Abstract
Background
High alpha-fetoprotein (AFP) expressions (>400 ng/mL) are associated with poor oncological characteristics for hepatocellular carcinoma (HCC). However, prognosis after liver resection for high-AFP HCC is poorly studied. To investigate long-term recurrence and survival after hepatectomy for high-AFP HCC, and to identify the predictive value of postoperative incomplete biomarker response (IBR) on overall survival (OS) and recurrence-free survival (RFS).
Methods
Patients undergoing curative resection for high-AFP HCC were analyzed. According to the decline magnitude of serum AFP as measured at first follow-up (4~6 weeks after surgery), all patients were divided into the complete biomarker response (CBR) and IBR groups. Characteristics, recurrence, and survival rates were compared. Univariate and Multivariate Cox-regression analyses were performed to identify independent predictors associated with poorer OS and RFS after liver resection for high-AFP HCC.
Results
Among 549 patients, the overall and early recurrence rates in patients with IBR were significantly higher than patients with CBR (97.8%vs.56.4%, and 92.5%vs.33.3%, both P<0.001). On multivariate analysis, postoperative IBR was the strongest risk factor with the highest hazard ratio in predicting poor OS (HR 2.97; 95% CI 2.49~3.45; P<0.001) and RFS (HR 4.29; 95% CI 3.31~5.55; P<0.001).
Conclusion
Postoperative biomarker response of serum AFP can be used in predicting recurrence and survival for high-AFP HCC patients. Once postoperative IBR was identified at first follow-up, subsequent enhanced recurrence surveillance and available treatments against recurrence should actively be considered.
Keywords: hepatocellular carcinoma, alpha-fetoprotein, hepatectomy, survival, recurrence
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent malignancies and the third leading cause of cancer-related deaths, making it a major public health issue globally.1 Surgical resection represents the mainstay approach for HCC and provides the possibility of cure for patients with HCC.2 Despite recent advances in surgical technique and multidisciplinary treatment, long-term prognosis after HCC resection remains dismal due to the high risk of tumor recurrence, with 40~50% of patients early relapsing within 2 years after surgery.3,4 Many tumor-related pathological characteristics, such as tumor size, tumor number, macrovascular and microvascular invasion, as well as preoperative serum levels of HCC biomarkers, such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin, have been recognized as independent predictors of long-term recurrence and survival after HCC resection.5–7 Postoperative HCC recurrence could be divided into early recurrence (less than 2 years) and late recurrence (more than 2 years) according to the time to recurrence since surgery.8 Early recurrence is most likely the consequence of occult metastasis from the initial tumor, whereas late recurrence after 2 years of surgery is often of clonal origin, which is different from the original tumor, suggesting a de novo second primary HCC. Numerous previous studies have showed that early recurrence is commonly associated with aggressive tumor pathological characteristics, while late recurrence is usually associated with underlying severity of liver disease, like cirrhosis and active hepatitis.5,8–10 Nevertheless, more potentially effective predictors for early and late recurrence still need identifying to improve long-term oncologic outcomes.
AFP is the most commonly used biomarker of HCC for detection, screening, surveillance, therapeutic efficacy measurement, and prognostic evaluation.11–14 When using a serum level of 20 ng/mL as the cut-off value, sensitivity and specificity for HCC diagnosis were 60~70% and 80~90%, respectively.12,14 When the cut-off value increases to 400 ng/mL, the diagnostic specificity for HCC can go up to almost 100%. In clinical settings, a high AFP expression (>400 ng/mL) represents a well-accepted indicator of poor oncological characteristics of HCC, which has been demonstrated to be closely associated with bad prognosis after various treatment modalities, including liver transplantation,14–17 transcatheter arterial chemoembolization (TACE),18,19 and systemic therapy.19–21 As for liver resection for HCC, previous studies have also identified that a high preoperative AFP level (>400 ng/mL) was independently associated with a high recurrence rate and poor survival when compared with a low preoperative AFP level (<400 ng/mL).22–25 To our knowledge, no prognostic studies have been published exclusively on liver resection for patients with HCC and high AFP expressions (>400 ng/mL).
The post-treatment biomarker response for malignant tumors has increasingly been recognized as a potentially effective and readily available tool in assessing therapeutic efficacy, predicting oncologic prognosis, as well as monitoring postoperative recurrence.26,27 The predictive value of post-treatment AFP response has been demonstrated in liver transplantation28,29 and in various non-curative treatments for HCC such as TACE, chemoradiotherapy, systemic therapies including chemotherapy, targeted therapy and immunotherapy.13,26,30–33 However, there have only been a few published reports on the predictive value of postoperative AFP response on long-term recurrence and survival following liver resection.34–40 These studies, however, failed to use a relatively unified definition for postoperative complete response of serum AFP, including time points used in preoperative and postoperative serum AFP measurement, and what constituted a significant magnitude of decline in postoperative AFP levels.34–40 The conclusions which can be drawn from these studies can hardly be used as reference values, let alone for clinical application.
Based on a large multicenter database with long-term follow-up, the present study aimed to study the clinical characteristics, long-term oncologic prognosis and prognostic factors after curative liver resection for HCC patients with high-AFP levels (>400 ng/mL). Pertinent definitions for postoperative complete and incomplete biomarker response of serum AFP were proposed so that the results of this study could be generalized. Attempts were made to identify the predictive value of postoperative AFP response on recurrence and survival after curative resection for high-AFP HCC to provide data to clinicians in planning for recurrence surveillance, and in decision-making on offering early treatments against recurrence for these patients.
Patients and Methods
Patient Selection
A multicenter study was conducted on patients who underwent open liver resection with curative-intent for HCC as an initial treatment at 11 hospitals in China between January 2008 and December 2015. The clinical data of these patients were retrospectively reviewed and analyzed. These 11 hospitals were Zhejiang Provincial People’s Hospital, Eastern Hepatobiliary Surgery Hospital, the First Affiliated Hospital of Harbin Medical University, Fuyang People’s Hospital, Ziyang First People’s Hospital, Meizhou People’s Hospital, Pu’er People’s Hospital, Tongji Hospital, Liuyang People’s Hospital, Mengchao Hepatobiliary Hospital, and Chongqing University Cancer Hospital. All patients had resectable tumors, adequate volumes of future liver remnant (FLR) with good liver function, and absence of distant metastasis. The diagnosis of HCC was confirmed by postoperative histopathological examinations. Curative resection was defined as complete removal of all tumor nodules with microscopic-negative margins (R0 resection). Patients were excluded with: 1) age less than 18 years, 2) no data on preoperative AFP within 1 week before surgery, 3) a preoperative AFP level ≤400 ng/mL, 4) R1 (microscopically positive) or R2 (macroscopically positive) resection, 5) postoperative 90-day mortality, 6) first follow-up evaluation in <4 or >8 weeks after surgery, 7) recurrent tumor(s) diagnosed by medical imaging at first follow-up within 8 weeks after operation, 8) no data of serum AFP measurement at the first postoperative follow-up, and 9) missing data on potentially important prognostic variables. The study was censored on December 31, 2019. Perioperative evaluation of the staging method and surgical procedures were generally consistent at each participating hospital.41 Resection criteria were constant over the study period, and the technical details of liver resection have been described previously.41 This study was performed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies. It was approved by the Institutional Review Board of the Eastern Hepatobiliary Surgery Hospital of Shanghai, China. Written informed consent was obtained to use their medical records for scientific research from all patients when they were admitted to hospital.
Clinicopathological Variables
The patients’ demographic and liver-related characteristics included age, sex, diabetes mellitus, body mass index, American Society of Anesthesiologists score, serological tests of serum hepatitis B surface antigen and anti-hepatitis C virus antibody, presence of cirrhosis and portal hypertension, and Child-Pugh grading. Liver fibrosis was assessed semi-quantitatively on a scale of 0–4: F0, absent; F1, portal fibrosis with no fibrosis beyond the portal tract; F2, portal fibrosis with a few septa; F3, portal fibrosis with numerous septa; and F4, cirrhosis. Portal hypertension was confirmed when there was either splenomegaly with a decreased platelet count (≤100×109/L) or gastroesophageal varices. Tumor-related characteristics consisted of preoperative AFP levels which were measured within 1 week before operation, maximum tumor diameter, tumor number, satellite nodules, tumor encapsulation, tumor differentiation, and macroscopic and microscopic vascular invasion. Operative variables included intraoperative blood loss, requirement of intraoperative blood transfusion, extent of liver resection (minor or major), type of hepatectomy (anatomical or non-anatomical), as well as resection margin (<1 or ≥ 1cm). Major hepatectomy referred to resection of three or more Couinaud’s liver segments. Anatomical and non-anatomical liver resections were defined according to the Brisbane 2000 nomenclature of Liver Anatomy and Resections. All continuous variables were divided into binaries as categorical variables based on those commonly used in previous studies.5,42 According to the commonly used cut-off values of serum AFP level at the vast majority of Chinese hospitals, a level <20 ng/mL was defined as negative, a level between 20 and 400 ng/mL was defined as positive but low, and a level >400 ng/mL was defined as high. Among the latter, a level between 401 and 1200 ng/mL was defined as intermediately high, while a level >1200 ng/mL was defined as extremely high.
Postoperative Follow-Up
After hospital discharge, all patients were regularly followed-up at each hospital. The postoperative surveillance strategies for recurrence were consistent across all participating hospitals. This consisted of history taking, physical examination, detection of serum AFP level, ultrasonography or contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the upper abdomen. In general, the first follow-up evaluation, either during hospitalization or at an outpatient setting, was performed at 4~6 weeks after surgery. The serum AFP measurement taken at this check-up was identified as the postoperative AFP level. These patients were subsequently followed-up at 2- or 3-monthly intervals for the first half-year after surgery, 3- to 4-monthly for the next 1.5 years, and then 3- to 6-monthly thereafter. When recurrence was suspected, enhanced CT or MRI, bone scan or positron emission tomography were performed as clinically indicated. Tumor recurrence was defined as new appearance of intrahepatic or extrahepatic tumor nodule(s), with typical imaging features which were consistent with HCC on contrast-enhanced CT or MRI, with or without a rise in serum AFP level. The diagnosis was made when dynamic CT or magnetic resonance imaging showed contrast enhancement in the arterial phase and washout in the venous phase or when hepatic angiography disclosed high tumor vascularity.5,43 Once tumor recurrence was suspected, patients were hospitalized to confirm diagnosis. Appropriate management for HCC recurrence included re-resection, liver transplantation, local ablation, TACE, systemic therapy, radiotherapy, and best supportive care. The dates of the initial HCC recurrence, last follow-up, and death, as well as patterns of initial recurrence and reasons of death, were recorded. The end follow-up time in this study was December 31, 2019.
Postoperative Biomarker Response of Serum AFP
Post-treatment biomarker response is an important variable that reflects the degree of tumor removal which is associated with long-term recurrence and overall survival outcomes after curative HCC resection. To allow clinical generalizability and improve feasibility, two postoperative biomarker responses of serum AFP after surgery were compared, ie, postoperative complete biomarker response (CBR) and incomplete biomarker conversion (IBR). Considering the half-life of AFP to be 5~7 days, and routine postoperative first check-up in most centers was performed from 1 month to 6 weeks (ie 30~42 days) after surgery, postoperative CBR was defined as normalization of postoperative AFP levels to <20 ng/mL in HCC patients with preoperative intermediately-high-AFP levels (401~1200 ng/mL), or to <1/60 (~26) of preoperative serum AFP level for patients with preoperative extremely-high-AFP levels (>1200 ng/mL). On the other hand, for patients with failure of preoperative AFP levels to decrease to the above-mentioned levels at first follow-up were defined to have postoperative IBR.
Study Endpoints
The primary endpoints of this study were overall survival (OS) and recurrence-free survival (RFS). OS was calculated from date of liver resection to either date of death or date of the last follow-up, while RFS was calculated from date of liver resection to date of first HCC recurrence, date of death, or date of the last follow-up. The secondary endpoints of this study were the rates of overall recurrence, early recurrence (within 2 years after surgery), late recurrence (beyond 2 years after surgery), and postoperative cancer-specific and non-cancer-specific mortality on follow-up. Note the percentage of late recurrence was calculated by the ratio of the number of patients who had recurrence after 2 years of surgery to the number of patients who were disease-free alive at 2 years of surgery.
Statistical Analysis
Categorical variables were expressed as proportions (%) or numbers (n) and compared using the χ2 test or Fisher’s exact test. Multivariate analyses were performed using logistic regression with a forward stepwise variable selection to identify independent predictors of IBR following curative liver resection for high-AFP HCC, with odds ratios (OR) and their 95% confidence intervals (CIs). Long-term oncologic outcomes after curative resection were compared between patients with postoperative IBR and CBR. The OS and RFS rates were calculated and compared using the Kaplan–Meier method generated by the Log rank test. Univariate and multivariate Cox regression analyses were performed to identify independent predictors associated with poor OS and RFS, with hazard ratios (HRs) and their 95% CI. Using a forward stepwise variable selection, variables that were significant at a P value <0.1 on univariate analyses were entered into the Cox proportional hazard model. All statistical analyses were performed using the SPSS software version 25.0 (SPSS, Chicago, IL, USA). A two-tailed P value <0.05 was considered to be statistically significant.
Results
Clinical Characteristics
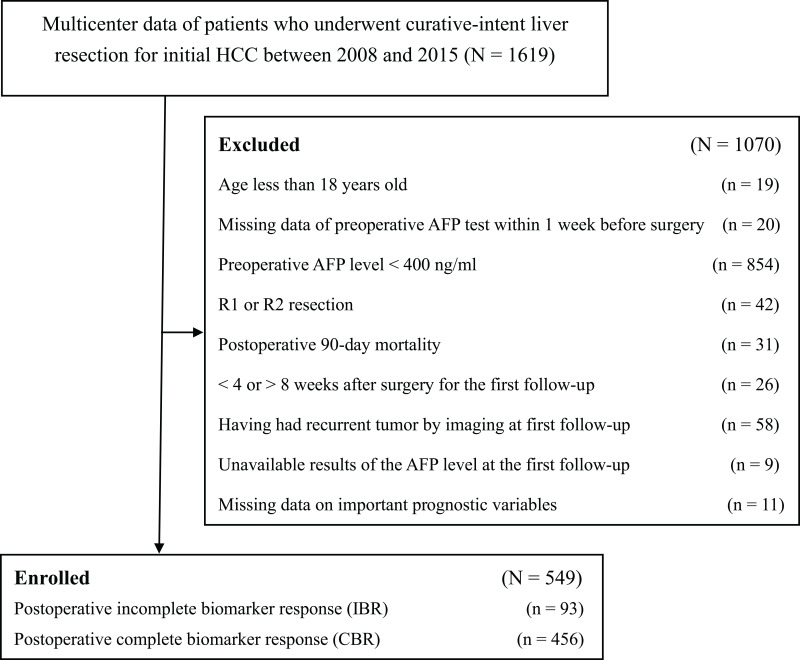
HCC patients with high-AFP levels (>400 ng/mL) after curative liver resection who met the inclusion criteria (n=549) formed the analytic cohort for this retrospective study (Figure 1). There were 469 (85.4%) males and 80 (14.6%) females. On the first postoperative follow-up (median: 35 days after surgery), 456 (83.1%) patients achieved a complete response in serum AFP level (the CBR group). However, 93 (16.9%) patients failed to achieve the predetermined levels (the IBR group). Comparison of clinical characteristics between the two groups of patients is summarized in Table 1, which shows no significant differences in all demographic and liver-related characteristics between the two groups. However, there were significant differences in most tumor-related characteristics and some operative variables, including preoperative tumor rupture, tumor size, macrovascular and microvascular invasion, satellite nodules, incomplete tumor encapsulation, intraoperative blood loss, and intraoperative blood transfusion (all P < 0.05). The proportion of patients with preoperative extremely high AFP levels (>1200 ng/mL) in the IBR group (77 [82.8%]) was significantly higher than those in the CBR group (316 [69.3%]) (P = 0.008).
Figure 1.
Flow chart of the cohort.
Abbreviations: HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein.
Table 1.
Comparisons of Patients’ Clinical Characteristics
| Variables (N [%]) | Total (N=549) | IBR (N=93) | CBR (N=456) | P |
|---|---|---|---|---|
| Male Sex | 469 (85.4) | 76 (81.7) | 393 (86.2) | 0.262 |
| Age > 60 years old | 83 (15.1) | 9 (9.7) | 74 (16.2) | 0.115 |
| Diabetes mellitus | 19 (3.5) | 3 (3.2) | 16 (3.5) | 0.892 |
| Overweight* | 206 (37.5) | 34 (36.6) | 172 (37.7) | 0.907 |
| ASA score > 2 | 58 (10.6) | 9 (9.7) | 49 (10.7) | 0.855 |
| HBsAg (+) | 511 (93.1) | 85 (91.4) | 426 (93.4) | 0.501 |
| Anti-HCV (+) | 8 (1.5) | 2 (2.2) | 6 (1.3) | 0.629 |
| Cirrhosis | 431 (78.5) | 71 (76.3) | 360 (78.9) | 0.581 |
| Portal hypertension | 127 (23.1) | 19 (20.4) | 108 (23.7) | 0.590 |
| Child-Pugh grade B | 55 (10.0) | 12 (12.9) | 43 (9.4) | 0.342 |
| Preoperative AFP level | ||||
| Intermediately-high (401~1200 ng/mL) | 156 (28.4) | 16 (17.2) | 140 (30.7) | 0.008 |
| Extremely-high (> 1200 ng/mL) | 393 (71.6) | 77 (82.8) | 316 (69.3) | |
| Preoperative tumor rupture | 21 (3.8) | 12 (12.9) | 9 (2.0) | < 0.001 |
| Maximum tumor size > 5.0 cm | 290 (52.8) | 68 (73.1) | 222 (48.7) | < 0.001 |
| Multiple tumors | 87 (15.8) | 16 (17.2) | 71 (15.6) | 0.755 |
| Macrovascular invasion | 47 (8.6) | 18 (19.4) | 29 (6.4) | < 0.001 |
| Microvascular invasion | 252 (45.9) | 53 (57.0) | 199 (43.6) | 0.022 |
| Satellite nodules | 118 (21.5) | 28 (30.1) | 90 (19.7) | 0.037 |
| Incomplete tumor encapsulation | 289 (52.6) | 60 (64.5) | 229 (50.2) | 0.012 |
| Poor tumor differentiation | 370 (67.4) | 60 (64.5) | 310 (68.0) | 0.545 |
| Intraoperative blood loss > 600 mL | 111 (20.2) | 26 (28.0) | 85 (18.6) | 0.048 |
| Intraoperative blood transfusion | 111 (20.2) | 28 (30.1) | 83 (18.2) | 0.015 |
| Major hepatectomy | 128 (23.3) | 33 (35.5) | 95 (20.8) | 0.004 |
| Anatomical resection | 406 (74.0) | 58 (62.4) | 348 (76.3) | 0.007 |
| Resection margin < 1.0 cm | 269 (49.0) | 66 (71.0) | 203 (44.5) | < 0.001 |
Note: *Body mass index >24.0 kg/m2.
Abbreviations: ASA, American Society of Anesthesiologists; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, alpha-fetoprotein; IBR, incomplete biomarker response; CBR, complete biomarker response.
Predictors of Postoperative IBR
On univariate analysis, variables which were found to be significant at a P value <0.1 between the IBR and CBR groups were entered into multivariate logistic regression analysis. Multivariate analysis showed preoperative tumor rupture (OR: 5.47, 95% CI: 2.14–14.03, P < 0.001), tumor size >5 cm (OR: 2.06, 95% CI: 1.22–3.46, P = 0.007), presence of macrovascular invasion (OR: 2.58, 95% CI: 1.31–5.06, P = 0.006) and resection margin <1 cm (OR: 2.25, 95% CI: 2.35–3.75, P = 0.002) to be independent predictors associated with postoperative IBR at first postoperative follow-up after curative resection for HCC patients with high-AFP levels.
Long-Term Oncologic Outcomes
At a median follow-up of 47.8 months for the entire cohort (n = 549), 290 (52.8%) patients had died, and 348 (63.4%) patients developed HCC recurrence, with 237 [68.1%] patients with recurrence occurring within 2 years, and 111 [31.9%] patients beyond 2 years after surgery. The 1-, 3-, and 5-year OS rates for the whole cohort were 92.2, 66.5, and 53.9%, respectively. The corresponding RFS rates were 70.7, 47.2, and 37.4%, respectively.
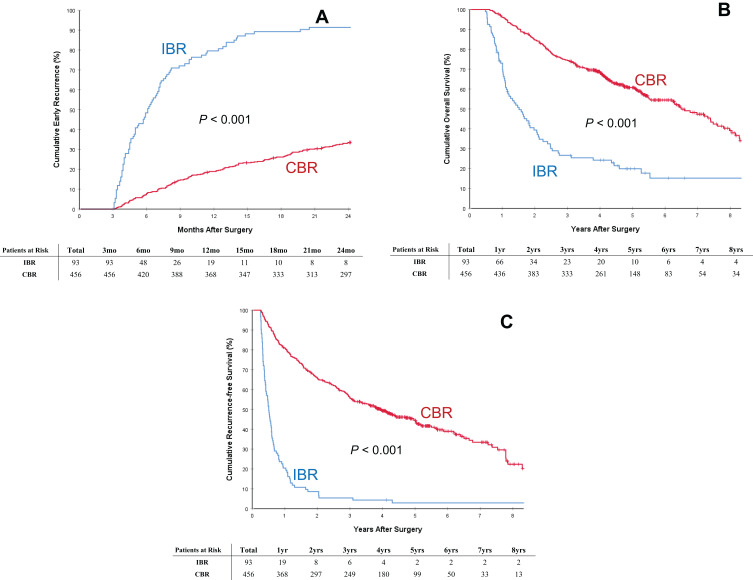
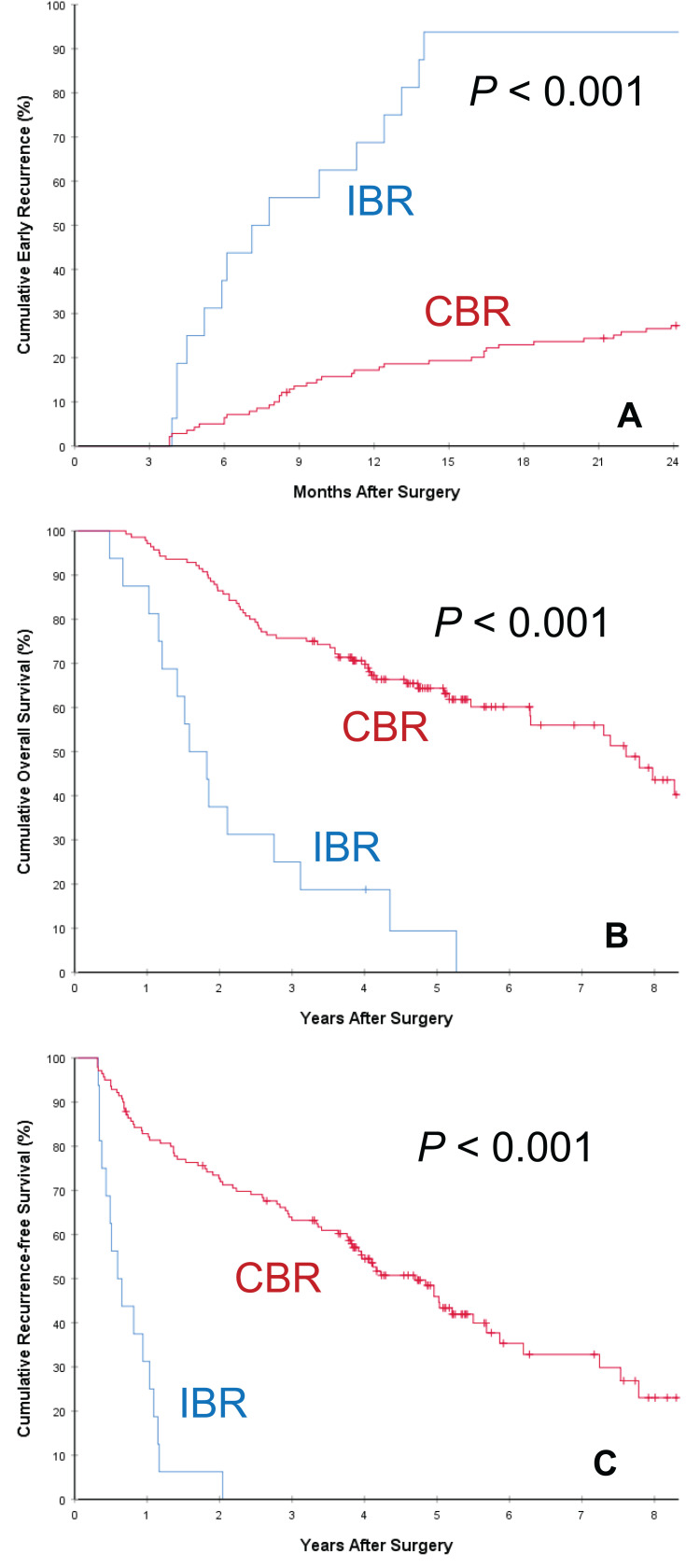
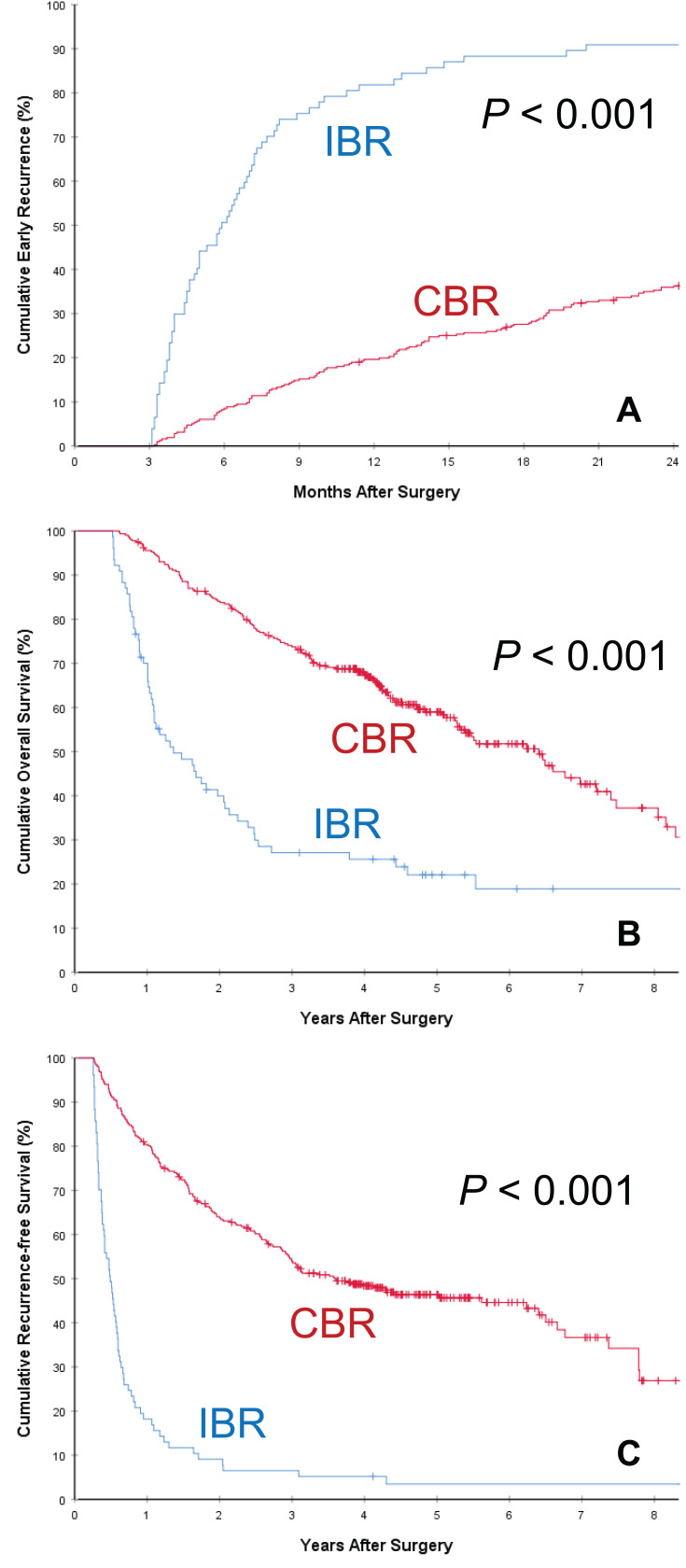
Comparison of long-term oncologic outcomes between the two groups of patients with postoperative IBR and CBR is listed in Table 2. The postoperative mortality and overall recurrence rates for patients in the IBR group were significantly higher than those in the CBR group (97.8% vs 56.4%, and 80.6% vs 47.1%, respectively, both P < 0.001). The rate of early HCC recurrence in the IBR group was also significantly higher than those in the CBR group (92.5% [86/93] vs 33.3% [152/456], P < 0.001), despite the two groups having comparable rates of late HCC recurrence (62.5% [5/8] vs 35.3% [105/297], P = 0.142). The cumulative rates of early HCC recurrence between the two groups of patients with IBR and CBR are depicted in Figure 2A. Similar results of early HCC recurrence with significant difference (both P < 0.001) were showed in the subgroup cohorts of patients with preoperative intermediately high (Figure 3A) and extremely high (Figure 4A) AFP level, respectively. The patterns of initial HCC recurrence also differed between the two groups of patients. While 70 (75.3%, 70/93) and 7 (7.5%, 7/93) patients in the IBR group developed sole intrahepatic and sole extrahepatic recurrences, respectively, the incidences were significantly higher than those in patients in the CBR group (45.4% and 2.4%, both P < 0.05). Furthermore, the incidence of cancer-specific deaths in patients with IBR was also significantly higher than those in patients with CBR (74.2% vs 36.4%, P < 0.001).
Table 2.
Comparisons of Long-Term Oncologic Outcomes
| Variables (N [%]) | Total (N=549) | IBR (N=93) | CBR (N=456) | P |
|---|---|---|---|---|
| Period of follow-up, months* | 47.8±27.3 | 27.7±25.6 | 51.9±25.8 | < 0.001 |
| Postoperative adjuvant TACE | 171 (31.1) | 29 (31.2) | 142 (31.1) | 1.000 |
| Recurrence during the follow-up | 348 (63.4) | 91 (97.8) | 257 (56.4) | < 0.001 |
| Early recurrence (within 2 years) | 237 (43.2) | 86 (92.5) | 152 (33.3) | < 0.001 |
| Later recurrence (beyond 2 years)** | 111 (36.4) | 5 (62.5) | 105 (35.3) | < 0.001 |
| Patterns of initial recurrence | ||||
| Intrahepatic recurrence | 277 (50.5) | 70 (75.3) | 207 (45.4) | < 0.001 |
| Extrahepatic recurrence | 18 (3.3) | 7 (7.5) | 11 (2.4) | 0.012 |
| Intra- and Extrahepatic recurrence | 53 (9.7) | 14 (15.1) | 39 (8.6) | 0.080 |
| Mortality during the follow-up | 290 (52.8) | 75 (80.6) | 215 (47.1) | < 0.001 |
| Cancer-specific mortality | 235 (42.8) | 69 (74.2) | 166 (36.4) | < 0.001 |
| Non-cancer-specific mortality | 55 (10.0) | 6 (6.5) | 49 (10.7) | 0.257 |
| Median OS, 95% CI, months | 65.6 (55.4~75.8) | 18.2 (12.8~23.6) | 77.9 (65.4~90.4) | < 0.001 |
| 1-year OS rate, % | 92.2 | 73.0 | 96.0 | |
| 3-year OS rate, % | 66.5 | 26.6 | 74.3 | |
| 5-year OS rate, % | 53.9 | 19.9 | 60.7 | |
| Median RFS, 95% CI, months | 33.5 (26.9~40.1) | 6.1 (5.1~7.1) | 47.5 (37.5~57.5) | < 0.001 |
| 1-year RFS rate, % | 70.7 | 20.4 | 80.9 | |
| 3-year RFS rate, % | 47.2 | 5.4 | 55.7 | |
| 5-year RFS rate, % | 37.4 | 2.9 | 44.5 |
Notes: *Values are mean ± standard deviation. **Numbers of patients who were disease-free alive at 2 years of surgery were 305, 8, and 297 in the overall, IBR, and CBR cohorts, respectively.
Abbreviations: CI, confidence interval; OS, overall survival; RFS, recurrence-free survival; TTR, time-to-recurrence; IBR, incomplete biomarker response; CBR, complete biomarker response; TACE, transcatheter arterial chemoembolization.
Figure 2.
Cumulative incidence of early recurrence (<2 years after surgery, (A)), overall survival (B) and recurrence-free survival (C) curves comparisons between patients with postoperative incomplete biomarker response (IBR) and complete biomarker response (CBR).
Figure 3.
Subgroup analyses of cumulative incidence of early recurrence (<2 years after surgery, (A)), overall survival (B) and recurrence-free survival (C) curves comparisons between patients with postoperative incomplete biomarker response (IBR) and complete biomarker response (CBR) in patients with preoperative intermediately high AFP level (401~1200 ng/mL).
Figure 4.
Subgroup analyses of cumulative incidence of early recurrence (<2 years after surgery, (A)), overall survival (B) and recurrence-free survival (C) curves comparisons between patients with postoperative incomplete biomarker response (IBR) and complete biomarker response (CBR) in patients with preoperative extremely high AFP level (>1200 ng/mL).
Comparison of OS and RFS Between the IBR and CBR Groups
The OS and RFS curves between patients with postoperative IBR and CBR are demonstrated in Figure 2B and C, respectively. As shown in Table 2, when compared with patients who had postoperative CBR, patients who had postoperative IBR had significantly poorer 1-, 3-, and 5-year OS (73.0, 26.6, and 19.9% vs 96.0, 74.3, and 60.7%, respectively, P<0.001) and RFS rates (20.4, 5.4 and 2.9% vs 80.9, 55.7 and 44.5%, respectively, P<0.001). The median overall and recurrence-free survival outcomes in patients with IBR were 18.2 and 6.1 months, respectively, which were significantly poorer than those in patients with CBR (77.9 and 47.5 months, respectively, both P<0.001). Similar results of OS and RFS with significant difference (both P < 0.001) were showed in the subgroup cohorts of patients with preoperative intermediately high (Figure 3B and C) and extremely high (Figure 4B and C) AFP level, respectively.
Independent Predictors of OS, RFS, and Early Recurrence
Tables 3 and 4 summarize the results of univariate and multivariate analyses of predictive factors which were associated with poor OS and RFS after curative liver resection for high-AFP HCC. Multivariate Cox-regression analyses revealed that preoperative tumor rupture, tumor size >5 cm, macrovascular invasion, presence of satellite nodules, intraoperative blood transfusion, resection margin <1cm, and postoperative IBR were independent risk factors associated with poor OS and RFS. Among all the independent predictors, postoperative IBR was the risk with the highest HR, and it was independently associated with poor OS (HR, 2.97; 95% CI, 2.49–3.45; P < 0.001) and poor RFS (HR, 4.29; 95% CI, 3.31–5.55; P < 0.001) after curative liver resection for high-AFP HCC. In addition, as shown in Table 5, multivariate Cox-regression analysis demonstrated that postoperative IBR was also independently associated with increased early recurrence rate following curative liver resection for high-AFP HCC (HR, 5.43; 95% CI, 4.08–7.25; P < 0.001).
Table 3.
Univariate and Multivariate Cox-Regression Analyses of Predictors Associated with Overall Survival After Curative Liver Resection for High-AFP Hepatocellular Carcinoma
| Variables | UV HR (95% CI) | UV P | MV HR (95% CI) | MV P* |
|---|---|---|---|---|
| Male sex | 1.01 (0.73–1.39) | 0.966 | ||
| Age > 60 years | 0.99 (0.71–1.38) | 0.957 | ||
| Diabetes mellitus | 1.27 (0.71–2.27) | 0.415 | ||
| Overweight | 1.15 (0.91–1.45) | 0.253 | ||
| ASA score > 2 | 1.12 (0.77–1.63) | 0.548 | ||
| HBsAg (+) | 0.83 (0.53–1.32) | 0.435 | ||
| Anti-HCV (+) | 1.49 (0.62–3.62) | 0.376 | ||
| Cirrhosis | 1.29 (1.02–1.73) | 0.041 | 1.43 (1.06–1.94) | 0.020 |
| Portal hypertension | 1.13 (0.86–1.47) | 0.373 | ||
| Child-Pugh grade B | 1.61 (1.14–2.27) | 0.006 | NA | 0.124 |
| Preoperative AFP > 1200 ng/mL | 1.22 (0.94–1.58) | 0.135 | ||
| Preoperative tumor rupture | 2.58 (1.58–4.22) | < 0.001 | 2.51 (1.51–4.18) | < 0.001 |
| Maximum tumor size > 5 cm | 2.07 (1.63–2.63) | < 0.001 | 1.45 (1.09–1.92) | 0.011 |
| Multiple tumors | 1.78 (1.35–2.38) | < 0.001 | NA | 0.765 |
| Macrovascular invasion | 3.98 (2.85–5.56) | < 0.001 | 2.08 (1.45–2.98) | < 0.001 |
| Microvascular invasion | 1.76 (1.39–2.22) | < 0.001 | 1.32 (1.01–1.73) | 0.044 |
| Satellite nodules | 2.36 (1.83–3.04) | < 0.001 | 1.47 (1.09–1.97) | 0.011 |
| Incomplete tumor encapsulation | 2.15 (1.69–2.74) | < 0.001 | NA | 0.234 |
| Poor tumor differentiation | 1.23 (0.94–1.60) | 0.130 | ||
| Intraoperative blood loss > 600 mL | 1.75 (1.34–2.28) | < 0.001 | NA | 0.109 |
| Intraoperative blood transfusion | 2.17 (1.67–2.81) | < 0.001 | 1.63 (1.24–2.13) | < 0.001 |
| Major hepatectomy | 1.89 (1.46–2.44) | < 0.001 | NA | 0.256 |
| Non-anatomical resection | 1.20 (0.93–1.55) | 0.170 | ||
| Resection margin < 1 cm | 2.38 (1.88–3.02) | < 0.001 | 1.97 (1.54–2.53) | < 0.001 |
| Postoperative adjuvant TACE | 0.78 (0.61–1.09) | 0.086 | NA | 0.118 |
| Postoperative IBR | 3.37 (2.58–4.41) | < 0.001 | 2.97 (2.49–3.45) | < 0.001 |
Note: *Those variables found significant at P < 0.1 in univariable analyses were entered into multivariable analyses.
Abbreviations: ASA, American Society of Anesthesiologists; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, alpha-fetoprotein; HR, hazard ratio; MV, multivariable; NA, not applicable; UV, univariable; CI, confidence interval; IBR, incomplete biomarker response; TACE, transcatheter arterial chemoembolization.
Table 4.
Univariate and Multivariate Cox-Regression Analyses of Predictors Associated with Recurrence-Free Survival After Curative Liver Resection for High-AFP Hepatocellular Carcinoma
| Variables | UV HR (95% CI) | UV P | MV HR (95% CI) | MV P* |
|---|---|---|---|---|
| Male sex | 0.97 (0.72–1.30) | 0.829 | ||
| Age > 60 years | 0.87 (0.64–1.18) | 0.367 | ||
| Diabetes mellitus | 1.32 (0.77–2.25) | 0.313 | ||
| Overweight | 1.09 (0.88–1.35) | 0.421 | ||
| ASA score > 2 | 0.91 (0.64–1.28) | 0.574 | ||
| HBsAg (+) | 0.78 (0.53–1.15) | 0.211 | ||
| Anti-HCV (+) | 1.39 (0.62–3.12) | 0.426 | ||
| Cirrhosis | 1.19 (0.92–1.54) | 0.198 | ||
| Portal hypertension | 1.06 (0.83–1.34) | 0.658 | ||
| Child-Pugh grade B | 1.38 (1.03–1.91) | 0.069 | NA | 0.331 |
| Preoperative AFP > 1200 ng/mL | 1.19 (0.94–1.49) | 0.148 | ||
| Preoperative tumor rupture | 2.26 (1.40–3.63) | 0.001 | 1.70 (1.05–2.77) | 0.032 |
| Maximum tumor size > 5 cm | 1.86 (1.51–2.30) | < 0.001 | 1.29 (1.03–1.62) | 0.026 |
| Multiple tumors | 1.89 (1.46–2.46) | < 0.001 | NA | 0.130 |
| Macrovascular invasion | 6.15 (4.45–8.50) | < 0.001 | 3.50 (2.50–4.88) | < 0.001 |
| Microvascular invasion | 1.63 (1.33–2.01) | < 0.001 | NA | 0.442 |
| Satellite nodules | 2.44 (1.94–3.09) | < 0.001 | 1.77 (1.39–2.27) | < 0.001 |
| Incomplete tumor encapsulation | 2.16 (1.74–2.67) | < 0.001 | 1.44 (1.14–1.83) | 0.003 |
| Poor tumor differentiation | 1.29 (1.02–1.62) | 0.031 | NA | 0.962 |
| Intraoperative blood loss > 600 mL | 1.69 (1.33–2.15) | < 0.001 | NA | 0.350 |
| Intraoperative blood transfusion | 1.90 (1.50–2.42) | < 0.001 | 1.42 (1.10–1.82) | 0.006 |
| Major hepatectomy | 1.85 (1.47–2.34) | < 0.001 | NA | 0.254 |
| Non-anatomical resection | 1.14 (0.90–1.44) | 0.281 | ||
| Resection margin < 1 cm | 2.19 (1.77–2.70) | < 0.001 | 1.80 (1.45–2.24) | < 0.001 |
| Postoperative adjuvant TACE | 1.03 (0.83–1.29) | 0.763 | ||
| Postoperative IBR | 5.73 (4.45–7.37) | < 0.001 | 4.29 (3.31–5.55) | < 0.001 |
Note: *Those variables found significant at P < 0.1 in univariable analyses were entered into multivariable analyses.
Abbreviations: ASA, American Society of Anesthesiologists; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, alpha-fetoprotein; HR, hazard ratio; MV, multivariable; NA, not applicable; UV, univariable; CI, confidence interval; IBR, incomplete biomarker response; TACE, transcatheter arterial chemoembolization.
Table 5.
Univariate and Multivariate Cox-Regression Analyses of Risk Factors Associated with Early Recurrence After Curative Liver Resection for High-AFP Hepatocellular Carcinoma
| Variables | UV HR (95% CI) | UV P | MV HR (95% CI) | MV P* |
|---|---|---|---|---|
| Male sex | 1.00 (0.70–1.44) | 0.986 | ||
| Age > 60 years | 0.83 (0.57–1.21) | 0.322 | ||
| Diabetes mellitus | 1.36 (0.72–2.57) | 0.337 | ||
| Overweight | 1.06 (0.81–1.37) | 0.686 | ||
| ASA score > 2 | 0.81 (0.52–1.26) | 0.345 | ||
| HBsAg (+) | 0.71 (0.45–1.11) | 0.128 | ||
| Anti-HCV (+) | 1.11 (0.41–2.99) | 0.831 | ||
| Cirrhosis | 1.12 (0.81–1.54) | 0.494 | ||
| Portal hypertension | 0.90 (0.66–1.23) | 0.507 | ||
| Child-Pugh grade B | 1.32 (0.89–1.95) | 0.173 | ||
| Preoperative AFP > 1200 ng/mL | 1.53 (1.23–2.08) | 0.006 | NA | 0.592 |
| Preoperative tumor rupture | 2.78 (1.67–4.63) | < 0.001 | NA | 0.072 |
| Maximum tumor size > 5 cm | 2.02 (1.55–2.64) | < 0.001 | NA | 0.153 |
| Multiple tumors | 2.08 (1.54–2.80) | < 0.001 | 1.72 (1.25–2.37) | 0.001 |
| Macrovascular invasion | 5.57 (3.96–7.82) | < 0.001 | 3.19 (2.25–4.51) | < 0.001 |
| Microvascular invasion | 1.65 (1.28–2.14) | < 0.001 | NA | 0.674 |
| Satellite nodules | 2.56 (1.96–3.35) | < 0.001 | NA | 0.166 |
| Incomplete tumor encapsulation | 2.26 (1.72–2.97) | < 0.001 | 1.44 (1.08–1.93) | 0.014 |
| Poor tumor differentiation | 1.18 (0.89–1.56) | 0.244 | ||
| Intraoperative blood loss > 600 mL | 1.75 (1.32–2.33) | < 0.001 | NA | 0.366 |
| Intraoperative blood transfusion | 2.13 (1.61–2.81) | < 0.001 | 1.69 (1.27–2.24) | < 0.001 |
| Major hepatectomy | 1.92 (1.46–2.52) | < 0.001 | NA | 0.095 |
| Non-anatomical resection | 1.31 (0.99–1.73) | 0.055 | NA | 0.247 |
| Resection margin < 1 cm | 2.49 (1.91–3.26) | < 0.001 | 1.95 (1.49–2.56) | < 0.001 |
| Postoperative adjuvant TACE | 1.01 (0.77–1.32) | 0.973 | ||
| Postoperative IBR | 7.12 (5.40–9.41) | < 0.001 | 5.43 (4.08–7.25) | < 0.001 |
Note: *Those variables found significant at P < 0.1 in univariable analyses were entered into multivariable analyses.
Abbreviations: ASA, American Society of Anesthesiologists; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, alpha-fetoprotein; HR, hazard ratio; MV, multivariable; NA, not applicable; UV, univariable; CI, confidence interval; IBR, incomplete biomarker response; TACE, transcatheter arterial chemoembolization.
Discussion
AFP is a widely used biomarker in the surveillance of patients at risk of developing HCC, as well as to assess the treatment response among patients with known HCC.44 Levels of AFP are also correlated with the tumor size, with higher AFP being associated with larger lesions. However, AFP as a prognostic indicator lacks consistency, as several authors reported limited correlation of AFP levels with disease factors such as tumor size, stage, or disease progression.45–47 Importantly, about 30~40% of HCCs are not AFP secretors.12 For these reasons, we did not include the data of those patients with negative (<20 ng/mL) and positive but low (20–400 ng/mL) AFP expressions into the present analyses. Importantly, a high AFP expression (>400 ng/mL) is a recognized manifestation of HCC with aggressive biological behavior, and it can also serve as a reflection of tumor burden.48,49 In the present study, clinical characteristics of a specific patient population with HCC with preoperative high AFP levels after curative liver resection were depicted, and their long-term oncologic prognosis and prognostic factors were evaluated. Using the magnitude in decline between preoperative and postoperative serum AFP levels, ie whether a postoperative biomarker response could completely be achieved at the first postoperative follow-up or not, the whole cohort (n=549 patients) was divided into the CBR group (n=456) and the IBR group (n=93). Despite comparable late recurrence rates, the rates of overall and early recurrence in patients with postoperative IBR were significantly higher than those in patients with postoperative CBR (97.8% vs 56.4%, and 92.5% vs 33.3%, both P < 0.001). Furthermore, the 5-year OS and RFS rates in patients with IBR were significantly poorer than those in patients with CBR (60.7% vs 19.9%, and 44.5% vs 2.9%, both P < 0.001). On multivariate Cox-regression analysis, postoperative IBR was shown to be the strongest risk associated with poorer OS (HR: 2.97) and poorer RFS (HR: 4.29), as well as increased early recurrence (HR: 5.43) after liver resection for patients with high-AFP HCC. To our knowledge, this is the first study that focused on clinical characteristics and postoperative oncologic prognosis exclusively for patients with high-AFP HCC (>400 ng/mL). The results of this study can be used as a useful guidance in planning for enhanced recurrence surveillance and decision-making on early adjuvant therapy for this specific group of patients.
The predictive value of postoperative serum AFP response to treatment has increasingly been used in locoregional and systemic therapies for HCC, especially in targeted therapy and immunotherapy.12,14,26,50 However, unlike the use of predictive value of AFP response to various non-curative treatments for HCC, published studies on its use to predict recurrence and survival after curative liver resection are very limited.34–40 There are a lot of challenges in designing and conducting such studies. On one hand, nearly 40% of HCCs do not express AFP, which limits the use of AFP response for patients with AFP-negative HCC.51 On the other hand, a clear, well-recognized and clinically relevant postoperative AFP response is difficult to define. A good definition requires precise preoperative and postoperative time points to measure serum AFP levels, and a reasonable magnitude of decline between pre- and post-operative AFP levels to determine a significant change in levels. Li et al38 measured serum AFP before surgery (AFP0) and 1 week after surgery (AFP7). They calculated the AFP response using the formula: log10AFP7/log10AFP0 to correlate the relationship between AFP response and long-term survival. They concluded that an AFP non-responder corresponded to an AFP response >0.8135. This formula is too complicated and difficult to be incorporated into routine use by clinicians. Moreover, in this contemporary era of enhanced recovery after surgery, patients are becoming more often to be discharged from hospitals within 1 week after HCC resection. The need to measure postoperative serum AFP level at 1 week after surgery is becoming less suitable in routine clinical practice. Another study by Zhou et al coming from the same hospital used a postoperative daily decrease in AFP of 9% as the cut-off value to evaluate AFP response. They showed this value to be useful in discriminating OS and RFS after HCC resection for patients having a preoperative AFP > 40 ng/mL. However, this algorithm, in addition to being inconvenient to use, could result in false judgments in HCC patients with a preoperative low-positive AFP level (41~100 ng/mL).35 Other studies by Shen et al34 and by Allard et al36 used around 90 days after surgery, and by Rungsakulkij et al39 around 180 days after surgery as postoperative measurement points of serum AFP levels. These studies all concluded that a serum AFP level that failed to decline to AFP negativity was independently associated with high recurrence rates and poor overall survival after curative resection of AFP-positive HCC. However, both 90 days and 180 days after surgery are too late to be clinically useful either in predicting early recurrence to offer an enhanced recurrence surveillance program, or to give early adjuvant treatments against recurrence.34,36,39
Thus, the time points used in defining postoperative CBR are critical in designing good studies on the predictive value of serum AFP in patients undergoing curative resection for AFP-positive HCC (>20 ng/mL), especially for those with preoperative extremely high AFP levels (>1200 ng/mL). As the first postoperative follow-up of around 4~6 weeks after surgery is most commonly carried out in centers worldwide, a serum AFP level measured at this time point was used in this study. As for the magnitude of AFP decline before and after operation, an easy-to-use formula to differentiate between postoperative CBR and IBR needs to be defined. Some studies defined AFP response as CBR to occur in patients with a preoperative positive status (≥20 ng/mL) which became negative (<20 ng/mL) within 1 month after surgery.37,40 However, as the half-life of AFP is 5~7 days, this definition is unreasonable for HCC patients with preoperative extremely-high-AFP levels of >1200 ng/mL. Even after complete resection of all tumors, the serum AFP levels will not decline to become negative (<20 ng/mL) within 30 days. Therefore, in the present study, the patients were classified into two groups according to their preoperative AFP levels: intermediately-high-AFP (401~1200 ng/mL) and extremely-high-AFP (>1200 ng/mL). CBR was defined as a decrease of postoperative AFP level to negative (<20 ng/mL) for patients with intermediately high AFP HCC, or to <1/60 (~26) of preoperative AFP level for patients with extremely-high-AFP HCC. Considering the half-life of AFP and the commonly used measurement time points after surgery, this classification is reasonable and practical.
Conventionally, curative HCC resection is defined either by postoperative histopathological examination of the resected specimen (R0 or R1 resection) or by radiologic imaging using contrast-enhanced CT or MRI at the first postoperative follow-up after operation. Neither of these two techniques can entirely rule out occult micro-metastasis or small metastatic HCCs in liver remnants, which are the most common sites for early HCC recurrence. In the past two decades, accurate prediction and early detection of tumor recurrence can be predicted by detecting circulating tumor cells, cell-free DNA, or microRNAs. However, these techniques suffer from the need for high technology and high costs which limit their generalizability.26,52–54 A special feature of high-AFP HCC being its tumor cells secreting AFP. If residual tumor cells were to exist in liver remnants or in patients after removal of all targeted tumors, as these cells continue to secrete AFP, the serum AFP levels fall slower than predicted and would hardly become negative. The present study confirmed the rationality of this hypothesis. In this study, the rate of early recurrence within 2 years after surgery among patients with postoperative IBR was as high as 92.5%, suggesting that these patients with high risks of early recurrence should be given close surveillance to detect early HCC recurrence and to consider offering early treatments against recurrence. Although there are currently no universally accepted effective treatments against early HCC recurrence, clinical trials for adjuvant therapy against HCC recurrence in this well-defined high-risk population should be considered.
Multivariate logistic regression analysis of this study showed many tumor-related characteristics to be independently associated with postoperative IBR, with preoperative tumor rupture being the strongest predictor of IBR (OR=5.47). Partial hepatectomy is usually considered as the most optimal treatment in selected patients with adequate liver function and resectable tumors,55 although intraperitoneal tumor dissemination remains a major obstacle in achieving good long-term survival outcomes after resection.56 In this study, patients with complete resection of ruptured HCC were strongly predicted to have IBR by postoperative AFP levels. The existence of peritoneal tumor seedings which persist to secret AFP can explain such findings.
This study had several limitations. First, this is a retrospective cohort study with its inherent defects. Second, most patients enrolled in this study had a history of chronic HBV infection. Therefore, the conclusions from this study may not be applicable to patients with HCC due to other etiologies, such as HCV and alcoholic liver disease. Third, antiviral therapy against HBV has been shown to reduce the baseline AFP level, thus affecting the diagnostic accuracy of AFP in patients who had chronic HBV infection.57 Additionally, high HBV-DNA load at the postoperative first follow-up might lead to serum mildly elevated AFP level. However, there was no relevant information regarding these above-mentioned issues in this study.
In conclusion, the present study revealed that HCC patients with high-AFP levels had a relatively poor postoperative oncologic prognosis. This study also identified postoperative IBR to be the strongest predictive risk factor of poor OS and RFS for these patients. For HCC patients with high-AFP levels, postoperative AFP response is an easy-to-use and a practical surrogate endpoint to identify patients who have a very high probability of early recurrence and poor long-term oncologic survival outcomes after liver resection. This study supports the use of postoperative AFP response in evaluating long-term oncologic prognosis of patients in clinical practice. Once postoperative IBR is identified at first follow-up visits, enhanced recurrence surveillance and available treatment options against recurrence need to be actively considered.
Summary
We investigated the association between postoperative biomarker response at first follow-up and long-term recurrence, and survival in patients undergoing curative resection for hepatocellular carcinoma (HCC) with high alpha-fetoprotein (AFP) expressions (>400 ng/mL). Postoperative incomplete biomarker response (IBR) predicted more recurrence, especially within 2 years after surgery, and poor long-term survival after liver resection for patients with high-AFP HCC. The results can provide useful guidance in planning for enhanced recurrence surveillance and decision-making on early adjuvant therapy for patients with postoperative IBR at first follow-up visits.
Acknowledgment
Thanks to Chao Li (Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University), Shanghai, China), Ying-Jian Liang (Department of Hepatobiliary Surgery, the First Affiliated Hospital of Harbin Medical University, Heilongjiang, China), Jie Li (Department of Hepatobiliary Surgery, Fuyang People’s Hospital, Anhui, China), Ting-Hao Chen (Department of General Surgery, Ziyang First People’s Hospital, Sichuan, China), Ya-Hao Zhou (Department of Hepatobiliary Surgery, Pu’er People’s Hospital, Yunnan, China), Hong Wang (Department of General Surgery, Liuyang People’s Hospital, Hunan, China), Yong-Yi Zeng (Department of Hepatobiliary Surgery, Mengchao Hepatobiliary Hospital, Fujian Medical University, Fujian, China), Yu Wang (Department of Hepatobiliary Surgery, Chongqing University Cancer Hospital, Chongqing, China), Yong-Kang Diao (Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Zhejiang, China), Hang-Dong Jia (Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College, Zhejiang, China), Hao Xing (Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University) for their contributions to the study design and data collection.
Funding Statement
This work was supported in part by the National Natural Science Foundation of China (No. 81702334 for Dr M. Wang and 81672699, 81972726 for Dr T. Yang) and Shanghai Sailing Program (17YF1424900, Dr M. Wang). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
HCC, Hepatocellular carcinoma; AFP, alpha-fetoprotein; IBR, incomplete biomarker response; CBR, complete biomarker response; CT, Computed tomography; MRI, Magnetic resonance imaging; OS, Overall survival; RFS, recurrence-free survival; SD, Standard deviation; HR, Hazard ratio; OR, odds ratios; MV, multivariable; UV, univariable; CI, Confidence interval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 2.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 3.Dhir M, Melin AA, Douaiher J, et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg. 2016;263(6):1112–1125. doi: 10.1097/SLA.0000000000001556 [DOI] [PubMed] [Google Scholar]
- 4.Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72(2):262–276. doi: 10.1016/j.jhep.2019.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi: 10.1001/jamasurg.2018.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toyoda H, Kumada T, Tada T, et al. Prognostic significance of a combination of pre- and post-treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J Hepatol. 2012;57(6):1251–1257. doi: 10.1016/j.jhep.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 7.Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253(4):656–665. doi: 10.1097/SLA.0b013e3181fc08ca [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi: 10.1016/S0168-8278(02)00360-4 [DOI] [PubMed] [Google Scholar]
- 9.Marasco G, Colecchia A, Colli A, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol. 2019;70(3):440–448. doi: 10.1016/j.jhep.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 10.Wang MD, Li C, Liang L, et al. Early and late recurrence of hepatitis B virus-associated hepatocellular carcinoma. Oncologist. 2020;25. doi: 10.1634/theoncologist.2019-0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trevisani F, Garuti F, Neri A. Alpha-fetoprotein for diagnosis, prognosis, and transplant selection. Semin Liver Dis. 2019;39(2):163–177 [DOI] [PubMed] [Google Scholar]
- 12.Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi: 10.1111/liv.14223 [DOI] [PubMed] [Google Scholar]
- 13.He C, Peng W, Liu X, Li C, Li X, Wen TF. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2019;98(31):e16557. doi: 10.1097/MD.0000000000016557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevisani F, Garuti F, Neri A. Alpha-fetoprotein for diagnosis, prognosis, and transplant selection. Semin Liver Dis. 2019;39(2):163–177. doi: 10.1055/s-0039-1677768 [DOI] [PubMed] [Google Scholar]
- 15.Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology. 2015;62(1):158–165. doi: 10.1002/hep.27787 [DOI] [PubMed] [Google Scholar]
- 16.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986. doi: 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128–139. doi: 10.1053/j.gastro.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 18.Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi: 10.1093/annonc/mdt247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M, Cheng AL, Park JW, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, Phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46. doi: 10.1016/S2468-1253(17)30290-X [DOI] [PubMed] [Google Scholar]
- 20.Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9 [DOI] [PubMed] [Google Scholar]
- 21.Zhu AX, Finn RS, Galle PR, Llovet JM, Kudo M. Ramucirumab in advanced hepatocellular carcinoma in REACH-2: the true value of α-fetoprotein. Lancet Oncol. 2019;20(4):e191. doi: 10.1016/S1470-2045(19)30165-2 [DOI] [PubMed] [Google Scholar]
- 22.Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. doi: 10.1186/1477-7819-11-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SL, Liu LP, Yang S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi: 10.1002/bjs.10093 [DOI] [PubMed] [Google Scholar]
- 24.Yu JJ, Shen F, Chen TH, et al. Multicentre study of the prognostic impact of preoperative bodyweight on long-term prognosis of hepatocellular carcinoma. Br J Surg. 2019;106(3):276–285. doi: 10.1002/bjs.10981 [DOI] [PubMed] [Google Scholar]
- 25.Chan MY, She WH, Dai WC, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy- an analysis of 1182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52. doi: 10.21037/tgh.2019.06.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nault JC, Villanueva A. Biomarkers for hepatobiliary cancers. Hepatology. 2020. doi: 10.1002/hep.31175 [DOI] [PubMed] [Google Scholar]
- 27.Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. J Clin Oncol. 2014;32(24):2611–2616. doi: 10.1200/JCO.2014.55.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from >1000 to <500 ng/mL in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology. 2019;69(3):1193–1205. doi: 10.1002/hep.30413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35(9):987–999. doi: 10.1111/j.1365-2036.2012.05060.x [DOI] [PubMed] [Google Scholar]
- 30.Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27(3):446–452. doi: 10.1200/JCO.2008.18.8151 [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Ouyang Q, Xia F, et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB (Oxford). 2019;21(1):107–113. doi: 10.1016/j.hpb.2018.06.1800 [DOI] [PubMed] [Google Scholar]
- 32.Personeni N, Bozzarelli S, Pressiani T, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57(1):101–107. doi: 10.1016/j.jhep.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 33.Kim BK, Ahn SH, Seong JS, et al. Early alpha-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma. Liver Int. 2011;31(3):369–376. doi: 10.1111/j.1478-3231.2010.02368.x [DOI] [PubMed] [Google Scholar]
- 34.Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res. 2017;209:102–111. doi: 10.1016/j.jss.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 35.Zhou PY, Yang CP, Tang Z, et al. Daily decrease of post-operative alpha-fetoprotein by 9% discriminates prognosis of HCC: a multicenter retrospective study. Aging (Albany NY). 2019;11(23):11111–11123. doi: 10.18632/aging.102513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allard MA, Sa CA, Ruiz A, et al. The postresection alpha-fetoprotein in cirrhotic patients with hepatocellular carcinoma. An independent predictor of outcome. J Gastrointest Surg. 2014;18(4):701–708. doi: 10.1007/s11605-013-2433-9 [DOI] [PubMed] [Google Scholar]
- 37.Nanashima A, Taura N, Abo T, et al. Tumor marker levels before and after curative treatment of hepatocellular carcinoma as predictors of patient survival. Dig Dis Sci. 2011;56(10):3086–3100. doi: 10.1007/s10620-011-1796-6 [DOI] [PubMed] [Google Scholar]
- 38.Li XL, Zhu XD, Cai H, et al. Postoperative α-fetoprotein response predicts tumor recurrence and survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. Surgery. 2019;165(6):1161–1167. doi: 10.1016/j.surg.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 39.Rungsakulkij N, Suragul W, Mingphruedhi S, Tangtawee P, Muangkaew P, Aeesoa S. Prognostic role of alpha-fetoprotein response after hepatocellular carcinoma resection. World J Clin Cases. 2018;6(6):110–120. doi: 10.12998/wjcc.v6.i6.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsukamoto M, Nitta H, Imai K, et al. Clinical significance of half-lives of tumor markers α-fetoprotein and des-γ-carboxy prothrombin after hepatectomy for hepatocellular carcinoma. Hepatol Res. 2018;48(3):E183–E193. doi: 10.1111/hepr.12942 [DOI] [PubMed] [Google Scholar]
- 41.Yang T, Lu JH, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: a propensity score matching analysis. J Hepatol. 2016;64(3):583–593. doi: 10.1016/j.jhep.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 42.Wang MD, Li C, Li J, et al. Long-term survival outcomes after liver resection for binodular hepatocellular carcinoma: a multicenter cohort study. Oncologist. 2019;24(8):e730–e739. doi: 10.1634/theoncologist.2018-0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayuso C, Rimola J, Vilana R, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72–81. doi: 10.1016/j.ejrad.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 44.Witjes CD, Polak WG, Verhoef C, et al. Increased alpha-fetoprotein serum level is predictive for survival and recurrence of hepatocellular carcinoma in non-cirrhotic livers. Dig Surg. 2012;29(6):522–528. doi: 10.1159/000348669 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Chen Y, Ge N, et al. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012;19:3540–3546. doi: 10.1245/s10434-012-2368-5 [DOI] [PubMed] [Google Scholar]
- 46.Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371–1379. doi: 10.1002/hep.25814 [DOI] [PubMed] [Google Scholar]
- 47.Burnett NP, Dunki-Jacobs EM, Callender GG, et al. Evaluation of alpha-fetoprotein staging system for hepatocellular carcinoma in noncirrhotic patients. Am Surg. 2013;79:716–722. doi: 10.1177/000313481307900717 [DOI] [PubMed] [Google Scholar]
- 48.Nishioka ST, Sato MM, Wong LL, Tiirikainen M, Kwee SA. Clinical and molecular sub-classification of hepatocellular carcinoma relative to alpha-fetoprotein level in an Asia-Pacific island cohort. Hepatoma Res. 2018;4:1. doi: 10.20517/2394-5079.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montal R, Andreu-Oller C, Bassaganyas L, et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br J Cancer. 2019;121(4):340–343. doi: 10.1038/s41416-019-0513-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Wang Q. Alpha-fetoprotein and hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol. 2018;2018:9049252. doi: 10.1155/2018/9049252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forner A, Reig M, Bruix J. Alpha-fetoprotein for hepatocellular carcinoma diagnosis: the demise of a brilliant star. Gastroenterology. 2009;137(1):26–29. doi: 10.1053/j.gastro.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 52.Jiao LR, Apostolopoulos C, Jacob J, et al. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol. 2009;27(36):6160–6165. doi: 10.1200/JCO.2009.24.5837 [DOI] [PubMed] [Google Scholar]
- 53.Xu RH, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–1161. doi: 10.1038/nmat4997 [DOI] [PubMed] [Google Scholar]
- 54.Ahn JC, Teng PC, Chen PJ, et al. Detection of circulating tumor cells and their implications as a novel biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2020. doi: 10.1002/hep.31165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang T, Sun YF, Zhang J, et al. Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg. 2013;100(8):1071–1079. doi: 10.1002/bjs.9167 [DOI] [PubMed] [Google Scholar]
- 56.Sahu SK, Chawla YK, Dhiman RK, et al. Rupture of hepatocellular carcinoma: a review of literature. J Clin Exp Hepatol. 2019;9(2):245–256. doi: 10.1016/j.jceh.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong GL, Chan HL, Tse YK, et al. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014;59(3):986–995. doi: 10.1002/hep.26739 [DOI] [PubMed] [Google Scholar]