Summary
TxNIP (Thioredoxin-interacting protein) is considered as a potential drug target for type 2 diabetes. Although TxNIP expression is correlated with hyperglycemia and glucotoxicity in pancreatic β cells, its regulation in liver cells has been less investigated. In the current study, we aim at providing a better understanding of Txnip regulation in hepatocytes in response to physiological stimuli and in the context of hyperglycemia in db/db mice. We focused on regulatory pathways governed by ChREBP (Carbohydrate Responsive Element Binding Protein) and FoxO1 (Forkhead box protein O1), transcription factors that play central roles in mediating the effects of glucose and fasting on gene expression, respectively. Studies using genetically modified mice reveal that hepatic TxNIP is up-regulated by both ChREBP and FoxO1 in liver cells and that its expression strongly correlates with fasting, suggesting a major role for this protein in the physiological adaptation to nutrient restriction.
Subject areas: Molecular Physiology, Molecular Biology, Endocrinology
Graphical abstract
Highlights
-
•
TxNIP is considered as a potential candidate drug target for type 2 diabetes
-
•
We provide better understanding of Txnip regulation and function in liver
-
•
Hepatic Txnip is up-regulated by both ChREBP and FoxO1 transcription factors
-
•
We suggest a role for TxNIP in the physiological adaptation to nutrient restriction
Molecular Physiology ; Molecular Biology ; Endocrinology
Introduction
Thioredoxin-interacting protein (TxNIP/VDUP1/TBP-2) originally discovered as a vitamin D3-inducible gene (Chen and DeLuca, 1994) has gained interest for being involved in glucose homeostasis, carcinogenesis, angiogenesis, or inflammation (Alhawiti et al., 2017; Yoshihara 2020). Structurally designated as part of the α-arrestin family, TxNIP contains two amino-terminal SH3-binding domains, whereas the carboxyl terminus contains two PPxY motifs and three SH3 domains (Patwari et al., 2009). TxNIP binds the anti-oxidant protein, thioredoxin and inhibits its disulfide reductase activity in vitro. In this context, TxNIP has been described as a possible link between cellular redox state and metabolism. Importantly, owing to its diverse array of functions in glucose and lipid metabolism in several cell types, TxNIP has been considered as a novel candidate drug target for type 2 diabetes (Thielen and Shalev, 2018). Interestingly, a study recently identified a novel anti-diabetic small molecule SRI-37330 that inhibits TxNIP expression and signaling in mouse and human islets (Thielen et al., 2020).
TxNIP expression and function have been extensively studied in pancreatic β cells (Shalev, 2014). In this cell type, Txnip is one of the most highly up-regulated genes in response to hyperglycemia (Cha-Molstad et al., 2009). As part of a negative-feedback loop, TxNIP was shown to inhibit glucose uptake and promote caspase-3 cleavage, contributing to glucose-dependent β cell death (Saxena et al., 2010). TxNIP also regulates pro-inflammatory gene expression through inflammasome activation via binding to NLRP3 (NOD-like receptor family pyrin domain containing 3) (Zhou et al., 2010). Altogether, TxNIP has emerged as an important factor in pancreatic β cell biology and tight regulation of TxNIP levels appears necessary for β cell survival. The mechanisms driving TxNIP expression in pancreatic β cells are complex and involve crosstalk between several transcription factors, including the glucose-sensitive transcription factor Carbohydrate Responsive Element Binding Protein (ChREBP) and the Forkhead boxO1 transcription factor (FoxO1). The Txnip promoter contains two carbohydrate response elements (ChoRE) for binding of ChREBP (Minn et al., 2005). FoxO1 was reported to up-regulate Txnip expression in neurons and endothelial cells (Li et al., 2009), whereas it is reported to significantly decrease Txnip expression in pancreatic β cells. Mechanistically, FoxO1 was reported to inhibit Txnip expression by reducing the glucose-induced binding of ChREBP on the Txnip promoter in β cells, suggesting that FoxO1 may antagonize ChREBP for binding to the Txnip promoter in the context of insulin-secreting cells (Kibbe et al., 2013).
The function and the mechanisms regulating Txnip expression are less well described in the liver. Key information, however, was gained from the analysis of Txnip-null mice. Txnip-null mice are hypoglycemic, hypoinsulinemic, and exhibit blunted glucose production following a glucagon challenge, a phenotype consistent with a defect in hepatic glucose metabolism (Chutkow et al., 2008). In vitro analysis confirmed that glucose release from isolated Txnip-null hepatocytes was lower than that from wild-type hepatocytes, supporting an intrinsic defect in hepatocyte glucose production. Although hepatocyte-specific gene deletion of Txnip did not alter glucose clearance compared with controls, Txnip expression in the liver is required for maintaining normal fasting glycemia and glucose production (Chutkow et al., 2008).
In this context, we sought to provide a better understanding of Txnip regulation in hepatocytes in response to physiological stimuli and in the context of diabetes. Owing to their previously reported implication, we focused on pathways governed by ChREBP and FoxO1. Studies using genetically modified mouse models of ChREBP and/or FoxO1 expression reveal that, in contrast to pancreatic β cells, hepatic TxNIP is stimulated by both ChREBP and FoxO1 and is strongly associated with the physiological response to fasting in liver.
Results
TxNIP expression is increased in liver of db/db mice
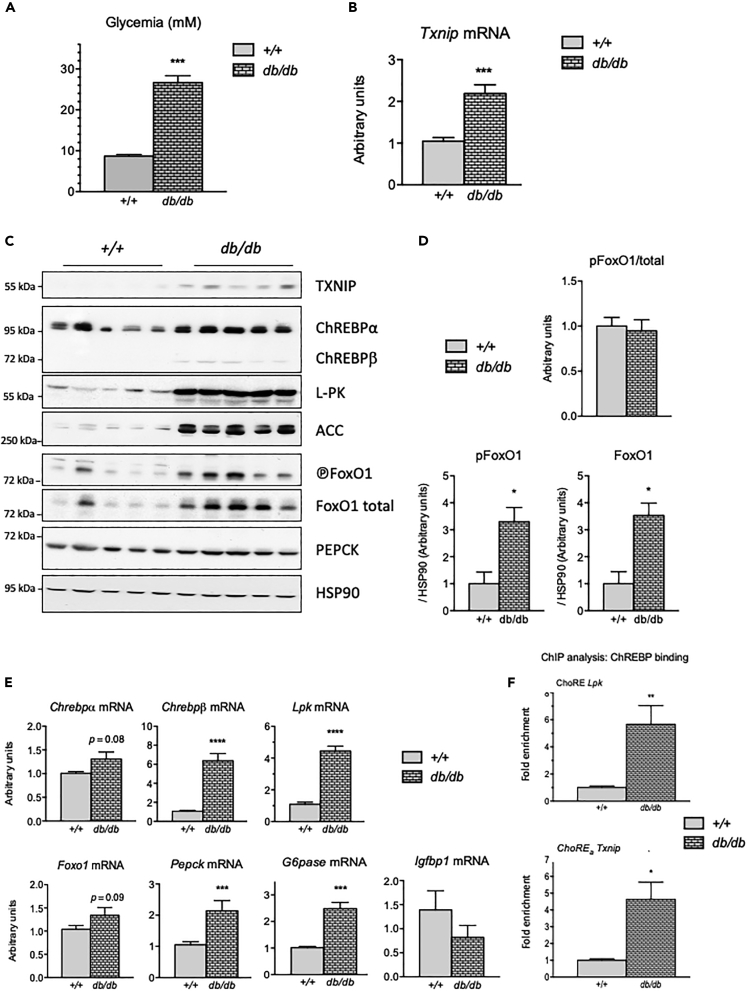
We first measured TxNIP expression in the liver of fed db/db mice (Figure 1). We chose db/db mice for their significant hyperglycemia and confirmed that their blood glucose concentrations were elevated compared with controls under our experimental conditions (Figure 1A). As previously reported (Jo et al., 2013), Txnip mRNA levels and TxNIP protein content were significantly increased in the liver of fed db/db mice compared with +/+ mice (Figures 1B and 1C). As Txnip was previously reported to be a direct target of ChREBP and/or FOXO1 depending on the cell type studied, we examined the expression and activity of these transcription factors. The ChREBP protein contains a low glucose inhibitory domain (LID) and a glucose responsive activation conserved element (GRACE) located in its N terminus (Li et al., 2006). Activation of the GRACE domain by glucose promotes ChREBP transcriptional activity and binding to the ChoRE element of its target genes. Another isoform of Chrebp, Chrebpβ, originating from an alternative first exon promoter, was identified in the adipose tissue and in liver (Herman et al., 2012). This alternative splicing results in a constitutively active and potent ChREBP isoform lacking the inhibitory LID domain (Herman et al., 2012). We observed that ChREBP protein content (both α and β isoforms) was elevated in the liver of db/db mice and paralleled a marked induction of LPK (L-pyruvate kinase) and ACC (acetyl coA carboxylase) protein levels (Figure 1C), two known ChREBP hepatic targets (Abdul-Wahed et al., 2017). A modest increase in Chrebpα mRNA levels was observed in the liver of db/db mice, whereas 6-fold induction in Chrebpβ expression was measured along with a 5-fold increase in Lpk mRNA levels (Figure 1E). Chromatin immunoprecipitation (ChIP) analysis revealed a significant increase in ChREBP binding on the ChoRE of the Lpk promoter in the liver of db/db mice compared with controls (+/+) (Figure 1F). Interestingly, increased binding of ChREBP to the ChoRE of the Txnip promoter was also observed (Figure 1F), suggesting direct control of Txnip expression by ChREBP in the liver of db/db mice.
Figure 1.
TxNIP expression is increased in the liver of db/db mice
Twelve-week-old C57BL/6J (+/+) and db/db male mice were fed ad libitum. Figures are presented as means ± SEM from 8 to 12 individual mice. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test. ∗p < 0.5, ∗∗p < 0.01, ∗∗∗p < 0.001; ∗∗∗∗p < 0.0005 when compared with (+/+) mice.
(A) Blood glucose (mM) recovered at the time of harvest from tail snip.
(B) Relative Txnip gene expression determined by qPCR.
(C) Western blot analysis of protein extracted from whole-liver lysate. HSP90 was used as loading control. Five representative samples are shown.
(D) Quantification of the ratio of phosphorylated FoxO1 corrected to total FoxO1 protein, of the ratio of phosphorylated FoxO1 corrected to HSP90, and of total FoxO1 corrected to HSP90.
(E) Relative Chrebpα, Chrebpβ, Lpk, Foxo1, Pepck, G6pase, and Igfbp1 gene expression determined by qPCR.
(F) ChIP analysis followed by qPCR of whole mouse liver tissue. Immunoprecipitation experiments conducted with ChREBP antibodies. The DNA regions of the Lpk and Txnip promoters were amplified using primers indicated in Table S2.
Interestingly, FoxO1 protein content was also elevated in the liver of db/db mice (Figure 1C). Of note, the level of phospho-FoxO1 was also increased, but in proportion to changes in total FoxO1 level (Figure 1D), indicating that levels of both phospho- (inactive) and nonphospho- (active) FoxO1 are likely increased in db/db liver. In contrast, the level of FoxO1 mRNA was not significantly altered in db/db mice, suggesting that differences in FoxO1 protein level are due to post-transcriptional mechanisms. A significant increase in the expression of several FoxO1 target genes (Pepck and G6pase) is consistent with increased FoxO1 activity in the liver of db/db mice (Figure 1E). Although we did not succeed in performing FoxO1 ChIP assays, our results, nevertheless, indicate that hepatic TxNIP content is increased in the liver of db/db mice and parallels with enhanced ChREBP and FoxO1 activity.
Direct effects of FoxO1 and ChREBP on Txnip expression in mouse hepatocytes
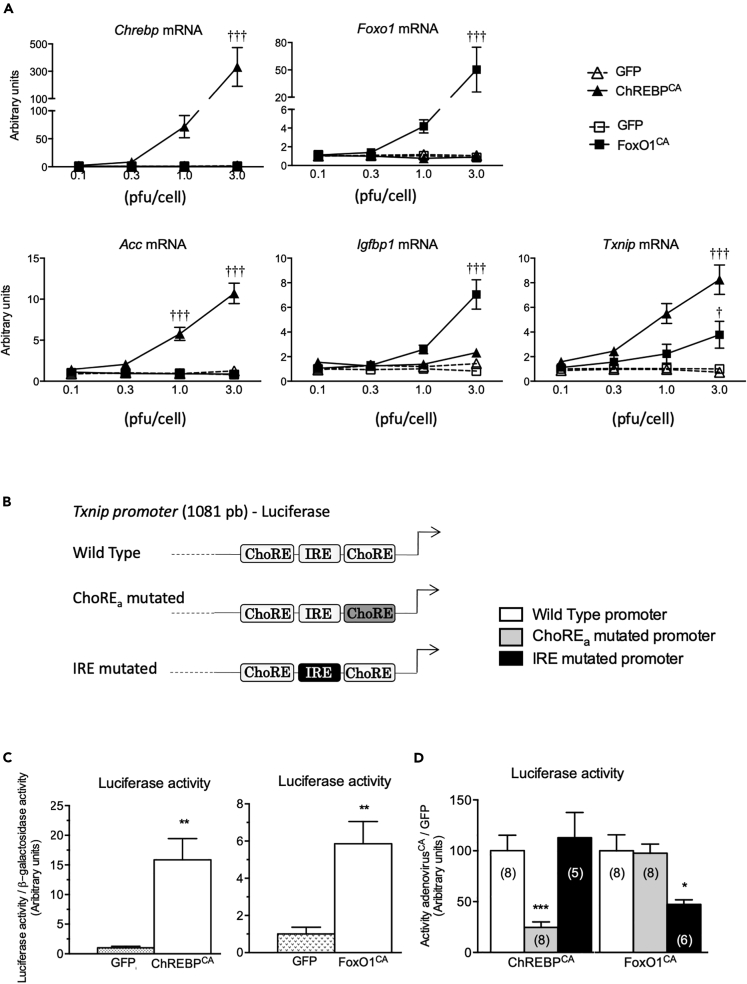
To investigate direct effects of ChREBP and/or FoxO1 on Txnip expression in liver cells, overexpression studies of ChREBP or FoxO1 were conducted using adenovirus strategies in vitro. Primary hepatocytes were transfected with increasing concentrations (from 0.1 to 3.0 plaque-forming unit [PFU]/cell) of an adenovirus expressing a constitutively active isoform of ChREBP (ChREBPCA) lacking the low glucose inhibitory domain (LID) (Li et al., 2006), a constitutively active form of FoxO1 (FoxO1CA) (Zhang et al., 2006), or green fluorescent protein (GFP) as a control. As shown in Figure 2A ChREBP overexpression (at 3 PFU/cell) increased mRNA levels of Acc, a known ChREBP target gene, but not of Igfbp1, which is regulated by FoxO1. Conversely, FoxO1 overexpression led to a significant increase in the expression of its target gene, Igfbp1, but not of Acc (Figure 2A), demonstrating gene-specific effects of ChREBP and FoxO1 in hepatocytes. Interestingly, both ChREBP and FoxO1 were able to induce Txnip expression. An 8-fold increase in response to ChREBPCA (3 PFU/cell) and a 4-fold stimulation in response to FoxO1CA (3 PFU/cell) were observed (Figure 2A), suggesting that ChREBP and FoxO1 are able to stimulate Txnip expression in hepatocytes in a cell-autonomous fashion.
Figure 2.
Effect of ChREBP and FoxO1 overexpression on Txnip expression and promoter activity in mouse hepatocytes
Primary hepatocytes derived from adult male mice were incubated under low glucose concentration (5 mM) with specific adenovirus as indicated (from 0.1 to 3.0 PFU/cell) for 24 h.
(A) Relative Chrebp, Foxo1, Acc, Igfbp1, and Txnip gene expression determined by qPCR. Figures are presented as means ± SEM from 6 to 8 independent cultures. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test †p < 0.05, ††p < 0.01, †††p < 0.001 when compared with GFP conditions.
(B) Schematic representation of the wild and mutated Txnip promoters (1,081 bp; the two ChREBP-binding sites [ChoRE] and the FoxO1-binding site [IRE] are indicated). Primers used for site-directed mutagenesis are indicated in Table S1.
(C) Luciferase activity of the wild-type Txnip promoter in primary hepatocytes in response to either ChREBPCA (3 PFU/cell) or FoxO1CA (3 PFU/cell). Figures are presented as means ± SEM from 6 to 8 independent cultures. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test. ∗∗p < 0.01 when compared with GFP conditions.
(D) Luciferase activity of the wild-type, ChoREa-mutated, or IRE-mutated Txnip promoter in primary hepatocytes in response to either ChREBPCA (3 PFU/cell) or FoxO1CA (3 PFU/cell). Figures are presented as means ± SEM from 5 to 8 independent cultures. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test ∗p < 0.05, ∗∗∗p < 0.001 when compared with wild-type promoter activity.
Next, Txnip promoter activity was measured by performing luciferase reporter gene assays (Figures 2C and 2D), using reporter gene constructs containing the wild-type Txnip promoter sequence or constructs in which ChREBP and/or FoxO1-binding sites are mutated (Figures 2B and Table S1). ChREBPCA stimulated by 15-fold wild-type Txnip promoter activity (Figure 2C), whereas a 6-fold effect was observed in response to FoxO1CA (Figure 2C). The stimulatory effect of ChREBPCA was significantly reduced when one ChREBP-binding site (ChoREa) was mutated but remained unchanged when the FoxO1-binding site (IRE) was mutated (Figure 2D). Similarly, the stimulatory effect of FoxO1CA was partially lost when the IRE was mutated on the Txnip promoter but remained unchanged when the ChoREa was mutated (Figure 2D). Together, these results indicate that Txnip expression is under the dual stimulatory control of ChREBP and FoxO1 operating through distinct cis-acting elements within the Txnip promoter in hepatocytes.
Correlation between TxNIP expression and blood glucose concentrations
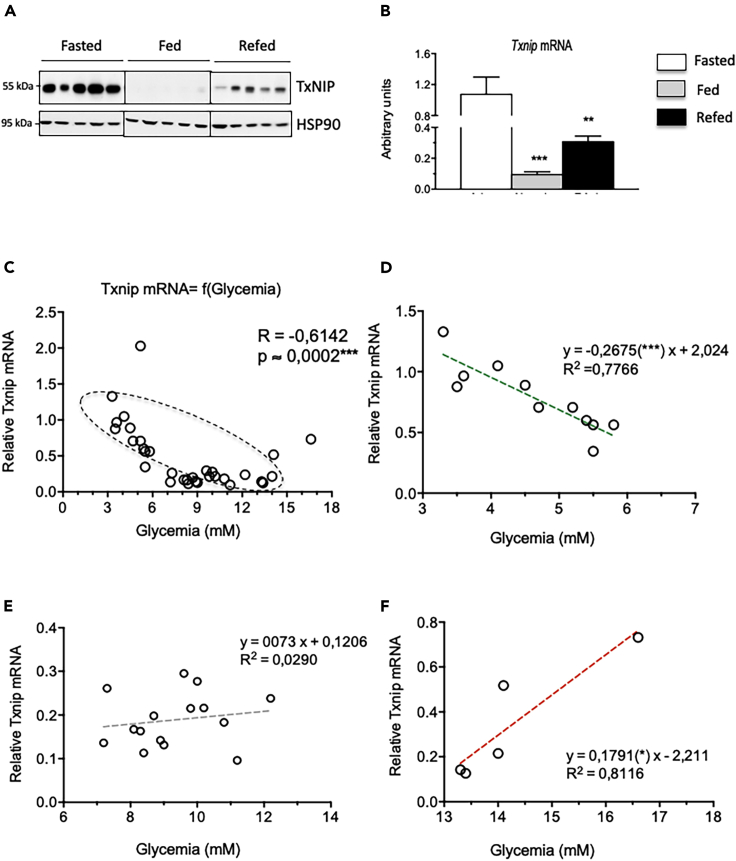
To characterize the physiological regulation of TxNIP in liver, its expression was measured in liver of fasted, freely fed, and refed mice (Figure 3). Interestingly, whereas TxNIP was described as a highly up-regulated gene in response to glucose (Cha-Molstad et al., 2009), TxNIP expression (both mRNA and protein levels) was lower in the liver of refed than in fasted mice (Figures 3A and 3B). To better understand the regulation of hepatic TxNIP expression depending on the nutritional status, we established correlations between the relative amount of Txnip expression as a function of glycemia. We plotted Txnip expression depending on blood glucose concentrations in 32 C57BL/6J mice and calculated the Spearman's correlation coefficient (R) (Figure 3C). Interestingly, we observed positive correlations between Txnip mRNA levels and low glucose concentrations below 6 mM (Figure 3D, n = 11), and also with elevated glucose concentrations above 13 mM (Figure 3F, n = 5). No positive correlation was found when glucose concentrations were in the normal range, between 6 and 13 mM (Figure 3E, n = 15). Taken together, our results suggest that hepatic Txnip expression correlates with glycemia under both low- and high-glucose conditions.
Figure 3.
Correlation between Txnip expression and blood glucose concentrations
(A and B) Twelve-week-old C57BL/6J (+/+) male mice were studied at the fed, fasted, and refed state. (A) Western blot analysis of protein extracted from whole-liver lysate. HSP90 was used as loading control. Five representative samples are shown. (B) Relative Txnip gene expression determined by qPCR. Figure is presented as means ± SEM from 8 to 12 individual mice. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test. ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with the fasted state.
(C–F) Representation of the relative amounts of Txnip mRNA levels as a function of glycemia. (C) Correlation using a set of C57BL/6J male mice (n = 32 points) measured at either the fasted, fed, and refed state. R, Spearman's correlation coefficient, ∗∗∗p < 0.001 (coefficient other than zero). Dashed oval, point cloud approximation. (D–F) Linear regressions for blood glucose concentrations below 6 mM (D, n = 11), between 6 and 13 mM (E, n = 15) and above 13 mM (F, n = 5). R2, coefficient R squared (adequacy of the points to the right); y = ax + b, equation of the regression line with a slope.
Regulation of TxNIP during fasting and refeeding
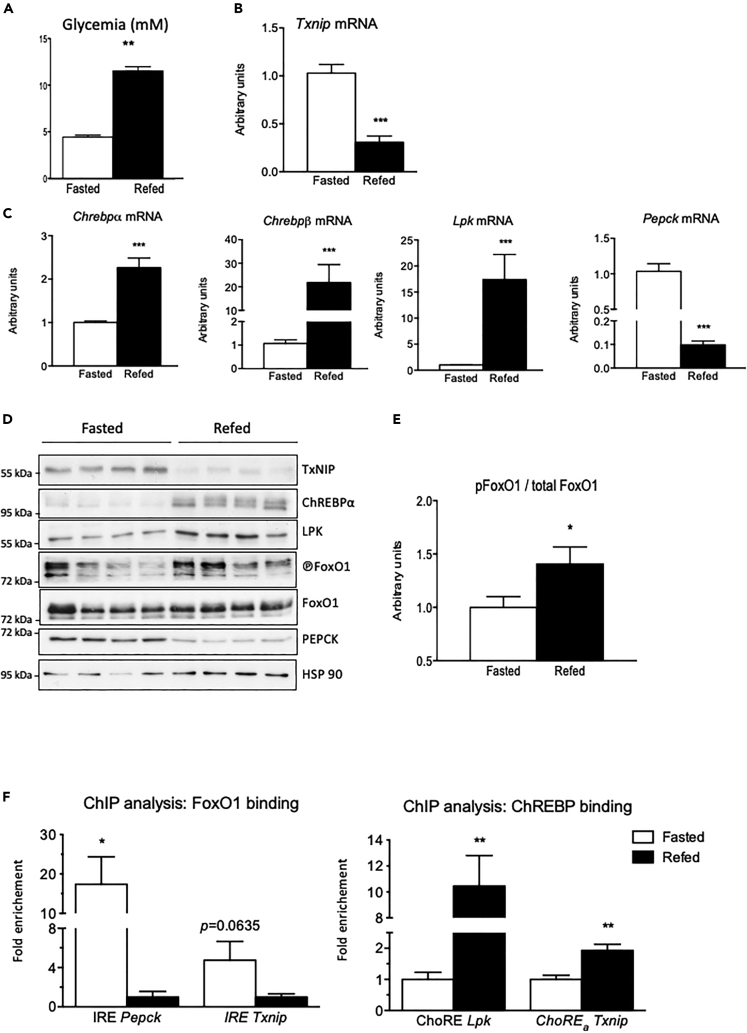
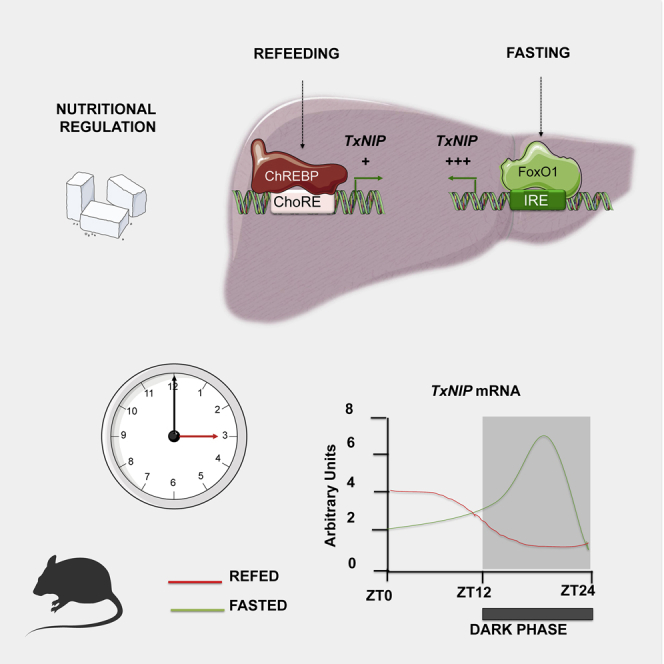
To further study the physiological regulation of TxNIP, we next focused on the fasting/refeeding transition, characterized by marked differences in blood glucose concentrations (Figure 4A) and in the ChREBP and FoxO1 activities (Figures 4C and 4D). As expected, the expression of ChREBPα and of its target genes Chrebpβ and Lpk were significantly induced in the liver of refed mice (Figures 4C and 4D). The refed state was also characterized by an increase in the ratio of phosphorylated FoxO1 to total FoxO1 (Figure 4E) and by decreased PEPCK expression (Figures 4C and 4D), consistent with a reduction in FoxO1 activity. We confirmed that TxNIP expression (both mRNA and protein levels) was lower in the liver of refed than of fasted mice (Figures 4B and 4D). To determine whether ChREBP or FoxO1 was recruited onto the Txnip promoter under these nutritional conditions, ChIP analysis was performed in the liver of fasted and refed mice (Figure 4F). Under fasted conditions, a significant enrichment of FoxO1 binding on the IRE of the Pepck promoter was observed. Although it did not reach significance, FoxO1 binding on the IRE of the Txnip promoter was also enriched compared with refed conditions (Figure 4F). As expected, under refed conditions, a marked increase in ChREBP binding was observed on the Lpk ChoRE. Interestingly, a significant enrichment in ChREBP binding was also observed on the ChoRE of the Txnip promoter (Figure 4F). Altogether, these results support the concept that FoxO1 and ChREBP contribute to the regulation of Txnip expression in the liver under fasting (FoxO1 active) and refed (ChREBP active) conditions.
Figure 4.
Differential regulation of TxNIP during fasting and refeeding. Adult C57BL/6J male mice were studied under fasting (24 h fast) or refed (a 18-h refeeding period) at ZT12
Data are expressed as means ± SEM, n = 6 to 8 individual mice/group. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with fasted conditions.
(A) Blood glucose (mM) recovered at the time of harvest from tail snip.
(B) Relative Txnip gene expression determined by qPCR.
(C) Relative Chrebpα, Chrebpβ, Lpk, and Pepck, gene expression determined by qPCR.
(D) Western blot analysis of protein extracted from whole-liver lysate. HSP90 was used as loading control. Four representative samples are shown.
(E) Quantification of the ratio of phosphorylated FoxO1 corrected to total FoxO1 protein is provided.
(F) ChIP analysis followed by qPCR of whole mouse liver tissue. Immunoprecipitation experiments conducted with FoxO1 and ChREBP antibodies. The DNA regions of the Pecpk, Lpk, and Txnip promoters were amplified using primers indicated in Table S1.
Data are expressed as means ± SEM, n = 6 to 8 individual mice/group. Significance is based on two-way ANOVA followed by a Bonferroni post hoc test ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with fasted conditions.
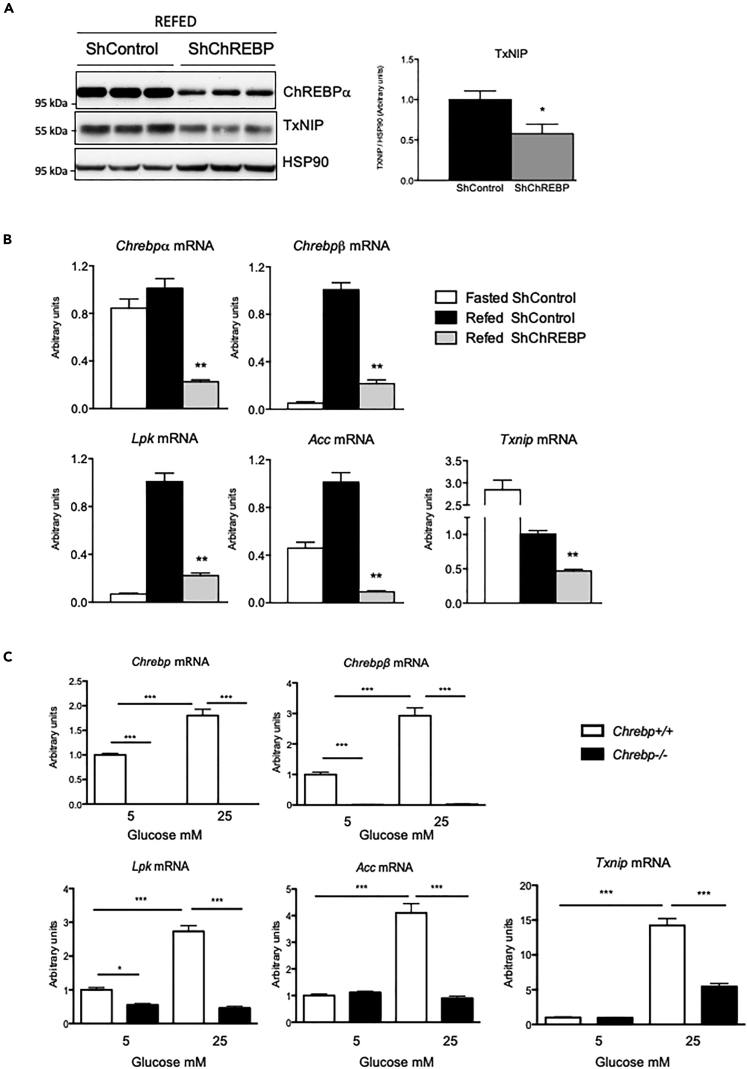
Glucose-mediated induction of Txnip requires ChREBP
To address the direct contribution of ChREBP to Txnip expression in the refed state, ChREBP was silenced in the liver of mice before fast-refeeding using a validated short hairpin RNA (shRNA) strategy (Dentin et al., 2004). We confirmed that ChREBP protein (Figure 5A) and mRNA (Figure 5B) levels were significantly decreased in the liver of refed ShChREBP mice compared with refed ShControl. Chrebp silencing led to 80% to 90% reduction in Lpk and Acc mRNA levels (Figure 5B). A 50% decrease in Txnip mRNA (Figure 5B) and protein (Figure 5A) levels was also was observed in the liver of refed ShChREBP mice, suggesting that ChREBP does contribute, at least partially, to Txnip expression under refeeding conditions.
Figure 5.
The glucose-dependent induction of Txnip requires ChREBP
C57Bl/6J male mice were injected intravenously with a single dose of 5 × 109 PFU of shCTRL or shChREBP adenovirus at Day1. Seven days later, mice were challenged to nutritional manipulations as indicated (fasted or refed).
(A) Western blot analysis of protein extracted from whole-liver lysate. HSP90 was used as loading control. Three representative samples are shown. Quantification of the ratio of TxNIP protein content corrected to HSP90 is shown. ∗p < 0.05 when compared with ShControl conditions.
(B) Relative gene expression of Chrebpα, Chrebpβ, Lpk, Acc, and Txnip gene was determined by qPCR. Figures are presented as means ± SEM from 6 individual mice. Significance is based on two-way ANOVA followed by Bonferroni post test, ∗p < 0.05, ∗∗p < 0.01, when compared with refed ShControl conditions.
(C) Primary hepatocytes from Chrebp−/- and Chrebp+/+ littermates were stimulated 1 day after platting for 24 h with cell culture medium containing 5 or 25 mM glucose. qPCR analysis of Chrebp, Chrebpβ, Lpk, Acc, and Txnip. Figures are presented as means ± SEM from 3 independent cultures done in triplicates. Significance is based on two-way ANOVA followed by Bonferroni post test, ∗p < 0.05, ∗∗∗p < 0.0 01.
Because ChREBP silencing was only partial using the shRNA strategy (Figure 5A), we also examined Txnip regulation in hepatocytes lacking both ChREBP isoforms (Chrebp−/-) (Iroz et al., 2017) (Figure 5C). We observed that Txnip expression was robustly increased in response to 25 mM glucose in wild-type hepatocytes (Chrepb+/+) and was comparable to the increase in Chrebpβ, Lpk, and Acc expression (Figure 5C). The stimulatory effect of glucose was totally prevented for Lpk and Acc in Chrebp−/- hepatocytes, and the induction of Txnip also was significantly reduced, although not completely disrupted, in response to 25 mM in these hepatocytes (Figure 5C). Together, these results confirm that ChREBP-independent mechanisms contribute to the glucose-mediated induction of Txnip in mouse hepatocytes.
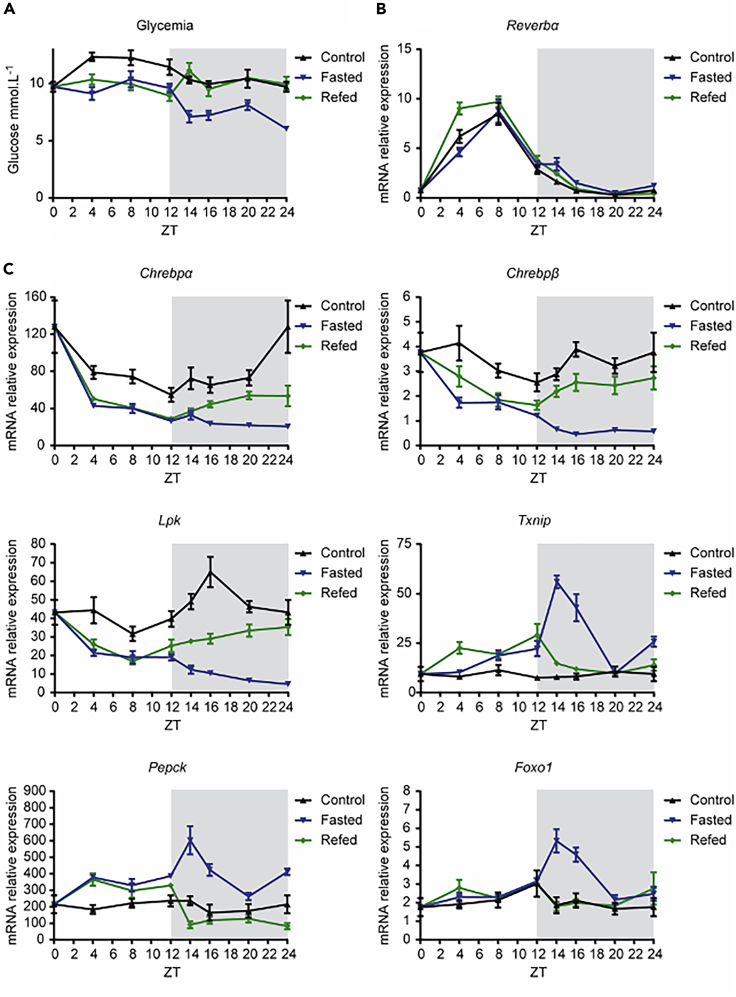
Daily rhythms of Txnip correlate with the one of FoxO1
To gain further insight into the physiological regulation of Txnip in liver, we measured its pattern of expression in the liver during daily rhythms under control, fasted, or refed conditions (Figure 6). Variations in blood glucose concentrations (Figure 6A) and in hepatic Reverbα (a well-known circadian-regulated gene) (Figure 6B) were measured to validate the experimental conditions used. Hepatic mRNA levels of Chrebpα, Chrebpβ, Lpk, Txnip, Pepck, and Foxo1 were measured at ZT0, ZT4, ZT8, ZT12, ZT16, ZT20, and ZT24 by qPCR (Figure 6C). The expression of Chrebpα, Chrebpβ, and Lpk increased in the dark phase in fed mice, when feeding largely occurs, but decreased in the dark under fasting conditions, indicating that diurnal changes in the expression of these genes coincide with changes in food intake. In contrast, the expression of Txnip underwent a different pattern with a major peak of induction at ZT14 in the dark phase under fasting conditions. Interestingly, the profile of Txnip paralleled the one of Foxo1 and of its target gene Pepck (Figure 6C), suggesting that food intake is necessary to prevent the induction and activation of FoxO1, Pepck, and Txnip expression during the transition from light to dark phases. These data support the concept that Txnip expression correlates with the expression of Foxo1 and Pepck in the transition to the fasting state in a physiological context.
Figure 6.
Daily rhythms of hepatic gene expression under control, fasted, and refed conditions
Adult C57BL/6J male mice were studied under control conditions (fed at libitum), fasted, or refed at ZT12. Mice were killed by cervical dislocation at several time points in a pairwise manner: ZT0, ZT4, ZT8, ZT12 ZT14, ZT16, ZT20, and ZT24 as indicated. Figures are presented as means ± SEM from 10 individual mice per time point.
(A) Blood glucose (mmol/L) recovered at time of harvest from tail snip.
(B) qPCR analysis of Reverbα.
(C) qPCR analysis of Chrebpα, Chrebpβ, Lpk, Txnip, Pepck, and Foxo1.
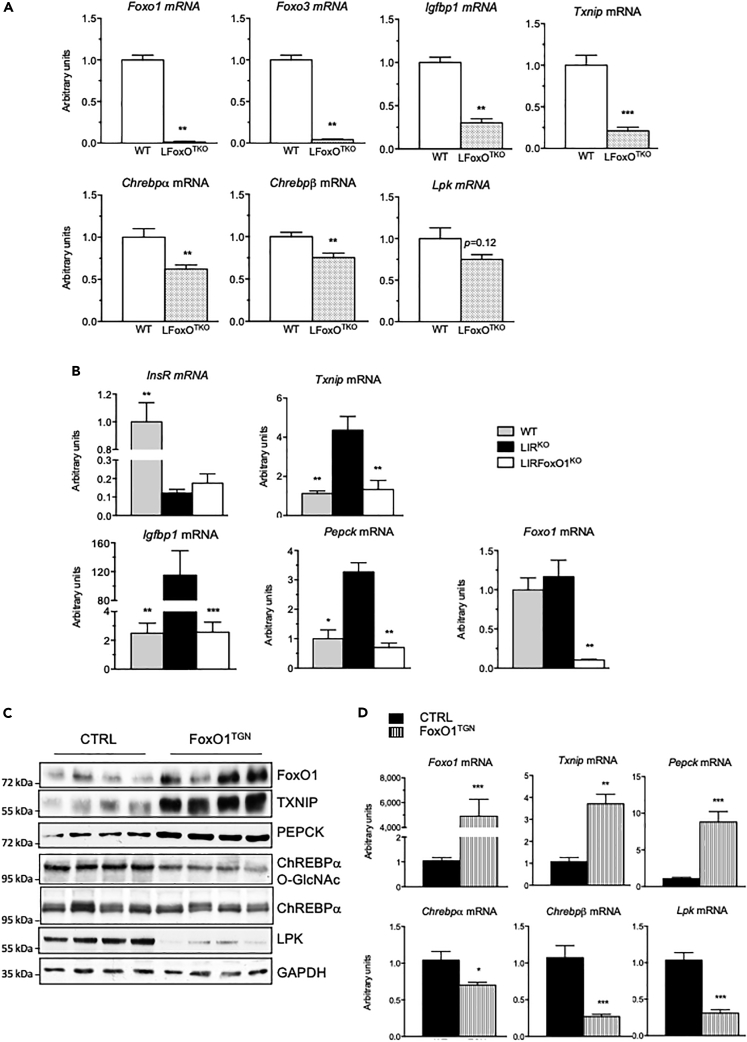
FoxO1 stimulates the expression of TxNIP in liver
To further characterize the contribution of FoxO1 to the regulation of Txnip in liver, we performed a series of experiments using mice with genetic modifications of the FoxO proteins in liver (Figure 7). First, Txnip expression was measured in the fasting state in the liver of mice with a liver-specific deficiency in the three FoxO proteins (FoxO1, FoxO3, and FoxO4) (LFoxOTKO mice) (Zhang et al., 2016). Txnip expression was significantly decreased along with the expression of Igfbp1, a well-known target of FoxO proteins, whereas the expression of Chrebpα, Chrebpβ, and Lpk was only modestly affected (Figure 7A). To address the specific role of FoxO1 on Txnip expression when insulin signaling is decreased, we measured the expression of Txnip in the liver of refed liver-specific insulin receptor knockout (LIRKO) and IR/FoxO1 double knockout (LIRFoxO1KO) mice (O-Sullivan et al., 2015) (Figure 7B). In the liver of LIRKO mice, where FoxO1 activity is enhanced (O-Sullivan et al., 2015), the expressions of Txnip as well as other FoxO1 targets (Igfbp1 and Pepck) were significantly increased and reversed when FoxO1, the predominant FoxO protein expressed in the liver (Zhang et al., 2016), was knocked out in this model (i.e., LIRFoxO1KO mice) (Figure 7B). These results confirm that endogenous FoxO1 promotes Txnip expression in the liver when insulin signaling is disrupted.
Figure 7.
FoxO1 stimulates the expression of TXNIP in liver
(A and B) (A) Wild-type (WT) and LFoxOTKO mice were fasted for 24 h qPCR analysis of Foxo1, Foxo3, Igfbp1, Txnip, Chrebpα, Chrebpβ, and Lpk. Data are expressed as means ± SEM, n = 6 to 8 individual mice/group. Significance is based on two-way ANOVA followed by Bonferroni post hoc test, ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with WT mice. (B) Wild-type (WT), LIRKO, and LIRFoxO1KO mice were studied at the fed state. qPCR analysis of InsR, Txnip, Igfbp1, Pecpk, and Foxo1. Data are expressed as means ± SEM, n = 6 to 8 individual mice/group. Significance is based on two-way ANOVA followed by Bonferroni post hoc test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with LIRKO mice.
(C and D) Control (CTRL) and transgenic mice overexpressing a constitutive active form of FoxO1 (FoxO1TGN) were studied at the fed state. (C) Western blot analysis of protein extracted from whole liver lysate. GAPDH was used as loading control. Four representative samples are shown. (D) qPCR analysis of Foxo1, Txnip, Pecpk Chrebpα, Chrebpβ, and Lpk. Data are expressed as means ± SEM, n = 6 individual mice/group. Significance is based on two-way ANOVA followed by Bonferroni post hoc test, Significance is based on two-way ANOVA followed by a Bonferroni post hoc test ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We also analyzed the expression of Txnip in the liver of transgenic mice that selectively express a constitutively active form of FoxO1 in the liver (FoxO1TGN) (Zhang et al., 2006). Txnip expression was markedly increased in the liver of FoxO1TGN mice and paralleled with changes in the expression of PEPCK at both protein and RNA levels (Figures 7C and 7D). In contrast, ChREBP activity was found to be reduced in the liver of these mice. Indeed, we observed that the expression of Chrebpβ and Lpk was decreased in the liver of FoxO1TGN mice. We hypothesized that this decrease could be due to reduced ChREBP O-GlcNAcylation (ChREBPα OGlcNAc) (Figure 7C). O-GlcNAcylation is a post-translational modification dependent on glucose metabolism that stimulates ChREBP transcriptional activity in the liver (Guinez et al., 2011). Interestingly, it was previously reported that ChREBPα OGlcNAcylation is reduced in response to FoxO1 in the liver (Ido-Kitamura et al., 2012), presumably due to the suppression of glucokinase expression and glucose utilization by FoxO1 (Zhang et al., 2006). Together these results indicate that FoxO1 is sufficient to promote Txnip expression in the liver, including under conditions where ChREBP expression and activity are reduced.
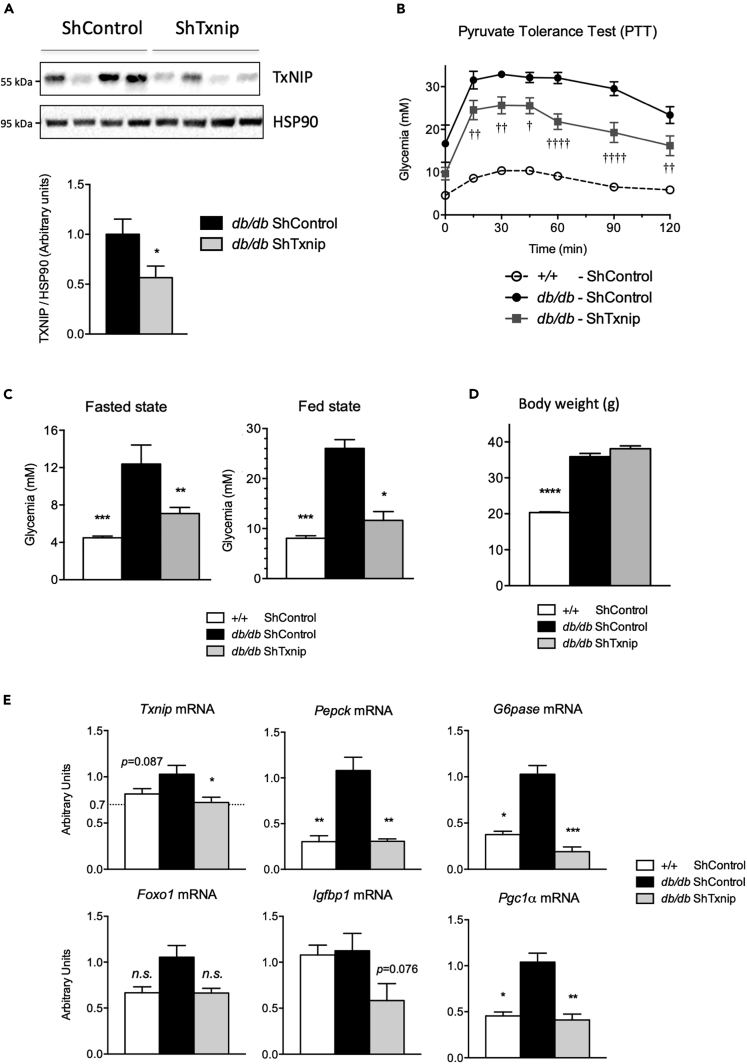
Txnip silencing in liver reduces hyperglycemia in db/db mice
We next evaluated the contribution of TxNIP to the hyperglycemic phenotype of db/db mice using a TxNIP shRNA strategy (Figure 8). TxNIP protein content was decreased by 50% in the liver of db/db mice treated with the ShTxnip adenovirus (Figure 8A). This decrease was associated with a significant improvement in the pyruvate tolerance test (PTT), which reflects production of glucose from exogenous pyruvate (Figure 8B). Blood glucose concentrations in db/db ShTxnip mice were significantly reduced compared with db/db ShControl under both fasted and fed states (Figure 8C). No change in body weight was observed upon TxNIP silencing in db/db mice (Figure 8D), indicating that effects of shTxnip on glucose levels and pyruvate tolerance were not due to modification in body weight. Txnip knockdown was associated with a significant decrease in Pepck, G6pase, and Pgc1α expression (Figure 8E).
Figure 8.
Txnip silencing in liver reduces hyperglycemia in db/db mice
Adult C57BL/6J (+/+) and db/db male mice were injected intravenously with a single dose of 5 × 109 PFU of ShControl or ShTxnip adenovirus (GeneCust) at Day1. Seven days later, mice were challenged to a pyruvate tolerance test (PTT) or to nutritional manipulations as indicated (a 24-h fast [Fasted] or analyzed at the fed state [Fed]). Data are expressed as means ± SEM, n = 6 to 8 individual mice/group. Significance is based on two-way ANOVA followed by Bonferroni post hoc test, (A) Western blot analysis of protein extracted from whole-liver lysate. HSP90 was used as loading control. Four representative samples are shown. Quantification of the ratio of TxNIP protein to HSP90 is shown. ∗p<0.05 when compared to db/db ShControl. (B) Pyruvate tolerance test (PTT) †p < 0.05, ††p < 0.01, †††p < 0.001, ††††p < 0.0005 when compared with db/db ShControl.. (C) Blood glucose concentrations under fasted and fed state. (D) Body weight at the time of the sacrifice. (E) qPCR analysis of Txnip, Pecpk G6Pase, Foxo1, and PGC1α. ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 when compared to db/db ShControl.
To determine whether TxNIP silencing also could affect glucose homeostasis in a physiological context, TxNIP was knocked down in the liver of C57Bl/6J mice (Figure S1A). We observed that the PTT was improved in ShTxNIP-treated mice compared with mice injected with a ShControl (Figure S1B). Blood glucose concentrations also were significantly decreased at the time of sacrifice (Figure S1C). qPCR analysis confirmed that Txnip mRNA levels were significantly decreased in the liver of ShTxNIP mice in parallel with reduced expression of gluconeogenic genes, including Pepck and G6pase and the co-activator Pgc1α (Figure S1D). Altogether, these results support a role for TxNIP in regulating hepatic glucose production and gluconeogenic gene expression under hyperglycemic and physiological conditions.
Discussion
In the current study, we sought to provide a better understanding of the mechanisms involved in the regulation of TxNIP in the liver under physiological and hyperglycemic conditions. In recent years, TxNIP has emerged as a key regulator of glucose and lipid metabolism and has been shown to influence metabolic regulation via multiple actions including insulin release from pancreatic β cells, glucose production by the liver, and glucose uptake in peripheral tissues including muscle and adipose tissue (Alhawiti et al., 2017). In addition, genetic and epigenetic variations in TxNIP are associated with chronic metabolic disorders including diabetes and hypertension (van Greevenbroek et al., 2007; Ferreira et al., 2012). Interestingly, anti-diabetic agents like insulin, metformin, glucagon-like peptide-1 (GLP-1) agonists, and resveratrol have been reported to inhibit TxNIP expression, which may contribute to their therapeutic efficacy in the treatment of diabetes (Chai et al., 2012; Bedarida et al., 2016; Nivet-Antoine et al., 2010; Shao et al., 2010). Interestingly, after a screen of more than 300,000 molecules, Thielen and co-workers recently identified a compound that downregulates TxNIP in mouse and human pancreatic islets. When given orally to mice, this small molecule (SRI-37330) lowered serum levels of glucagon, prevented fatty liver, and inhibited glucose production by the liver (Thielen et al., 2020). In this context, TxNIP continues to generate significant interest as a potential therapeutic target for the management of diabetes and other metabolic disorders (Yoshihara, 2020). We report in the present study that TxNIP is dually regulated in the liver by the transcription factors ChREBP and FoxO1 under specific nutritional conditions.
TxNIP can be induced by glucose in a ChREBP-dependent fashion in a variety of cell types and the TxNIP promoter contains two ChREBP response elements (Cha-Molstad et al., 2009). Txnip is one of the most highly up-regulated genes in response to glucose in pancreatic β cells (Cha-Molstad et al., 2009) where its induction by glucose was previously reported to be ChREBP dependent (Minn et al., 2005). Our study shows that Txnip expression also is significantly up-regulated by high glucose concentrations in primary hepatocytes and that the glucose-sensitive transcription factor ChREBP contributes to glucose-mediated induction of Txnip in hepatocytes. Although the results demonstrate that ChREBP does contribute to the induction of TxNIP in refed conditions, our data also suggest that other molecular mechanisms may be involved. Indeed, a significant (although reduced) glucose effect was maintained on Txnip in Chrebp−/− hepatocytes challenged with high glucose concentrations. One possible candidate for mediating this effect could be MondoA, the paralog of ChREBP (Richards et al., 2017), which was shown to be important for Txnip expression in skeletal muscles (Ahn et al., 2016). Interestingly, we also reported that glucose still induces Txnip expression in islets from Chrebp−/− mice and that this effect does in fact require MondoA in human β cells (Richards et al., 2018). While MondoA functions were predominantly reported in skeletal muscle and proliferative cells (Wilde and Ayer, 2015), a role for MondoA in hepatocytes should not be excluded. In fact, the contribution of MondoA to liver metabolism was previously evidenced by its direct control of the glycogen-targeting protein (PTG) in response to glucose (Petrie et al., 2013). The potential contribution of MondoA to the Chrebp−/−phenotype remains to be determined.
Surprisingly, unlike other ChREBP targets, our study reveals that liver Txnip expression is higher under fasting than under refed conditions. Interestingly, correlation studies established that Txnip mRNA levels correlate with both low glucose concentrations (below 6 mM) and elevated glucose concentrations (above 13 mM). In the liver of hyperglycemic db/db mice, we confirmed that Txnip expression is elevated and that ChREBP binding is enriched on the ChoRE of the Txnip promoter. In this mouse model, we cannot exclude that elevated Txnip expression also could result from enhanced glucocorticoid signaling or other factors. Indeed, several studies have suggested that chronic corticosterone treatment may upregulate Txnip by targeting the glucocorticoid receptor (Bharti et al., 2018; Reich et al., 2012). Glucocorticoids have been shown to regulate TxNIP in neuronal cells (Bharti et al., 2018) and often function cooperatively to regulate the expression of FoxO1 target genes in the liver, including Pepck, G6pase, and IGFBP-1 (Goswami et al., 1994). Interestingly, our study suggests that FoxO1 activity may be enhanced in the liver of db/db mice, where FoxO1 is known to play a significant role in promoting hyperglycemia (Zhang et al., 2012). Although we were not able to address the direct contribution of FoxO1 to the expression of Txnip in the liver of db/db mice due to unresolved technical problems, we were able to demonstrate that FoxO1 plays a key role in promoting Txnip expression in the liver, based on complementary approaches in vitro and in vivo.
To date, the role of FoxO1 on Txnip expression has been rather complex. FoxO1 was reported to stimulate Txnip expression in neurons (Al-Mubarak et al., 2009) and glucose-treated endothelial cells (Li et al., 2009), and to down-regulate its expression in liver tumor cells (de Candia et al., 2008). A study also reported that the expression of FoxO1 and TxNIP is inversely correlated in alcohol-induced hepatitis. However, this study did not examine the direct effect of FoxO1 on TxNIP expression in liver cells in this pathophysiological context (Heo et al., 2019). Here, we report that FoxO proteins contribute to the regulation of Txnip in the liver under a variety of physiological conditions. Using liver-specific knockout mice, we demonstrate that Txnip expression is strongly induced during the fasting state in a FoxO-dependent manner, and that FoxO1, the major FoxO protein expressed in the liver (Zhang et al., 2016), plays a crucial role in promoting Txnip expression when insulin signaling is disrupted in the liver of LIR-KO mice. Studies in transgenic mice expressing a constitutively active form of FoxO1 in the liver also demonstrated that FoxO1 promotes the expression of Txnip in the liver, and studies with adenoviral vectors demonstrated that FoxO1 stimulates hepatic Txnip expression in primary hepatocytes in a cell-autonomous fashion. Reporter gene studies and ChIP analysis showed that this effect of FoxO1 is mediated through the cis-acting FoxO1 target site located in the Txnip promoter, supporting the concept that TxNIP is a direct downstream target of FoxO1 in the liver (Zhang et al., 2012). Together, these data provide strong support for the concept that FoxO1 promotes TxNIP expression in the liver under conditions where insulin levels are low (fasting) or insulin signaling is impaired.
Crosstalk between FoxO1 and ChREBP was previously reported in pancreatic β cells (Kibbe et al., 2013). In these cells, FoxO1 was reported to inhibit the ability of ChREBP to stimulate Txnip expression, and it was suggested that FoxO1 might exert this effect by binding to a FoxO target site overlapping a nearby ChoRE in the Txnip promoter, thereby displacing ChREBP from its ChoRE (Kibbe et al., 2013). In addition, FoxO1 has been reported to suppress the expression of glucokinase in pancreatic β cells (Buteau et al., 2007) similar to the liver, and might therefore limit ChREBP activation to Txnip promoter by an indirect mechanism. In the present study, we found that FoxO1 and ChREBP are able to both stimulate the expression of Txnip in hepatocytes. Although this apparent discrepancy might reflect tissue-specific differences in FoxO1 and ChREBP function, it is important to note that FoxO1 and ChREBP activity are regulated very differently under physiological conditions in the liver. FoxO1 activity is inhibited by insulin due to its phosphorylation by Akt, so that it is most active when insulin levels are low (i.e., in fasting) or insulin signaling is disrupted; conversely, FoxO1 activity is suppressed when insulin levels are high and Akt is activated (i.e., in the fed state). Conversely, ChREBP is activated in the postprandial state, where glucose utilization is increased, and its activity is suppressed when glucose utilization is limited in the liver (e.g., fasting). Based on these observations, we suggest that FoxO1 and ChREBP act coordinately to maintain and promote the expression of TxNIP under both fasting (FoxO1 active, ChREBP inactive) and fed (ChREBP active, FoxO1 inactive) conditions.
It is important to note that other factors also may contribute to the coordinated regulation of TxNIP in the response to fasting and feeding and during daily rhythms, such as PPARα, Indeed, while a degenerate sequence rather than the consensus AGGTCA-N-AGGTCA sequence was identified on the Txnip promoter (AGGACA-G-AGGGGG) (Tzeng et al., 2015), functional evidence reveals that the expression of Txnip is significantly reduced in the liver of fasted hepatocyte-specific Pparα deficient mice (Régnier et al., 2018). Interestingly, a novel PPARα-dependent regulatory axis involving Gm15441, a long non-coding RNA, was recently identified (Brocker et al., 2020). In this study, Gm15441 was shown to be transcribed in a liver-specific, PPARα-dependent manner and protect against metabolic stress by suppressing the expression of TxNIP and thereby suppressing TxNIP-mediated NLRP3 inflammasome activation.
Like its regulation, the physiological functions of the TXNIP protein also appear to be complex when considered in a tissue-specific manner. Several studies, including the current one, demonstrate that TxNIP plays a critical role in the regulation of hepatic glucose production. HcB-19 mice, which contain a nonsense mutation of the Txnip gene, and Txnip knockout mice have an inherent defect in maintaining blood glucose levels through glucose production, as well as other defects in lipid and glucose metabolism (Hui et al., 2004). Moreover, liver-specific Txnip knockout mice suffer from fasting hypoglycemia and elicit a diminished response to glucagon (Chutkow et al., 2008). Hepatocytes isolated from TxNIP-deficient animals produce significantly less glucose when compared with hepatocytes obtained from their wild-type littermates. In the current study, we report that acute suppression of TxNIP in the liver of hyperglycemic db/db mice leads to normalization of blood glucose concentrations under both fasted and fed states and is associated with normalized expression of key genes that promote hepatic production, including Foxo1 and FoxO1-regulated genes. Thus, in addition to its expression being positively regulated by FoxO1, Txnip also appears to support the expression and function of FoxO1 and its downstream targets on glucose production by hepatocytes, suggesting that these factors may function in a feedforward fashion. The induction of Txnip also may provide other protective functions during prolonged fasting. Interestingly, it was reported that the expression of Txnip is induced to a great extent in torpid animals (both fasting-induced and natural torpor) (Hand et al., 2013). The fact that Txnip expression is increased in both the hypothalamus and peripheral tissues (including liver) of torpid mice suggests that it may play an essential role in adapting to major shifts in energy supply in hypometabolic states. In agreement with this concept, mice lacking Txnip die during a prolonged fast (Oka et al., 2006).
In summary, we report Txnip to be up-regulated in the liver by two master nutritional regulators, ChREBP and FoxO1, in response to glucose/refeeding and fasting, respectively. Txnip expression is inducible by glucose through a ChREBP-dependent manner, whereas Txnip expression is greater and rapidly switched on during fasting in the liver in a FoxO-dependent manner, suggesting a major role for this protein in the liver in the physiological adaptation to nutrient restriction.
Limitations of the study
In our study, we have provided a molecular basis to explain the dual regulation of TxNIP in response to fasting and refeeding in liver, via the transcription factors FoxO1 and ChREBP, respectively. However, we have not clearly determined which of these two factors contributes to enhanced TxNIP expression in the liver of hyperglycemic db/db mice. In addition, although we report that TxNIP silencing reduces blood glucose concentrations and improves PTTs in C57Bl/6J and db/db mice, the mechanism(s) involved was not elucidated.
Resource availability
Lead contact
Further information, requests, and inquiries should be directed to and will be fulfilled by the lead contact, Catherine Postic (catherine.postic@inserm.fr).
Materials availability
All tables and figures are included in the text and supplemental information.
Data and code availability
The published article includes all data generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
The authors thank E. Anthony (U1016-Institut Cochin), C. Naylies (GeT-TRiX, INRA ToxAlim), and G. Michel for providing excellent technical assistance. The authors would like to thank R. Dentin and F. Levavasseur (U1016-Institut Cochin) for the use of the ChREBP knockout mouse model. We also thank the animal facility staff (EZOP, INRA ToxAlim) for their excellent work. Postic's lab (U1016-Institut Cochin) is supported by grants from Servier Laboratories, FRM (Foundation for the Medical Research, DEQ20150331744), EFSD-Novonordisk (European Foundation for the Study of Diabetes), and National Agency for Research (ANR) (ANR-15-CE14-0026-Hepatokind). Unterman's lab is supported by VA Merit Review Grant (IO1BX001968).
Author contributions
B.N., F.B., I.O.-S., W.Z., G.F., A.M., A.P., and S.M. designed experiments, performed experiments, and analyzed the data. F.B., A.-F.B., S.G., T.I., H.G., and C.B. contributed to the critical review of the manuscript. C.P. and T.U. analyzed the data and wrote the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102218.
Contributor Information
Terry Unterman, Email: unterman@uic.edu.
Catherine Postic, Email: catherine.postic@inserm.fr.
Supplemental information
References
- Abdul-Wahed A., Guilmeau S., Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Ahn B., Soundarapandian M.M., Sessions H., Peddibhotla S., Roth G.P., Li J.L., Sugarman E., Koo A., Malany S., Wang M. MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling. J. Clin. Invest. 2016;126:3567–3579. doi: 10.1172/JCI87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mubarak B., Soriano F.X., Hardingham G.E. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009;3:233–238. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhawiti N.M., Al Mahri S., Aziz M.A., Malik S.S., Mohammad S. TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr. Drug Targets. 2017;18:1095–1103. doi: 10.2174/1389450118666170130145514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedarida T., Baron S., Vibert F., Ayer A., Henrion D., Thioulouse E., Marchiol C., Beaudeux J.L., Cottart C.H., Nivet-Antoine V. Resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2016;71:720–729. doi: 10.1093/gerona/glv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti V., Tan H., Chow D., Wang Y., Nagakannan P., Eftekharpour E., Wang J.F. Glucocorticoid upregulates thioredoxin-interacting protein in cultured neuronal cells. Neuroscience. 2018;384:375–383. doi: 10.1016/j.neuroscience.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Brocker C.N., Kim D., Melia T., Karri K., Velenosi T.J., Takahashi S., Aibara D., Bonzo J.A., Levi M., Waxman D.J. Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat. Commun. 2020;11:5847–5863. doi: 10.1038/s41467-020-19554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J., Shlien A., Foisy S., Accili D. Metabolic diapause in pancreatic beta-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J. Biol. Chem. 2007;282:287–293. doi: 10.1074/jbc.M606118200. [DOI] [PubMed] [Google Scholar]
- de Candia P., Blekhman R., Chabot A.E., Oshlack A., Gilad Y. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS One. 2008;3:e1670. doi: 10.1371/journal.pone.0001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha-Molstad H., Saxena G., Chen J., Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J. Biol. Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T.F., Hong S.Y., He H., Zheng L., Hagen T., Luo Y., Yu F.X. A potential mechanism of metformin-mediated regulation of glucose homeostasis: inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cell Signal. 2012;24:1700–1705. doi: 10.1016/j.cellsig.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Chen K.S., DeLuca H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Chutkow W.A.,, Patwari P., Yoshioka J., Lee R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008;283:2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- Dentin R., Pégorier J.P., Benhamed F., Foufelle F., Ferré P., Fauveau V., Magnuson M.A., Girard J., Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- Ferreira N.E., Omae S., Pereira A., Rodrigues M.V., Miyakawa A.A., Campos L.C., Santos P.C., Dallan L.A., Martinez T.L., Santos R.D. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis. 2012;221:131–136. doi: 10.1016/j.atherosclerosis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Goswami R., Lacson R., Yang E., Sam R., Unterman T. Functional analysis of glucocorticoid and insulin response sequences in the rat insulin-like growth factor-binding protein-1 promoter. Endocrinology. 1994;134:736–743. doi: 10.1210/endo.134.2.7507835. [DOI] [PubMed] [Google Scholar]
- van Greevenbroek M.M., Vermeulen V.M., Feskens E.J., Evelo C.T., Kruijshoop M., Hoebee B., van der Kallen C.J., de Bruin T.W. Genetic variation in thioredoxin interacting protein (TXNIP) is associated with hypertriglyceridaemia and blood pressure in diabetes mellitus. Diabet Med. 2007;24:498–504. doi: 10.1111/j.1464-5491.2007.02109.x. [DOI] [PubMed] [Google Scholar]
- Guinez C., Filhoulaud G., Rayah-Benhamed F., Marmier S., Dubuquoy C., Dentin R., Moldes M., Burnol A.F., Yang X., Lefebvre T. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand L.E., Saer B.R., Hui S.T., Jinnah H.A., Steinlechner S., Loudon A.S., Bechtold D.A. Induction of the metabolic regulator Txnip in fasting-induced and natural torpor. Endocrinology. 2013;154:2081–2091. doi: 10.1210/en.2012-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo M.J., Kim T.H., You J.S., Blaya D., Sancho-Bru P., Kim S.G. Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression. Gut. 2019;68:708–720. doi: 10.1136/gutjnl-2017-315123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M.A., Peroni O.D., Villoria J., Schön M.R., Abumrad N.A., Blüher M., Klein S., Kahn B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui T.Y., Sheth S.S., Diffley J.M., Potter D.W., Lusis A.J., Attie A.D., Davis R.A. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J. Biol. Chem. 2004;279:24387–24393. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- Ido-Kitamura Y., Sasaki T., Kobayashi M., Kim H.J., Lee Y.S., Kikuchi O., Yokota-Hashimoto H., Iizuka K., Accili D., Kitamura T. Hepatic FoxO1 integrates glucose utilization and lipid synthesis through regulation of Chrebp O-glycosylation. PLoS One. 2012;7:e47231. doi: 10.1371/journal.pone.0047231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iroz A., Montagner A., Benhamed F., Levavasseur F., Polizzi A., Anthony E., Régnier M., Fouché E., Lukowicz C., Cauzac M. A specific ChREBP and PPARα cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 2017;21:403–416. doi: 10.1016/j.celrep.2017.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S.H., Kim M.Y., Park J.M., Kim T.H., Ahn Y.H. Txnip contributes to impaired glucose tolerance by upregulating the expression of genes involved in hepatic gluconeogenesis in mice. Diabetologia. 2013;56:2723–2732. doi: 10.1007/s00125-013-3050-6. [DOI] [PubMed] [Google Scholar]
- Kibbe C., Chen J., Xu G., Jing G., Shalev A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. J. Biol. Chem. 2013;288:23194–23202. doi: 10.1074/jbc.M113.473082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.V., Chang B., Imamura M., Poungvarin N., Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006;55:1179–1189. doi: 10.2337/db05-0822. [DOI] [PubMed] [Google Scholar]
- Li X., Rong Y., Zhang M., Wang X.L., LeMaire S.A., Coselli J.S., Zhang Y., Shen Y.H. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem. Biophys. Res. Commun. 2009;381:660–665. doi: 10.1016/j.bbrc.2009.02.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- Nivet-Antoine V., Cottart C.H., Lemaréchal H., Vamy M., Margaill I., Beaudeux J.L., Bonnefont-Rousselot D., Borderie D. Trans-Resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie. 2010;92:1766–1771. doi: 10.1016/j.biochi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- O-Sullivan I., Zhang W., Wasserman D.H., Liew C.W., Liu J., Paik J.,, DePinho R.A., Stolz D.B., Kahn C.R., Schwartz M.W., Unterman T.G. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015;6:7861. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S., Liu W., Masutani H., Hirata H., Shin-kai Y., Yamada S., Yoshida T., Nakamur H., Yodoi J. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. FASEB J. 2006;20:121–123. [Google Scholar]
- Patwari P., Chutkow W.A., Cummings K., Verstraeten V.L.R.M., Lammerding J., Schreiter E.R., Richard T.L. Thioredoxin-independent regulation of metabolism by the α-arrestin proteins. J. Biol. Chem. 2009;284:24996. doi: 10.1074/jbc.M109.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie J.L., Al-Oanzi Z.H., Arden C., Tudhope S.J., Mann J., Kieswich J., Yaqoob M.M., Towle H.C., Agius L. Glucose induces protein targeting to glycogen in hepatocytes by fructose 2,6-bisphosphate-mediated recruitment of MondoA to the promoter. Mol. Cell Biol. 2013;33:725–738. doi: 10.1128/MCB.01576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier M., Polizzi A., Lippi Y., Fouché E., Michel G., Lukowicz C., Smati S., Marrot A., Lasserre F., Naylies C. Insights into the role of hepatocyte PPARα activity in response to fasting. Mol. Cell Endocrinol. 2018;471:75–88. doi: 10.1016/j.mce.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Reich E., Tamary A., Sionov R.V., Melloul D. Involvement of thioredoxin-interacting protein (TXNIP)in glucocorticoid-mediated beta cell death. Diabetologia. 2012;55:1048–1057. doi: 10.1007/s00125-011-2422-z. [DOI] [PubMed] [Google Scholar]
- Richards P., Ourabah S., Montagne J., Burnol A.F., Postic C., Guilmeau S. MondoA/ChREBP: the usual suspects of transcriptional glucose sensing; Implication in pathophysiology. Metab. Clin. Exp. 2017;70:133–151. doi: 10.1016/j.metabol.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Richards P., Rachdi L., Oshima M., Marchetti P., Bugliani M., Armanet M., Postic C., Guilmeau S., Scharfmann R. MondoA is an essential glucose-responsive transcription factor in human pancreatic β-cells. Diabetes. 2018;67:461–472. doi: 10.2337/db17-0595. [DOI] [PubMed] [Google Scholar]
- Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A. Minireview: thioredoxin-interacting protein: regulation and function in the pancreatic β-cell. Mol. Endocrinol. 2014;28:1211–1220. doi: 10.1210/me.2014-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W., Yu Z., Fantus I.G., Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal. 2010;22:1240–1246. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Thielen L.A., Shalev A. Diabetes pathogenic mechanisms and potential new therapies based upon a novel target called TXNIP. Curr. Opin. Endocrinol. Diabetes Obes. 2018;25:75–80. doi: 10.1097/MED.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen L.A., Chen J., Jing G., Moukha-Chafiq O., Xu G., Jo S., Grayson T.B., Lu B., Li P., Augelli-Szafran C.E. Identification of an anti-diabetic, orally available small molecule that regulates TXNIP expression and glucagon action. Cell Metab. 2020;32:353–365. doi: 10.1016/j.cmet.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng J., Byun J., Park J.Y., Yamamoto T., Schesing K., Tian B., Sadoshima J., Oka S. An ideal PPAR response element Bound to and activated by PPARα. PLoS One. 2015;10:e0134996. doi: 10.1371/journal.pone.0134996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde B.R., Ayer D.E. Interactions between Myc and MondoA transcriptionfactorsin metabolism and tumourigenesis. Br. J. Cancer. 2015;113:1529–1533. doi: 10.1038/bjc.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E. TXNIP/TBP-2: a master regulator for glucose homeostasis. Antioxidants. 2020;9:765. doi: 10.3390/antiox9080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Patil S., Chauhan B., Guo S., Powell D.R., Le J., Klotsas A., Matika R., Xiao X., Franks R. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- Zhang K., Li L., Qi Y., Zhu X., Gan B., DePinho R.A., Averitt T., Guo S. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Bu S.Y., Mashek M.T., O-Sullivan I., Sibai Z., Khan S.A., Ilkayeva O., Newgard C.B., Mashek D.G., Unterman T.G. Integrated regulation of hepatic lipid and glucose metabolism by adipose triacylglycerol lipase and FoxO proteins. Cell Rep. 2016;15:349–359. doi: 10.1016/j.celrep.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data generated or analyzed during this study.