Highlights
-
•
We determined C5/C6/C7 myotomes from patients with MRI-confirmed single-root radiculopathy.
-
•
The brachioradialis was mainly innervated by C5.
-
•
C6 muscles were the pronator teres and extensor carpi radialis brevis.
Keywords: Myotome, Cervical spondylotic radiculopathy, Brachioradialis, Pronator teres, Extensor carpi radialis longus, Extensor carpi radialis brevis
Abstract
Objective
There are many myotome charts in the literature, but few studies have presented actual data to support their identification. We aimed to determine C5/C6/C7 myotomes based on clinical and EMG data of patients with cervical spondylotic radiculopathy (CSR) having a single-root lesion confirmed by MRI.
Methods
Medical Research Council (MRC) scores and EMG findings were retrospectively reviewed for patients enrolled from our EMG database.
Results
Enrolled were 25 patients (10 C5, 6 C6, and 9 C7 CSR). In C5 CSR, weakness or denervation potentials in EMG, or both, were observed in the deltoid (Del) and infraspinatus (Isp) muscles for all patients, and in the biceps brachii (BB) and brachioradialis (BR) muscles for 9/10 and 8/9 patients, respectively. In C6 CSR, weakness of the wrist extensor and/or denervation of the extensor carpi radialis longus (ECRL)/extensor carpi radialis brevis (ECRB), and those of the pronator teres (PT) were observed for all patients. Weakness was not observed for any other muscle in C6 CSR. Denervation potentials of ECRL were found in 5/8 and 3/5 patients with C5 and C6 CSR, respectively, whereas those of ECRB were found in 1/5, 6/6, and 2/5 patients with C5, C6 and C7 CSR, respectively. In C7 CSR, weakness/denervation of the triceps brachii (TB) and denervation potentials of the flexor carpi radialis (FCR) were observed for all patients. Denervation potentials in PT and weakness/denervation of the extensor digitorum (ED) were observed in 2/9 and 4/9 patients, respectively.
Conclusion
Suggested dominant myotomes are: C5 for the Del, Isp, BB, and BR, C5/6 for the ECRL, C6 > C7 for the ECRB and PT, and C7 for the TB and FCR.
Significance
The current study identified dominant myotomes that differ from the existing literature.
1. Introduction
Myotome charts play an important role in the clinical and electrodiagnosis of neuromuscular disorders. There are many charts in the literature, all differing from one another. However, the source of the identification of myotomes is not clear in most charts (Brendler, 1968). Few studies have presented actual data supporting their identification (Yoss et al., 1957, Brendler, 1968, Levin et al., 1996, Chiba et al., 2015). Levin et al. (1996) provided strong evidence by investigating electromyography (EMG) findings in patients with surgically confirmed single-root lesions. However, C6-innervated muscles were less well defined in their study. In our previous study, we clarified C8 and T1 innervations of the forearm muscles (Chiba et al., 2015).
In this study, we investigated the C5, C6 and C7 myotomes by reviewing clinical (manual muscle testing; MMT) and EMG findings of patients with cervical spondylotic radiculopathy (CSR) and evidence of a single-root lesion confirmed by magnetic resonance imaging (MRI).
2. Methods
2.1. Inclusion criteria and subjects
Subjects were retrospectively enrolled from our EMG database from 2009 to 2018. Inclusion criteria were: 1) Symptoms and signs that are typical for CSR, such as radicular pain, back pain, segmental weakness, segmental sensory disturbance, reflex changes, and Spurling’s sign, 2) One month or more from the symptom onset (this restriction was set because an acute lesion may not cause denervation in the affected muscles), 3) Needle EMG showing abnormality in at least one muscle, 4) Needle EMG localizing the lesion at the root level, e.g. denervation at the cervical paraspinal muscles (PSM) or involvement of two muscles innervated by different peripheral nerves and different plexus components, 5) Cervical MRI documenting an isolated unilateral C5, C6, or C7 root lesion that may well cause radiculopathy, and the affected root coinciding with the symptoms and signs of the patient such as segmental sensory disturbance, reflex changes, or the site of shoulder or back pain (Mizutamari et al., 2010). Patients with polyradiculopathy, myelopathy evident from clinical or MRI findings, or both, previous neck surgery, or other disorders that might affect clinical signs or EMG results were excluded. Medical Research Council (MRC) scores and needle EMG findings of the upper-limb muscles were retrospectively reviewed.
The study design was approved by the ethics committee of Teikyo University (approval number: 17–147)
2.2. MRC score
The MRC scores during routine neurological examinations of the patient were used as parameters in this study. The evaluated muscles were the deltoid (Del), infraspinatus (Isp), biceps brachii (BB), brachioradialis (BR), pronator teres (PT), triceps brachii (TB), and extensor digitorum (ED). The wrist extensor (WE) was evaluated as a muscle group (extensor carpi radialis longus (ECRL)/extensor carpi radialis brevis (ECRB) and extensor carpi ulnaris). Similarly, the wrist flexor (WF) was evaluated as a muscle group (flexor carpi radialis (FCR) and flexor carpi ulnaris (FCU)). All MRC scores were evaluated by a single skilled examiner (MS; Sonoo, 2018). The MRC score was graded by the standard method except that a modified scale was adopted for muscles that are free from the effect of gravity (Brandsma et al., 1995, Sonoo, 2018).
2.3. Needle EMG
Needle EMG was conducted as a routine diagnostic evaluation for CSR patients in our laboratory as recommended by existing guidelines (American Association of Electrodiagnostic Medicine, 1999). We reviewed the EMG findings for the Del, BB, BR, ECRL, ECRB, PT, TB, FCR, ED, FCU, and PSM. EMG findings were scored as previously described (Chiba et al., 2015). Fibrillation potentials and positive sharp waves (fib/psw) were graded from 0 (none) to 3 (profuse). Voluntary activities were graded based on the qualitative assessment of the recruitment pattern (Nomenclature Committee of American Association of Electromyography and Electrodiagnosis, 1987, Chiba et al., 2015) from 0 (normal) to 3. In the PSM, only fib/psw were evaluated. All EMG examinations were conducted by MS or under his close supervision. Positive fib/psw were considered definite abnormalities, whereas abnormal voluntary activities were considered supplementary findings.
3. Results
3.1. Enrolled subjects
In all, 25 subjects were enrolled. They consisted of 10 patients with C5 CSR (9 men and 1 woman, age 65.6 ± 16.8 years, range 29–86), 6 patients with C6 CSR (4 men and 2 women, age 48.2 ± 5.9 years, range 43–56), and 9 patients with C7 CSR (7 men and 2 women, age 50.7 ± 6.5 years, range 38–59). Duration of illness was 2 to 24 months (6.9 ± 5.2, median 5 months). The symptoms and signs were unilateral in all patients. Weakness was present in 24/25 patients. Pain was present in 21/25 patients, neck pain in 2/10, 4/6 and 1/9 and back pain in 2/10, 0/6 and 9/9 patients with C5, C6, and C7 CSR, respectively. Subjective tingling was noted in 18/21, and objective sensory disturbance was observed in 14/22 patients. Spurling’s sign was positive in 21/25 patients.
3.2. MRI findings
Cervical MRI documented a lesion that likely caused unilateral root compression at a single level of either C4/5, C5/6 or C6/7, which coincided with the symptomatic side and the corresponding level inferred from the symptoms and signs. An evident protrusion into the spinal canal or intervertebral foramen was observed for all cases. The nature of the protrusion was likely either disc or bony spur (hard disc), but the precise differentiation between the two could not be determined as computed tomography (CT) was not performed. The location of the protrusion was lateral in 9 patients and foraminal in 16 patients (Yamano, 1985, Hamasaki et al., 2005). No patients showed lesions at the central or paracentral region.
3.3. MRC scores
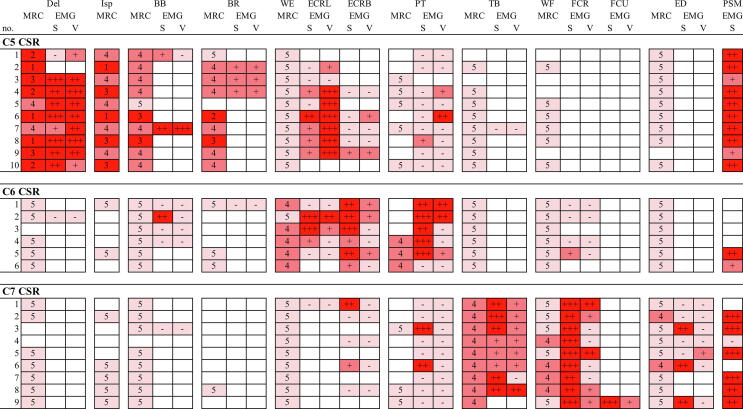
MRC scores of evaluated muscles for individual subjects are shown in Fig. 1. For C5 CSR, weakness was found in all 10 patients in the Del and Isp muscles, and in 9/10 and 8/9 patients in the BB and BR muscles. For C6 CSR, weakness of the WE and PT muscles was observed in 5/6 and 3/3 patients, respectively. For C7 CSR, weakness of the TB, WF and ED muscles was observed in 9/9, 4/9 and 2/9 patients, respectively. These relations were specific for each muscle or segment as follows. Weakness of the Del, Isp, BB and BR muscles was observed only in C5 CSR but in no patients with C6 or C7 CSR. Similarly, weakness of the WE and PT was observed only in C6 CSR, and weakness of the TB, WF and ED was observed only in C7 CSR.
Fig. 1.
MRC scores and EMG grades for evaluated muscles of individual patients. MRC score: 5; 4; ≤3; no data. Grades for spontaneous activities (S): 0, none; 1, few; 2 moderate (observed following about half of insertions) and 3 (observed following almost every insertion); no data. Grades for voluntary activities (V): 0, normal; 1, reduced; 2 discrete and 3 single oscillation; no data. Del, deltoid; Isp, infraspinatus; BB, biceps brachii; BR, brachioradialis; WE, wrist extensors; ECRL, extensor carpi radialis longus; ECRB, extensor carpi radialis brevis; PT, pronator teres; TB, triceps brachii; WF, wrist flexors; FCR, flexor carpi radialis; FCU, flexor carpi ulnaris; ED, extensor digitorum; PSM, paraspinal muscles; S, spontaneous activities; V, voluntary activities; MRC, Medical Research Council; CSR, cervical spondylotic radiculopathy.
3.4. EMG findings
EMG grades of evaluated muscles for individual patients are also shown in Fig. 1. For C5 CSR, the Del, BB, BR and ECRL were abnormal (positive fib/psw) in 8/9, 2/2, 3/3 and 5/8 patients, respectively, whereas the ECRB and PT was abnormal only in 1/5 and 1/9 patients, respectively. For C6 CSR, the ECRB and PT most frequently showed abnormalities (7/7 and 6/7 patients, respectively), followed by the ECRL (3/5 patients). For C7 CSR, the TB and FCR were abnormal in all patients examined, but the ED, ECRB and PT showed denervation in limited cases (3/7, 2/5 and 2/9 patients, respectively). The FCU showed profuse denervation in a patient examined. The PSM showed denervation in 18/19 patients examined.
4. Discussion
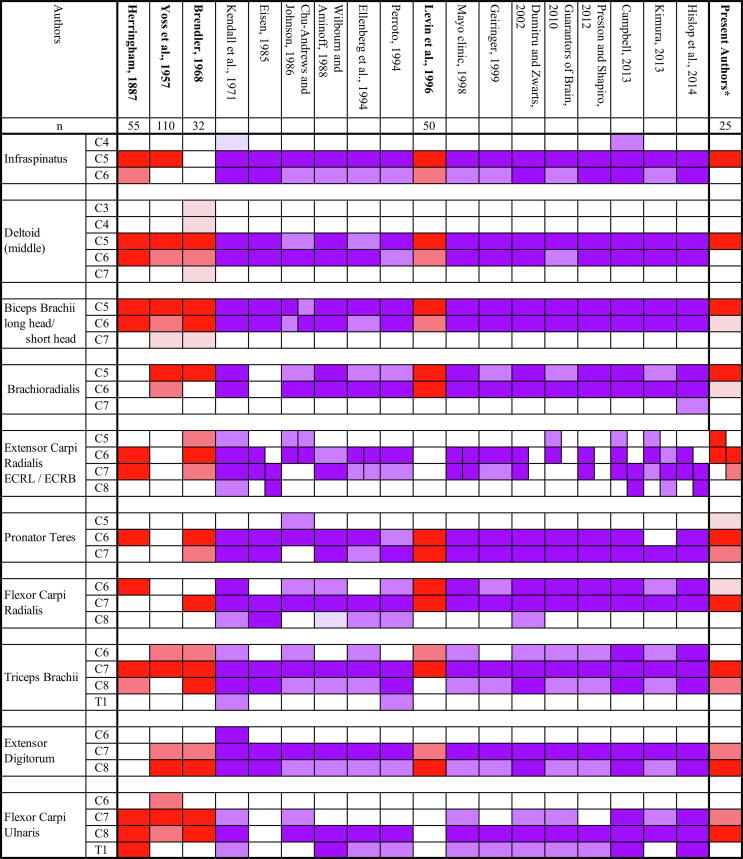
Existing literature on the typical myotome charts of the muscles described in the current study are summarized in Fig. 2. Only a few presented raw data of anatomical, clinical, electromyographical, or intraoperative findings (Herringham, 1887, Yoss et al., 1957, Brendler, 1968, Levin et al., 1996). In contrast to the most recent study by Levin et al. (1996) which referred to surgically confirmed cases, we used MRI to determine the affected root. There may be criticisms in adopting this approach due to the non-specific nature of MRI findings (Nardin et al., 1999), but we believe that the findings from our study is reliable since we strictly selected patients whose symptoms and signs coincided with the single root lesion documented by MRI. Katirji et al. (1988) also correlated EMG findings with imaging studies (metrizamide CT), without intending to revise the myotome chart. MRI with its better resolution should be used more extensively to update the myotome charts.
Fig. 2.
Typical myotome charts from the literature, together with our identification. Authors presenting raw data as the basis for their identification are written in bold letters, together with the number of patients included in each study (n). Note that this is the total number of patients and the number of patients having lesion of a specific root or receiving stimulation of a specific root is smaller. Dominant innervation; lesser contributions (studies with raw data). Dominant innervation; lesser contributions (studies without raw data). *For the present authors, results of Chiba et al. (2015) and this study were combined, and were graded semi-quantitatively as follows according to the percentage of patients showing abnormal results, except when we had too few data. In the latter situation, the grade was sometimes modified by other experiences of the authors or with reference to past studies. 60% or more; between 20 and 60%; 20% or less. ECRL, extensor carpi radialis longus; ECRB, extensor carpi radialis brevis.
This study suggested that the Del, Isp, BB, and BR are mainly innervated by C5, the ECRL by both C5 and C6, the ECRB and PT mainly by C6, and the TB and FCR mainly by C7. C7 contribution to ED and FCU has also been suggested, but it has been established that the main innervation to these two muscles is C8 (Chiba et al., 2015). Therefore, these two muscles probably receive C7 to a lesser extent than C8 innervation. The present results largely coincided with existing charts for several muscles (Del, Isp, BB, TB, and FCR), but were different from most conventional charts for other muscles (Fig. 2). The BR has usually been attributed to C6 or C5/C6. However, this study suggested C5-dominant innervation, which coincided with two old studies based on raw data (Yoss et al., 1957, Brendler, 1968). Levin et al. (1996) stated that C6 may not have definite innervation. However, we identified at least two muscles that were dominantly innervated by C6, which were PT and ECRB. Regarding PT, most authors have suggested double innervation by C6 and C7, sometimes dominant in C7. Only two authors concurred with our findings, C6 greater than C7 innervation (Brendler, 1968, Ellenberg et al., 1994). Our study also suggested dissociation between the ECRL and ECRB. Similar dissociation, ECRL rostral and ECRB caudal, has been reported by a few authors (Eisen, 1985, Campbell, 2013, Kimura, 2013), although they attributed the ECRL to C6 or C6/7 and the ECRB to C7 or C7/8. Our conclusion is more rostral, ECRL by C5/C6 and ECRB by C6 greater than C7.
The previously published myotome charts listed in Fig. 2 that were not based on the raw data were established in several ways. Several authors stated that their chart was based on their own experiences, which were not explicitly presented (Chu-Andrews and Johnson, 1986, Wilbourn and Aminoff, 1988, Ellenberg et al., 1994, Geiringer, 1999). Two authors stated that they referred to other specific charts (Kendall et al., 1971, Dumitru and Zwarts, 2002). The reasons were less clear in others (Eisen, 1985, Perotto, 1994, Members of the Mayo Clinic Department of Neurology, 1998, Guarantors of Brain, 2010, Preston and Shapiro, 2012, Kimura, 2013, Hislop et al., 2014). As Brendler (1968) discussed, the basis of the conventional myotome charts might be animal studies or very old anatomical study of human dissection (Herringham, 1887), which could not accurately trace the nerve fiber course (Sharrard, 1964). Fig. 2 indicates that our identifications are generally in good agreement with studies providing raw data (red boxes), typically in the BR, PT, or ED.
There are several limitations in this study. First, the examiner was not blinded to other information. The MRI findings were sometimes known prior to the examination. More importantly, if the examiner came to believe that the patient had, e.g., a C6 lesion during the MMT and other neurological examinations, then the overall findings may have been biased. However, EMG abnormalities, especially fib/psw, are objective findings and not subject to bias. The EMG results overall agreed well with MRC scores, which supports the reliability of our MRC scores. Second, MRC scores of certain movements cannot separate the actions of individual muscles. MMTs of the BB and BR are such examples. It has been proposed that the BB and BR can be separately evaluated by changing the forearm position for pronation/supination (Hislop et al., 2014), but we feel that it is difficult to definitely separate the actions of the BB and BR. In this regard, normal BB may have masked the BR dysfunction in C6 lesion. Normal EMG in the BR in C6 CSR was confirmed in only one patient. However, we often observed that the BR was extremely atrophic in patients with C5 lesion, which supports the C5-dominant innervation of the BR. Lastly, normal strength of a muscle does not exclude minor innervation from the affected root. EMG is sensitive to detecting subclinical abnormalities (Wilbourn and Aminoff, 1988), but in order to not cause further discomfort, we performed limited EMG in muscles of normal strength. Therefore, we could not evaluate minor contributions from a specific root when EMG was not done. These include C6 contribution to the Del, Isp, BR and TB. In such situations, this study just revealed major innervations to relevant muscles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Takahiro Nakayama (Department of Neurology, Yokohama Rosai Hospital), Dr. Hiromasa Matsuno (Department of Neurology, Jikei University School of Medicine), and Drs. Fumiaki Katada, Yoko Tomoda, Yoshito Arakaki and Toshio Fukutake (Department of Neurology, Kameda Medical Center) for cooperating with this study. This study was partly supported by Grants-in-Aid for Scientific Research (19K07966) from the Ministry of Education, Science, Sports and Culture of Japan, and by AMED, Japan under Grant Number 19ek0109252h0003.
References
- American Association of Electrodiagnostic Medicine Guidelines in electrodiagnostic medicine. Practice parameter for needle electromyographic evaluation of patients with suspected cervical radiculopathy. Muscle Nerve Suppl. 1999;8:S209–S221. [PubMed] [Google Scholar]
- Brandsma J.W., Schreuders T.A.R., Birke J.A., Piefer A., Oostendorp R. Manual muscle strength testing: intraobserver and interobserver reliabilities for the intrinsic muscles of the hand. J. Hand Therapy. 1995;8(3):185–190. doi: 10.1016/S0894-1130(12)80014-7. [DOI] [PubMed] [Google Scholar]
- Brendler S.J. The human cervical myotomes: functional anatomy studied at operation. J. Neurosurg. 1968;28:105–111. doi: 10.3171/jns.1968.28.2.0105. [DOI] [PubMed] [Google Scholar]
- Campbell W.W. 7th ed. Wolters Kluwer; Philadelphia: 2013. Dejong's the Neurologic Examination. [Google Scholar]
- Chiba T., Konoeda F., Higashihara M., Kamiya H., Oishi C., Hatanaka Y., Sonoo M. C8 and T1 innervation of forearm muscles. Clin. Neurophysiol. 2015;126(4):837–842. doi: 10.1016/j.clinph.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Chu-Andrews J., Johnson R.J. J. B. Lippincott Company; Philadelphia: 1986. Electrodiagnosis: An Anatomical And Clinical Approach. [Google Scholar]
- Dumitru D., Zwarts M.J. Radiculopathies. In: Dumitru D., Amato A.A., Zwarts M.J., editors. Electrodiagnostic Medicine. second ed. Hanley & Belfus Inc; Philadelphia: 2002. pp. 713–776. [Google Scholar]
- Eisen A. Electrodiagnosis of radiculopathies. Neurol. Clin. 1985;3(3):495–510. doi: 10.1016/S0733-8619(18)31018-1. [DOI] [PubMed] [Google Scholar]
- Ellenberg M.R., Honet J.C., Treanor W.J. Cervical radiculopathy. Arch. Phys. Med. Rehabil. 1994;75(3):342–352. doi: 10.1016/0003-9993(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Geiringer S.R. second ed. Hanley & Belfus Inc; Philadelphia: 1999. Anatomic Localization For Needle Electromyography. [Google Scholar]
- Guarantors of Brain . 5th ed. Saunders Elsevier; Edinburgh: 2010. Aids to the Examination of the Peripheral Nervous System. [Google Scholar]
- Hamasaki T., Baba I., Tanaka S., Sumida T., Manabe H., Tanaka N., Ochi M. Clinical characterizations and radiologic findings of pure foraminal-type cervical disc herniation: CT discography as a useful adjuvant in its precise diagnosis. Spine. 2005;30(20):E591–E596. doi: 10.1097/01.brs.0000179310.39568.29. [DOI] [PubMed] [Google Scholar]
- Herringham W.P. The minute anatomy of the brachial plexus. Proc. R Soc. 1887;41:423–441. [Google Scholar]
- Hislop H.J., Avers D., Brown M. 9th ed. W. B. Saunders; Philadelphia: 2014. Daniels and Worthingham's Muscle Testing: Techniques of Manual Examination and Performance Testing. [Google Scholar]
- Katirji M.B., Agrawal R., Kantra T.A. The human cervical myotomes: an anatomical correlation between electromyography and CT/myelography. Muscle Nerve. 1988;11:1070–1073. doi: 10.1002/mus.880111010. [DOI] [PubMed] [Google Scholar]
- Kendall H.O., Kendall F.P., Wadsworth G.E. second ed. The Williams and Wilkins Company; Baltimore: 1971. Muscles: Testing and Function. [Google Scholar]
- Kimura . 4th ed. Oxford University Press; New York: 2013. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. [Google Scholar]
- Levin K.H., Maggiano H.J., Wilbourn A.J. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46:1022–1025. doi: 10.1212/wnl.46.4.1022. [DOI] [PubMed] [Google Scholar]
- Members of the Mayo Clinic Department of Neurology . Mosby; St. Louis: 1998. Mayo Clinic Examinations in Neurology. [Google Scholar]
- Mizutamari Masaya, Sei Akira, Tokiyoshi Akinari, Fujimoto Toru, Taniwaki Takuya, Togami Wakana, Mizuta Hiroshi. Corresponding scapular pain with the nerve root involved in cervical radiculopathy. J. Orthop. Surg. (Hong Kong) 2010;18(3):356–360. doi: 10.1177/230949901001800320. [DOI] [PubMed] [Google Scholar]
- Nardin R.A., Patel M.R., Gudas T.F., Rutkove S.B., Raynor E.M. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22(2):151–155. doi: 10.1002/(sici)1097-4598(199902)22:2<151::aid-mus2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Nomenclature Committee of American Association of Electromyography and Electrodiagnosis AAEE glossary of terms in clinical electromyography. Muscle Nerve. 1987;10:G1–G60. [PubMed] [Google Scholar]
- Perotto A.O. third ed. Charles C. Thomas; Springfield: 1994. Anatomical Guide For The Electromyographer. The Limbs And Trunk. (translated by Kayamori R) [Google Scholar]
- Preston D.C., Shapiro B.E. third ed. Elsevier Saunders; London: 2012. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. [Google Scholar]
- Sharrard W.J.W. The segmental innervation of the lower limb muscles in man. Ann. R Coll. Surg. 1964;35:106–122. [PMC free article] [PubMed] [Google Scholar]
- Sonoo M. Chugai Igakusya; Tokyo: 2018. Guidebook for MMT and needle EMG. [Google Scholar]
- Wilbourn A.J., Aminoff M.J. The electrophysiologic examination in patients with radiculopathies. Muscle Nerve. 1988;11:1099–1114. doi: 10.1002/mus.880111102. [DOI] [PubMed] [Google Scholar]
- Yamano Yoshiki. Soft disc herniation of the cervical spine. Int. Orthop. 1985;9(1):19–27. doi: 10.1007/BF00267033. [DOI] [PubMed] [Google Scholar]
- Yoss R.E., Corbin K.B., MacCarty C.S., Love J.G. Significance of symptoms and signs in localization of involved root in cervical disk protrusion. Neurology. 1957;7:673–683. doi: 10.1212/wnl.7.10.673. [DOI] [PubMed] [Google Scholar]