Abstract
Introduction
Iron profiles in patients with type 2 diabetes (T2D) are inconsistent. In this study, we assessed the levels of hepcidin, a regulatory protein involved in iron homoeostasis, in patients with T2D. We further evaluated the surrogate markers of hepcidin action, particularly those associated with erythropoiesis.
Methods
This systematic review and meta-analysis was reported following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. We searched for relevant studies in electronic databases from inception until 31 October 2020 without any language restriction. The random effects model was used to calculate effect estimates, and outcomes were reported as either standardised mean difference (SMD) or mean differences (MD), 95 percent confidence interval (95% CI).
Results
Eleven studies involving 2 620 participants were included in this study. Patients with T2D had a slight increase in hepcidin levels when compared to controls SMD: 0.07 [95% CI: -0.30, 0.44]. The subgroup analysis showed that studies involving patients with T2D who were overweight reported elevated hepcidin levels SMD: 0.35 [95% CI: 0.07, 0.62] whilst those with grade I obesity described reduced levels SMD: -0.42 [95% CI: -1.21, 0.38]. All T2D patients had low levels of haemoglobin MD: -0.23 g/dl [95% CI: -0.46, -0.01] irrespective of body weight.
Conclusion
The levels of hepcidin are altered in patients with T2D and are disproportionately influenced by weight. Moreover, patients with T2D present with subclinical anaemia despite elevated iron stores. The regulation of hepcidin in patients with T2D is dependent on several factors and vary greatly, thus its sole use in clinical settings may be less beneficial.
Keywords: Hepcidin, Haemoglobin, Anaemia, Iron profiles, Obesity, Type 2 diabetes
Hepcidin; Haemoglobin, Anaemia; Iron profiles; Obesity; Type 2 diabetes.
1. Introduction
Obesity is an independent risk-factor for several non-communicable disease (NCD) which includes type 2 diabetes (T2D) [1]. An obese state in patients with T2D is associated with exacerbated systemic inflammation and an increased degree of insulin resistance [2, 3]. T2D is also considered as a chronic inflammatory condition that is characterised by insulin resistance and hyperglycaemia [4]. A growing body of evidence linking dysregulated iron metabolism and T2D has been described over the years [5, 6, 7]. Whereby, both conditions of iron deficiency anaemia [8, 9] and in some instances iron overload [6, 10, 11] have been widely reported in patients with T2D. The reported different relationships between poor glucose control and iron metabolism sparked interest in investigating iron metabolism in T2D, particularity on the modulators of iron intake, release, and transportation. One of these regulators is hepcidin, an acute phase protein synthesised in the liver and encoded by the HAMP gene, that inhibits iron release from macrophages and uptake from intestinal cells through its interaction with ferroportin, an iron importer protein [12, 13]. The synthesis of hepcidin is induced by the janus kinase/signal transducer and activator of transcription (JAK/STAT) and bone morphogenetic protein/s-mothers against decapentaplegic (BMP/SMAD) signalling pathways mediated by interleukin (IL)-6 and BMP-6 in response to inflammation and iron status, respectively [14, 15].
Hepcidin plays a pivotal role in regulating iron homeostasis and indirectly modulates red cell production [16]. In that context, the release of erythropoietin, a major regulator of erythropoiesis, blocks hepcidin secretion via erythroferrone action leading to increased iron supply to the bone marrow for erythropoiesis [16, 17]. Consequently, aberrant hepcidin levels are associated with anaemia of chronic disease (ACD), a common feature of chronic inflammatory diseases such as T2D [17]. Interestingly, this mild to moderate ACD is attributed to increased release of pro-inflammatory IL-6 which in addition to promoting hepcidin synthesis, has anti-erythropoietic effects induced by inhibiting the proliferation of erythroid precursor cells and erythropoietin action [9, 14]. The reduction in hepcidin expression may also cause ineffective erythropoiesis, leading to iron-loading anaemia deficiency as previously reported elsewhere [18]. The dysregulation of hepcidin levels is also associated with non-alcoholic fatty liver disease (NAFLD), a metabolic disorder that is closely associated with hyperglycaemia and insulin resistance [19, 20]. The elevated levels of hepcidin in NALFD leads to the excessive accumulation of hepatic iron stores coupled with a deficiency in serum levels [21]. Notably, this increase in hepcidin levels is attributed to obesity [22] and consequently, NALFD has become a growing complication of diabetes with a prevalence of 54% in T2D cases [23]. The resulting dysmetabolic iron overload syndrome (DIOS) in NAFLD and T2D is driven by insulin resistance and low-grade inflammation [21, 22, 24].
Although it is apparent that hepcidin is an important regulator of iron metabolism, its levels together with its surrogate in patients with T2D remain elusive. For instance, elevated [5, 6, 7, 25] and comparable [10, 26, 27, 28] levels of hepcidin between patients with T2D and healthy controls have been previously shown [10, 11, 27, 29, 30]. Whilst others have reported a marked reduction in the levels of hepcidin in patients with T2D [11, 29, 30]. Therefore, these findings suggest the presence of other underlying factors besides poor glucose control in these patients that may influence hepcidin levels. These include the severity of obesity (which is associated with increased insulin resistance and IL-6 levels [27, 31]) and DIOS, but also the presence of hemochromatosis and beta-thalassemia major (which is associated with increased beta-cell damage and impaired insulin synthesis and action) [22, 32, 33]. Therefore, inferences on the levels of hepcidin in poor glucose control will be important in the risk stratification of T2D and the development of its associated complications that are mediated by altered iron metabolism. In this systematic review and meta-analysis, we comprehensively assessed available literature reporting on the expression of hepcidin in patients with T2D and further explored how obesity impacts these levels. Lastly, we assessed the levels of surrogate markers of iron metabolism influenced by hepcidin action.
2. Methods
This systematic review and meta-analysis was reported following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [34]. A detailed MOOSE checklist is provided in Table 1S. A protocol was designed for this study and was agreed upon by all authors before conducting the qualitative and quantitative synthesis. However, it was not registered and therefore the protocol does not have a registration number.
2.1. Information sources and search strategy
A comprehensive search strategy was designed by two independent reviewers (FN and PVD) with the help of an experienced librarian. The MEDLINE electronic database, and Google Scholar as well as grey literature including preprints, were searched from inception until 31 October 2020. The reviewers used the following Medical Subject-Heading (MeSH) terms and text words to retrieve relevant studies; “hepcidin” AND ‘type 2 diabetes mellitus’. The reference lists of included studies were further scanned for additional relevant studies. No language restrictions were applied, and a detailed MEDLINE search strategy using PubMed search engine is shown in Table 2S. We retrieved studies to address the following research questions;
-
1.
Are there differences in the levels of regulatory proteins involved in iron homoeostasis in patients with T2D?
-
2.
Does the degree of obesity in these patients influence the levels of iron regulatory proteins?
2.2. Eligibility criteria and study selection
Studies were independently screened and selected by two reviewers (FN and BBN) using a pre-defined inclusion and exclusion criteria. In cases of disagreements, third reviewer (TMN) was consulted for arbitration. We included experimental and observational studies that reported on hepcidin levels in patients with T2D irrespective of the age. We excluded reviews, case studies, letters to the editor and animal studies.
2.2.1. Participants
Patients living with T2D and individuals with normal glucose control (normoglycaemics).
2.2.2. Intervention and comparator
No intervention was considered in this study and the comparators included normoglycaemics (controls).
2.2.3. Outcomes
The primary outcome of this study was to determines the level of regulatory proteins involved in iron homoeostasis. The secondary outcome involved iron profiles modulated by iron regulatory proteins.
2.3. Data extraction and management
Two independent reviewers (FN and TMN) extracted detailed study information and characteristics using a predefined data extraction form adapted from the Cochrane Consumers and Communication Review Group data extraction for included studies template [35]. The following data were extracted from each study, author's name and year of publication, country, number of participants, number of males, age and body mass index (BMI) as well as effect measures (levels of hepcidin, iron, haemoglobin, ferritin and hepcidin:ferritin ratio) and main findings. Discrepancies in the extracted data items were resolved through discussions or consultating the third reviewer (BBN).
2.4. Risk of bias and confidence in the cumulative evidence
The risk of bias in the included studies was assessed by two independent reviewers (FN and EPN) using the modified Newcastle-Ottawa scale, adapted for observational studies [36]. Briefly, a star system was used to appraise studies based on three domains (selection, comparability, and outcome ascertainment). A study was rated unsatisfactory if the score was between 0-4, satisfactory if 5–6, good if 7–8 and very good if 9–10. Inconsistencies in the scores were resolved by consulting the third reviewer (TMN). The quality of the included studies was assessed used the Grading of Recommendations Assessment, development, and Evaluation (GRADE) approach to evaluate cumulative evidence quality [37].
2.5. Statistical analysis
The random-effects model was used to estimate the effect size and the I2 was used to test for statistical heterogeneity. The effect estimates were reported as the standardised mean difference (SMD) or mean differences (MD), 95 percent confidence interval (95% CI) depending on the reported effect measure and units of measurement, and the Cohen's d method was used to interpret the calculated pooled estimates [38]. We performed a subgroup-analysis based on the reported BMI (overweight vs. obesity), which may have an influence on hepcidin levels [25]. We further conducted a sensitivity analysis to explore the sources of unexplained statistical heterogeneity amongst the included studies and to evaluate the robustness of the reported overall effect estimates. The Cohen's Kappa (κ) was used to assess inter-rate reliability on study selection and risk of bias assessment [39]. A p-value<0.05 was considered statistically significant whilst a p-value of less than 0.1 was considered statistically significant in the subgroup analysis [40]. Publication bias was assessed using visual inspection of funnel plots. All statistical analysis was performed using Review Manager (RevMan V.5.3) software.
3. Results
3.1. Selected studies
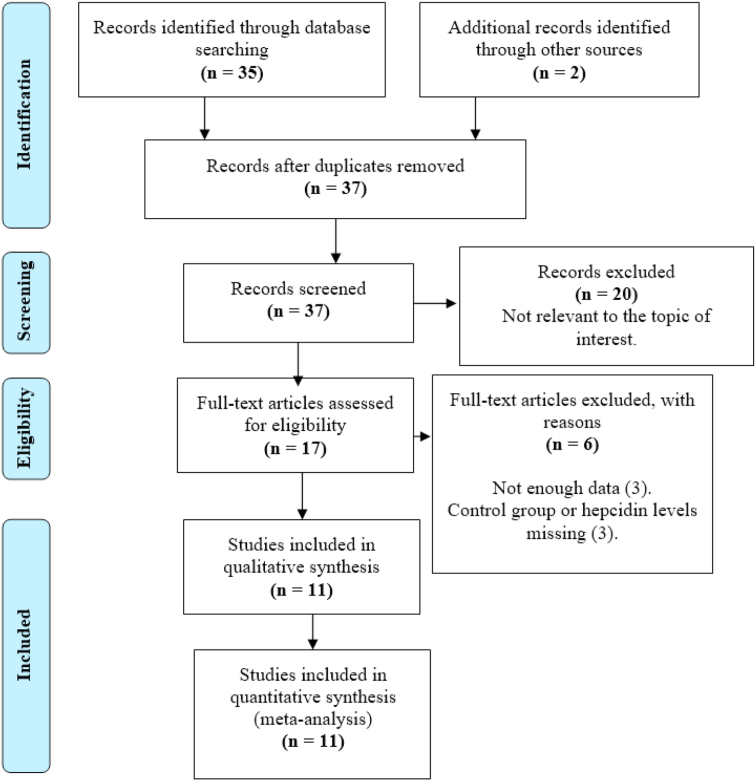
We identified a total of 37 citations, of which thirty-five were retrieved from PubMed (n = 35) and two from other sources (n = 2). After the abstract screening phase, we excluded twenty studies (n = 20) as these were not relevant to the topic of interest. Furthermore, six citations (n = 6) were excluded upon the full-text screening phase due to not reporting on suitable control group or hepcidin levels (n = 3) or not reporting adequate study-level data (n = 3). Therefore, only 11 studies met the inclusion criteria and had enough data for the qualitative and quantitaive synthesis (Figure 1).
Figure 1.
PRISMA flow diagram showing the study selection process.
3.2. Characteristics of included studies
All included studies [5, 6, 7, 10, 11, 25, 26, 27, 28, 29, 30] were observational studies published between 2011 and 2018, and were from Egypt [5], Colombia [30], Germany [11], Chile [25], Finland [29], Israel [28] and the rest from China (n = 4) [6, 7, 10, 26]. However, one study by Vela and colleagues [27] did not indicate the country where the study was conducted. This systematic review and meta-analysis included a total of 2 620 participants, of which 1276 (49%) had T2D and a mean age of 60.49 ± 10.93 years. The remaining 1344 (51%) were healthy individuals, with a mean age of 54.03 ± 12.24 years, and a male to female ratio of 0.98. Amongst the T2D group, 899 individuals were overweight (BMI>25 but less than 30 kg/m2) and were reported in 5 of the included studies [6, 7, 10, 26, 27] whilst 337 individuals had grade I obesity (BMI>30 but less than 35 kg/m2) and were reported in 4 of the included studies [11, 28, 29, 30]. One study reported both overweight and obese T2D patients [25] while the other did not specify the degree of obesity [5] (Table 1).
Table 1.
Characteristics of included studies reporting on hepcidin levels and iron profiles in patients with type 2 diabetes (T2D) (n = 11).
| Study | Country | Study size | Male, n (%) | Age (years) BMI [kg/m2] |
Reported effect measures of iron metabolism in serum | Main findings |
|---|---|---|---|---|---|---|
| Jiang et al., 2011 [6] | China | 64 participants (34 T2D and 30 controls) | 35 (55%) | T2D (60.88 ± 10.99) [25.72 ± 3.28] Control (60.19 ± 6.74) [22.52 ± 2.44] |
Hepcidin, haemoglobin, ferritin and erythropoietin | The levels of hepcidin, ferritin and erythropoietin were elevated in T2D patients, whilst haemoglobin levels were lower than in healthy controls. The levels of hepcidin positively correlated with ferritin levels among T2D individuals and the controls. |
| Zheng et al., 2011 [10] | China | 238 participants (168 T2D and 70 controls) | 77 (32%) | T2D (62.7 ± 8.7) [25.4 ± 3.65] Control (52.94 ± 8.03) [24.28 ± 1.25] |
Hepcidin, iron, haemoglobin, and ferritin | Levels of hepcidin and iron were comparable between T2D patients and controls. However, the levels of ferritin and haemoglobin were elevated in patients with T2D. |
| Guo et al., 2013 [26] | China | 1259 participants (555 T2D and 704 controls) | 422 (34%) | T2D (64.45 ± 9.16) [25.3 ± 3.49] Control (58.55 ± 9.56) [24.46 ± 3.33] |
Hepcidin, ferritin and haemoglobin | The levels of hepcidin were comparable between patients with T2D versus controls. However, the levels of ferritin and haemoglobin were elevated in the T2D group. A significant positive correlation was found between hepcidin and haemoglobin levels in patients with T2D. |
| Sam et al., 2013 [29] | Finland | 66 participants (33T2D and 33 controls) | 46 (70%) | T2D (53.34 ± 3.69) [32.23 ± 1.09] Control (49.63 ± 6.50) [31.03 ± 1.82] |
Hepcidin, ferritin, hepcidin: ferritin ratio, haemoglobin | Patients with T2D had lower levels of hepcidin, hepcidin:ferritin ratio and haemoglobin levels when compared to controls. On the other hand, ferritin levels were increased in T2D patients when compared to controls. |
| Andrews et al., 2015 [25] | Chile | 312 participants (166 T2D and 146 controls) | 312 (100%) | T2D# (59.38 ± 9.04) [29.23 ± 3.73] Control (51.4 ± 15.7) [24.6 ± 2.2] |
Hepcidin, iron, ferritin and haemoglobin | Increased levels of hepcidin, iron and ferritin levels were reported in T2D patients in comparison to controls. These increments were further exacerbated by the presence of obesity in T2D patients. Haemoglobin levels were decreased in T2D patients compared to controls. Notably, increased hepcidin levels were associated with iron and increased risk of developing T2D independent of weight. |
| Suarez-Ortegon et al., 2015 [30] | Colombia | 304 participants (65 T2D and 239 controls) | 148 (49%) | T2D (53.1 ± 8.3) [30.4 ± 5.4] Control (45.5 ± 7.7) [26.0 ± 3.6] |
Hepcidin, hepcidin: ferritin ratio, ferritin | Hepcidin levels and hepcidin: ferritin ratio were significantly decreased in patients with T2D in comparison to controls. However, ferritin levels were comparable between T2D and control. |
| Vela et al., 2017 [27] | Not indicated | 80 participants (60 T2D and 20 controls) | 57 (71%) | T2D (55.6 ± 6.1) [28.4 ± 3.7] Control (58.1 ± 9.3) [27.2 ± 3.4] |
Hepcidin, hepcidin: ferritin ratio, ferritin, iron, haemoglobin, haematocrit, and red blood cell count | Hepcidin and hepcidin:ferritin ratios were comparable between T2D patients and control group. However, ferritin and iron levels were increased in T2D patients whilst the levels of haemoglobin, haematocrit and red blood cells count were decreased. There was no significant association between hepcidin levels and glucose or haematological parameters. |
| Altamura et al., 2017 [11] | Germany | 155 Participants (115 T2D and40 Controls) |
113 (73%) | T2D (60.2 ± 6.9) [33.1 ± 5.5] Control (57.9 ± 11.7) [28.0 ± 4.4] |
Hepcidin, iron, ferritin and haemoglobin and haematocrit. | Patients with T2D had reduced hepcidin levels when compared to control group. However, the levels of iron and ferritin were increased in T2D patients whilst haemoglobin and haematocrit levels were comparable. Notably, a significant positive association was found between iron and ferritin levels among patients with T2D. |
| Atyia et al., 2018 [5] | Egypt | 60 participants (40 T2D and 20 controls) | Not reported | T2D (49.23 ± 5.62) [Not reported] Control (48.55 ± 7.76) [Not reported] |
Hepcidin, ferritin, iron and hepcidin: ferritin ratio. | Hepcidin and ferritin levels were elevated in patients with T2D when compared to controls, whilst iron levels were comparable between T2D and control group with T2D patients exhibiting lower hepcidin: ferritin ratio. Elevated iron levels were associated with high risk of T2D. |
| Guo et al., 2018 [7] | China | 44 participants (22 T2D and 22 Controls) | 29 (73%) | T2D (56.45 ± 11.80) [25.48 ± 3.45] Control (52.45 ± 10.01) [24.43 ± 2.33] |
Hepcidin, ferritin and hepcidin: ferritin ratio | Hepcidin and ferritin levels were elevated whilst hepcidin: ferritin ratio was decreased in T2D patients in comparison to the controls. |
| Shalitin et al., 2018 [28] | Israel | 38 participants (18 T2D and 20 controls) | 25 (66%) | T2D (15.1 ± 3.1) [2.45 ± 0.43]∗ Control (13.1 ± 3.0) [2.39 ± 0.36] |
Hepcidin. | There were no differences in hepcidin levels between T2D the control group. |
T2D group comprised of lean [Age: 61.3 ± 10.0; BMI: 25.4 ± 2.1] and obese [Age: 58.3 ± 8.3; BMI: 31.4 ± 2.5] groups.
: BMIZ scores.
3.3. Study quality and publication bias
The quality assessment for included studies was rated using the Newcastle-Ottawa scale (Table 3S). The median score range of included studies was 7 (5–9) out of total 10 scores. Four of the studies were rated as satisfactory [5, 25, 27, 29] and 6 as good [6, 7, 11, 26, 28, 30] with only one study as very good [10]. Briefly, the selection domain for included studies had a median of 3 (2–4) out of 5 overall score (overall agreement 86.36%, kappa = 0.73), comparability median of 1 (1–2) out of 2 maximum stars (overall agreement 54.55%, kappa = 0.09) and outcome median of 2 (2–3) out of 3 possible stars (overall agreement 95.46%, kappa = 0.91) Table 2S. Visual assessment of funnel plots indicated no potential publication bias (Figure 1S).
3.4. Data synthesis
3.4.1. Reported metabolic parameters in included studies
Fasting blood glucose levels were reported in 9 (82%) of the included studies [5, 6, 7, 10, 11, 26, 27, 28, 30]. Overall, the pooled effect estimates of fasting blood glucose levels, showed a large effect size between patients with T2D and controls (SMD: 2.19 [95% CI: 1.41, 2.98]; I2 = 97%, pH<0.00001) (Figure 2aS). A total of 4 (36%) studies reported on insulin levels [10, 27, 28, 30] and pooled effect showed increased insulin levels in patients with T2D when compared to controls (SMD: 1.34 [ 95% CI: 0.07, 2.60]; I2 = 97%, pH<0.00001) (Figure 2bS). Glycated haemoglobin levels were reported in 8 (73%) of the included studies [5, 6, 10, 11, 26, 27, 28, 29] and were increased in patients with T2D when compared to controls (MD: 2.30% [95% CI: 1.82, 2.77]; I2 = 97%, pH<0.00001) (Figure 2cS.
3.4.2. Primary findings on hepcidin levels in patients with T2D
A total of 11 (100%) studies reported on hepcidin levels in T2D patients. The qualitative synthesis of 4 included studies (36%) [5, 6, 7, 25] showed elevated levels of hepcidin in T2D when compared to controls, whilst 3 studies (27%) [11, 29, 30] described decreased hepcidin levels in T2D patients in comparison to controls. In contrast, the other 4 studies (36%) [10, 26, 27, 28] reported on comparable hepcidin levels between T2D and control groups (Table 1). Nonetheless, the pooled effect estimates showed a slight increase in hepcidin levels between patients with T2D and controls (SMD: 0.07 [95% CI: -0.30, 0.44]; I2 = 93%, pH<0.00001) (Figure 3S).
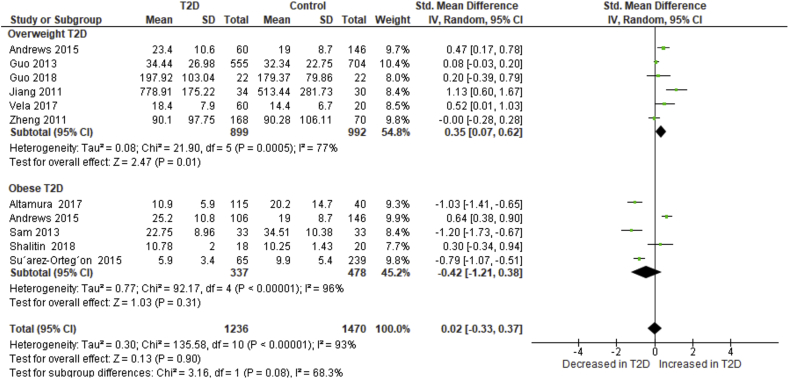
To investigate sources of heterogeneity amongst the 11 studies, we performed a subgroup analysis based on the classification of weight status by BMI. Normal weight is considered as BMI<25 kg/m2, overweight as BMI ≥25 but less than 30 kg/m2 whilst class I, II and III obesity as BMI >30 but less than 35 kg/m2, BMI ≥35 but less than 40 kg/m2 and BMI ≥40 kg/m2, respectively. In this subgroup analysis, we included 10 studies [6, 7, 10, 11, 25, 26, 27, 28, 29, 30] and we omitted the study by Atyia and colleagues [5] as they did not specify the degree of obesity amongst the included participants. Subsequently, the test for subgroup differences showed a significant subgroup effect (p = 0.08) [40]. Thus, the overall classification of body weights based on the reported BMI modified the overall effect of hepcidin levels. Studies that reported on T2D patients who were overweight showed significantly elevated hepcidin levels in comparison to controls (SMD: 0.35 [95% CI: 0.07, 0.62]; I2 = 77%, pH = 0.0005) (Figure 2). In contrast, studies that involved T2D patients with grade I obesity described reduced levels of hepcidin (SMD: -0.42 [95% CI: -1.21, 0.38]; I2 = 96%, pH<0.00001) (Figure 2). Notably, the sources of heterogeneity remained high in the subgroup analysis. To further investigate sources of heterogeneity and the robustness of our results, we performed a sensitivity analysis based on treatment, age and C-reactive protein levels which are known to influence hepcidin levels. The SMDs did not change direction nor the magnitude of our effect size and the levels of heterogeneity remained substantial (Table 4S).
Figure 2.
A subgroup analysis of hepcidin levels in type 2 diabetes (T2D) patients based on degree of obesity, between overweight T2D (BMI ≥25 but less than 30 kg/m2) and grade I obese T2D (BMI>30 but less than 35 kg/m2). Hepcidin levels were significantly increased in overweight T2D when compared to patients with grade 1 obesity.
3.4.3. Surrogate markers of hepcidin
3.4.3.1. Serum iron levels
Overall, 45% of the included studies (n = 5) reported on iron levels [5, 10, 11, 25, 27]. Of these, 3 studies [5, 10, 41] reported comparable iron levels between T2D and controls. The other 2 studies [11, 25] described elevated levels in T2D when compared to controls (Table 1). However, pooled effect estimates showed a slight increase in iron levels in patients with T2D when compared to controls (SMD: 0.06 [95% CI: -0.26, 0.39]; I2 = 80%, pH = 0.0005) (Table 2). Although the test for subgroup differences was not significant (p = 0.29), it is important to note that in the overweight T2D, the iron levels were lower than healthy controls.
Table 2.
Pooled effect estimates of surrogate markers of hepcidin in patents with type 2 diabetes (T2D).
| Effect Measure | Number of Studies | Number of participants | Effect Estimate |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | MD | SMD | 95% CI | I2, p-value | Z, p-value | ||||
| Iron levels | Overall | 5 [10,11,25,27] | 931 | RE | - | 0.06 | -0.26 to 0.39 | 80%, pH=0.0005 | 0.38, p = 0.70 |
| Overweight T2D | 3 [10,25,27] | 524 | - | -0.11 | -0.57 to 0.36 | 81%, pH = 0.005 | 0.46, p = 0.65 | ||
| Obese T2D | 2 [11,25] | 407 | - | 0.30 | -0.30 to 0.90 | 86%, pH = 0.007 | 0.98, p = 0.33 | ||
| Haemoglobin levels | Overall | 8 [6,10,11,25,26,27,29] | 2320 | RE | -0.23 | - | -0.46 to -0.01 | 85%, pH<0.00001 | 2.02, p = 0.04 |
| Overweight T2D | 5 [6,10,[25], [26], [27]] | 1847 | -0.43 | - | -0.94 to 0.07 | 87%, pH <0.00001 | 1.68, p = 0.09 | ||
| Obese T2D | 3 [11,25,29] | 473 | -0.15 | - | -0.27 to -0.03 | 0%, pH = 0.37 | 2.44, p = 0.01 | ||
| Ferritin levels | Overall | 10 [6,7,10,11,25,26,27,29,30] | 2668 | RE | - | 0.60 | 0.32 to 0.88 | 88%, pH<0.00001 | 4.15, p < 0.00001 |
| Overweight T2D | 6 [6,7,10,25,26,27] | 1891 | - | 0.57 | 0.31 to 0.83 | 74%, pH = 0.02 | 4.28, p < 0.0001 | ||
| Obese T2D | 4 [11,25,29,30] | 777 | - | 0.65 | -0.08 to 1.37 | 95%, pH <0.00001 | 1.75, p = 0.08 | ||
| Hepcidin:ferritin ratio | Overall | 4 [7,27,29,30] | 494 | RE | -0.19 | - | -0.46 to 0.08 | 96%, pH<0.00001 | 1.35, p = 0.18 |
| Overweight | 2 [7,27] | 124 | 0.01 | - | -0.08 to 0.09 | 0%, pH = 0.91 | 0.16, p = 0.87 | ||
| T2D Obese T2D | 2 [29,30]) | 370 | -0.37 | - | -0.70 to -0.05 | 96%, pH <0.00001 | 2.23. p = 0.03 | ||
T2D groups were compared to heathy controls.
3.4.3.2. Haemoglobin levels
A total of 8 of the included studies reported on haemoglobin levels. Two citations [10, 26] reported increased haemoglobin levels in T2D when compared to the control group, whilst the other 5 studies described decreased [6, 25, 27, 29] and comparable [11] haemoglobin levels (Table 1). The pooled estimates showed, significantly decreased levels of haemoglobin in patients with T2D when compared to healthy controls (MD: -0.23 g/dl [95% CI: -0.46, -0.01]; I2 = 85%, pH<0.00001) (Table 2). Although the test for subgroup differences was nonsignificant (p = 0.28), the reduction of haemoglobin levels in the obese subgroup was suggestive of subclinical anaemia in these patients (p = 0.01).
3.4.3.3. Ferritin levels
Approximately 91% of the included studies (n = 10) reported on ferritin levels, with 9 of these [5, 6, 7, 10, 11, 25, 26, 27, 29] showing elevated levels of ferritin in T2D when compared to controls, whilst one study described comparable levels [30]. The pooled effect estimates showed significantly increased ferritin levels in patients with T2D when compared to healthy controls (SMD: 0.60 [95% CI: 0.32, 0.88]; I2 = 88%, pH<0.00001) (Table 2). The test for subgroup analysis was insignificant (p = 0.85), thus iron stores were not influenced by body weight.
3.4.3.4. Hepcidin:ferritin ratio
A total of 5 of the included studies reported on the hepcidin: ferritin ratio. The hepcidin:ferritin ratio was increased in patients with T2D compared to controls [5, 7]. Whilst the other 3 studies described decreased [29, 30] and comparable [27] ratios (Table 1). The meta-analysis revealed a slight decrease in the hepcidin:ferritin ratio in patients with T2D when compared to healthy controls (SMD: -0.19 [95% CI: -0.46, 0.88]; I2 = 96%, pH<0.00001) (Table 2). The test for subgroup differences was significant (0.03) and we therefore performed a subgroup analysis based on weights. The overweight T2D subgroup had comparable hepcidin:ferritin ratios whilst it was significantly decreased in the obese group (p = 0.03).
4. Discussion
Patients with T2D are known to have altered liver function, which impacts on the synthesis of regulator proteins involved in iron metabolism. As a consequence, dysregulated iron metabolism may lead to ACD or iron-loading anaemia deficiency [42, 43]. In this study, we aimed at evaluating the levels of hepcidin in patients with T2D as well as the surrogate markers of iron synthesis that are influenced by hepcidin action, particularly those that are associated with erythropoiesis. Overall, hepcidin levels were slightly elevated in T2D patients and this was concomitant with a modest reduction in haemoglobin levels and a slight increase in iron and ferritin levels. Interestingly, the levels of hepcidin were dependent on body weight, such that overweight was associated with elevated levels whilst class I obesity was characterised by a reduction in its concentration. The decrease in haemoglobin levels were independent of hepcidin levels, thus patients with T2D present with subclinical anaemia. A summary of findings is provided in Table 3.
Table 3.
Summary of findings table.
| Type 2 diabetes compared to healthy controls | ||||||
|---|---|---|---|---|---|---|
| Patient or population and Exposure: Individuals with T2D Comparison: Healthy controls (normoglycaemics) Outcome: Hepcidin levels and iron overload | ||||||
| Outcomes | Absolute effects∗ (95% CI) |
Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk in T2D patients | |||||
|
Iron regulator proteins Measured by the levels of hepcidin |
- | The standardised MD in the exposure group was 0.07 higher (-0.30 to 0.44) | - | 2 620 (11 observational studies) | ⨁⨁OO LOW |
|
|
Iron profiles Measured by haemoglobin levels |
- | The mean level in the exposure group was -0.23 mg/dl lower (-0.46 to -0.01) | - | 2 320 (8 observational studies) | ⨁⨁OO LOW |
|
CI: Confidence interval; MD: Mean difference; OR: Odds ratio; NE: Not estimable.
GRADE Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The accumulation of visceral fat in obesity promotes insulin resistance and the secretion of pro-inflammatory cytokines from the adipose tissues, such as IL-6 and TNF-α [44, 45]. In addition to inhibiting the proliferation of erythroid precursor cells, these cytokines can inhibit renal erythropoietin synthesis, which is essential for erythropoiesis [9, 14, 46]. On the other hand, IL-6 via the JAK/STAT signalling pathway may induce hepatic hepcidin synthesis [41, 47] which promotes iron supply to the bone marrow for erythropoiesis. Therefore, a tight regulation is essential in maintaining a fine balance between hepcidin synthesis and erythropoiesis. However, the alteration in the cytokine milieu in T2D dysregulates this balance. The reduced levels of hepcidin in the obese group may be attributed to modulatory effects of insulin on STAT signalling and hepcidin synthesis [41, 48]. Since the relationship between circulating iron levels and hepcidin levels is directly proportional, an elevation in iron levels is expected to induce hepcidin synthesis via the BMP/SMAD signalling pathway. However, this mechanism seems to be altered in patients with T2D. This may be ascribed to increased PI3K/AKT activation, a regulator of glucose metabolism, which outweighs BMP/SMAD signalling as previously reported [11]. Moreover, the decreased levels of hepcidin may also be attributed to the suppression of hepcidin synthesis through nutrient-dependent mechanistic target of rapamycin (mTOR) signalling, which is well-established to be elevated in both obesity and T2D [49, 50]. The suppression of hepatic glucose production through the activation of adenosine-monophosphate-activated protein kinase (AMPK) renders metformin its anti-hyperglycaemic effects [51]. Notably, the phosphorylation of AMPK mediates the suppression of STAT3 signalling and hepcidin synthesis [52]. On the other hand, the use of insulin therapy is an effective strategy to improve glucose control in older patients with T2D, who mostly present with poor insulin secretion capacity and are usually lean [53]. Interestingly, this therapy is closely associated with hypoglycaemia and hyperinsulinemia [53, 54], with the latter known to directly promote and induce hepcidin synthesis [27, 48]. Therefore, different treatment strategies may be a contributing factor to the variation in hepcidin levels in patients with T2D.
Anaemia, is common in T2D patients and is directly associated with the development of microvascular complications [55]. Normocytic-normochromic ACD, that is characterised by decreased levels of haemoglobin and haematocrit has been reported in patients with T2D [9]. Our study showed a reduction in haemoglobin levels in the T2D group in comparison to healthy individuals. Although this reduction did not constitute overt anaemia, it was suggestive of subclinical anaemia. In fact, the reduction in haemoglobin levels was more pronounced in the obese subgroup. In patients with ACD, elevated ferritin levels are expected due to increased iron retention in the reticuloendothelial cells and inflammation-induced production [42]. Our study showed that included patients with T2D presenting with subclinical anaemia had elevated ferritin levels, irrespective of body weights. This increment is responsible for a lower hepcidin:ferritin ratio in patients with T2D.
Our systematic review and meta-analysis had a few limitations. Firstly, there were substantial levels of unexplained statistical heterogeneity in the included studies. Moreover, most studies did not control for confounders such as inflammation and obesity which have an impact on hepcidin levels. Most of the included studies did not report on the treatment that these patients were on which could have modified the hepcidin levels. For instance, metformin, the standard drug used in obese T2D cases is known to cause anaemia due to vitamin B12 deficiency [56]. Lastly, the quality of cumulative evidence in this study was low due to the observational nature of the included studies which are associated with a high risk of selection bias due to lack of randomisation. Therefore, caution should be taken when extrapolating these findings. Nonetheless, our current study has significant strengths. A previous systematic review and meta-analysis by Karamzad and colleagues demonstrated a slight increase in hepcidin levels in patients with T2D [57]. In agreement with this finding, our current meta-analysis further explored the impact body weight on hepcidin levels. Other strengths included the robustness of our results and the methods used as indicated by the sensitivity analysis and high inter-rate reliability, respectively. Lastly, this study showed that hepcidin regulation in poor glucose control is a complex phenomenon that is influenced by several factors. Therefore, its sole diagnostic and clinical utility in overweight or obese cases of T2D may lead to variable patient outcomes. However, its use in the hepcidin:ferritin ratio is a reliable and better maker for T2D risk stratification [57].
5. Conclusion
Patients with T2D present with iron overload which is congruent with impaired metabolic function and elevated levels of hepcidin. While hepcidin levels were elevated in overweight patients, they were decreased in patients with grade I obesity, suggesting that the degree of obesity can greatly impact iron metabolism in patients with T2D. Despite the body weights, patients with T2D generally present with subclinical anaemia marked by a reduction in haemoglobin levels despite an increase in iron stores. Overall, the synthesis of hepcidin and its impact on iron metabolism in patients with T2D is a quite complex relationship that is depended on multifactorial factors.
Declarations
Author contribution statement
Fransina Ndevahoma and Tawanda Maurice Nyambuya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Munyaradzi Mukesi: Conceived and designed the experiments; Wrote the paper.
Phiwayinkosi V. Dludla: Analyzed and interpreted the data; Wrote the paper.
Bongani B. Nkambule: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Elina P. Nepolo: Performed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Daousi C. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad. Med. 2006;82:280–284. doi: 10.1136/pmj.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyambuya T.M., Dludla P.V., Mxinwa V., Nkambule B.B. Obesity-induced inflammation and insulin resistance: a mini-review on T-cells. Metab. Open. 2019;3:100015. doi: 10.1016/j.metop.2019.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014 doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Hameed I. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J. Diabetes. 2015;6:598. doi: 10.4239/wjd.v6.i4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atyia F.T.F., Gawaly A.M., El-Bar E.S.A., Eissa A.E.-M.T. Hepcidin level changes in type 2 diabetes. Med. J. Cairo Univ. 2018;86:3077–3082. [Google Scholar]
- 6.Jiang F., Sun Z.Z., Tang Y.T., Xu C., Jiao X.Y. Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res. Clin. Pract. 2011;93:43–48. doi: 10.1016/j.diabres.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Guo L.N., Yang Y.Z., Feng Y.Z. Serum and salivary ferritin and Hepcidin levels in patients with chronic periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2018;18:1–9. doi: 10.1186/s12903-018-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlDallal S.M., Jena N. Prevalence of anemia in type 2 diabetic patients. J. Hematol. 2018;7:57–61. doi: 10.14740/jh411w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbieri J. Anemia in patients with type 2 diabetes mellitus. World Appl. Sci. J. 2015;13:1–6. [Google Scholar]
- 10.Zheng X. Hepatic iron stores are increased as assessed by magnetic resonance imaging in a Chinese population with altered glucose homeostasis. Am. J. Clin. Nutr. 2011;94:1012–1019. doi: 10.3945/ajcn.111.015743. [DOI] [PubMed] [Google Scholar]
- 11.Altamura S. Uncoupled iron homeostasis in type 2 diabetes mellitus. J. Mol. Med. 2017;95:1387–1398. doi: 10.1007/s00109-017-1596-3. [DOI] [PubMed] [Google Scholar]
- 12.Collins J.F., Wessling-Resnick M., Knutson M.D. Hepcidin regulation of iron transport. J. Nutr. 2008;138:2284–2288. doi: 10.3945/jn.108.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth E. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 14.Sangkhae V., Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. An Int. Rev. J. 2017;8:126–136. doi: 10.3945/an.116.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vela D. Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 2018;16:1–18. doi: 10.1186/s12967-018-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak M., Lopez M.A., Gabayan V., Ganz T., Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagani A., Nai A., Silvestri L., Camaschella C. Hepcidin and anemia: a tight relationship. Front. Physiol. 2019;10:1–7. doi: 10.3389/fphys.2019.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R., Musallam K.M., Taher A.T., Rivella S. Ineffective erythropoiesis: anemia and iron overload. Hematol. Oncol. Clin. N. Am. 2018;32:213–221. doi: 10.1016/j.hoc.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao M., Ye Z., Qin Y., Wu T. Abnormal metabolic processes involved in the pathogenesis of non-alcoholic fatty liver disease (Review) Exp. Ther. Med. 2020;20 doi: 10.3892/etm.2020.9154. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim S., Oh T.J., Koh K.K. Mechanistic link between nonalcoholic fatty liver disease and cardiometabolic disorders. Int. J. Cardiol. 2015;201:408–414. doi: 10.1016/j.ijcard.2015.08.107. [DOI] [PubMed] [Google Scholar]
- 21.Britton L.J., Subramaniam V.N., Crawford D.H.G. Iron and non-alcoholic fatty liver disease. World J. Gastroenterol. 2016;22:8112–8122. doi: 10.3748/wjg.v22.i36.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raju K., Venkataramappa S. Primary hemochromatosis presenting as Type 2 diabetes mellitus: a case report with review of literature. Int. J. Appl. Basic Med. Res. 2018;8:57. doi: 10.4103/ijabmr.IJABMR_402_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amiri N. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol. Hepatol. From Bed to Bench. 2017;10:S1–S7. [PMC free article] [PubMed] [Google Scholar]
- 24.Dongiovanni P., Fracanzani A.L., Fargion S., Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J. Hepatol. 2011;55:920–932. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Andrews M., Soto N., Arredondo-Olguín M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition. 2015;31:51–57. doi: 10.1016/j.nut.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Guo X. Associations between serum hepcidin, ferritin and Hb concentrations and type 2 diabetes risks in a Han Chinese population. Br. J. Nutr. 2013;110:2180–2185. doi: 10.1017/S0007114513001827. [DOI] [PubMed] [Google Scholar]
- 27.Vela D. The role of insulin therapy in correcting hepcidin levels in patients with type 2 diabetes mellitus. Oman Med. J. 2017;32:195–200. doi: 10.5001/omj.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shalitin S., Deutsch V., Tauman R. Hepcidin, soluble transferrin receptor and IL-6 levels in obese children and adolescents with and without type 2 diabetes mellitus/impaired glucose tolerance and their association with obstructive sleep apnea. J. Endocrinol. Invest. 2018;41:969–975. doi: 10.1007/s40618-017-0823-7. [DOI] [PubMed] [Google Scholar]
- 29.Sam A.H. Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet. Med. 2013;30:1495–1499. doi: 10.1111/dme.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suárez-Ortegón M.F. Circulating hepcidin in type 2 diabetes: a multivariate analysis and double blind evaluation of metformin effects. Mol. Nutr. Food Res. 2015;59:2460–2470. doi: 10.1002/mnfr.201500310. [DOI] [PubMed] [Google Scholar]
- 31.Le Guenno G., Chanséaume E., Ruivard M., Morio B., Mazur A. Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res. Clin. Pract. 2007;77:363–370. doi: 10.1016/j.diabres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Simcox Judith A., McClain Donald.A. 2013. Iron and diabetes risk. Bone. 2013;23:1–7. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merlin C., Richard J., Con T., George J., P D. Unrecognized anemia in patients with diabetes. Diabetes Care. 2003;26:1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood D.C. Meta-analysis of observational studies. Mod. Methods Epidemiol. 2012:173–189. [Google Scholar]
- 35.Ryan R., Synnot A., Prictor M., Hill S. 2016. Cochrane Consumers and Communication Group Data Extraction Template for Included Studies; pp. 1–25. [Google Scholar]
- 36.G.A. Wells, B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos, P. Tugwell, G. Wells, B. Shea, D. O’Connell, et al. The Newcastle -Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 37.Balshem H. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan G.M., Feinn R. Using effect size—or why the P value is not enough. J. Grad. Med. Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landis J.R., Koch G.G. Landis amd Koch1977_agreement of categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 40.Richardson M., Garner P., Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin. Epidemiol. Glob. Heal. 2019;7:192–198. [Google Scholar]
- 41.Vela D., Sopi R.B., Mladenov M. Low hepcidin in type 2 diabetes mellitus: examining the molecular links and their clinical implications. Can. J. Diabetes. 2018;42:179–187. doi: 10.1016/j.jcjd.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Madu A.J., Ughasoro M.D. Anaemia of chronic disease: an in-depth review. Med. Princ. Pract. 2017;26:1–9. doi: 10.1159/000452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theurl I. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 44.Fuster J.J., Ouchi N., Gokce N., Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. World Patent Inf. 2016;118:11. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:1–12. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morceau F., Dicato M., Diederich M. Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediat. Inflamm. 2009;2009 doi: 10.1155/2009/405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wrighting D., Andrews N. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes. 2014;63:1506–1518. doi: 10.2337/db13-1195. [DOI] [PubMed] [Google Scholar]
- 49.Mleczko-Sanecka K. Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood. 2014;123:1574–1585. doi: 10.1182/blood-2013-07-515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao Z., Zhang W. Role of mTOR in glucose and lipid metabolism. Int. J. Mol. Sci. 2018;19:1–14. doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarrouk M., Finlay D.K., Foretz M., Viollet B., Cantrell D.A. Adenosine-mono-phosphate-activated protein kinase- independent effects of metformin in T cells. PloS One. 2014;9 doi: 10.1371/journal.pone.0106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M. AMPK serves as a therapeutic target against anemia of inflammation. Antioxidants Redox Signal. 2017;27:251–268. doi: 10.1089/ars.2016.6846. [DOI] [PubMed] [Google Scholar]
- 53.Brunetti P. The lean patient with type 2 diabetes: characteristics and therapy challenge. Int. J. Clin. Pract. 2007;61:3–9. doi: 10.1111/j.1742-1241.2007.01359.x. [DOI] [PubMed] [Google Scholar]
- 54.Home P. Insulin therapy in people with type 2 diabetes: opportunities and challenges. Diabetes Care. 2014;37:1499–1508. doi: 10.2337/dc13-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee M.K. High hemoglobin levels are associated with decreased risk of diabetic retinopathy in Korean type 2 diabetes. Sci. Rep. 2018;8:1–8. doi: 10.1038/s41598-018-23905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ko S.H. Association of vitamin B12 deficiency and metformin use in patients with type 2 diabetes. J. Kor. Med. Sci. 2014;29:965–972. doi: 10.3346/jkms.2014.29.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karamzad N. Serum hepcidin, the hepcidin/ferritin ratio and the risk of type 2 diabetes: a systematic review and meta-analysis. Curr. Med. Chem. 2020;27:1–2. doi: 10.2174/0929867327666200207120158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.