete sequence of Leishmania RNA virus LRV2-1, a virus of an Old World parasite strain. Virology. 1995;212(1):84–90. doi: 10.1006/viro.1995.1456. [DOI] [PubMed] [Google Scholar]
- 12.Mondal D, Bern C, Ghosh D, Rashid M, Molina R, Chowdhury R, Nath R, Ghosh P, Chapman LAC, Alim A, Bilbe G, Alvar J. Quantifying the infectiousness of post-kala-azar dermal leishmaniasis toward sand flies. Clin Infect Dis. 2019;69(2):251–258. doi: 10.1093/cid/ciy891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Rutte EA, Zijlstra EE, de Vlas SJ. Post-kala-azar dermal leishmaniasis as a reservoir for visceral leishmaniasis transmission. Trends Parasitol. 2019;35(8):590–592. doi: 10.1016/j.pt.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.•Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–70. A concise overview of the major aspects of caring for persons with leishmaniasis. [DOI] [PubMed]
- 15.Davidson R, Croft S. Visceral leishmaniasis in Africa. Afr Health. 1992;14(5):18–19. [PubMed] [Google Scholar]
- 16.Faye B, Bañuls AL, Bucheton B, Dione MM, Bassanganam O, Hide M, Dereure J, Choisy M, Ndiaye JL, Konaté O, Claire M, Senghor MW, Faye MN, Sy I, Niang AA, Molez JF, Victoir K, Marty P, Delaunay P, Knecht R, Mellul S, Diedhiou S, Gaye O. Canine visceral leishmaniasis caused by Leishmania infantum in Senegal: risk of emergence in humans? Microbes Infect. 2010;12(14-15):1219–1225. doi: 10.1016/j.micinf.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 18.Scott P, Novais FO. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nat Rev Immunol. 2016;16(9):581–592. doi: 10.1038/nri.2016.72. [DOI] [PubMed] [Google Scholar]
- 19.John E. Bennett RD MBJB. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases: 8th ed. Philadelphia: Elsevier/Saunders, [2015]; 2015.
- 20.Herwaldt BL. Leishmaniasis. Lancet. 1999;354(9185):1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 21.Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24(4):684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 22.Olivo Freites C, Gundacker ND, Pascale JM, Saldaña A, Diaz-Suarez R, Jimenez G, et al. First case of diffuse leishmaniasis associated with Leishmania panamensis. Open Forum Infect Dis. 2018;5(11):ofy281. doi: 10.1093/ofid/ofy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.•Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2017;96(1):24–45. One of the primary guidelines about leishmaniasis. [DOI] [PMC free article] [PubMed]
- 24.Weller PF, Durand ML, Pilch BZ. Case 4-2005. New England Journal of Medicine. 2005;352(6):609–615. doi: 10.1056/NEJMcpc049035. [DOI] [PubMed] [Google Scholar]
- 25.Scope A, Trau H, Bakon M, Yarom N, Nasereddin A, Schwartz E. Imported mucosal leishmaniasis in a traveler. Clinical Infectious Diseases. 2003;37(6):e83–ee7. doi: 10.1086/377045. [DOI] [PubMed] [Google Scholar]
- 26.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Trans R Soc Trop Med Hyg. 1986;80(6):859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 27.Walton BC, Chinel LV, Eguia Y Eguia O. Onset of espundia after many years of occult infection with Leishmania braziliensis. Am J Trop Med Hyg. 1973;22(6):696–698. doi: 10.4269/ajtmh.1973.22.696. [DOI] [PubMed] [Google Scholar]
- 28.Viscerotropic leishmaniasis in persons returning from Operation Desert Storm--1990-1991. MMWR Morb Mortal Wkly Rep. 1992;41(8):131-4. [PubMed]
- 29.Seaman J, Mercer AJ, Sondorp HE, Herwaldt BL. Epidemic visceral leishmaniasis in southern Sudan: treatment of severely debilitated patients under wartime conditions and with limited resources. Ann Intern Med. 1996;124(7):664–672. doi: 10.7326/0003-4819-124-7-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Report on the Consultative Meeting on Leishmania/HIV Co-Infection Rome 6–7 September 1994 1995 [Available from: https://www.who.int/leishmaniasis/resources/who_leish_95.35/en/.
- 31.Mukhopadhyay D, Dalton JE, Kaye PM, Chatterjee M. Post kala-azar dermal leishmaniasis: an unresolved mystery. Trends Parasitol. 2014;30(2):65–74. doi: 10.1016/j.pt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33(1):101–17. This article explains some of the more recent developments in leishmaniasis diagnosis and treatment. [DOI] [PubMed]
- 33.WHO. Control of the leishmaniases: report of a meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22-26 March 2010. World Health Organization. 2010 [Available from: https://apps.who.int/iris/handle/10665/44412.
- 34.Georgiadou SP, Makaritsis KP, Dalekos GN. Leishmaniasis revisited: current aspects on epidemiology, diagnosis and treatment. J Transl Int Med. 2015;3(2):43–50. doi: 10.1515/jtim-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jorgensen JH, Pfaller MA, Carroll KC. American Society for Manual of clinical microbiology 2015.
- 36.CDC. American Trypanosomiasis [Available from: https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html].
- 37.Magill A, Meyers W, Neafie R, Klassen-Fischer M. Cutaneous leishmaniasis. 2011:18.
- 38.Cañavate C, Herrero M, Nieto J, Cruz I, Chicharro C, Aparicio P, et al. Evaluation of two rK39 dipstick tests, direct agglutination test, and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in highland Ethiopia. Am J Trop Med Hyg. 2011;84(1):102–106. doi: 10.4269/ajtmh.2011.10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundar S, Reed SG, Singh VP, Kumar PC, Murray HW. Rapid accurate field diagnosis of Indian visceral leishmaniasis. Lancet. 1998;351(9102):563–565. doi: 10.1016/S0140-6736(97)04350-X. [DOI] [PubMed] [Google Scholar]
- 40.Guimarães Carvalho SF, Lemos EM, Corey R, Dietze R. Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg. 2003;68(3):321–324. doi: 10.4269/ajtmh.2003.68.321. [DOI] [PubMed] [Google Scholar]
- 41.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. Bmj. 2006;333(7571):723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO. Guideline for Diagnosis, treatment and prevention of visceral leishmaniasis in South Sudan [Available from: https://www.who.int/leishmaniasis/burden/Guidelines_for_diagnosis_treatment_and_prevention_of_VL_in_South_Sudan.pdf].
- 43.National vector borne disease control program GoI. Guideline on use of rK39.
- 44.Diro E, Techane Y, Tefera T, Assefa Y, Kebede T, Genetu A, Kebede Y, Tesfaye A, Ergicho B, Gebre-Yohannes A, Mengistu G, Engers H, Aseffa A, Desjeux P, Boelaert M, Hailu A. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Trans R Soc Trop Med Hyg. 2007;101(9):908–914. doi: 10.1016/j.trstmh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Guideline for the diagnosis, treatment, and prevention of leishmaniasis in Ethiopia 2013 [Available from: https://www.afrikadia.org/wp-content/uploads/2018/08/VL_Guidelines_Ethiopia_2013.pdf].
- 46.Vallur AC, Tutterrow YL, Mohamath R, Pattabhi S, Hailu A, Abdoun AO, Ahmed AE, Mukhtar M, Salam MA, Almeida ML, Almeida RP, Mondal D, Albertini A, Ghalib H, Duthie MS, Reed SG. Development and comparative evaluation of two antigen detection tests for Visceral Leishmaniasis. BMC Infect Dis. 2015;15:384. doi: 10.1186/s12879-015-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh P, Bhaskar KRH, Hossain F, Khan MAA, Vallur AC, Duthie MS, et al. Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis. Parasit Vectors. 2016;9(1):383. doi: 10.1186/s13071-016-1667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryceson AD. Diffuse cutaneous leishmaniasis in Ethiopia. I. The clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63(6):708–737. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- 49.Salman SM, Rubeiz NG, Kibbi AG. Cutaneous leishmaniasis: clinical features and diagnosis. Clin Dermatol. 1999;17(3):291–296. doi: 10.1016/S0738-081X(99)00047-4. [DOI] [PubMed] [Google Scholar]
- 50.Gonçalves S, Costa CHN. Treatment of cutaneous leishmaniasis with thermotherapy in Brazil: an efficacy and safety study. An Bras Dermatol. 2018;93(3):347–355. doi: 10.1590/abd1806-4841.20186415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann S, Phupitakphol T, Davis B, Newman S, Suarez JA, Henao-Martínez A, Franco-Paredes C. Case Report: Cutaneous leishmaniasis due to Leishmania (Viannia) panamensis in two travelers successfully treated with miltefosine. Am J Trop Med Hyg. 2020;103(3):1081–1084. doi: 10.4269/ajtmh.20-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundar S, Thakur BB, Tandon AK, Agrawal NR, Mishra CP, Mahapatra TM, et al. Clinicoepidemiological study of drug resistance in Indian kala-azar. Bmj. 1994;308(6924):307. doi: 10.1136/bmj.308.6924.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deep DK, Singh R, Bhandari V, Verma A, Sharma V, Wajid S, Sundar S, Ramesh V, Dujardin JC, Salotra P. Increased miltefosine tolerance in clinical isolates of Leishmania donovani is associated with reduced drug accumulation, increased infectivity and resistance to oxidative stress. PLOS Neglected Tropical Diseases. 2017;11(6):e0005641. doi: 10.1371/journal.pntd.0005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kedzierski L. Leishmaniasis vaccine: where are we today? J Glob Infect Dis. 2010;2(2):177–185. doi: 10.4103/0974-777X.62881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34(26):2992–2995. doi: 10.1016/j.vaccine.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 56.•Moafi M, Rezvan H, Sherkat R, Taleban R. Leishmania vaccines entered in clinical trials: a review of literature. Int J Prev Med. 2019;10:95. A recent article describing the major vaccine candidates. [DOI] [PMC free article] [PubMed]
- 57.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, Rolf T, Misquith A, Laurance JD, Raman VS, Bailor HR, Cauwelaert ND, Reed SJ, Vallur A, Favila M, Orr MT, Ashman J, Ghosh P, Mondal D, Reed SG. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clinical & Translational Immunology. 2015;4(4):e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, Wiggins R, di Marco S, Colloca S, Siani L, Cortese R, Smith DF, Aebischer T, Kaye PM, Lacey CJ. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis. 2017;11(5):e0005527. doi: 10.1371/journal.pntd.0005527. [DOI] [PMC free article] [PubMed] [Google Scholar]