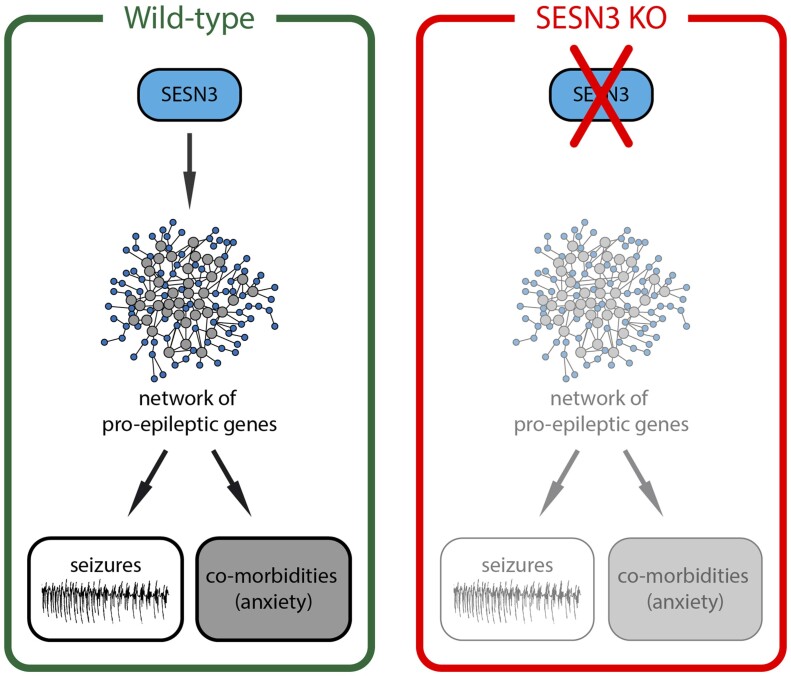
Abstract
Epilepsy is a serious neurological disorder affecting about 1% of the population worldwide. Epilepsy may arise as a result of acquired brain injury, or as a consequence of genetic predisposition. To date, genome-wide association studies and exome sequencing approaches have provided limited insights into the mechanisms of acquired brain injury. We have previously reported a pro-epileptic gene network, which is conserved across species, encoding inflammatory processes and positively regulated by sestrin3 (SESN3). In this study, we investigated the phenotype of SESN3 knock-out rats in terms of susceptibility to seizures and observed a significant delay in status epilepticus onset in SESN3 knock-out compared to control rats. This finding confirms previous in vitro and in vivo evidence indicating that SESN3 may favour occurrence and/or severity of seizures. We also analysed the phenotype of SESN3 knock-out rats for common comorbidities of epilepsy, i.e., anxiety, depression and cognitive impairment. SESN3 knock-out rats proved less anxious compared to control rats in a selection of behavioural tests. Taken together, the present results suggest that SESN3 may regulate mechanisms involved in the pathogenesis of epilepsy and its comorbidities.
Keywords: sestrins, epilepsy, anxiety, depression
We previously identified a pro-epileptic gene network positively regulated by sestrin3 (SESN3). Here, we found that SESN3 knock-out rats have decreased susceptibility to seizures and reduced propensity to anxiety, suggesting that SESN3 may regulate mechanisms causally involved in the pathogenesis of both epilepsy and its comorbidities.
Graphical Abstract
Introduction
Epilepsies are a complex group of neurological diseases, each differing in many aspects: aetiology (genetic, lesional/metabolic or unknown), localization of the epileptic focus, symptomatology. The common feature in epilepsy is the occurrence of spontaneous seizures, triggered by a paroxysmal excitatory activity in neuronal networks. However, whilst recurrent seizures are a core feature of epilepsy, people with epilepsy report additional comorbidities (Gaitatzis et al., 2012; Pitkanen et al., 2019), including neuropsychiatric disorders such as anxiety, depression and cognitive impairment. These comorbidities have a major impact on everyday social life (Taylor et al., 2011).
The burden of epilepsy comorbidities is high: ∼50% of adult patients suffer from atleast one comorbidity (Keezer et al., 2016). Similarly in animal models of epilepsy, where models of mesial temporal lobe epilepsy develop a depression-like state (Steimer, 2011; Chen et al., 2016) and anxiety-related behaviour (Hesdorffer et al., 2012; Falcicchia et al., 2018; Paolone et al., 2019). These observations highlight that shared pathogenic mechanisms may underlie both epileptic seizures and some of the neuropsychiatric comorbidities of epilepsy (Kanner, 2016).
Several causative mechanisms linking epilepsy and depression have been proposed, including a decrease in function of specific neurotransmitters (serotonin, noradrenaline, dopamine and GABA), tissue atrophy, morphological alterations in limbic areas and higher cytokine concentrations (IL-1beta) (Kanner, 2016; Rider et al., 2018). Comorbidities represent an important issue also for the healthcare system because they require additional, expensive medical interventions and can also aggravate the severity of epilepsy or the side effects of anti-epileptic drugs. The identification of pathophysiological mechanisms underlying both comorbidities and epilepsy therefore offer the potential for novel therapeutic interventions with potential health benefits beyond seizure control.
Recently, we used a systems biology approach to investigate transcriptional networks and pathways within the hippocampus of mesial temporal lobe epilepsy patients who underwent surgical resection of the epileptic focus, and we identified a transcription program that is overexpressed in the mesial temporal lobe epilepsy hippocampus and promotes the expression of epileptogenic signalling pathways (Johnson et al., 2015). The sestrin3 (SESN3) gene, belonging to the sestrin family of stress-inducible proteins, was identified as an activator of this transcriptional program. The pro-epileptic gene network regulated by SESN3 was initially identified using the pilocarpine mouse model of acquired epilepsy and subsequently shown to be conserved in the human epileptic hippocampus (Johnson et al., 2015). This observation was actually unexpected, because other sestrins may exert anti-epileptic effects. By upregulating the nuclear factor erythroid 2-related factor 2 signalling, SESN1 and SESN2 are thought to inhibit the mechanistic target of rapamycin complex 1 (mTORC1), attenuating reactive oxygen species accumulation (Rhee and Bae, 2015). mTORC1 inhibition, as obtained through injection of the prototype inhibitor rapamycin, can decrease seizure susceptibility (Citraro et al., 2016; Talos et al., 2018). However, mTORC1 activation seems to be required for ketamine-induced anti-depressant effects (Li et al., 2010), indicating that SESN1 and 2 may worsen epilepsy comorbidities.
In this work, we asked if SESN3 has a similar double-edged effect on epilepsy and epilepsy comorbidities. To explore this concept, we generated SESN3 knock-out (SESN3 KO) rats and studied in detail their phenotype, not only in terms of susceptibility to seizures but also to anxiety and depression.
Materials and methods
Animals
SESN3-KO and wild-type (WT) rats in a Sprague-Dawley (SD) background (Harlan, Italy) were used for all experiments. They were housed under standard conditions: constant temperature (22–24°C) and humidity (55–65%), 12 h light/dark cycle, water and food ad libitum. The Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines have been followed. Procedures involving animals and their care were carried out in accordance with European Community, national and local guidelines, laws and policies. Experimental protocols for animal experimentation were approved by the University of Ferrara Ethical Committee and the Italian Ministry of Health (n.953/2016-PR). All animals were euthanized by anaesthetic overdose.
SESN3-KO rat generation
SESN3-KO rats were generated at the Imperial College, London, exploiting a zinc finger nucleases engineered plasmid (Sigma Aldrich) transfected into single-cell SD embryos. The zinc finger nucleases technique allowed to cut the SESN3 gene at a specific site, leading to its complete knockdown (Supplementary Fig. 1). Heterozygous rats were then mated to give birth to WT and SESN3-KO rats.
The foster females aged 8–12 weeks have been injected intraperitoneally (i.p.) with 40 μg (0.2 ml) of Luteinizing Hormone–Releasing Hormone agonist 4 days prior to mating. On the day of mating, the recipients were individually placed with males. The following day, the females were de-mated and examined for the presence of copulation plug. The females presenting a plug have been used as embryo recipient for embryo transfer surgery.
SD females aged 4–6 weeks have been used as embryo donors. Two days before mating, the females were injected with 30 IU of pregnant mare’s serum gonadotropin (i.p.). On the day of mating, the females have been injected with human pregnancy urine chorionic gonadotropin (40 IU, i.p.) and placed individually with SD males. The following day, the females were separated, killed and the oviducts removed. The embryos were then collected and left in an incubator at 37°C, 5% CO2 until used for pronuclear injections.
The embryo recipients were anaesthetized with a mix of ketamine and xylazine (1:1, i.p.) and a painkiller (Carprofen, 5 mg/kg, s.c.). The female rats were placed under the microscope, the incision area between flank and ribcage was shaved and an incision was made through the skin and peritoneal wall. After the implantation of the embryos, the peritoneal wall was sutured. The female rats were then placed back in their cages on a heating pad at 37°C, until recovery from anaesthesia.
After having generated the first colony of rats, animals were sent to the laboratory of the University of Ferrara, where the breeding was conducted in heterozygosis, by mating SESN3-KO males and SD females.
SESN3-KO rats genotyping
Genotyping was conducted via PCR analysis. DNA was obtained by harvesting ear punch biopsies from 21-day-old rats, both female and male, and put into 1.5 ml tubes. Samples were then dipped in 25 µl OB protease and 180 µl lysis buffer (55°C, overnight) to enhance tissue digestion.
The day after, DNA was extracted with Tissue DNA Kit (Omega bio-tek) and the zinc finger nucleases target region was PCR-amplified. After amplification, a specific nuclease was added to the DNA samples (IDT Surveyor Mutation Detection Kit), enabling to recognize the cutting sites of the DNA chain of samples from SESN3-KO rats. Finally, samples were run in a 2% agarose gel: WT bands were observed at 400 kb, SESN3-KO bands at 200 kb.
WT and SESN3-KO homozygous littermates were employed for all experiments.
Behavioural testing in pups
Observational screens and simple behavioural tests were performed 7 days after birth to assess general health and developmental stages. We rapidly screened rats for the appearance of the fur, whiskers and posture, and we tested them for neurological and sensory reflexes and non-complex motor activities through a battery of simple tests: the postural flexion test, the righting reflex test, the response to the fear test, the negative geotaxis test and the placing responses test.
Postural flexion test
Pups were lifted by the tail at 30 cm height from a horizontal plan. If the proprioceptive system is functioning correctly, pups perceive the gravity and turn around in the natural posture. We recorded the time it took pups to turn around, with a cut-off of 60 s, and the side by which they turned.
Righting reflex test
Pups were laid supine on a horizontal plan. The time required to turn procumbent and the side by which the pup turned was noted by the experimenter.
Response to fear test
Pups were placed near the edge of a plan, facing the empty space. The time required for turning to the opposite side or for evaluating the intention to explore the empty (i.e. jumping from the plan) was recorded, with a 60 s cut-off.
Negative geotaxis test
Pups were put facing down on a 30° inclined plane, and the experimenters noted the time employed to turn up, with a cut-off of 60 s. Pups that failed to turn face up, or that slipped down, were assigned 60 s.
Response to stimulus test
This test evaluates the capacity of the pups to react to an external stimulus. The experimenter, leaving its paws free to move, holds the animal. Using an iron spatula, the experimenter touches both the right and the left anterior part of the rat’s paw and notes if the animal moves the paw after the stimulus.
Behavioural testing in adults
A battery of behavioural tests was conducted in adult (8 weeks old) WT and SESN3 KO male rats, to assess anxiety and depressive-related behaviour, motor and cognitive functions. We employed the following tests: Elevated Plus Maze (EPM), Open Field (OF), Novel Object Recognition (NOR) and Rotarod and Forced Swimming. Since the forced swimming test causes a high level of stress, only a subgroup of rats (WT n = 11, SESN3-KO n = 8) was tested and was not used in further experiments. Animals performed just one test per morning, under artificial diffused red light. Behavioural experiments were conducted in a soundproof room, into which animals were moved at least 30 min before testing. Each rat was used to be handled by researchers before being evaluated in behavioural tests.
Elevated plus maze test
The test was performed as described by Tchekalarova et al. (2015). The apparatus consisted of two open arms (50 cm × 10 cm) and two closed arms (50 cm × 10 cm × 50 cm) connected through a central platform (10 cm × 10 cm). The apparatus was 50 cm above floor level. Luminosity was checked using a luminometer and found to be nearly 1 in the open arms and close to 0 in the closed arms. At the beginning of the test, rats were placed in the central part of the platform, facing an open arm. The test lasted 5 min. The calculated measures were: number of entries in open arms; number of entries in closed arms; time spent in open arms; time spent in closed arms; number of stretched-attend postures, i.e., how many times the rat looked at the open arms while its body being in a closed arm; number of head dippings (HD), i.e., how many times the rat looked down from the open arms of the EPM apparatus; and number of rearings, i.e., how many times the rat looked up leaning against the walls of the closed arms. After each trial, the EPM apparatus was cleaned with 0.1% ethanol solution.
Open field test
The test was performed as described before (Tchekalarova et al., 2015). The apparatus consisted of a grey box (100 cm × 100 cm) divided into two compartments: outer (periphery) and inner (centre). The rat was placed in the centre of the box and was allowed to explore it for 20 min. The calculated measures were: total distance travelled (in cm); time spent in the centre; number of entries in the centre; time spent in the corners; and number of entries in the corners. After each trial, the OF apparatus was thoroughly cleaned with 0.1% ethanol solution. The test was recorded using a video camera infra-red (IR) (DSS1000 video recording system V4.7.0041FD, AverMedia Technologies, USA), and then videos were analysed using the Any Maze software (Ugo Basile S.R.L., Gemonio VA, Italy).
Forced swimming test
The forced swimming testwas employed to investigate despair-like behaviour (Porsolt, 1979). The test was performed using a plexiglass cylinder (50 cm × 20 cm) filled by three-fourth with water kept at 25 ± 1°C. Two swim sessions were conducted: 15 min of training followed (24 h later) by a 5-min test session. After each test, the rat was wiped and kept warm by a heating device for 10 min. Two different experienced observers recorded (i) the time of immobility, which occurred when the rat floated in the water without struggling and (ii) the time of climbing or movements made by the rat to keep its head above the water. The time spent immobile has been related to a depressive behaviour (D'Aquila et al., 2004).
Novel object recognition test
The NOR test was performed as described by Ennaceur and Delacour (1988). The test consisted of three phases: habituation, acquisition and test. The OF test, conducted the day before NOR, was used as habituation phase. The day after habituation, the acquisition trial was conducted by placing the rat in the field, in which two identical objects were positioned at the corners of the arena, ∼10 cm from the walls. Rats were free to explore the two objects for 5 min, and exploratory activity (i.e. the time spent exploring each object) was recorded. After 2 h, rats were placed again in the arena, in which one object was substituted with a novel one, not used in the acquisition phase. Again, the time that each animal spent exploring each object was measured. We considered a valid exploratory behaviour when the rat directly interacted with the object. The choice of objects as a novel or familiar was carried out in a random way, and the position of each object was also alternated between trials. After each test, the arena and the objects were thoroughly cleaned with 0.1% ethanol solution.
Rotarod test
The test has been run in a 3-day trial. The rotarod apparatus is composed of a rotating cylinder, divided into different compartments, one for each animal. The cylinder rotates and the speed is increased by steps of 5 rpm every 3 min. The maximum duration of the test was 30 min. The operator recorded every fault, i.e., when rats fell from the cylinder or hung to the cylinder without running. The test was considered finished if the animal made four continuous errors.
Models of epilepsy
Animals underwent seizure susceptibility tests at least 2 weeks after behavioural testing (i.e. at 10–14 weeks of age).
Pilocarpine
Both WT and SESN3-KO animals were randomly assigned to two groups: pilocarpine treated and controls. The former received a single injection of methyl-scopolamine (1 mg/kg, s.c.) 30 min prior to pilocarpine (370 mg/kg, i.p.), whereas the latter received a single injection of methyl-scopolamine 30 min prior to vehicle (0.9% NaCl solution). Thereafter, experienced researchers observed the animals, taking note of convulsive activity based on the Racine scoring scale (Racine et al., 1972). Usually, about 80% of WT rats develop seizures evolving into convulsive status epilepticus (SE) within the first 20–25 min after pilocarpine injection (Roncon et al., 2015). The experimenters also recorded the time required to enter convulsive SE. SE has been interrupted 3 h after its onset by the administration of diazepam (20 mg/kg, i.p.).
Intra-hippocampal kainic acid
We also assessed the susceptibility to SE in the intra-hippocampal kainic acid (KA) model (Raedt et al., 2009). Because KA was administered in awake animals, to allow proper observation of SE, guide cannulas were implanted 1 week before KA administration in the right ventral hippocampus for insertion of the injecting needle.
Stereotaxic surgery was thus performed to implant 20 G guide cannulas (PlasticsOne, USA) in the ventral hippocampus of both WT and SESN3-KO rats, following Paxinos’ Atlas (coordinates from bregma Antero-Posterior (AP) = −5.6; ML = +4.5; Dorso Ventral (DV) = +3.5). Anaesthesia was induced with ketamine/xylazine (respectively 87 and 13 mg/kg, i.p.) and maintained with isoflurane (1.5% in oxygen). After placing the rat in the stereotaxic frame, an incision made the skull exposed and a burr hole was drilled after at the above coordinates. Six other smaller holes were drilled to position screws. The guide cannula was then inserted, dental cement was used to fix cannula and screws to the skull, and the scalp was sutured with stitches. Animals were treated with tramadol and antibiotics before and after the stereotaxic surgery and then checked daily to monitor their well-being.
After a week of recovery, rats were injected with KA (0.4 µg/0.2 µl) using a 30 G needle connected to a 5 µl Hamilton syringe. After KA administration, rats were video-monitored and SE severity was recorded using the Racine’s scale. If SE did not resolve within 2.5 h, diazepam (10 mg/kg, i.p.) was administered to stop seizure activity.
Kindling
Rats were anaesthetized with mixed ketamine (Imalgene) and medetomidine (Domitor) in water (v/v; 50 mg/kg and 0.5 mg/kg, respectively; intramuscular injection, 2 ml/kg) and implanted with a bipolar stimulation/recording electrode into the right basolateral amygdala with the following coordinates: AP = −2.3, ML = −4.8, DV = +8.5, all measured from bregma. The electrode consisted of two twisted Teflon-coated stainless-steel wires. An electrode in the left occipital cortex served as the indifferent reference electrode. Bipolar, reference and ground electrodes were connected to plugs and the electrode assembly and anchor screws were held in place with dental acrylic cement (Grip Cement Liquid, Dentsply International Inc., USA) applied to the exposed skull surface. After 50 min, awakening was facilitated with an intramuscular administration (2.5 ml/kg) of atipamezole (Antisedan), 1.25 mg/kg, prepared at the final concentration of 10% in water. Following surgery, the rats were kept individually in Makrolon cages (Model 4, 480 mm × 375 mm × 210 mm) and were allowed ad libitum access to standard dry pellet food and tap water before random assignment to experimental groups.
After a post-operative period of 3 weeks, rats were placed in individual boxes and stimulated in the amygdala with increasing stimulation intensities, 1 ms monophasic square wave pulses, 50 Hz for 1 s. We started at a sub-maximal electrical intensity to determine the afterdischarge seizures threshold for each animal. We used a range of stimulation intensities from 20 µA to 444 µA with increasing steps of 20%.
Three days after initial threshold determination, rats were stimulated once daily, 5 days per week, in the amygdala with 500 µA. Kindling was defined as the appearance of 10 consecutive stages 4 or 5 seizures according to the scale of Racine where:
0 = no reaction
1 = stereotype mouthing, eye blinking and mild facial clonus
2 = head nodding and severe facial clonus
3 = myoclonic jerks in the forelimbs
4 = clonic convulsions in the forelimbs with rearing
5 = generalized clonic convulsions associated with loss of balance
Scores 0–2 were considered to reflect the focal phase and scores 3–5 the generalized phase of the motor seizures.
Structure analysis and immunofluorescence
Rats were killed by decapitation after an anaesthetic overdose. Brains were removed and immersed in 10% formalin for 48 h. They were then processed using a standard protocol (VTP 300, Bio-Optica, Milan, Italy) and paraffin embedded. Coronal sections of 7 µm were cut across the hippocampus and mounted onto polarized slides (Superfrost slides, Diapath Martinengo, BG, Italy). Sections were dewaxed with two washes in xylol (10 min each), 5 min in ethanol 100% and rehydrated in ethanol 95%, ethanol 80% and phosphate-buffered saline 1× (5 min each).
To analyse potential differences in tissue architecture, we performed a haematoxylin/eosin staining. Sections were immersed in haematoxylin for 5 min, washed in tap water, then stained with eosin for 2 min and washed again. For immunofluorescence, antigens were unmasked with a solution of citric acid and sodium citrate in a microwave oven at 750 W (3 min), then at 350 W (2 cycles of 5 min). Sections were incubated with 5% bovine serum albumin and 5% serum of the species in which the secondary antibody was produced, for 30 min at room temperature. They were then incubated in a humid plastic box with primary antibodies, overnight at 4°C. The primary antibodies were: anti-IBA-1, diluted 1:200 (rabbit monoclonal, #234003, Synaptic System, Gottingen, Germany) for microglia staining; anti-glial fibrillary acidic protein (GFAP) 1:100 (mouse monoclonal, #MAB12029, Immunological Science, Rome, Italy) for astrocyte staining; anti-NeuN/Fox-3 1:100 (mouse monoclonal, #MAB-90228 Immunological Science, Rome, Italy) to label neuronal nuclei and cytoplasm; anti-β3-tubulin 1:400 (mouse monoclonal, #4466X, Cell Signaling Technologies, MA, USA) for neuron staining. Two washes in 1× phosphate-buffered saline and a 30 min incubation in 0.3% Triton X-100 were performed before applying the secondary antibody, 488 or 594 Alexa-Fluor anti-mouse or anti-rabbit (diluted 1:250 in 1× phosphate-buffered saline) depending on the species of the primary antibody. Sections were left in a dark chamber under controlled humidity conditions for 3 h before proceeding with DAPI staining (0.0001% in 1× phosphate-buffered saline for 15 min; Santa Cruz, TX, USA) to label nuclei. Coverslips were mounted using an aqueous anti-fading mounting gel (Sigma).
Image analysis was performed on 6-µm paraffin-embedded coronal brain sections cut across the dorsal hippocampus (plates 21/22, Paxinos and Watson, 2007). Images were captured using a Leica microscope (DMRA2, Leica). Iba1- and GFAP-positive cells in the hippocampus were counted by automatic image analysis through a batch process in the Fiji (ImageJ) open-source software (Schindelin et al., 2012), according to the following algorithm: (i) auto-brightness/contrast to enhance signal/background ratio; (ii) reduce noise from image with mean filter; (iii) convert image to an 8-bit black and white; (iv) auto thresholding according to the MaxEntropy method (Kapur et al., 1985); (v) analyse and count thresholded particles excluding artefacts (out of defined size-range, perfectly linear and perfectly round ones); (vi) summarize count results for each image and save in the custom table. NeuN-positive pixel was measured using the same software and an algorithm tailored to measure per cent of supra-threshold pixels according to the IsoData method (Ridler and Calvard, 1978). The complete macros are shown in Supplementary Fig. 2. Average values of three sections per animal were employed for statistical analysis.
Real-time PCR
Total RNA was extracted from frozen hippocampi of WT and SESN3-KO rats, by using the RNeasy Lipid Tissue Mini Kit (Exiqon, #74804), following the manufacturer’s instructions. RNA was quantified using Nanodrop and diluted to 150 ng/µl, then retro-transcribed to cDNA using the SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific, #18091050) and stocked at −20°C. Real-time PCR was run to evaluate the expression of IL-1β, TNFα, c-fos, c-jun and Sesn1. Data were normalized to alpha-tubulin. The reverse transcription protocol was set up following Biorad Kit indications, while the primers recognizing the gene sequencing for the final quantification were purchased from Exiqon.
Statistical analysis
All experiments were run and analysed by blinded experimenters. Animals have been assigned randomly during the behavioural tests: only during NOR test, the researchers followed a pre-imposed order because following the correct timing and order of animals is pivotal for the test to succeed.
To test for statistical significance between two groups (i.e. SESN3-KO versus WT rats) a two-tailed unpaired t-test has been applied, and a two-tailed Mann–Whitney U-test has been used for non-normally distributed data, as indicated. The Welch’s correction has been used when data showed different standard deviations between the two samples. Fold change difference analysis after real-time PCR was performed using the Livorak formula.
Statistical test analyses have been performed and graphs prepared with GraphPad Prism (6.0 version).
Data availability
Data supporting the findings of this study are available from the corresponding author on request.
Results
Pups behaviour
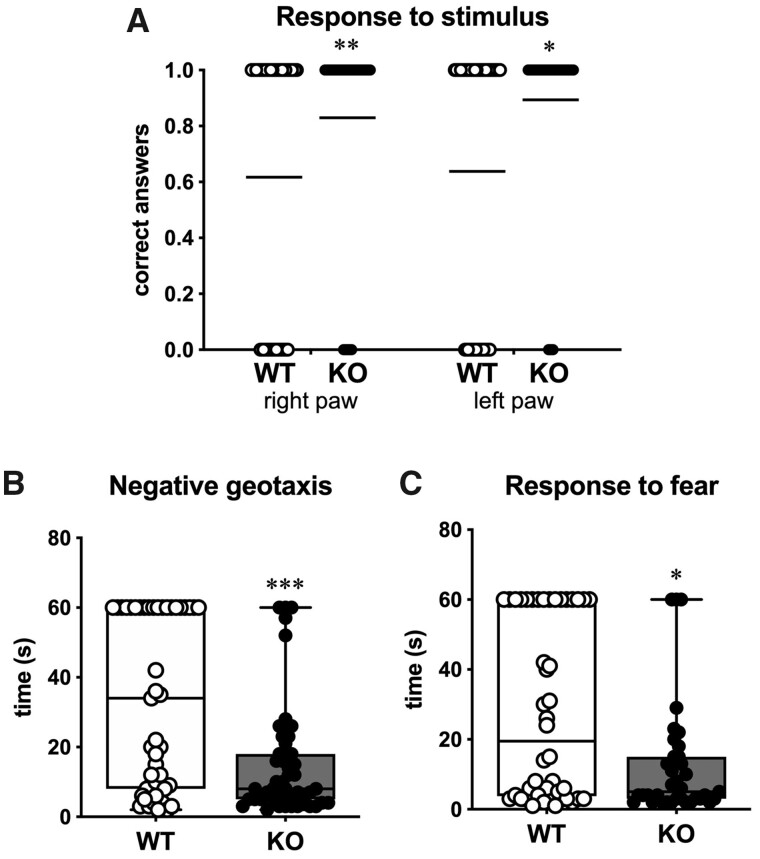
To investigate the neurological development of SESN3-KO rats, we performed a battery of behavioural tests on KO and WT littermate pups, that provide information on proprioception, movement execution, general neurological development and fear. Seven days after birth, both male and female rats were evaluated in all tests. First, pups were tested for postural flexion and righting reflex to test proprioceptive and cognitive functions. These two tests explore the capacity of the rat to return to its normal position after being posed in an unnatural position by the operator. WT pups spent less than 10 s to complete the tasks: in the postural flexion test, they spent 9.6 ± 1.0 s to rotate by 45° when suspended by the tail (Supplementary Fig. 3A), whereas in the righting reflex test they spent 5.0 ± 0.5 s to rotate from a supine to prone position (Supplementary Fig. 3B). KO pups performed in a similar manner (no significant differences), as shown in Supplementary Fig. 3. Next, we tested the ability to respond to an external stimulus represented by a touch with a small metal spatula to the left and the right front paw. Pups should retrieve their paw when touched. KO rats displayed a significantly higher percentage of correct answers (86.2%) compared to the WT littermates (62.8%; P = 0.003; power = 0.77; Fig. 1A).
Figure 1.
Behavioural testing on pups. (A) Response to stimulus. Scatter dot plot of all responses. Shown are the correct (1) or wrong (0) responses following a stimulus to the right and left anterior paw of WT (open circles) and of the SESN3-KO rats (solid circles) (n = 46 rats). Horizontal lines indicate the percentage of correct responses. Statistical analysis: Mann–Whitney U-test. (B) Negative geotaxis test. Time spent by WT and SESN3-KO rats to turn from down to up on the 45° inclined plan (n = 46 rats). (C) Response to fear. Time spent by WT and SESN3-KO rats to turn from the edge to the centre of the plan (n = 46 rats). Statistical analysis in B and C: unpaired t-test with Welch’s correction. *P < 0.05; **P < 0.01; ***P < 0.001.
Finally, we performed two tests to analyse fear and anxiety-related behaviour. In both tests, the pup is forced to an unnatural position, i.e., positioned facing down on a 45° inclined plane in the negative geotaxis test, and on the edge of a table facing the empty space, in the response to fear test. In the negative geotaxis test, SESN3-KO rats were much faster in completing their task compared to WT (P < 0.001; power = 0.99; Fig. 1B). Interestingly, 20 of 46 WT and 3 of 46 SESN3-KO pups froze and stayed immobile for more than 60 s (the cut-off time). These rats were included in the analysis with a time of 60 s. We observed a similar situation in the response to the fear test. Indeed, SESN3-KO pups returned in the middle of the table, escaping from the empty space, significantly faster than the WT (P = 0.008; power = 0.92; Fig. 1C). However, 11 KO but only 2 WT pups (out of 46 per group) attempted to jump off the table. Together, these data highlight a fearless-like attitude of SESN3-KO pups compared to WT littermates that, instead, displayed a passive reaction to the tests. This behaviour may be linked to a less anxious phenotype.
Adult rats’ behaviour
Elevated plus maze
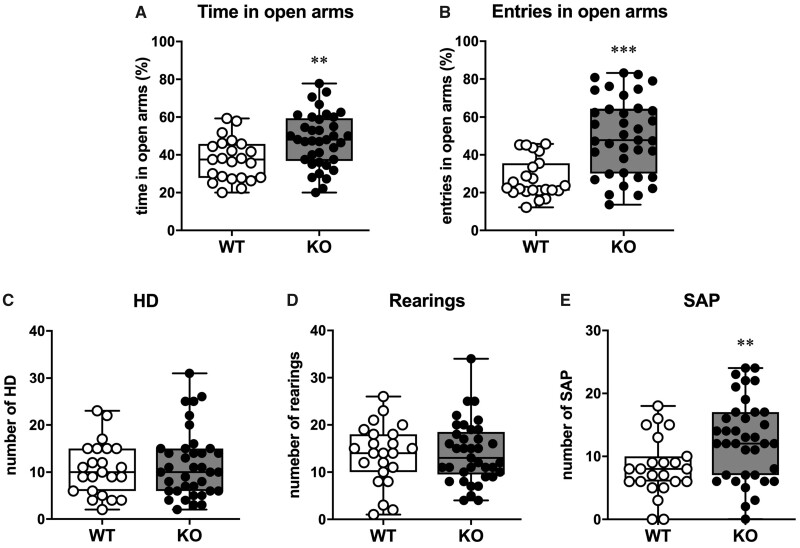
The EPM test aimed to evaluate anxiety. During this test, rodents normally prefer the most protected and safest areas of the apparatus, i.e., the closed arms. Thus, the more time they spend in the open arms, the less they can be considered anxious. In this test, we observed a significant difference between the two groups both in terms of time spent (P = 0.004) and entries (P < 0.001; power = 0.97) in the open arms, as SESN3-KO rats spent more time (about +30%; Fig. 2A) and entered more frequently in the open arms (about +80%; Fig. 2B).
Figure 2.
EPT test. (A) Per cent of time spent exploring the open arms over the total time of exploration. (B) Per cent of entries in the open arms over the total number of entries. (C) Number of head dipping during the 5 min of testing. (D) Number of rearings during the 5 min of testing. (E) Number of stretched-attend postures during the 5 min of testing. Statistical significance has been calculated with the Unpaired t-test with the Welch’s correction. In all panels, open circles represent WT (n = 23 rats) and solid circles represent SESN3-KO rats (n = 37 rats). Statistical analysis in A and B: Mann–Whitney U-test. Statistical analysis in B and C: unpaired t-test with Welch’s correction. **P < 0.01; ***P < 0.001.
During the 5-min testing, we also explored other behavioural traits, such as the number of stretched-attended postures, the number of head dipping and the number of rearings. The comparison between SESN3-KO and WT rats showed a similar number of head dipping and rearings (Fig. 2C and D), whereas a significantly increased number of stretched-attend postures was observed in the KO group (P = 0.003, power = 0.76; Fig. 2E). These findings may reflect a proactive attitude towards danger situations of SESN3-KO rats, in line with the behaviour observed in the pups in the response to fear.
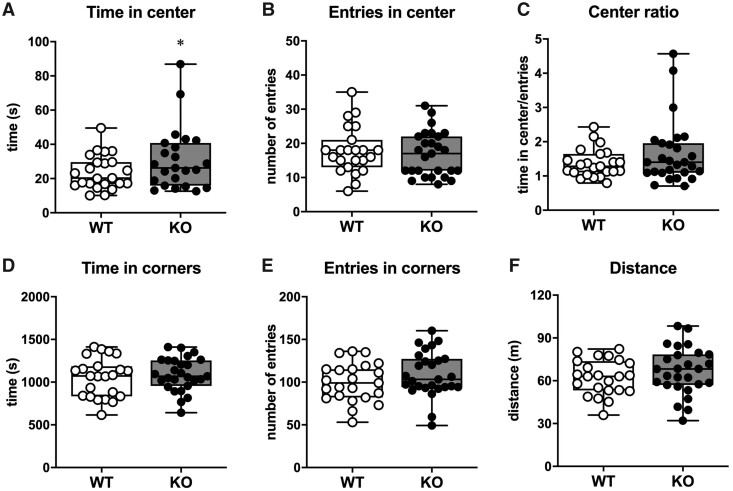
Open field
In order to better characterize such less anxious phenotype of SESN3-KO rats, we tested the two groups in the OF test. This test provides additional information on susceptibility to anxiety, based on the number of entries and the time spent in the central area of the arena, that is perceived as a dangerous area of the apparatus. The SESN3-KO rats spent slightly more time in the central area compared to WT littermates (P = 0.023, power = 0.64, Fig. 3A), while entering the central area the same number of times as WT (Fig. 3B). However, the average time per entry was slightly, but not significantly increased in SESN3-KO rats (Fig. 3C). Both groups spent a similar amount of time (Fig. 3E) and entered a similar number of times in the corners (Fig. 3F). Importantly, the behavioural profile observed in the EPM and OF cannot be due to increased motor activity, because SESN3-KO and WT rats walked similar total distances in the OF arena during the test (Fig. 3F).
Figure 3.
OF test. (A) Time, in seconds, spent in the centre of the arena. (B) Number of entries in the centre of the arena. (C) Time spent in the centre of the arena per entry. (D) Time spent in the corners of the arena. (E) Number of entries in any of the four corners of the arena. (F) Total distance in metres run by the rats. In all panels, open circles represent WT (n = 23 rats) and solid circles represent SESN3-KO rats (n = 27 rats). Statistical analysis: unpaired t-test with Welch’s correction. *P < 0.05.
Forced swim test
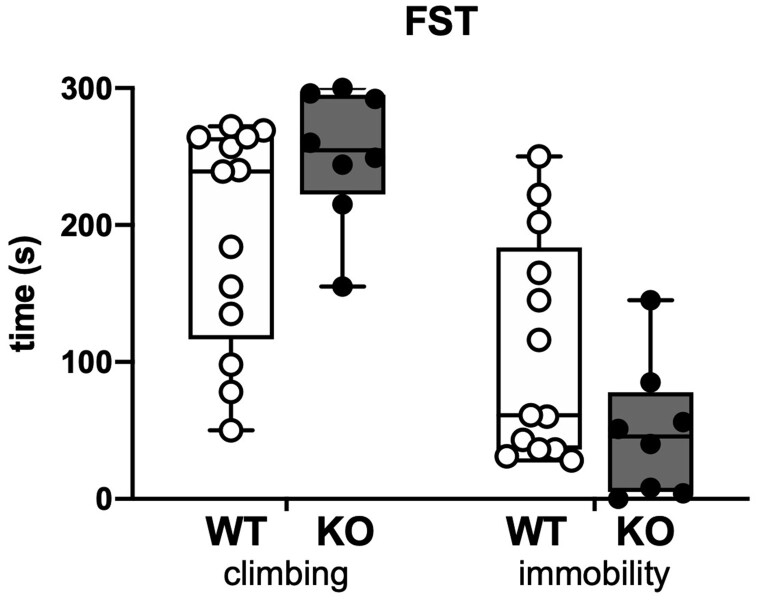
The two parameters taken into consideration were the climbing and the immobility time (Fig. 4). During the 5 min testing, SESN3-KO rats displayed a slight, but non-significantly different tendency to keep trying to escape from the water cylinder and to stay less immobile compared to WT rats. Increasing sample size might have permitted reaching significance. However, we had to limit numbers for this highly stressful test.
Figure 4.
Forced swimming test. The plot shows the time spent by the rats in trying to climb the cylinder or swimming in an attempt to find an escape (climbing) and the time spent floating immobile (immobility). Open circles represent WT (n = 13 rats) and solid circles represent SESN3-KO rats (n = 8 rats).
Novel object recognition
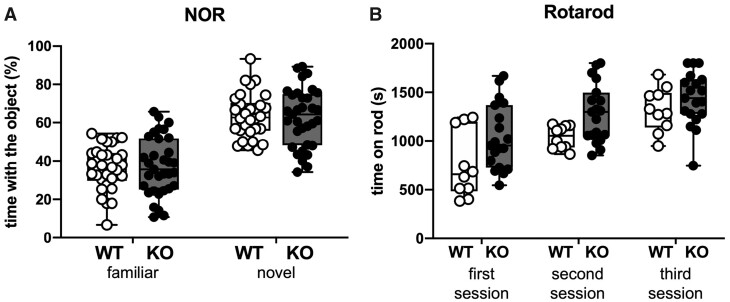
We performed the NOR test to evaluate if a memory impairment was also present in SESN3-KO rats. During the testing trial, in which one object (familiar) was substituted by a novel one, both WT and SESN3-KO rats explored for a longer time the novel than the familiar object. No significant differences between the two groups could be observed (Fig. 5A).
Figure 5.
Novel object recognition and rotarod tests. (A) Novel object recognition. Percentage of time spent interacting with the familiar and the novel object in 30 WT and 33 SESN3-KO rats. (B) Rotarod. Time spent on the rods during in three trials of testing. Open circles represent WT (n = 10 rats), and solid circles represent SESN3-KO rats (n = 19 rats).
Rotarod
Finally, to further verify that changes in motor activity could account for the findings in the EPM and OF, we performed the Rotarod test. No differences in motor coordination were observed between the two groups (Fig. 5B).
Seizure susceptibility
Systemic pilocarpine
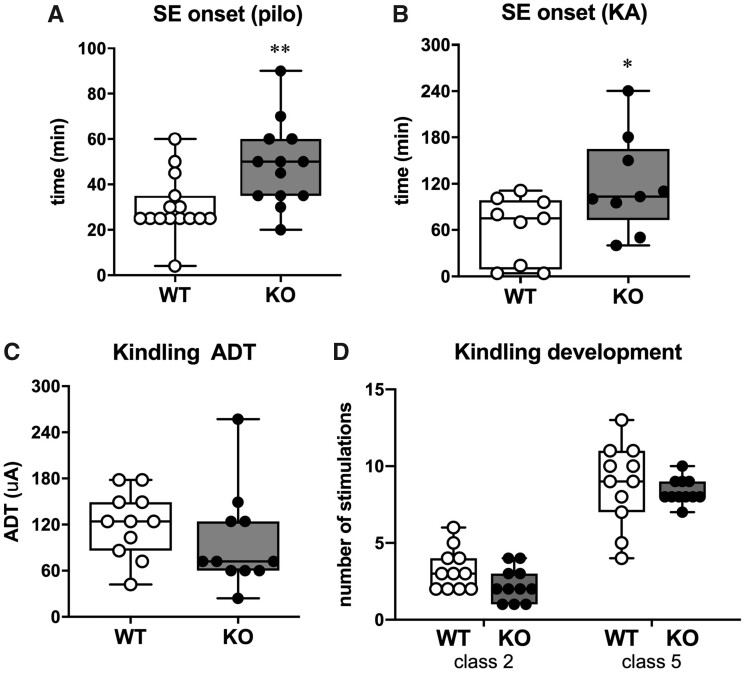
The epileptic phenotype was first explored by inducing SE by i.p. administration of pilocarpine. Eleven of 13 SESN3-KO rats (85%) did not develop SE within 30 min after pilocarpine injection and were administered a second half dose of the chemoconvulsant. In contrast, only 4 of 15 WT rats (27%) did not develop SE within the first 30 min and received a second half dose. Altogether, SESN3-KO rats needed significantly more time (and higher doses of pilocarpine) to develop the SE compared to WT (P = 0.008; power= 0.92; Fig. 6A).
Figure 6.
Seizure susceptibility. (A) Time to SE onset after i.p. pilocarpine injection onset in 15 WT and 13 SESN3-KO rats. (B) Time to SE onset after intra-hippocampal injection of KA in 9 WT rats and 9 SESN3-KO rats. (C) after discharge threshold (ADT) associated with minimal behavioural symptoms (i.e. 1–2 on Racine scale score, n = 11 rats per genotype) (D) Kindling development. Days to reach Class 2 or Class 5 seizures according to the Racine’s scoring scale, in 11 animals per genotype. In all panels, open circles represent WT and solid circles represent SESN3-KO rats. Statistical analysis: unpaired t-test with Welch’s correction. *P < 0.05; **P < 0.01.
Intra-hippocampal KA
Because of a progressive accumulation of fat tissue, SESN3-KO animals tend to have a greater body weight in adulthood compared with WT rats. This may affect the distribution of peripherally administered drugs, including pilocarpine. Therefore, we decided to verify the above findings using a SE model in which the chemoconvulsant is directly administered in the brain, namely intra-hippocampal KA. Consistent with the findings in the pilocarpine model, the onset of generalized convulsive SE was delayed in SESN3-KO in comparison with WT rats (P = 0.041; power= 0.61; Fig. 6B).
Kindling
Because both the pilocarpine and the KA model are chemically induced, we decided to investigate also the electrical kindling model. Contrary to the expectations, we did not find any difference in afterdischarge threshold or kindling development between SESN3-KO and WT rats (Fig. 6C and D).
Morphology and immunohistochemistry
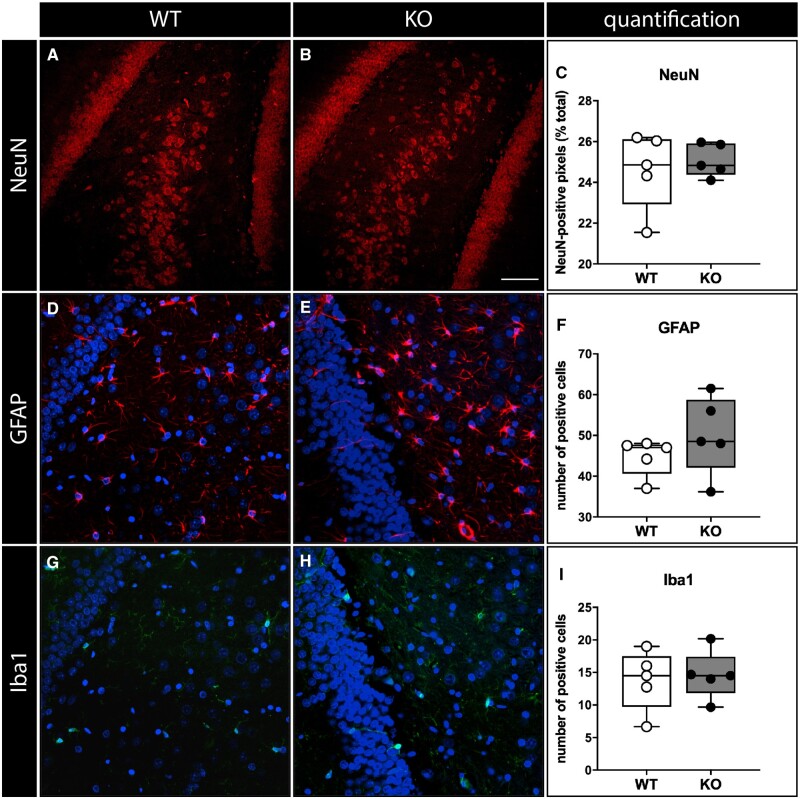
Histological analysis was focused on the hippocampus, the region where SESN3 was originally identified to regulate a proconvulsive module of genes (Johnson et al., 2015). Based on haematoxylin–eosin staining, no differences in the structure of the hippocampus were detected (data not shown). The number and morphology of neurons, astrocytes and microglial cells were also not obviously altered (Fig. 7 and Supplementary Fig. 4).
Figure 7.
Immunohistochemical analysis. Neurons (A–C), labelled in red with a NeuN antibody, astrocytes (D–F, labelled in red with a GFAP antibody), microglial cells (G–I, labelled in green with an Iba-1 antibody). Shown are representative sections of the dentate gyrus area from naïve WT (A, D, G) and SESN3-KO rats (B, E, H). DAPI staining in blue. Data quantification in C, F, I. In all panels, open circles represent WT and solid circles represent SESN3-KO rats. Horizontal bar in panel B (for all panels) = 100 µm.
Real-time PCR
Expression levels of selected Module-1 genes (IL-1β, TNFα, c-fos, c-jun), which we previously show to be regulated by SESN3 in the hippocampus (Johnson et al., 2015), has been quantified as cycle threshold number using the Bio-Rad software. No significant differences between the two groups were observed. We therefore hypothesized that compensatory mechanisms may be set in motion by knocking-out SESN3. In line with this idea, although not a demonstration of it, SESN1 expression levels were slightly higher in SESN3-KO animals than in WT controls, even if not in a significant manner (data not shown).
Discussion
The extensive analysis of the phenotype of SESN3-KO rats presented here supports the notion that SESN3 not only promotes seizures as previously reported (Johnson et al., 2015) but may also impact the appearance of certain comorbidities of epilepsy (e.g. anxiety), while not interfering with others (e.g. cognitive impairment).
SESN3-KO rats and their WT littermates were subject to a battery of behavioural tests assessing anxiety, depression, cognition and motor function. The most striking result was that both pups and adult SESN3-KO rats manifested increased ability to cope with novelty and stress, such as the exploration of the empty space at the edge of a table, or when being placed into the open space of an arena. These findings indicate that these animals are less prone to the fear and anxiety that normally guide animals to stay away from situations that are perceived as potentially dangerous. Importantly, this is not due to an unspecific stimulation of motor activity, because the distance walked in the OF during a test session and the performance on the Rotarod test were identical in KO and WT rats. Moreover, because behavioural analyses were made in the same animals, when pups and then as adults, these behavioural traits do not appear to undergo compensation in the course of life.
Based on these findings, we hypothesized that SESN3-KO rats would have been also less prone to depression. This idea was tested using the Forced Swimming test (FST), a rather stressful test that induces a state of desperation due to the impossibility to escape a life-threatening situation. While a lower tendency to desperation (i.e. continued attempts to escape and less immobility) was observed in SESN3-KO rats, this was not statistically significant. In addition, we did not observe any improvement in cognitive tests like novel object recognition.
Next, we thought to confirm in mammals the observation previously made in a Zebrafish model, that knocking down SESN3 can reduce seizure susceptibility (Johnson et al., 2015). As a first study test we employed the pilocarpine model, and found that, in keeping with the previous results, susceptibility to SE was reduced in SESN3-KO rats, because they required higher doses and more time than WT to develop SE. In our WT group, these parameters were identical to those previously reported in the literature (Curia et al., 2008). However, a limitation in this experiment is that the pharmacokinetics of pilocarpine may be altered in adult SESN3-KO rats, because these animals tend to gain weight and, in particular, accumulate fatty tissue (Narasimhan et al., 2009a, b; Dalina et al., 2018). In addition, other authors have reported the opposite result that knocking down SESN3 using a different approach (namely, intra-hippocampal injection of lentivectors expressing a small interfering RNA against SESN3) aggravates pilocarpine-induced acute seizures (Huang et al., 2018). Therefore, we thought to employ a model in which a convulsant agent (KA) is directly injected in the brain, thus avoiding the pharmacokinetics confounding factor (Bragin et al., 2004). This approach confirmed our data from the pilocarpine model. In addition, we also decided to test an electrical model of epilepsy, amygdala kindling. However, we did not observe any difference in afterdischarge threshold or kindling development between the two groups, suggesting a prominent role of SESN3 on seizure generation and generalization, rather than on epileptogenesis.
Finally, similar to SESN2 KOs (Kallenborn-Gerhardt et al., 2013), we did not find differences in morphology or number of neuronal cells populations nor significant activation of astrocytes or microglia between SESN3-KO and WT rats.
Overall, these data suggest the presence of a phenotype characterized by reduced anxiety and fear, as well as lower susceptibility to seizures. However, only some of these traits are clearly evident in SESN3 KO rats, while others are more subtle and not confirmed when using additional tests. One possible interpretation of these inconsistencies may be the constitutive gene knock out, which could trigger compensatory mechanisms during development.
As stated in the Introduction section, the finding that SESN1 and 2 inhibit mTORC1 suggest an anti-epileptic but also a pro-depressant effect (Li et al., 2010; Citraro et al., 2016; Talos et al., 2018). On the other hand, our present data support the notion that increased SESN3 activity may have both a pro-epileptic effect [confirming our previous findings (Johnson et al., 2015)], and an anxiogenic effect. Whereas the former effect is likely due to activation of expression of a module of pro-epileptic genes (Johnson et al., 2015), the second remains to be investigated. SESN3 is primarily regulated by FOXO1 and FOXO3 (Budanov, 2011). FOXO proteins are expressed in the limbic system, in particular in the hippocampus, amygdala and nucleus accumbens, areas implicated in the regulation of mood and emotions (Wang et al., 2015). Indeed, FOXO3 was hypothesized to be a pro-depression gene, because FOXO3-deficient mice are less prone to develop depressive-like behaviour (Polter et al., 2009). These effects of FOXO3 may be mediated by activation of SESN3 expression that, in turn, will increase expression of the genes in the pro-epileptic module, many of which (for example IL-1b may exert anxiogenic and/or depressant effect (Pineda et al., 2012). In neurons, FoxO3a activation is involved in protection against excitotoxic and proteotoxic insults (Mojsilovic-Petrovic et al., 2009). Furthermore, activation of FoxO3a has been associated with increased neuronal excitability (Howlett et al., 2008). Differential role of FoxO3a has been suggested depending on the duration of seizures, meaning brief and repetitive or prolonged and harmful (Caballero-Caballero et al., 2013); this may explain why we did not highlight an impact of SESN3 KO in the kindling model which induces limited neuronal death in comparison to SE models.
In conclusion, the present data suggest that SESN3 may represent a common mechanism of epilepsy and anxiety (maybe depression), i.e., some comorbidities of epilepsy. Identifying common mechanisms of epilepsy and its comorbidities would be important to develop therapies that are not purely anti-seizure and, in some cases, may even worsen comorbidities (Kanner, 2016). The present findings are informative in that direction and will prompt more detailed investigations. However, the limitations of this study should be also carefully considered. First, behavioural testing was performed in normal (naïve) animals. It will be important in the future to extend it to chronically epileptic animals in which anxiety, depression and cognitive impairment are already present (Hesdorffer et al., 2012; Falcicchia et al., 2018; Paolone et al., 2019). Second, part of the phenotype may have been obscured in our experiments by compensatory mechanisms that occurred during development in constitutive KO. Therefore, another important future goal will be to modulate SESN3 expression in key brain areas and at specific time points. These limitations notwithstanding, the present study identifies a possible target for the treatments of both epilepsy and its psychiatric comorbidities. Strategies to pursue this aim may include antisense oligonucleotides, gene therapy vectors expressing small interfering RNAs (siRNAs) against SESN3, or even small molecules. Molecules activating other sestrins have been developed already (Kato et al., 2019), suggesting that it may also be possible in the future to identify SESN3-selective antagonists.
Funding
European Union Seventh Framework Programme (FP7/2007-2013), Grant/Award Number: 602102 (Epitarget) to M.J., E.P. and M.S.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- EPM =
elevated plus maze
- KA =
kainic acid
- KO =
knock out
- mTORC1 =
mechanistic target of rapamycin complex 1
- NOR =
novel object recognition
- OF =
open field
- ROS =
reactive oxygen species
- SE =
status epilepticus
- SESN =
sestrin
- WT =
wild type
References
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J.. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 2004; 45: 1017–23. [DOI] [PubMed] [Google Scholar]
- Budanov AV.Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid Redox Signal 2011; 15: 1679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Caballero A, Engel T, Martinez-Villarreal J, Sanz-Rodriguez A, Chang P, Dunleavy M, et al. Mitochondrial localization of the forkhead box class O transcription factor FOXO3a in brain. J Neurochem 2013; 124: 749–56. [DOI] [PubMed] [Google Scholar]
- Chen S-D, Wang Y-L, Liang S-F, Shaw F-Z.. Rapid amygdala kindling causes motor seizure and comorbidity of anxiety- and depression-like behaviors in rats. Front Behav Neurosci 2016; 10: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R, Leo A, Constanti A, Russo E, De Sarro G.. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res 2016; 107: 333–43. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RSG, Avoli M.. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 2008; 172: 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila PS, Panin F, Serra G.. Long-term imipramine withdrawal induces a depressive-like behaviour in the forced swimming test. Eur J Pharmacol 2004; 492: 61–3. [DOI] [PubMed] [Google Scholar]
- Dalina AA, Kovaleva IE, Budanov AV.. Sestrins are gatekeepers in the way from stress to aging and disease. Mol Biol 2018; 52: 823–962. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J.. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 1988; 31: 47–59. [DOI] [PubMed] [Google Scholar]
- Falcicchia C, Paolone G, Emerich DF, Lovisari F, Bell WJ, Fradet T, et al. Seizure-suppressant and neuroprotective effects of encapsulated BDNF-producing cells in a rat model of temporal lobe epilepsy. Mol Ther Methods Clin Dev 2018; 9: 211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitatzis A, Sisodiya SM, Sander JW.. The somatic comorbidity of epilepsy: a weighty but often unrecognized burden. Epilepsia 2012; 53: 1282–93. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA, et al. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol 2012; 72: 184–91. [DOI] [PubMed] [Google Scholar]
- Howlett E, Lin CC-J, Lavery W, Stern M.. A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet 2008; 4: e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LG, Zou J, Lu QC.. Silencing rno-miR-155-5p in rat temporal lobe epilepsy model reduces pathophysiological features and cell apoptosis by activating Sestrin-3. Brain Res 2018; 1689: 109–22. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Behmoaras J, Bottolo L, Krishnan ML, Pernhorst K, Santoscoy PLM, et al. Systems genetics identifies Sestrin 3 as a regulator of a proconvulsant gene network in human epileptic hippocampus. Nat Commun 2015; 6: 6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenborn-Gerhardt W, Lu R, Syhr KMJ, Heidler J, von Melchner H, Geisslinger G, et al. Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury. Antioxid Redox Signal 2013; 19: 2013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AM.Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol 2016; 12: 106–16. [DOI] [PubMed] [Google Scholar]
- Kapur JN, Sahoo PK, Wong AKC.. A new method for gray-level picture thresholding using the entropy of the histogram. Comp Vis Graph Image Proc 1985; 29: 273–85. [Google Scholar]
- Kato T, Pothula S, Liu R-J, Duman CH, Terwilliger R, Vlasuk GP, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest 2019; 129: 2542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keezer MR, Sisodiya SM, Sander JW.. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 2016; 15: 106–15. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, Nedelsky N, Boccitto M, Mano I, Georgiades SN, Zhou W, et al. FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci 2009; 29: 8236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Mukhopadhyay A, Tissenbaum HA.. InAKTivation of insulin/IGF-1 signaling by dephosphorylation. Cell Cycle 2009. a; 8: 3878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA.. Converging pathways in lifespan regulation. Curr Biol 2009. b; 19: R657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Falcicchia C, Lovisari F, Kokaia M, Bell WJ, Fradet T, et al. Long-term, targeted delivery of GDNF from encapsulated cells is neuroprotective and reduces seizures in the pilocarpine model of epilepsy. J Neurosci 2019; 39: 2144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C.. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier Academic; 2007. [Google Scholar]
- Pineda EA, Hensler JG, Sankar R, Shin D, Burke TF, Mazarati AM, et al. Interleukin-1beta causes fluoxetine resistance in an animal model of epilepsy-associated depression. Neurotherapeutics 2012; 9: 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, et al. Epilepsy biomarkers - toward etiology and pathology specificity. Neurobiol Dis 2019; 123: 42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A, Yang S, Zmijewska AA, van Groen T, Paik J-H, DePinho RA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry 2009; 65: 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD.Animal model of depression. Biomedicine 1979; 30: 139–40. [PubMed] [Google Scholar]
- Racine RJ, Gartner JG, Burnham WM.. Epileptiform activity and neural plasticity in limbic structures. Brain Res 1972; 47: 262–8. [DOI] [PubMed] [Google Scholar]
- Raedt R, Van Dycke A, Van Melkebeke D, De Smedt T, Claeys P, Wyckhuys T, et al. Seizures in the intrahippocampal kainic acid epilepsy model: characterization using long-term video-EEG monitoring in the rat. Acta Neurol Scand 2009; 119: 293–303. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae SH.. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med 2015; 88: 205–11. [DOI] [PubMed] [Google Scholar]
- Rider FK, Danilenko OA, Grishkina MN, Kustov GV, Akzhigitov RG, Lebedeva AV, et al. Depression and epilepsy: comorbidity, pathogenetic similarity, and principles of treatment. Neurosci Behav Physiol 2018; 48: 78–82. [Google Scholar]
- Ridler T, Calvard S.. Picture thresholding using an iterative selection method. IEEE Trans Syst Man Cyber 1978; 8: 630–2. [Google Scholar]
- Roncon P, Soukupovà M, Binaschi A, Falcicchia C, Zucchini S, Ferracin M, et al. MicroRNA profiles in hippocampal granule cells and plasma of rats with pilocarpine-induced epilepsy-comparison with human epileptic samples. Sci Rep 2015; 5: 14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T.Animal models of anxiety disorders in rats and mice: some conceptual issues. Dialogues Clin Neurosci 2011; 13: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Jacobs LM, Gourmaud S, Coto CA, Sun H, Lim K-C, et al. Mechanistic target of rapamycin complex 1 and 2 in human temporal lobe epilepsy. Ann Neurol 2018; 83: 311–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RS, Sander JW, Taylor RJ, Baker GA.. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia 2011; 52: 2168–80. [DOI] [PubMed] [Google Scholar]
- Tchekalarova J, Moyanova S, Fusco AD, Ngomba RT.. The role of the melatoninergic system in epilepsy and comorbid psychiatric disorders. Brain Res Bull 2015; 119: 80–92. [DOI] [PubMed] [Google Scholar]
- Wang H, Quirion R, Little PJ, Cheng Y, Feng Z-P, Sun H-S, et al. Forkhead box O transcription factors as possible mediators in the development of major depression. Neuropharmacology 2015; 99: 527–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on request.