Abstract
The inability to control or inhibit emotional distractors characterizes a range of psychiatric disorders. Despite the use of a variety of task paradigms to determine the mechanisms underlying the control of emotional interference, a precise characterization of the brain regions and networks that support emotional interference processing remains elusive. Here, we performed coordinate-based and functional connectivity meta-analyses to determine the brain networks underlying emotional interference. Paradigms addressing interference processing in the cognitive or emotional domain were included in the metaanalyses, particularly the Stroop, Flanker, and Simon tasks. Our results revealed a consistent involvement of the bilateral dorsal anterior cingulate cortex, anterior insula, left inferior frontal gyrus, and superior parietal lobule during emotional interference. Follow-up conjunction analyses identified correspondence in these regions between emotional and cognitive interference processing. Finally, the patterns of functional connectivity of these regions were examined using resting-state functional connectivity and meta-analytic connectivity modeling. These regions were strongly connected as a distributed system, primarily mapping onto fronto-parietal control, ventral attention, and dorsal attention networks. Together, the present findings indicate that a domain-general neural system is engaged across multiple types of interference processing and that regulating emotional and cognitive interference depends on interactions between large-scale distributed brain networks.
Keywords: Emotional interference, Cognitive control, Activation likelihood estimation (ALE), Meta-analysis, Meta-analytic connectivity modeling (MACM), Resting-state functional connectivity (RSFC), Large-scale network, Functional decoding
Introduction
Goal-directed behavior often involves regulating the interference between competing response tendencies via inhibiting task-irrelevant but predominant responses and maintaining task-relevant processes. The cognitive control of interference has been extensively examined using validated task paradigms, particularly the classical Stroop (MacLeod 1991; Stroop 1935), Simon (Simon et al. 1971), and Flanker tasks (Eriksen and Eriksen 1974). Across these tasks, the responses to a target stimulus are typically slower and less accurate when the stimulus and response are incongruent (e.g., RED printed in green) than when they are congruent (e.g., RED printed in red), i.e., an interference effect. At the neural level, interference control is supported by a neural circuit encompassing the lateral prefrontal cortex (PFC), anterior insula (AI), dorsal anterior cingulate cortex (dACC), and parietal cortex (Cieslik et al. 2015; Derrfuss et al. 2005; Laird et al. 2005b; Nee et al. 2007; Cole and Schneider 2007).
While classical paradigms mainly target non-emotional interference processes, conflicts in daily life often relate to emotional events (e.g., smiling in an angry situation). The inability to control or inhibit emotional distracters furthermore characterizes a variety of psychiatric disorders, ranging from anxiety to depressive, trauma-related, and addictive disorders (Bishop et al. 2004; Etkin et al. 2010; Wang et al. 2008; Froeliger et al. 2012). Accordingly, the past decade has witnessed a surge of interest in examining the neurobiological foundation underlying the control of emotional distracters (Etkin et al. 2006; Egner et al. 2008; Ochsner et al. 2009; Kanske and Kotz 2010; Dolan et al. 2001; Beall and Herbert 2008; Xue et al. 2016; Klasen et al. 2011; Chiew and Braver 2011; Chen et al. 2014, 2016).
These studies usually employ paradigms that allow for a measure of emotional interference comparable to the cognitive interference assessed in the classical paradigms. For instance, in an emotional variant of the Stroop task, participants are presented with facial expressions (e.g., fearful or happy) overlaid with congruent or incongruent emotional labels (e.g., “fearful” or “happy”) and are required to indicate the facial expressions while ignoring the distracter of emotional word labels across the face (Houwer and Hermans 1994; Stenberg et al. 1998; Etkin et al. 2006). In such cases, the emotional interference is based on incompatible response tendencies between emotional expressions and word labels (Krug and Carter 2012; Etkin et al. 2006; Bang et al. 2016; Stenberg et al. 1998; Haas et al. 2006). Similarly, another paradigm requires participants to indicate the valence of emotional prosody in the presence of congruent or incongruent semantic cues (Mitchell 2006a, b, 2013; Rota et al. 2008; Schirmer et al. 2004; Wittfoth et al. 2010). Furthermore, in the emotion-word Flanker task, participants are required to indicate the valence (positive or negative) of a central target word while ignoring flanking emotional words mapped onto either the congruent or incongruent valence of the target (Ochsner et al. 2009; Samanez-Larkin et al. 2009). In these paradigms, incongruent trials in comparison with congruent trials typically induce an emotional interference effect similar to that probed in the classical paradigms (Vanderhasselt et al. 2013; Stenberg et al. 1998). Using these paradigms in combination with neuroimaging methods, numerous studies have investigated the neural signatures underlying emotional interference and attempted to determine the common and specific neural substrates of emotional and cognitive interference processing.
However, previous neuroimaging studies have revealed inconsistent results regarding the specific neural systems that mediate emotional interference. On one hand, many studies have reported that emotional interference involves a domain-general cognitive control network comprising the dACC and lateral PFC, which are also strongly engaged in non-emotional interference processing (Chechko et al. 2009, 2012, 2013; Krug and Carter 2010, 2012; Ochsner et al. 2009; Torres-Quesada et al. 2014; Frühholz et al. 2009; Godinez et al. 2016; Rey et al. 2014; Kühn et al. 2010; Müller et al. 2011). Accordingly, recent meta-analyses on emotional variants of the Stroop task demonstrated a robust engagement of cognitive control regions including the dACC and lateral PFC (Song et al. 2017; Xu et al. 2016). In line with the involvement of this domain-general network, several studies have linked inter-individual variations in emotional interference to neural activity in the dACC and lateral PFC (Chechko et al. 2012; Ovaysikia et al. 2011). Moreover, similar regions have been consistently identified in tasks involving the regulation of emotional experiences or emotion–cognition interactions (Ochsner et al. 2002; Buhle et al. 2014; Cromheeke and Mueller 2014). These findings together prompt a domain-general account holding that a domain-general cognitive control network may be responsible for controlling interference induced by different types of stimuli (Buhle et al. 2014; Ochsner et al. 2009; Ochsner and Gross 2005; Collignon et al. 2008; De Gelder and Vroomen 2000).
On the other hand, it has been demonstrated that emotional interference involves brain regions that are particularly sensitive to emotional stimuli, particularly the amygdala and rostral ACC (rACC) (Etkin et al. 2006, 2010, 2011; Park et al. 2008; Mitchell et al. 2003; Mitchell 2006b; Watson et al. 2013; Eugène et al. 2010). For instance, the amygdala and rACC have been specifically identified during emotional interference processing and have been directly related to the behavioral interference effects in emotional context but not in non-emotional context (Ochsner et al. 2009; Etkin et al. 2006). Likewise, rACC lesions have been specifically associated with impairments in emotional interference resolution, while cognitive interference resolution remained intact (Maier and Di Pellegrino 2012). Moreover, individuals with disorders characterized by emotional dysregulation, such as anxiety or depression, exhibit rACC perturbations in response to emotional interference (Etkin and Schatzberg 2011), which have been linked to the severity of symptoms (Offringa et al. 2013; Etkin et al. 2010; Fonzo et al. 2017). Finally, functional connectivity findings revealed that control of cognitive interference recruits a cognitive control circuit that engages the dACC, lateral PFC, and sensory cortex, whereas control of emotional interference relies on an affective control circuit that encompasses the dACC, rACC, and amygdala (Egner et al. 2008). These findings resonate with a domain-specific account suggesting that the rACC and amygdala specifically mediate emotional interference (Etkin et al. 2011, 2015). Importantly, however, the domain-general and domain-specific accounts are not necessarily mutually exclusive given that common and distinct neural bases of the emotional and cognitive interference are highly conceivable (Zaki et al. 2010; Fleury et al. 2014; Comte et al. 2014). Indeed, a recent meta-analytic study revealed both common and distinct networks for the control of emotion (i.e., emotion regulation) and action (Langner et al. 2018).
Against this background, the present study aimed to achieve a more fine-grained characterization of the neural networks implicated in emotional interference based on meta-analytic and data-driven analyses. First, we utilized a coordinate-based meta-analysis to quantitatively synthesize previous neuroimaging findings on emotional interference with the goal of determining the underlying neural architecture of the phenomenon. We next assessed the correspondence between emotional and cognitive interference to determine the common and distinct neural circuits underlying different types of interference. Second, we examined both task-dependent and task-independent functional connectivity profiles of the identified regions with meta-analytic connectivity modeling (MACM) and resting-state functional connectivity (RSFC), respectively. Third, large-scale network correlates of the identified regions and corresponding functional connectivity profiles were determined by mapping them to seven canonical functional networks covering the whole brain (Yeo et al. 2011; Choi et al. 2012). Finally, the functions of the identified domain-general network were decoded using the BrainMap database, allowing a data-driven quantitative inference on mental processes associated with the network (Laird et al. 2009b).
Together, the complementary analytical strategies employed in the current study not only allowed us to localize brain regions associated with emotional interference, as in previous work (Song et al. 2017; Xu et al. 2016), but also allowed us to first characterize network-level mechanisms underlying emotional and cognitive interference as well as to decode the functional significance of the underlying network (Zhang et al. 2017). In the context of previous work suggesting that the regulation of emotional and cognitive interference might depend on extensive interactions between distributed brain networks (Pessoa 2008; Mesulam 1990), the present large-scale network analysis further supports a system-level understanding of these behavioral domains.
Materials and methods
Meta-analysis
Literature search and selection
A systematic online database search was performed in April 2017 using PubMed, ISI Web of Science and Google Scholar by entering various combinations of relevant search items [e.g., (‘affective conflict’ OR ‘emotional conflict’ OR ‘affective interference’ OR ‘emotional interference’ OR ‘affective compatibility’ OR ‘emotional compatibility’ OR ‘affective stroop’ OR ‘emotional stroop’) AND (‘fMRI’ OR ‘magnetic resonance imaging’ OR ‘neuroimaging’)]. In addition, we explored several other sources, including (1) the BrainMap database (http://brainmap.org), (2) the bibliography and citation indices of the pre-selected articles, and (3) direct searches on the names of frequently occurring authors. The obtained studies were further assessed according to the following criteria: (i) subjects were free from psychiatric or neurological diagnoses; (ii) subjects performed tasks in which they were exposed to interference induced by incompatible emotional events. This included but was not limited to the emotional Stroop (e.g., Chechko et al. 2014) and emotional Flanker tasks (e.g., Ochsner et al. 2009) commonly employed in the literature. For instance, in the approach–avoidance task, participants were asked to respond to stimuli associated with both positive and negative (and thus conflicting) outcomes (Aupperle et al. 2015). Notably, the following frequently used paradigms were excluded in the current meta-analysis: (a) traditional emotion-word Stroop tasks were not included because the stimuli used in this task did not induce incompatible response tendencies (see also Feng et al. 2018; Etkin et al. 2006); and (b) cognitive interference tasks (e.g., a classical Stroop task) performed in the context of emotional stimuli (e.g., Kanske and Kotz 2010; Hart et al. 2010) were not included, since interference in these tasks is induced by conflicts in the cognitive rather than emotional domain. (iii) fMRI was used as the imaging modality; (iv) whole-brain general-linear-model-based analyses [rather than region of interest (ROI) analyses] were applied; (v) fMRI results were derived from a general-linear model based on either a binary contrast or parametric analyses; and (vi) activations were presented in a standardized stereotaxic space (Talairach or Montreal Neurological Institute, MNI). Note that for studies reporting Talairach coordinates, a conversion to the MNI coordinates was employed, as implemented in the GingerALE software with Brett’s mni2tal algorithm (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
Filtering search results according to the inclusion/exclusion criteria yielded a total of 48 “incongruent > congruent” or “incongruent > neutral/baseline” contrasts (503 foci, 1125 subjects) (Table 1). In line with previous meta-analytic studies (Fan et al. 2011; Bartra et al. 2013; Luo et al. 2018; Vartanian and Skov 2014; Fouragnan et al. 2018), activation peaks from binary contrast and continuous parametric analyses were included because both analysis strategies aim to determine brain regions engaged during interference processing. Second, we allowed for variations in contrasts rather than restricting our analyses to a single contrast, as long as the different contrasts (e.g., incongruent > congruent, or incongruent > baseline) carried information about interference processing. This is an advantage provided by neuroimaging meta-analytic methods (see also Wager et al. 2007), such that brain regions consistently reported in different contrasts are detected while those that vary across contrasts (due to such factors as participants, stimuli, and specific contrasts) are likely canceled out. Similar ideas allowed us to compare emotional interference with cognitive interference, the findings of which were derived from different tasks and stimuli.
Table 1.
Summary of the studies included in the meta-analysis focusing on emotional interference
| Study | N | Paradigm | Stimuli | Task description | Contrast | No. of foci |
|---|---|---|---|---|---|---|
| Aupperle et al. (2015) | 15 | Approach–avoidance conflict task | Emotional pictures | In non-conflict trials, participants decided to approach reward or avoid threat; In conflict trials, participants decided to approach/avoid stimuli resulting in both negative and positive outcomes | Incongruent > congruent | 1 |
| Bang et al. (2016) | 43 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 9 |
| Chechko et al. (2009) | 18 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 4 |
| Chechko et al. (2012) | 24 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 24 |
| Chechko et al. (2012) | 24 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 27 |
| Chechko et al. (2013) | 18 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 20 |
| Chechko et al. (2014) | 24 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 16 |
| Comte et al. (2014) | 33 | Emotional Stroop task | Facial expressions and pictures | Participants indicated valence of pictures/overlaid faces while ignoring faces/pictures | Incongruent > congruent | 43 |
| Egner et al. (2008) | 22 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 2 |
| Fleury et al. (2014) | 12 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 15 |
| Fleury et al. (2014) | 12 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 15 |
| Fleury et al. (2014) | 12 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 16 |
| Fruhholz et al. (2009) | 20 | Emotional interference task | Facial expressions | Participants indicated the valence of facial expressions while ignoring colors associated with congruent or incongruent valence | Incongruent > congruent | 23 |
| Fruhholz et al. (2009) | 20 | Emotional interference task | Facial expressions | Participants indicated the valence of facial expressions while ignoring colors associated with congruent or incongruent valence | Incongruent > congruent | 33 |
| Fruhholz et al. (2009) | 20 | Emotional interference task | Facial expressions | Participants indicated the valence of facial expressions while ignoring colors associated with congruent or incongruent valence | Incongruent > congruent | 2 |
| Fruhholz et al. (2009) | 20 | Emotional interference task | Facial expressions | Participants indicated the valence of facial expressions while ignoring colors associated with congruent or incongruent valence | Incongruent > congruent | 18 |
| Godinez et al. (2016) | 9 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the emotional words while ignoring congruent or incongruent facial expression as background | Incongruent > congruent | 5 |
| Huang et al. (2013) | 28 | Emotional interference task | Video clips of facial expressions and sentences | Participants were presented with congruent or incongruent dynamic facial expressions and sentences. Participants indicated gender of faces | Incongruent > congruent | 18 |
| Jarcho et al. (2013) | 35 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the emotional words while ignoring congruent or incongruent facial expression as background | Incongruent > congruent | 2 |
| Krug and Carter (2012) | 42 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the emotional words while ignoring congruent or incongruent facial expression as background | Incongruent > congruent | 21 |
| Krug and Carter (2012) | 42 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the emotional words while ignoring congruent or incongruent facial expression as background | Incongruent > congruent | 20 |
| Kuhn et al. (2010) | 16 | Emotional interference task | Facial expressions | Participants were presented with facial expressions that are congruent or incongruent with their own expressions, and indicated how close they felt with the target | Incongruent > congruent | 4 |
| Lee et al. (2007) | 32 | Emotion expression interference | Video clips of facial expressions | Participants were instructed to make frowning or smiling expressions that are congruent or incongruent with expressions in the video clips | Incongruent > congruent | 11 |
| Lee et al. (2007) | 32 | Emotion expression interference | Video clips of facial expressions | Participants were instructed to make frowning or smiling expressions that are congruent or incongruent with expressions in the video clips | Incongruent > congruent | 11 |
| Lee et al. (2007) | 32 | Emotion expression interference | Video clips of facial expressions | Participants were instructed to make frowning or smiling expressions that are congruent or incongruent with expressions in the video clips | Incongruent > congruent | 9 |
| Mitchell et al. (2006a, b) | 28 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | Incongruent > baseline | 8 |
| Mitchell et al. (2003) | 13 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | Incongruent > baseline | 3 |
| Mitchell et al. (2003) | 13 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | Incongruent > neutral | 1 |
| Mitchell (2013) | 33 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | Parametric analysis with incongruent level | 1 |
| Mitchell (2013) | 33 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | 60% incongruent > congruent | 4 |
| Mitchell (2013) | 33 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | 80% incongruent > congruent | 2 |
| Mitchell (2013) | 33 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | 100% incongruent > congruent | 8 |
| Muller et al. (2011) | 40 | Emotional interference task | Facial expressions and emotional sounds | Participants indicated the facial expressions while ignoring congruent or incongruent emotional sounds | Incongruent > congruent | 4 |
| Ochsner et al. (2009) | 16 | Emotional Flanker task | Emotional words | Participants indicated the valence of a central target word while ignoring flanking stimuli that have either congruent or incongruent valence | Incongruent > congruent | 13 |
| Offringa et al. (2013) | 18 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 6 |
| Ovaysikia et al. (2011) | 10 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated valence of faces/overlaid words while ignoring words/faces | Incongruent > congruent | 16 |
| Ovaysikia et al. (2011) | 10 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated valence of faces/overlaid words while ignoring words/faces | Incongruent > congruent | 10 |
| Park et al. (2008) | 14 | Emotional Stroop task | Affective pictures and emotional words | Participants indicated the emotional words while ignoring congruent or incongruent affective pictures as background | Incongruent > congruent | 2 |
| Rey et al. (2014) | 12 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 12 |
| Samanez-Larkin et al. (2009) | 24 | Emotional Flanker task | Emotional words | Participants indicated the valence of a central target word while ignoring flanking stimuli that have either congruent or incongruent valence | Parametric analysis with behavioral interference | 10 |
| Schirmer et al. (2004) | 25 | Emotional interference task | Auditory words | Participants indicated the valence of word/prosody while ignoring congruent or incongruent prosody/word | Incongruent > congruent | 2 |
| Torres-Quesada et al. (2014) | 21 | Emotional Stroop task | Facial expressions and emotional words | Participants indicated the facial expressions while ignoring overlaid congruent or incongruent emotional words | Incongruent > congruent | 4 |
| Vanderhasselt et al. (2013) | 30 | Emotional interference task | Facial expressions | Participants were instructed to press the button that is consistent or inconsistent with the presented expressions | Incongruent > congruent | 5 |
| Vrticka et al. (2014) | 33 | Emotional interference task | Facial expressions and emotional words | Participants performed a counting task and were presented with feedback comprising congruent or incongruent facial expressions and emotional words | Incongruent > congruent | 2 |
| Watanabe et al. (2013) | 27 | Emotional interference task | Video clips | Participants were presented with video clips in which verbal information and facial expressions of an actor were either congruent or incongruent, and indicated whether the actor was a friend or foe | Incongruent > congruent | 5 |
| Watson et al. (2013) | 18 | Emotional interference task | Video clips of facial expressions and emotional sounds | Participants indicated valence of stimuli comprising congruent or incongruent facial expressions and emotional sounds | Parametric analysis with incongruent level | 2 |
| Wittfoth et al. (2010) | 20 | Emotional interference task | Auditory sentences | Participants listened to emotional sentences that are congruent or incongruent in emotions conveyed by lexico-semantic and prosodic elements, and indicated the emotional tone of the sentence | Incongruent > congruent | 3 |
| Zaki et al. (2010) | 16 | Emotional interference task | Video clips | Participants were presented with video clips in which verbal information and facial expressions of an actor were either congruent or incongruent, and indicated the feelings of the actor | Incongruent > congruent | 11 |
Another meta-analysis on neuroimaging studies of cognitive interference processing was conducted based on one of our recent publications (Cieslik et al. 2015). In particular, we searched for neuroimaging studies that employed the Stroop, Simon and Flanker tasks to identify brain regions consistently involved in cognitive interference processing. The search and selection procedures resulted in 96 “incongruent > congruent” or “incongruent > neutral/baseline” contrasts (1022 foci, 1689 subjects) (Table S1). Importantly, tasks involving an emotional component were excluded from the meta-analysis of cognitive interference (see also Cieslik et al. 2015). For studies that employed both cognitive and emotional versions of the interference task, coordinates were separately coded for the meta-analysis of emotional and cognitive interference (e.g., Ochsner et al. 2009). Furthermore, the selection criteria used for cognitive studies were similar to those for emotional studies, such that only results from healthy subjects and whole-brain analyses were included.
Main activation likelihood estimation (ALE) analysis
A coordinate-based meta-analysis of reported fMRI studies was conducted, employing the ALE algorithm (in-house MATLAB scripts) (Eickhoff et al. 2009). ALE determines the convergence of foci reported from different functional [e.g., blood-oxygen-level dependent (BOLD) contrast imaging] or structural (e.g., voxel-based morphometry) neuroimaging studies with published foci in Talairach or MNI space (Laird et al. 2005a; Turkeltaub et al. 2002). ALE interprets reported foci as spatial probability distributions, whose widths are based on empirical estimates of the spatial uncertainty due to the between-subject and between-template variability of the neuroimaging data (Eickhoff et al. 2009). The ALE algorithm weights the between-subject variability based on the number of subjects analyzed in the studies, modeling larger sample sizes with smaller Gaussian distributions and thus presupposing more reliable approximations of the ‘true’ activation for larger sample sizes (Eickhoff et al. 2009).
The union of the individual modulated activation maps first created from the maximum probability associated with any one focus (always the closest one) for each voxel (Turkeltaub et al. 2012) is then calculated to obtain an ALE map across studies. Then, using a non-linear histogram integration algorithm, this ALE map is assessed against a null distribution of random spatial association between studies (Eickhoff et al. 2012; Turkeltaub et al. 2012). In addition, the average non-linear contribution of each experiment for each cluster was calculated from the fraction of the ALE values at the cluster with and without the experiment in question (Eickhoff et al. 2017). Based on the calculated contribution, we employed additional criteria to select significant clusters: (1) the contributions for one cluster were from at least two experiments, so that the finding was not driven by a single experiment; and (2) the average contribution of the most dominant experiment (MDE) did not exceed 50%, and the average contribution of the two most dominant experiments (2MDEs) did not exceed 80% (Eickhoff et al. 2017).
Main conjunction/contrast analysis
After obtaining the consistent maxima separately for emotional and cognitive interference, a conjunction analysis between them was conducted to assess the correspondence. In addition, differences between emotional and cognitive interference were tested by first performing separate ALE analyses for each condition and then computing the voxel-wise difference between the ensuing ALE maps (Eickhoff et al. 2011).
Validation analysis
We implemented additional analyses to validate findings derived from conventional ALE meta-analysis and conjunction analyses. First, we implemented a leave-one-experiment-out (LOEO) analysis for the ALE meta-analyses on the emotional interference. On each fold, one contrast was excluded, and the ALE meta-analysis was conducted on the remaining N – 1 contrasts (N – 1 = 47). Afterwards, we conducted a conjunction analysis on the ALE results of all folds to identify the brain regions that were robustly engaged in emotional interference. These analyses were employed to make sure that our main ALE meta-analysis findings on emotional interference were not driven by a single contrast/experiment.
Second, the unbalanced number of contrasts between emotional and cognitive interference (48 vs. 96) could lead to differences in statistical power for the contrasts to be compared. To address this issue, we employed a re-subsampling approach for the meta-analysis of cognitive interference. The re-subsampling was repeated 1000 rounds. On each round, 47 contrasts of cognitive interference were randomly selected to match the number of contrasts in emotional interference. The ALE meta-analysis was then conducted for the selected contrasts of cognitive interference. Afterwards, the ALE results of all rounds were averaged to represent the re-subsampling findings. These analyses were employed to validate our main ALE meta-analysis findings on cognitive interference.
Third, we aimed to validate our main conjunction findings with the following analyses: we computed the overlap between regions that were most robustly engaged in emotional interference (i.e., the voxel-wise probabilities of being declared active in all folds of the ALE-LOEO analysis) and those in re-subsampling analyses for cognitive interference. Importantly, this conjunction analysis was based on equal numbers of contrasts associated with emotional and cognitive interference.
Finally, the ALE approach treats different contrasts within a study as distinct experiments (Laird et al. 2009a). This approach (i) ensures that all relevant information from each study is accounted for in the meta-analysis and (ii) avoids the selection of a single contrast based on subjective selection criteria. However, a disadvantage of this approach is that multiple contrasts from a single study could be related. Although this potential issue could be partly addressed by the LOEO and resampling methods mentioned above, we implemented an additional supplementary analysis by combining different contrasts from the same publication into a single experiment. In this way, the approach prevented multiple non-independent contrasts from a single publication from influencing the results of the meta-analyses (Turkeltaub et al. 2012). Combining within-study contrasts resulted in 34 contrasts for emotional interference and 84 contrasts for cognitive interference.
All maps were thresholded using a cluster-level family-wise error (cFWE) correction (P < 0.05) with a clusterforming threshold of P < 0.001 using 10,000 permutations for correcting multiple comparisons. Notably, the current analyses were implemented using key functions of the most recent version of GingerALE (version 2.3.6), which uses valid multiple-comparison corrections (Eickhoff et al. 2017).
Task-based connectivity: MACM analyses
To examine the co-activation patterns of the bilateral dACC, AI, left inferior frontal gyrus (IFG) and superior parietal lobe (SPL) commonly recruited by emotional and cognitive interference processing, we conducted MACM analyses—with these regions as ROIs—using the BrainMap Database (Laird et al. 2009a). MACM delineates patterns of coactivation across thousands of studies using neuroimaging databases and produces data-driven functional connectivity maps based on pre-defined ROIs (Langner et al. 2014). The BrainMap database (http://www.brainmap.org/) was used, which at the time of assessment contained coordinates of reported activation foci and associated meta-data of more than 8400 neuroimaging experiments. For our analysis, only whole-brain neuroimaging studies that report activation in standard stereotaxic space and recruit a healthy population were included, while other studies investigating differences in age, gender, handedness, and training effects or clinical populations were excluded. For the dACC, 333 experimental contrasts and 4910 foci from 4555 participants were identified; for the left AI, 302 experimental contrasts and 5068 foci from 4845 participants; for the right AI, 264 experimental contrasts and 4381 foci from 4035 participants; for the left IFG, 219 experimental contrasts and 3250 foci from 3367 participants; and for the left SPL, 168 experimental contrasts and 2893 foci from 2728 participants. First, whole-brain peak coordinates of all those studies from BrainMap were downloaded if the study reported at least one focus of activation within each ROI. Next, coordinates were analyzed with the ALE algorithm (as described above) to detect areas of convergence of coactivations with each seed. Finally, the ALE maps were family-wise error (FWE) corrected at a threshold of P < 0.05 (corresponding to Z = 5.03) at the voxel-level and converted into Z scores for display.
Task-free connectivity: RSFC analyses
Neuroimaging data
To complement task-based connectivity derived from MACM analyses, whole-brain RSFC of the bilateral dACC, AI, left IFG and SPL as ROIs was assessed. Specifically, resting-state fMRI images of 192 healthy volunteers were obtained from the Enhanced Nathan Kline Institute-Rock-land Sample (http://fcon_1000.projects.nitrc.org/indi/enhanced/) (Nooner et al. 2012). During resting-state acquisition, subjects were instructed to look at a fixation cross, not to think about anything in particular and not to fall asleep (which was confirmed by post-scan debriefing). For each subject, 260 resting-state echo planar imaging (EPI) data were acquired on a Siemens TimTrio 3T scanner using the following parameters: repetition time (TR) = 1.4 s, echo time (TE) = 30 ms, flip angle = 65°, voxel size = 2.0 mm × 2.0 mm × 2.0 mm, 64 slices.
Image preprocessing
Physiological and movement artifacts were removed from the resting-sate data using FIX [FMRIB’s independent component analysis (ICA)-based Xnoiseifier, version 1.061 as implemented in FSL 5.0.9] (Salimi-Khorshidi et al. 2014; Griffanti et al. 2014). Unique variance related to the identified artifactual independent components was then regressed from the data together with 24 movement parameters (including derivatives and second-order effects as previously described and evaluated) (Satterthwaite et al. 2013). Data were further preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, London) and in-house Matlab scripts. The first four scans were excluded prior to further analyses. The remaining EPI images were corrected for head movement using a two-pass (alignment to the initial volume followed by alignment to the mean after the first pass) affine registration. The mean EPI image for each subject was then spatially normalized to the ICBM-152 reference space using the “unified segmentation” approach (Ashburner and Friston 2005). The resulting deformation was applied to the individual EPI volumes, which were subsequently smoothed with a Gaussian kernel with a full width at half maximum (FWHM) of 5 mm to improve the signal-to-noise ratio and to compensate for residual anatomic variations.
Seed-to-voxel connectivity
Implementing a seed-based analysis, the functional connectivity (bivariate correction) between the average BOLD signals from given seed regions (the bilateral dACC, AI, left IFG and SPL) and all other voxels in the brain was computed. The voxel-wise correlation coefficients were then transformed into Fisher’s Z scores and tested for consistency across subjects by a second-level analysis of variance (ANOVA, including appropriate nonsphericity correlation). Consistent with our MACM analyses, results were FEW-corrected at a threshold of P < 0.05 (corresponding to T = 4.92) at the voxel-level.
Large-scale network analysis
To assess the underlying large-scale network correlates, clusters that were revealed by meta-analysis, MACM, and RSFC analyses were overlaid onto seven canonical functional networks covering the cerebral cortex and striatum (Choi et al. 2012; Yeo et al. 2011). Canonical networks include the fronto-parietal network (FPN), dorsal attention network (DAN), ventral attention network (VAN), somatomotor network (SMN), visual network (VN), affective network (AFN), nd default mode network (DMN). The relative distribution was computed by the proportion of activated voxels of a given network vs. all activated voxels, while the absolute distribution was calculated by the proportion of activated voxels of a given network vs. voxels of that template network (Zhang et al. 2017).
Functional decoding
After identifying a domain-general network in the ALE meta-analysis, and the MACM and RSFC analyses, we decoded the potential functions of the network based on the BrainMap database (http://www.brainmap.org/). In particular, we performed the functional characterization of the network based on the behavioral domain meta-data categories available for each neuroimaging experiment included in the BrainMap database. Behavioral domains included the main categories cognition, action, perception, emotion, and interoception, as well as related sub-categories (Turner and Laird 2012) (see http://brainmap.org/scribe/).
We determined the individual functional profile corresponding to the domain-general network using forward and reverse inference approaches. Forward inference refers to the probability of identifying activity in a brain network given knowledge of the psychological process, whereas reverse inference refers to the probability of a psychological process being present given knowledge of a particular brain network. In the forward inference approach, a network’s functional profile was determined by identifying taxonomic labels, for which the probability of finding activation in the respective network was significantly higher than the overall chance (across the entire database) of finding activation in that particular network. In particular, we assessed whether the conditional probability of activation given a particular label [P (Activation|Task)] was higher than the baseline probability of activation in the network in question per se [P (Activation)]. In the reverse inference approach, a network’s functional profile was determined by identifying the most likely behavioral domains given activation in a particular network. This likelihood [P (Activation|Task)] can be derived from [P (Activation|Task)] as well as [P (Task)] and [P (Activation)] using Bayes rule. Significance (at P < 0.05, corrected for multiple comparisons using the FDR method) was then assessed by means of a Chi-square test.
Results
Main ALE findings
For emotional interference, the ALE meta-analysis revealed significant convergence of activity in the bilateral dACC/SMA, AI, left IFG, SPL and inferior temporal gyrus (Fig. 1a; Table 2). Twenty-five out of 48 contrasts contributed to the cluster in the dACC/SMA (MDE = 10.84%; 2MDE = 20.96%, Table 3). Eleven out of 48 contrasts contributed to the cluster in the left AI (MDE = 22.07%; 2MDE = 36.63%, Table 3). Twenty-five out of 48 contrasts contributed to the cluster in the right AI (MDE = 10.08%; 2MDE = 16.42%, Table 3). Sixteen out of 48 contrasts contributed to the cluster in the left IFG (MDE = 18.50%; 2MDE = 31.89%, Table 3). Twelve out of 48 contrasts contributed to the cluster in the left SPL (MDE = 15.79%; 2MDE = 31.48%, Table 3). Seven out of 48 contrasts contributed to the cluster in the left inferior temporal gyrus (MDE = 26.04%; 2MDE = 50.97%, Table 3).
Fig. 1.

Significant clusters from the main coordinate-based activation likelihood estimation (ALE) meta-analysis (cluster-level family-wise error correction (P <0.05) with a cluster-forming threshold of P <0.001 using 10,000 permutations) for emotional interference, cognitive interference, and the common clusters for both. a Consistent maximum for emotional interference was found in the bilateral dACC/SMA, AI, left IFG, SPL and inferior temporal gyrus. b Consistent maximum for cognitive interference was found in the bilateral dACC/SMA, AI, left IFG, SPL/IPL, middle frontal gyrus and middle occipital gyrus. c Consistent maximum for the conjunction of emotional interference and cognitive interference was found in the bilateral AI, dACC/SMA, left IFG and SPL. L left, R right
Table 2.
ALE meta-analysis results for emotional interference and cognitive interference
| Laterality | Cluster no. | Brain regions | BA | MNI coordinates (mm) |
Peak Z score | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Emotional interference | ||||||||
| L | 1 | Inferior temporal gyrus | 37/20 | −46 | −50 | −18 | 4.62 | 976 |
| R | 2 | Anterior insula | 13/47 | 34 | 26 | −4 | 6.27 | 6008 |
| L | 3 | Anterior insula | 13/47 | −32 | 22 | −4 | 4.62 | 1216 |
| L | 4 | Inferior frontal gyrus | 9/46 | −46 | 16 | 22 | 5.39 | 2360 |
| R/L | 5 | dACC/SMA | 6/8/32 | −6 | 10 | 56 | 6.35 | 5736 |
| L | 6 | Superior parietal lobule | 7 | −26 | −62 | 44 | 4.98 | 1552 |
| Cognitive interference | ||||||||
| L | 1 | Middle occipital gyrus | 37 | −44 | −68 | −10 | 4.88 | 1504 |
| R | 2 | Anterior insula | 13/47 | 36 | 24 | −4 | 6.51 | 10,648 |
| L | 3 | Anterior insula | 13/47 | −32 | 18 | 0 | 6.75 | 6296 |
| L | 4 | Inferior frontal gyrus | 9/46 | −42 | 4 | 34 | 7.82 | 7136 |
| L | 5 | Inferior parietal lobule | 7/40 | −26 | −70 | 40 | 6.66 | 10,552 |
| R/L | 6 | dACC/SMA | 8/32 | −2 | 14 | 50 | 8.27 | 9560 |
| R | 7 | Inferior parietal lobule | 7/40 | 40 | −48 | 50 | 6.02 | 4016 |
| L | 8 | Middle frontal gyrus | 6 | −26 | −12 | 58 | 4.46 | 1144 |
| Conjunction between emotional and cognitive interference | ||||||||
| R | 1 | Anterior insula | 13/47 | 36 | 26 | −4 | 5.97 | 2176 |
| L | 2 | Anterior insula | 13/47 | −32 | 22 | −4 | 4.63 | 1008 |
| L | 3 | Inferior frontal gyrus | 9/46 | −44 | 16 | 24 | 4.85 | 944 |
| L/R | 4 | dACC/SMA | 6/32 | −4 | 12 | 54 | 6.17 | 3232 |
| L | 5 | Superior parietal lobule | 7 | −26 | −62 | 42 | 4.82 | 1048 |
P(FWE) <0.05 at the cluster level with a cluster-forming threshold of P <0.001 using 10,000 permutations
Table 3.
Average contribution of each experimental contrast for significant clusters identified for the meta-analysis of emotional interference
| Cluster no. | Study | N | Contrast | Stimuli | Average contribution (%) |
|---|---|---|---|---|---|
| 1 | Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 26.04 |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 24.93 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 19.82 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 18.32 | |
| Chechko et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 9.63 | |
| Fleury et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 0.76 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions and emotional words | 0.49 | |
| 2 | Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 10.08 |
| Lee et al. (2007) | 32 | Incongruent > congruent | Video clips of facial expressions | 6.34 | |
| Chechko et al. (2014) | 24 | Incongruent > congruent | Facial expressions and emotional words | 6.25 | |
| Chechko et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 6 | |
| Mitchell (2013) | 33 | 100% incongruent > congruent | Auditory sentences | 5.72 | |
| Lee et al. (2007) | 32 | Incongruent > congruent | Video clips of facial expressions | 5.52 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 5.36 | |
| Lee et al. (2007) | 32 | Incongruent > congruent | Video clips of facial expressions | 4.7 | |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 4.61 | |
| Schirmer et al. (2004) | 25 | Incongruent > congruent | Auditory words | 4.38 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions and emotional words | 4.36 | |
| Fleury et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 4.34 | |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 4.24 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 4.21 | |
| Torres-Quesada et al. (2014) | 21 | Incongruent > congruent | Facial expressions and emotional words | 3.78 | |
| Zaki et al. (2010) | 16 | Incongruent > congruent | Video clips | 3.74 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions and emotional words | 3.6 | |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 3.45 | |
| Ochsner et al. (2009) | 16 | Incongruent > congruent | Emotional words | 2.82 | |
| Bang et al. (2016) | 43 | Incongruent > congruent | Facial expressions and emotional words | 2.35 | |
| Samanez-Larkin et al. (2009) | 24 | Parametric analysis with behavioral interference | Emotional words | 2.15 | |
| Fleury et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 0.87 | |
| Jarcho et al. (2013) | 35 | Incongruent > congruent | Facial expressions and emotional words | 0.68 | |
| Comte et al. (2014) | 33 | Incongruent > congruent | Facial expressions and pictures | 0.22 | |
| Vrticka et al. (2014) | 33 | Incongruent > congruent | Facial expressions and emotional words | 0.1 | |
| 3 | Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 22.07 |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 14.56 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions and emotional words | 13.56 | |
| Bang et al. (2016) | 43 | Incongruent > congruent | Facial expressions and emotional words | 12.23 | |
| Aupperle et al. (2015) | 15 | Incongruent > congruent | Emotional pictures | 11.26 | |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 7.45 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 6.17 | |
| Ochsner et al. (2009) | 16 | Incongruent > congruent | Emotional words | 4.7 | |
| Samanez-Larkin et al. (2009) | 24 | Parametric analysis with behavioral interference | Emotional words | 3.93 | |
| Comte et al. (2014) | 33 | Incongruent > congruent | Facial expressions and pictures | 3.4 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 0.4 | |
| 4 | Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 18.5 |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 13.39 | |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 10.94 | |
| Chechko et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 9.12 | |
| Samanez-Larkin et al. (2009) | 24 | Parametric analysis with behavioral interference | Emotional words | 8.74 | |
| Chechko et al. (2014) | 24 | Incongruent > congruent | Facial expressions and emotional words | 6.32 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 6.16 | |
| Mitchell et al. (2006a, b) | 28 | Incongruent > baseline | Auditory sentences | 5.85 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 5.13 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 5.02 | |
| Schirmer et al. (2004) | 25 | Incongruent > congruent | Auditory words | 4.11 | |
| Bang et al. (2016) | 43 | Incongruent > congruent | Facial expressions and emotional words | 4.01 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 1.41 | |
| Godinez et al. (2016) | 9 | Incongruent > congruent | Facial expressions and emotional words | 0.85 | |
| Chechko et al. (2009) | 18 | Incongruent > congruent | Facial expressions and emotional words | 0.22 | |
| Comte et al. (2014) | 33 | Incongruent > congruent | Facial expressions and pictures | 0.16 | |
| 5 | Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 10.84 |
| Chechko et al. (2014) | 24 | Incongruent > congruent | Facial expressions and emotional words | 10.12 | |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 8.82 | |
| Chechko et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 7.52 | |
| Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 7.05 | |
| Ochsner et al. (2009) | 16 | Incongruent > congruent | Emotional words | 5.88 | |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 4.34 | |
| Bang et al. (2016) | 43 | Incongruent > congruent | Facial expressions and emotional words | 5.88 | |
| Torres-Quesada et al. (2014) | 21 | Incongruent > congruent | Facial expressions and emotional words | 3.97 | |
| Lee et al. (2007) | 32 | Incongruent > congruent | Video clips of facial expressions | 3.77 | |
| Lee et al. (2007) | 32 | Incongruent > congruent | Video clips of facial expressions | 3.71 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 3.58 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 3.5 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 3.41 | |
| Chechko et al. (2009) | 18 | Incongruent > congruent | Facial expressions and emotional words | 3.1 | |
| Mitchell (2013) | 33 | 80% incongruent > congruent | Auditory sentences | 3.03 | |
| Muller et al. (2011) | 40 | Incongruent > congruent | Facial expressions and emotional sounds | 2.96 | |
| Samanez-Larkin et al. (2009) | 24 | Parametric analysis with behavioral | Emotional words interference | 2.36 | |
| Ovaysikia et al. (2011) | 10 | Incongruent > congruent | Facial expressions and emotional words | 2.06 | |
| Offringa et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 1.75 | |
| Fleury et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 0.69 | |
| Zaki et al. (2010) | 16 | Incongruent > congruent | Video clips | 0.31 | |
| Mitchell (2013) | 33 | Parametric analysis with incongruent level | Auditory sentences | 0.18 | |
| Mitchell (2013) | 33 | 60% incongruent > congruent | Auditory sentences | 0.05 | |
| 6 | Chechko et al. (2012) | 24 | Incongruent > congruent | Facial expressions and emotional words | 15.79 |
| Chechko et al. (2014) | 24 | Incongruent > congruent | Facial expressions and emotional words | 15.69 | |
| Chechko et al. (2013) | 18 | Incongruent > congruent | Facial expressions and emotional words | 12.27 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 11.15 | |
| Comte et al. (2014) | 33 | Incongruent > congruent | Facial expressions and pictures | 10.51 | |
| Chechko et al. (2009) | 18 | Incongruent > congruent | Facial expressions and emotional words | 9.27 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 9.22 | |
| Jarcho et al. (2013) | 35 | Incongruent > congruent | Facial expressions and emotional words | 8.4 | |
| Fruhholz et al. (2009) | 20 | Incongruent > congruent | Facial expressions | 4.37 | |
| Krug and Carter (2012) | 42 | Incongruent > congruent | Facial expressions and emotional words | 1.64 | |
| Fleury et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 1.36 | |
| Rey et al. (2014) | 12 | Incongruent > congruent | Facial expressions and emotional words | 0.24 |
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the VAN (relative: 22.67%; absolute: 4.24%), DAN (relative: 21.57%; absolute: 3.59%), FPN (relative: 18.94%; absolute: 2.49%), and VN (relative: 17.15%; absolute: 1.97%) (Fig. 2a, b).
Fig. 2.
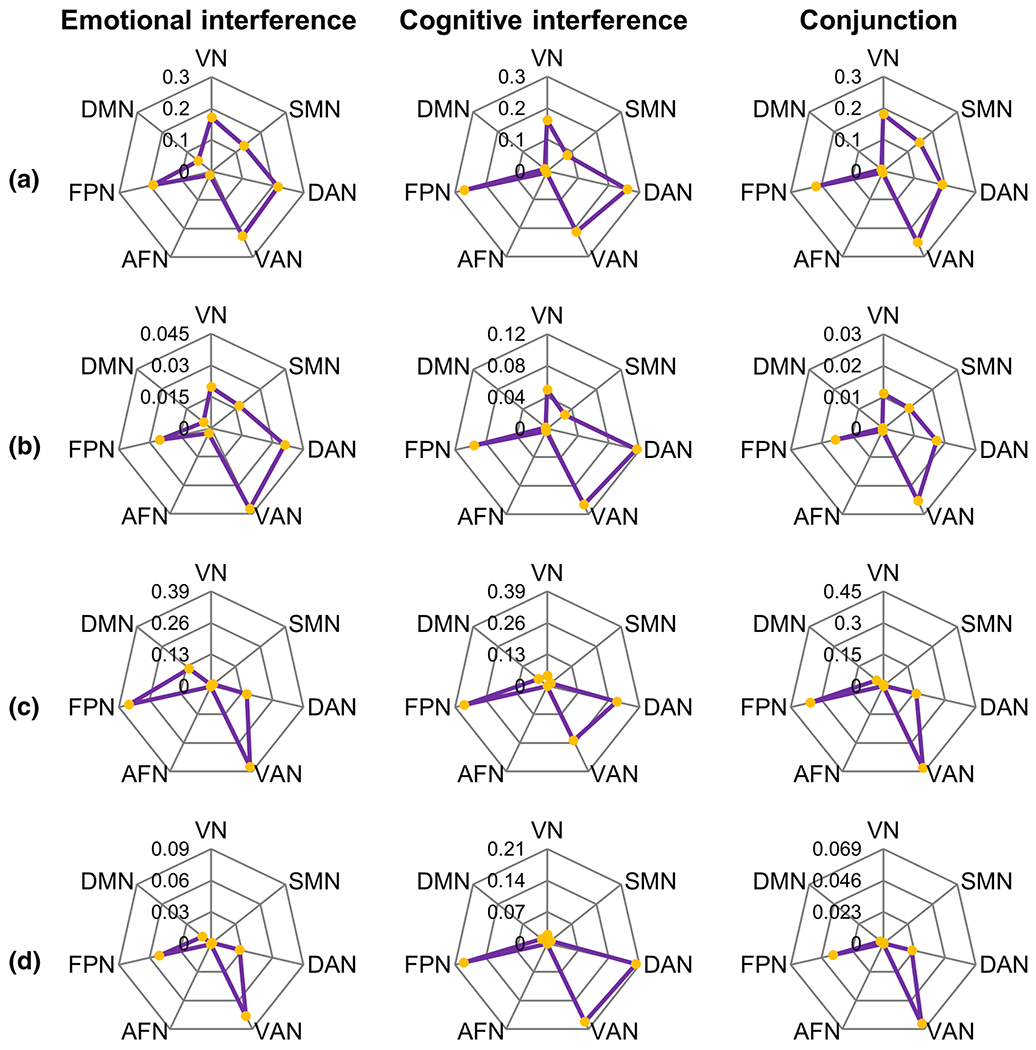
Network distribution of significant clusters for emotional interference, cognitive conflict, and the common clusters for both. a Relative network distribution of clusters from primary meta-analyses. b Absolute network distribution of clusters from primary meta-analyses. c Relative network distribution of clusters from validation meta-analyses. d Absolute network distribution of clusters from validation meta-analyses. VN visual network, SMN somatomotor network, DAN dorsal attention network, VAN ventral attention network, AFN affective network, FPN fronto-parietal network, DMN default-mode network
The ALE meta-analysis on the cognitive interference revealed significant convergence of activity in the bilateral dACC/SMA, AI, left IFG, SPL/IPL, middle frontal gyrus and middle occipital gyrus (Fig. 1b; Table 2). Fifty-four out of 96 contrasts contributed to the cluster in the right dACC/SMA (MDE = 5.00%; 2MDE = 9.68%, Table S2). Forty-seven out of 96 contrasts contributed to the cluster in the left AI (MDE = 5.92%; 2MDE = 11.22%, Table S2). Fifty-six out of 96 contrasts contributed to the cluster in the right AI (MDE = 6.42%; 2MDE = 11.94%, Table S2). Forty-five out of 96 contrasts contributed to the cluster in the left IFG (MDE = 7.05%; 2MDE = 12.44%, Table S2). Sixty-two out of 96 contrasts contributed to the first cluster in the left SPL/IPL (MDE = 5.25%; 2MDE = 9.97%, Table S2). Thirty-three out of 96 contrasts contributed to the second cluster in left SPL/IPL (MDE = 7.30%; 2MDE = 12.51%, Table S2). Twenty-two out of 96 contrasts contributed to the cluster in the left middle frontal gyrus (MDE = 11.67%; 2MDE = 22.24%, Table S2). Twenty-four out of 96 contrasts contributed to the cluster in the left middle occipital gyrus (MDE = 13.62%; 2MDE = 25.07%, Table S2).
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the FPN (relative: 26.92%; absolute: 9.50%), DAN (relative: 26.00%; absolute: 11.61%), VAN (relative: 21.29%; absolute: 10.68%), and VN (relative: 16.07%; absolute: 4.94%) (Fig. 2a, b).
Main conjunction/contrast findings
A conjunction analysis revealed a common activation maximum in the bilateral AI, dACC/SMA, left IFG and SPL for emotional and cognitive interference (Fig. 1c; Table 2).
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the VAN (relative: 24.90%; absolute: 2.53%), FPN (relative: 21.87%; absolute: 1.56%), DAN (relative: 19.10%; absolute: 1.73%), and VN (relative: 18.05%; absolute: 1.13%) (Fig. 2a, b).
Finally, contrast results (Table 2) showed that the right lateral orbital frontal cortex (lOFC) and right IFG were more activated in the emotional interference than cognitive interference. In contrast, the left AI, IFG, inferior parietal lobule, right middle frontal gyrus and bilateral dACC/SMA were more activated in the cognitive interference than emotional interference. Notably, regions revealed by the contrast analyses were adjacent to those revealed by the conjunction analysis (Fig. S1).
Validation of main findings
LOEO findings
Consistent activation maxima for the emotional interference were found in the bilateral dACC, AI/IFG, left SPL and inferior temporal gyrus (Fig. 3a; Table 4). Therefore, the results of the LOEO approach corroborated the findings of the standard ALE meta-analysis.
Fig. 3.
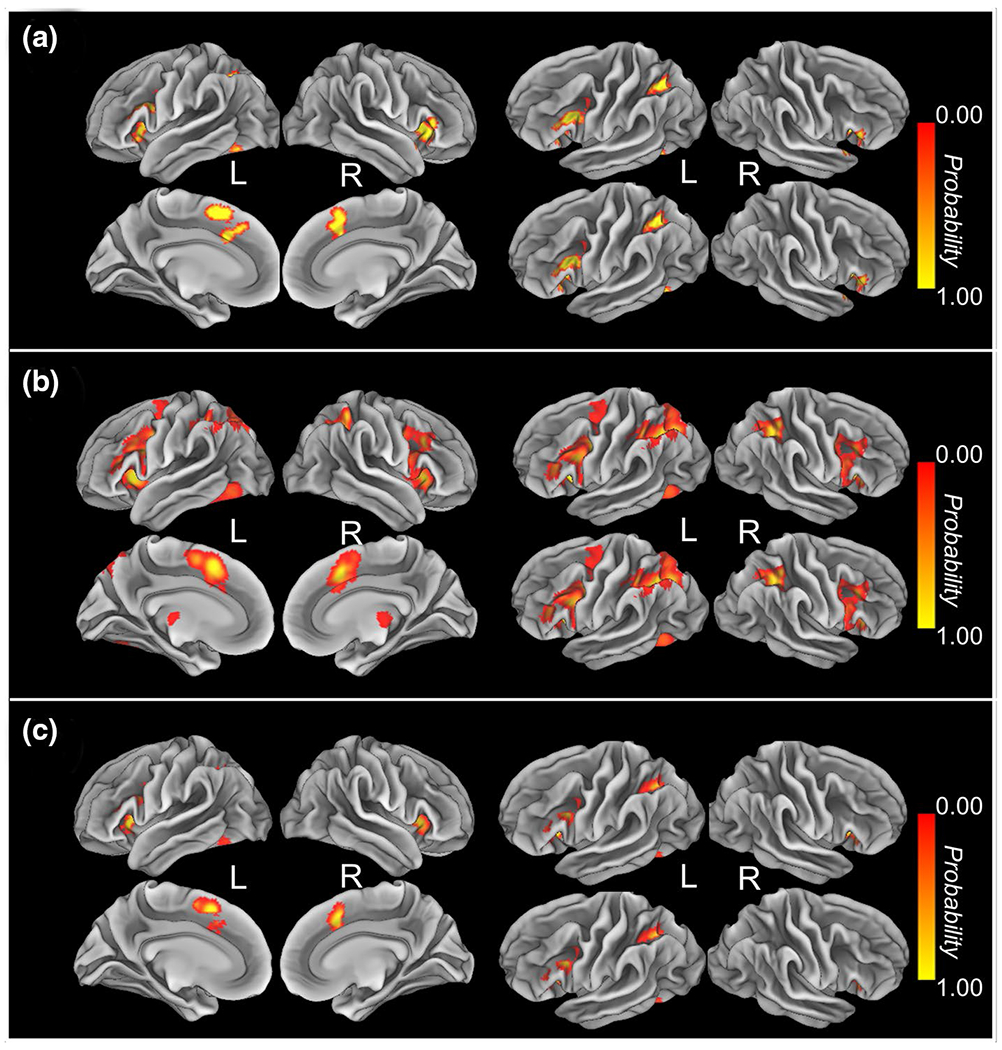
Significant clusters from the leave-one-experiment-out (LOEO) analysis for the emotional interference, the re-subsampling results for the cognitive interference, and the common clusters for both (cluster-level family-wise error correction (P <0.05) with a cluster-forming threshold of P <0.001 using 10,000 permutations). a Consistent maximum for emotional interference was found in the bilateral dACC, AI/IFG, left SPL and inferior temporal gyrus. b Consistent maximum for cognitive interference was found in the bilateral dACC, AI/IFG, SPL, thalamus, left dlPFC, middle occipital gyrus and right caudate. c Consistent maximum was found in the bilateral dACC, AI, IFG, left SPL and middle occipital gyrus when overlapping average results of LOEO for emotional interference and re-subsampling ALE results for the cognitive interference. L left, R right
Table 4.
Validation of main meta-analyses and conjunction analyses derived from leave-one-experiment-out and re-subsampling approaches
| Laterality | Cluster no. | Brain regions | BA | MNI coordinates (mm) |
Probability | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Emotional interference | ||||||||
| L | 1 | Inferior temporal gyrus | 37/20 | −44 | −50 | −22 | 0.91 | 1072 |
| R | 2 | Superior temporal gyrus/inferior frontal gyrus/anterior insula | 13/47/38 | 50 | 20 | −14 | 1.00 | 5624 |
| L | 3 | Anterior insula | 13/47 | −32 | 20 | −6 | 0.96 | 1344 |
| L | 4 | Inferior frontal gyrus | 9/46 | −46 | 16 | 18 | 1 | 2432 |
| L/R | 5 | dACC/SMA | 6/8/32 | 4 | 18 | 46 | 1 | 4632 |
| L | 6 | dACC | 32/24 | −4 | 18 | 32 | 0.96 | 1280 |
| L | 7 | Superior parietal lobule | 7 | −26 | −64 | 40 | 1.00 | 1752 |
| Cognitive interference | ||||||||
| L | 1 | Middle occipital gyrus | 37 | −42 | −66 | −10 | 0.27 | 3864 |
| R | 2 | Anterior insula/inferior frontal gyrus | 13/47 | 46 | 10 | 34 | 0.81 | 17,672 |
| L | 3 | Anterior insula/inferior frontal gyrus | 13/47 | −32 | 20 | 2 | 0.96 | 20,072 |
| L/R | 4 | Thalamus | – | 6 | −18 | −6 | 0.02 | 1072 |
| L | 5 | Thalamus | – | −10 | −24 | −2 | 0.01 | 792 |
| R | 6 | Caudate | – | 10 | 2 | 10 | 0.02 | 1488 |
| R | 7 | Superior parietal lobule | 7/40 | −26 | −68 | 40 | 0.94 | 17,048 |
| L/R | 8 | dACC/SMA | 6/32 | −2 | 14 | 48 | 0.99 | 13,712 |
| R | 9 | Superior parietal lobule | 7/40 | 40 | −48 | 50 | 0.84 | 6168 |
| L | 10 | Middle frontal gyrus | 6 | −26 | −10 | 58 | 0.18 | 3192 |
| Conjunction between emotional and cognitive interference | ||||||||
| L | 1 | Middle occipital gyrus | 37 | −46 | −66 | −14 | 0.22 | 656 |
| R | 2 | Anterior insula | 13/47 | 36 | 22 | −2 | 0.75 | 3072 |
| L | 3 | Anterior insula | 13/47 | −34 | 22 | 0 | 0.95 | 1184 |
| L | 4 | Inferior frontal gyrus | 46 | −46 | 32 | 14 | 0.33 | 160 |
| L | 5 | Inferior frontal gyrus | 9/46 | −44 | 12 | 28 | 0.88 | 1408 |
| L/R | 6 | dACC/SMA | 6/32 | −2 | 14 | 48 | 0.99 | 3872 |
| L/R | 7 | dACC | 32 | −4 | 18 | 40 | 0.64 | 504 |
| L | 8 | Superior parietal lobule | 7 | −26 | −68 | 40 | 0.94 | 1464 |
P(FWE) <0.05 at the cluster level with a cluster-forming threshold of P <0.001 using 10,000 permutations
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the VAN (relative: 36.93%; absolute: 7.65%), FPN (relative: 34.70%; absolute: 5.05%), and DAN (relative: 15.01%; absolute: 2.77%) (Fig. 2c, d).
Re-subsampling findings
The re-subsampling ALE meta-analysis on the cognitive interference revealed consistent maxima in the bilateral dACC, AI/IFG, SPL, thalamus, left dlPFC, middle occipital gyrus and right caudate (Fig. 3b; Table 4).
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the FPN (relative: 35.04%; absolute: 19.01%), DAN (relative: 29.34%; absolute: 20.15%), and VAN (relative: 24.90%; absolute: 19.20%) (Fig. 2c, d).
Conjunctions between LOEO and re-subsampling findings
The minimum conjunction between the probability map for emotional interference and the cognitive interference processing was identified in the bilateral dACC, AI, IFG, left SPL and middle occipital gyrus (Fig. 3c; Table 4).
By overlapping these clusters with the seven functional networks, it was revealed that the identified areas were primarily distributed in the VAN (relative: 43.29%; absolute: 6.46%), FPN (relative: 35.70%; absolute: 3.75%), and DAN (relative: 16.02%; absolute: 2.13%) (Fig. 2c, d).
Within-study contrasts combined
This analysis revealed essentially the same findings as those from the main ALE meta-analyses and conjunction analysis (Figure S2 & Table S3), further validating our findings.
MACM and RSFC results
We conducted task-based (MACM) and task-free (RSFC) connectivity analyses to further investigate the functional characterization and connectivity of brain regions commonly recruited by emotional interference and cognitive interference processing, including the bilateral dACC, AI, left IFG and SPL. MACM and RSFC analyses showed remarkably similar results for the functional connectivity of these seed regions (Fig. 4).
Fig. 4.

Results for the conjunction of task-based connectivity analysis (MACM) and task-free connectivity analysis (RSFC) for the regions commonly involved in the emotional and cognitive interference (voxel-wise P(FWE) <0.05). a Conjunction of MACM and RSFC for each seed region. b Relative network distribution of clusters from conjunction of MACM and RSFC for each seed region. c Absolute network distribution of clusters from conjunction of MACM and RSFC for each seed region. L left, R right, dACC dorsal anterior cingulate cortex, AI anterior insula, IFG inferior frontal gyrus, SPL superior parietal lobule, MCAM meta-analytic connectivity modeling, RSFC resting-state functional connectivity
For the dACC (Fig. S3 & S4 & Table S4), both MACM and RSFC analyses revealed functional connectivity with the medial frontal gyrus, AI/IFG, SPL, putamen, thalamus, middle frontal gyrus, precentral gyrus and cerebellum, which were primarily distributed in the FPN (relative: 32.32%; absolute: 10.57%), VAN (relative: 29.82%; absolute: 13.86%), and DAN (relative: 16.74%; absolute: 6.93%).
For the left AI (Figs. S3 & S4 & Table S5), both MACM and RSFC analyses revealed functional connectivity with the dACC/SMA, AI/IFG, SPL, thalamus and middle frontal gyrus, which were primarily distributed in the FPN (relative: 39.77%; absolute: 13.69%), VAN (relative: 31.58%; absolute: 15.45%), and DAN (relative: 16.90%; absolute: 7.36%).
For the right AI (Figs. S3 & S4 & Table S6), both MACM and RSFC analyses revealed functional connectivity with the dACC/SMA, AI/IFG, SPL, caudate, thalamus, precentral gyrus and middle frontal gyrus. These regions were primarily distributed in the FPN (relative: 42.72%; absolute: 14.10%), VAN (relative: 30.90%; absolute: 14.49%), and DAN (relative: 17.47%; absolute: 7.30%).
For the left IFG (Figs. S3 & S4 & Table S7), both MACM and RSFC analyses revealed functional connectivity with the dACC/SMA, AI/IFG, SPL, middle temporal gyrus, precentral gyrus and cerebellum, which were primarily distributed in the FPN (relative: 43.25%; absolute: 13.80%), DAN (relative: 24.12%; absolute: 9.74%), DMN (relative: 15.56%; absolute: 3.20%) and VAN (relative: 14.79%; absolute: 6.70%).
For the left SPL (Fig. S3 & S4 & Table S8), both MACM and RSFC analyses revealed functional connectivity with the dACC/SMA, AI/IFG, SPL, middle frontal gyrus, inferior occipital gyrus and cerebellum, which were primarily distributed in the DAN (relative: 51.13%; absolute: 15.11%) and FPN (relative: 38.61%; absolute: 9.01%).
Functional decoding of the domain-general network
A conjunction analysis across meta-analysis, MACM, and RSFC revealed a consistent network comprising the bilateral AI, dACC/SMA, left IFG and SPL (Fig. 5). Afterwards, functional characterization according to the BrainMap metadata was performed for the network. Forward and reverse inference alike indicated a significant association of the network with language-related cognition (especially phonology, semantics, and orthography) and working memory (Fig. 5). Reverse inference additionally revealed an association with attention, pain, and activation inhibition (Fig. 5).
Fig. 5.

The domain-general network and functional decoding. a Conjunction across meta-analyses, MACM and RSFC analyses. b Quantitative forward inference on the domain-general network. c Quantitative reverse inference on the domain-general network. L left, R right
Discussion
The past decade has witnessed an explosion of interest in examining neural signatures of emotional interference (Huang et al. 2013; Comte et al. 2014; Chen et al. 2014, 2016; Chiew and Braver 2011). Neuroscientific studies on this topic often utilize paradigms that induce emotional interference comparable to cognitive interference and assess the neuropsychological underpinnings of the interference effect (e.g., Etkin et al. 2006; Ochsner et al. 2009). The objective of the current work was to quantitatively synthesize the results of previous fMRI studies on emotional interference and to characterize large-scale network correlates of brain regions consistently involved in emotional interference. Our results identified a convergence of reported activation foci in neural circuits important in cognitive control (MacDonald et al. 2000; Ridderinkhof et al. 2004). Specifically, consistent involvement of the bilateral dACC, AI, left IFG, and SPL was identified for emotional interference, and conjunction analyses demonstrated correspondence in these regions between emotional and cognitive interference. These meta-analytic findings were further validated using LOEO and re-subsampling analyses. Furthermore, characterization of connectivity profiles revealed that these regions were extensively connected to each other in both task and resting states, in addition to connectivity with other regions. Finally, large-scale network analyses indicate that these regions as well as their functional connectivity profiles were primarily mapped onto the fronto-parietal network, the ventral attention network, and the dorsal attention network. These findings together suggest that there is a domain-general neural system recruited by different types of interference and that control of emotional and cognitive interference depends on interactions between distributed brain networks.
In line with the domain-general account, the current meta-analysis demonstrated overlap in cognitive control systems consisting of the dACC, AI, IFG and SPL between emotional and cognitive interference. Specifically, the dACC and AI have been implicated in the detection of interference induced by both emotional and non-emotional stimuli (Chechko et al. 2012; Egner et al. 2008; Aupperle et al. 2015; Wittfoth et al. 2010). Furthermore, these regions have been consistently activated by the expectancy violations (Garrison et al. 2013; Wu et al. 2016; Feng et al. 2015) and by events salient to current goals (Seeley et al. 2007; Menon and Uddin 2010). Accordingly, the dACC and AI have been thought to contribute to generic monitoring processes that signal the need for adjustment in cognitive control (Menon and Uddin 2010; Menon 2011). The IFG is frequently engaged by response inhibition and interference suppression, as revealed in different lines of previous neuroimaging studies (Bunge et al. 2002; Levy and Wagner 2011; Aron et al. 2004). The activity of the IFG has thus been attributed to inhibitory control of prepotent responses and information from semantic memory (Swick et al. 2008; Aron et al. 2004; Whitney et al. 2010; Thompson-Schill et al. 1998). Finally, the SPL plays critical roles in selective attention and working memory (Behrmann et al. 2004; Koenigs et al. 2009; Yantis and Serences 2003). For instance, the SPL is usually engaged in the condition where attention needs to be shifted to goal-related features (Yantis et al. 2002; Liu et al. 2003). Considering the consistent co-activation of these regions across emotional and cognitive interference, it is possible that they work together with each other as a connected network to support different aspects of interference control (Spielberg et al. 2015). Specifically, different types of interference might be registered in the dACC and AI as generic control signals driving subsequent increases in cognitive control implemented in the IFG and SPL, among other regions. Those conflicts are in turn resolved by inhibiting task-irrelevant responses (Ovaysikia et al. 2011; Egner et al. 2008; Etkin et al. 2006) and/or by directing attention to task-relevant features (Lee et al. 2007; Chechko et al. 2014).
In accordance with the current findings, many studies have indicated that control of emotion consistently recruits cognitive control systems. For instance, a domain-general control network consisting of prefrontal and parietal regions is often activated in emotion regulation tasks in which participants are explicitly asked to regulate their emotions (Buhle et al. 2014; Kohn et al. 2014; Kalisch 2009; Ochsner et al. 2002, 2012). The regulatory role of these regions is further supported by growing evidence demonstrating that neural activity in the dACC and lateral PFC is directly related to emotion regulation success, presumably via their regulatory control over limbic emotion processing regions (Wager et al. 2008; Kober et al. 2010; Lee et al. 2012; Silvers et al. 2017). Moreover, reliable increases in the dACC and lateral PFC activity are accompanied by successful treatment of patients with mood disorder (Delaveau et al. 2011). Finally, the presence of emotional distractors in cognitive tasks consistently enhances the engagement of cognitive control systems, especially when emotional information impairs ongoing task performance (Cromheeke and Mueller 2014). Considering these data in conjunction with the current observations, implicates a potential neuropsychological mechanism in the control of emotional processing with respect to the pivotal role of the domain-general cognitive control system.
Our connectivity and network analyses further revealed that these regions were connected to each other and mainly engaged the fronto-parietal network, the ventral attention network, and the dorsal attention network. These findings are in line with the domain-general account, considering that the engaged networks have demonstrated functional generality across diverse cognitive challenges, ranging from the control of attention to memory and response (Duncan and Owen 2000; Duncan 2010, 2013; Lückmann et al. 2014; Vincent et al. 2008; Dosenbach et al. 2006). For instance, the fronto-parietal network is thought to contribute to task-set maintaining, long-term planning, and response suppression and selection, among other high-order control processes (Menon 2011; Seeley et al. 2007; Cole et al. 2014; Miller and Cohen 2001). The dorsal attention network presumably implements top-down attention orientation to task-relevant features, whereas the ventral attention network is recruited by the detection of salient stimuli (Corbetta et al. 2000; Kim 2014; Cieslik et al. 2010). Other studies have distinguished the functional roles of these networks as initiating/adjusting control or providing stable maintenance of task sets (Dosenbach et al. 2007, 2008). Engagement of those distinct large-scale networks by emotional and cognitive interference is in line with hypothesis that human emotion and cognition emerge from dynamic interactions between spatially distributed and functionally dissociable brain networks (Barrett and Satpute 2013; Pessoa 2008; Mesulam 1990; Sha et al. 2017). Complementary to the present meta-analytic findings, recent studies employing network analyses have demonstrated that the dACC, AI, lateral PFC, and SPL form coherent large-scale networks, which change their topological properties and dynamic interactions as a function of the level of interference (Spielberg et al. 2015).
Finally, we revealed additional possible functions of the identified domain-general network using the functional decoding analysis. The network was associated with higher-level cognitive domains such as working memory, attention, language, and action inhibition. Therefore, the identified network is associated with functions or processes representing facets of cognitive control, which aligns with our meta-analytic results indicating an involvement of these regions in top-down control across emotional and cognitive interference. Notably, the network is also associated with emotion (pain) processing. These findings are consistent with the idea that interference might be in and of itself affective (Botvinick 2007; Dreisbach and Fischer 2012, 2015). This conjecture has been supported by evidence from both behavioral and brain imaging studies (Schouppe et al. 2015; Dreisbach and Fischer 2015; Braem et al. 2017; Pan et al. 2016).
The current study is the first to employ meta-analytic connectivity modeling, resting-state functional connectivity, and functional decoding to characterize brain networks associated with emotional and cognitive interference. Accordingly, the current study extends beyond localizing brain regions of interference processing (for recent meta-analyses, see also Song et al. 2017; Xu et al. 2016) and unveiled the underlying neural networks and their functional profiles, which could contribute to a better understanding of interference processing. Moreover, the current work is the first to apply functional decoding to characterize the network associated with emotional and cognitive interference, pointing to possible novel functions of brain systems not necessarily addressed in individual studies. Furthermore, this study included the largest number of datasets (48 experiments) and utilized advanced methods (LOEO and resubsampling) that allowed a more precise estimation of the effects than previous ALE meta-analyses of 14 (Xu et al. 2016) or 5 experiments (Song et al. 2017). Relatedly, the current meta-analysis was not restricted to the word-face Stroop paradigm, which has attracted the most attention in the field and was, therefore, the focus of the two previous meta-analyses. Instead, our study attempted to bring together a variety of tasks developed for examining emotional interference with the aim of providing a larger scope and determining inference-related networks across different paradigms.
Although the current findings provide converging evidence for a domain-general neural network involved in emotional and cognitive interference, several important issues remain open. First, contrast analyses revealed that brain regions showing differences in emotional and cognitive interference are adjacent to those regions consistently engaged across both domains. On the one hand, these findings suggest that emotional and cognitive interference processing engage similar regions but to different extents. On the other hand, it is possible that these adjacent regions are engaged in different functions. Indeed, adjacent or overlapping brain activations do not necessarily imply identical functions for all neurons within these regions (Haxby et al. 2001). Further studies are needed to examine the functions of the adjacent/overlapping regions with more fine-grained techniques or specifically designed task paradigms. For instance, advanced imaging techniques (e.g., multi-voxel pattern analysis) provide promising approaches to compare the neural patterns of adjacent/overlapping regions at the sub-voxel level (Norman et al. 2006; Peelen and Downing 2007).
Second, an alternative strategy to test the functional significance of the identified network is to examine the specificity of adaptation of the network across emotional and cognitive interference. That is, it would be interesting to examine whether the presence of emotional interference in the current trial reduces the neural responses (i.e., adaptation effects) of the network in the subsequent trial. Similar paradigms have frequently been used to test the specificity of interference adaptation across different cognitive tasks in the behavioral domain (Braem et al. 2014; Egner 2008, 2014). Further studies are needed to test whether interference adaptation transfers across emotional and cognitive domains at both the behavioral and neural levels.
Third, the current work focused on the contrast of incongruent vs. congruent, which is most frequently reported in the literature. Therefore, our study did not allow for the distinctions between different processes involved in interference processing, such as conflict monitoring and conflict resolution. This distinction requires experimental design with conflict adaptation manipulations, but these have been employed in only few previous studies (e.g., Egner et al. 2008; Etkin et al. 2006, 2010; Etkin and Schatzberg 2011; Chechko et al. 2009, 2014; Jarcho et al. 2013). Employing conflict adaptation manipulations, previous studies have identified domain-specific regions in emotional interference processing, e.g., the amygdala is involved in conflict monitoring (Etkin et al. 2006), whereas the rACC is involved in conflict resolution by dampening activity in the amygdala or dACC (Etkin et al. 2006; Egner and Hirsch 2005). It is thus possible that these emotion-related regions are involved in emotional interference processing but require more sensitive experimental designs to detect them (but see also Chechko et al. 2009, 2014; Krug and Carter 2010). Alternatively, it is possible that the rACC engagement in the emotional interference is further modulated by personality attributes, such as anxiety or depression (Krug and Carter 2010; Rey et al. 2014; Haas et al. 2007).
In either case, previous studies employing adaptation paradigms indicate that emotional and cognitive interference might also depend on domain-specific neural circuits. Indeed, the domain-general network identified in the current meta-analysis does not preclude the possibility that they depend on additional domain-specific processes. This conjecture fits with recent findings that control of emotion and action recruits both common and distinct brain systems (Langner et al. 2018). In line with the current findings, Langner et al. (2018) identified a core network—consisting of the dACC, AI, and SPL—commonly involved in emotion regulation and action control. However, the authors further revealed specific networks for each domain (Langner et al. 2018). Discrepant findings between the present meta-analysis and Langner et al.’s study may be related to the disparity in the topics covered. In the current study, we compared the interference in emotional and cognitive domains, which are similar to each other in terms of topic, tasks, and contrasts (although variations exist). In contrast, the two fields compared by Langner et al. (2018) often employed very different tasks (e.g., “enhancing your emotional experience while viewing the picture” vs. “responding to the color of the word while ignoring meaning of the words”) and stimuli (e.g., arousing affective pictures vs. neutral words), and they concerned related but distinct contrasts (e.g., “enhancing emotion > passive viewing” vs. “incongruent > congruent”). For these reasons, it is not surprising that Langner et al. (2018) revealed more distinct processes between two domains.
Fourth, the limited number of studies for each type of paradigm (e.g., emotional Flanker, emotional Stroop) and stimulus modality (e.g., visual, auditory) did not allow for the examination of neural substrates within each task and modality (e.g., Watanabe et al. 2013), but an accumulating number of neuroimaging studies on emotional interference will enable such an investigation in future meta-analyses.
In summary, our findings indicate that interference induced by emotional stimuli modulates neural mechanisms that strongly resemble those for cognitive interference at both the regional and network levels. These findings coincide with the domain-general hypothesis holding that different types of interference share similar neural mechanisms implemented in domain-general cognitive control systems (Buhle et al. 2014; Ochsner et al. 2009; Ochsner and Gross 2005). Our findings might have significant clinical implications with respect to the critical roles of domain-general cognitive systems in psychiatric distress. That is, our results suggest that cognitive control deficits could account for the etiology and symptoms (e.g., emotion regulation deficits) of a variety of mental illness. In line with this conjecture, a recent meta-analysis of the gray matter volume identified common structural perturbations in the dACC and AI across psychopathologies (Goodkind et al. 2015). In a transdiagnostic meta-analysis of neuroimaging studies on cognitive control tasks (e.g., classical Stroop, Go/No-Go task, working memory), McTeague et al. (2017) identified a common neural circuit disruption across psychiatric diagnoses that parallels the currently observed neural network, including the dACC, lateral PFC, and parietal cortex. The current results complement previous observations by suggesting that disruptions in cognitive control systems could impair not only cognitive functions, but also appropriate control of emotional distress.
Supplementary Material
Acknowledgments
Funding This work was supported by the National Postdoctoral Program for Innovative Talents under Grant agreement no. BX201600019 (to C.F.), the China Postdoctoral Science Foundation under Grant agreement nos. 2017M610055 (to C.F.) and 2013M530401 (to T.C.), the National Natural Science Foundation of China under Grant agreement nos. 81401398 (to T.C.), 91632117 (to B.B.), and 31500920 (to C.F.), and the National Institute of Mental Health (R01-MH074457, to E.S.), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement no. 7202070 (HBP SGA1, to E.S.).
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00429-018-1727-9) contains supplementary material, which is available to authorized users.
Conflict of interest The authors are unaware of any conflicts of interest, financial or otherwise.
Ethical approval The study was approved by the Ethics Committee of Beijing Normal University.
Informed consent Not applicable. This is a meta-analytic study.
References
- Aron AR, Robbins TW, Poldrack RA (2004) Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8(4):170–177 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851 [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB (2015) Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp 36(2):449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang L, Rø Ø, Endestad T (2016) Amygdala alterations during an emotional conflict task in women recovered from anorexia nervosa. Psychiatry Res Neuroimaging 248:126–133 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB (2013) Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol 23(3):361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013) The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall PM, Herbert AM (2008) The face wins: stronger automatic processing of affect in facial expressions than words in a modified Stroop task. Cogn Emot 22(8):1613–1642 [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S (2004) Parietal cortex and attention. Curr Opin Neurobiol 14(2):212–217 [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Lawrence AD (2004) Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7(2):184–188. 10.1038/Nn1173 [DOI] [PubMed] [Google Scholar]
- Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7(4):356–366 [DOI] [PubMed] [Google Scholar]
- Braem S, Abrahamse EL, Duthoo W, Notebaert W (2014) What determines the specificity of conflict adaptation? A review, critical analysis, and proposed synthesis. Front Psychol 5:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S, King JA, Korb FM, Krebs RM, Notebaert W, Egner T (2017) The role of anterior cingulate cortex in the affective evaluation of conflict. J Cogn Neurosci 29(1):137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN (2014) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24(11):2981–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD (2002) Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17(3):1562–1571 [DOI] [PubMed] [Google Scholar]
- Chechko N, Wehrle R, Erhardt A, Holsboer F, Czisch M, Sämann PG (2009) Unstable prefrontal response to emotional conflict and activation of lower limbic structures and brainstem in remitted panic disorder. PLoS One 4(5):e5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechko N, Kellermann T, Zvyagintsev M, Augustin M, Schneider F, Habel U (2012) Brain circuitries involved in semantic interference by demands of emotional and non-emotional distractors. PLoS One 7(5):e38155. 10.1371/journal.pone.0038155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechko N, Augustin M, Zvyagintsev M, Schneider F, Habel U, Kellermann T (2013) Brain circuitries involved in emotional interference task in major depression disorder. J Affect Disord 149(1):136–145 [DOI] [PubMed] [Google Scholar]
- Chechko N, Kellermann T, Schneider F, Habel U (2014) Conflict adaptation in emotional task underlies the amplification of target. Emotion 14(2):321. [DOI] [PubMed] [Google Scholar]
- Chen T, Kendrick KM, Feng C, Yang S, Wang X, Yang X, Lei D, Wu M, Huang X, Gong Q (2014) Opposite effect of conflict context modulation on neural mechanisms of cognitive and affective control. Psychophysiology 51(5):478–488 [DOI] [PubMed] [Google Scholar]
- Chen T, Kendrick KM, Feng C, Sun S, Yang X, Wang X, Luo W, Yang S, Huang X, Valdés-Sosa PA (2016) Dissociable early attentional control mechanisms underlying cognitive and affective conflicts. Sci Rep 6:37633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS (2011) Neural circuitry of emotional and cognitive conflict revealed through facial expressions. PLoS One 6(3):e17635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL (2012) The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108(8):2242–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB (2010) Dissociating bottom-up and top-down processes in a manual stimulus-response compatibility task. J Neurophysiol 104(3):1472–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB (2015) Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev 48:22–34. 10.1016/j.neubiorev.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W (2007) The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37(1):343–360 [DOI] [PubMed] [Google Scholar]
- Cole MW, Repovš G, Anticevic A (2014) The frontoparietal control system: a central role in mental health. Neuroscientist 20(6):652–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon O, Girard S, Gosselin F, Roy S, Saint-Amour D, Lassonde M, Lepore F (2008) Audio-visual integration of emotion expression. Brain Res 1242:126–135 [DOI] [PubMed] [Google Scholar]
- Comte M, Schön D, Coull JT, Reynaud E, Khalfa S, Belzeaux R, Ibrahim EC, Guedj E, Blin O, Weinberger DR (2014) Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing. Cereb Cortex 26(1):144–155 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3(3):292–297 [DOI] [PubMed] [Google Scholar]
- Cromheeke S, Mueller SC (2014) Probing emotional influences on cognitive control: an ALE meta-analysis of cognition emotion interactions. Brain Struct Funct 219(3):995–1008 [DOI] [PubMed] [Google Scholar]
- De Gelder B, Vroomen J (2000) The perception of emotions by ear and by eye. Cogn Emot 14(3):289–311 [Google Scholar]
- Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P (2011) Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord 130(1):66–74 [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY (2005) Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp 25(1):22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Morris JS, de Gelder B (2001) Crossmodal binding of fear in voice and face. Proc Natl Acad Sci 98(17):10006–10010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006) A core system for the implementation of task sets. Neuron 50(5):799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 104(26):11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008) A dual-networks architecture of top-down control. Trends Cogn Sci 12(3):99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R (2012) Conflicts as aversive signals. Brain Cogn 78(2):94–98 [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Fischer R (2015) Conflicts as aversive signals for control adaptation. Curr Dir Psychol Sci 24(4):255–260 [Google Scholar]
- Duncan J (2010) The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14(4):172–179 [DOI] [PubMed] [Google Scholar]
- Duncan J (2013) The structure of cognition: attentional episodes in mind and brain. Neuron 80(1):35–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000) Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23(10):475–483 [DOI] [PubMed] [Google Scholar]
- Egner T (2008) Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci 12(10):374–380 [DOI] [PubMed] [Google Scholar]
- Egner T (2014) Creatures of habit (and control): a multi-level learning perspective on the modulation of congruency effects. Front Psychol 5:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J (2005) Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci 8(12):1784–1790 [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J (2008) Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex 18(6):1475–1484. 10.1093/cercor/bhm179 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30(9):2907–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011) Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57(3):938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59(3):2349–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT (2017) Implementation errors in the GingerALE Software: description and recommendations. Hum Brain Mapp 38(1):7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B, Eriksen C (1974) Effects of noise letters upon the identification of a target letter in a nonsearch task. Atten Percept Psychophys 16(1):143–149. 10.3758/bf03203267 [DOI] [Google Scholar]
- Etkin A, Schatzberg AF (2011) Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry 168(9):968–978 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51(6):871–882 [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF (2010) Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 167(5):545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15(2):85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ (2015) The neural bases of emotion regulation. Nat Rev Neurosci 16(11):693. [DOI] [PubMed] [Google Scholar]
- Eugène F, Joormann J, Cooney RE, Atlas LY, Gotlib IH (2010) Neural correlates of inhibitory deficits in depression. Psychiatry Res Neuroimaging 181(1):30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G (2011) Is there a core neural network in empathy? An fMRI based quantitative metaanalysis. Neurosci Biobehav Rev 35(3):903–911 [DOI] [PubMed] [Google Scholar]
- Feng C, Luo YJ, Krueger F (2015) Neural signatures of fairness-related normative decision making in the ultimatum game: a coordinate-based meta-analysis. Hum Brain Mapp 36(2):591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Becker B, Huang W, Wu X, Eickhoff SB, Chen T (2018) Neural substrates of the emotion-word and emotional counting Stroop tasks in healthy and clinical populations: a meta-analysis of functional brain imaging studies. NeuroImage 173:258–274 [DOI] [PubMed] [Google Scholar]
- Fleury V, Cousin E, Czernecki V, Schmitt E, Lhommée E, Poncet A, Fraix V, Troprès I, Pollak P, Krainik A (2014) Dopaminergic modulation of emotional conflict in Parkinson’s disease. Front Aging Neurosci 6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Lindley SE (2017) PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am J Psychiatry 174(12):1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouragnan E, Retzler C, Philiastides MG (2018) Separate neural representations of prediction error valence and surprise: evidence from an fMRI meta-analysis. Hum Brain Mapp 39(7):2887–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, McClernon FJ (2012) Smoking abstinence and depressive symptoms modulate the executive control system during emotional information processing. Addict Biol 17(3):668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühholz S, Fehr T, Herrmann M (2009) Interference control during recognition of facial affect enhances the processing of expression specific properties—an event-related fMRI study. Brain Res 1269:143–157 [DOI] [PubMed] [Google Scholar]
- Garrison J, Erdeniz B, Done J (2013) Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37(7):1297–1310 [DOI] [PubMed] [Google Scholar]
- Godinez DA, McRae K, Andrews-Hanna JR, Smolker H, Banich MT (2016) Differences in frontal and limbic brain activation in a small sample of monozygotic twin pairs discordant for severe stressful life events. Neurobiol Stress 5:26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS (2015) Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72(4):305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE (2014) ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 95:232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T (2006) Interference produced by emotional conflict associated with anterior cingulate activation. Cogn Affect Behav Neurosci 6(2):152–156 [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T (2007) Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci 121(2):249. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Green SR, Casp M, Belger A (2010) Emotional priming effects during Stroop task performance. Neuroimage 49(3):2662–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P (2001) Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293(5539):2425–2430 [DOI] [PubMed] [Google Scholar]
- Houwer JD, Hermans D (1994) Differences in the affective processing of words and pictures. Cogn Emot 8(1):1–20 [Google Scholar]
- Huang J, Wang Y, Jin Z, Di X, Yang T, Gur RC, Gur RE, Shum DH, Cheung EF, Chan RC (2013) Happy facial expression processing with different social interaction cues: an fMRI study of individuals with schizotypal personality traits. Prog Neuropsychopharmacol Biol Psychiatry 44:108–117 [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, Ernst M (2013) The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol Psychol 92(2):306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R (2009) The functional neuroanatomy of reappraisal: time matters. Neurosci Biobehav Rev 33(8):1215–1226 [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA (2010) Emotion triggers executive attention: anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum Brain Mapp 32(2):198–208. 10.1002/hbm.21012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2014) Involvement of the dorsal and ventral attention networks in oddball stimulus processing: a meta-analysis. Hum Brain Mapp 35(5):2265–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen M, Kenworthy CA, Mathiak KA, Kircher TT, Mathiak K (2011) Supramodal representation of emotions. J Neurosci 31(38):13635–13643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010) Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci 107(33):14811–14816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J (2009) Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29(47):14980–14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014) Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage 87:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Carter C (2010) Adding fear to conflict: a general purpose cognitive control network is modulated by trait anxiety. Cogn Affect Behav Neurosci 10(3):357–371. 10.3758/cabn.10.3.357 [DOI] [PubMed] [Google Scholar]
- Krug MK, Carter CS (2012) Proactive and reactive control during emotional interference and its relationship to trait anxiety. Brain Res 1481:13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Müller BC, van der Leij A, Dijksterhuis A, Brass M, van Baaren RB (2010) Neural correlates of emotional synchrony. Soc Cogn Affect Neurosci 6(3):368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005a) ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25(1):155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT (2005b) A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp 25(1):6–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT (2009a) ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT (2009b) Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci 29(46):14496–14505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Rottschy C, Laird AR, Fox PT, Eickhoff SB (2014) Meta-analytic connectivity modeling revisited: controlling for activation base rates. NeuroImage 99:559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Leiberg S, Hoffstaedter F, Eickhoff SB (2018) Towards a human self-regulation system: common and distinct neural signatures of emotional and behavioural control. Neurosci Biobehav Rev 90:400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-W, Dolan RJ, Critchley HD (2007) Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cereb Cortex 18(1):104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Heller AS, Van Reekum CM, Nelson B, Davidson RJ (2012) Amygdala–prefrontal coupling underlies individual differences in emotion regulation. Neuroimage 62(3):1575–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011) Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224(1):40–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S (2003) Cortical mechanisms of feature-based attentional control. Cereb Cortex 13(12):1334–1343 [DOI] [PubMed] [Google Scholar]
- Lückmann HC, Jacobs HI, Sack AT (2014) The cross-functional role of frontoparietal regions in cognition: internal attention as the overarching mechanism. Prog Neurobiol 116:66–86 [DOI] [PubMed] [Google Scholar]
- Luo Y, Eickhoff SB, Hétu S, Feng C (2018) Social comparison in the brain: a coordinate-based meta-analysis of functional brain imaging studies on the downward and upward comparisons. Hum Brain Mapp 39(1):440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288(5472):1835–1838 [DOI] [PubMed] [Google Scholar]
- MacLeod CM (1991) Half a century of research on the Stroop effect: an integrative review. Psychol Bull 109(2):163. [DOI] [PubMed] [Google Scholar]
- Maier ME, Di Pellegrino G (2012) Impaired conflict adaptation in an emotional task context following rostral anterior cingulate cortex lesions in humans. J Cogn Neurosci 24(10):2070–2079 [DOI] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A (2017) Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry 174(7):676–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15(10):483–506 [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M (1990) Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28(5):597–613 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24(1):167–202 [DOI] [PubMed] [Google Scholar]
- Mitchell RL (2006a) Does incongruence of lexicosemantic and prosodic information cause discernible cognitive conflict? Cogn Affect Behav Neurosci 6(4):298–305 [DOI] [PubMed] [Google Scholar]
- Mitchell RL (2006b) How does the brain mediate interpretation of incongruent auditory emotions? The neural response to prosody in the presence of conflicting lexico-semantic cues. Eur J Neurosci 24(12):3611–3618 [DOI] [PubMed] [Google Scholar]
- Mitchell RL (2013) Further characterisation of the functional neuroanatomy associated with prosodic emotion decoding. Cortex J Devot Study Nerv Syst Behav 49(6):1722–1732 [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Elliott R, Barry M, Cruttenden A, Woodruff PW (2003) The neural response to emotional prosody, as revealed by functional magnetic resonance imaging. Neuropsychologia 41(10):1410–1421 [DOI] [PubMed] [Google Scholar]
- Müller VI, Habel U, Derntl B, Schneider F, Zilles K, Turetsky BI, Eickhoff SB (2011) Incongruence effects in crossmodal emotional integration. Neuroimage 54(3):2257–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J (2007) Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7(1):1–17 [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q (2012) The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front Neurosci 6:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV (2006) Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci 10(9):424–430 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci 9(5):242–249 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002) Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14(8):1215–1229 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD (2009) Neural systems supporting the control of affective and cognitive conflicts. J Cogn Neurosci 21(9):1842–1855. 10.1162/jocn.2009.21129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251(1): E1–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offringa R, Brohawn KH, Staples LK, Dubois SJ, Hughes KC, Pfaff DL, VanElzakker MB, Davis FC, Shin LM (2013) Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biol Mood Anxiety Disord 3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovaysikia S, Chan JL, Tahir K, DeSouza JF (2011) Word wins over face: emotional Stroop effect activates the frontal cortical network. Front Hum Neurosci 4:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Shi L, Lu Q, Wu X, Xue S, Li Q (2016) The negative priming effect in cognitive conflict processing. Neurosci Lett 628:35–39 [DOI] [PubMed] [Google Scholar]
- Park IH, Park H-J, Chun J-W, Kim EY, Kim J-J (2008) Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neurosci Lett 440(2):119–124 [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE (2007) Using multi-voxel pattern analysis of fMRI data to interpret overlapping functional activations. Trends Cogn Sci 11(1):4–5 [DOI] [PubMed] [Google Scholar]
- Pessoa L (2008) On the relationship between emotion and cognition. Nat Rev Neurosci 9(2):148–158 [DOI] [PubMed] [Google Scholar]
- Rey G, Desseilles M, Favre S, Dayer A, Piguet C, Aubry J-M, Vuilleumier P (2014) Modulation of brain response to emotional conflict as a function of current mood in bipolar disorder: preliminary findings from a follow-up state-based fMRI study. Psychiatry Res Neuroimaging 223(2):84–93 [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Van Den Wildenberg WP, Segalowitz SJ, Carter CS (2004) Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56(2):129–140 [DOI] [PubMed] [Google Scholar]
- Rota G, Veit R, Nardo D, Weiskopf N, Birbaumer N, Dogil G (2008) Processing of inconsistent emotional information: an fMRI study. Exp Brain Res 186(3):401–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM (2014) Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90:449–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Robertson ER, Mikels JA, Carstensen LL, Gotlib IH (2009) Selective attention to emotion in the aging brain. Psychol Aging 24(3):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64:240–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, von Cramon DY (2004) Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage 21(3):1114–1123 [DOI] [PubMed] [Google Scholar]
- Schouppe N, Braem S, De Houwer J, Silvetti M, Verguts T, Ridderinkhof KR, Notebaert W (2015) No pain, no gain: the affective valence of congruency conditions changes following a successful response. Cogn Affect Behav Neurosci 15(1):251–261 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Xia M, Lin Q, Cao M, Tang Y, Xu K, Song H, Wang Z, Wang F, Fox PT (2017) Meta-connectomic analysis reveals commonly disrupted functional architectures in network modules and connectors across brain disorders. Cereb Cortex. 10.1093/cercor/bhx273 [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey BJ, Ochsner KN (2017) vlPFC–vmPFC–Amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex 27(7):3502–3514. 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Craft JL, Small AM (1971) Reactions toward the apparent source of an auditory stimulus. J Exp Psychol 89(1):203–206. 10.1037/h0031164 [DOI] [PubMed] [Google Scholar]
- Song S, Zilverstand A, Song H, Uquillas FdO, Wang Y, Xie C, Cheng L, Zou Z (2017) The influence of emotional interference on cognitive control: a meta-analysis of neuroimaging studies using the emotional Stroop task. Sci Rep 7(1):2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Heller W, Banich MT (2015) Flexible brain network reconfiguration supporting inhibitory control. Proc Natl Acad Sci 112(32):10020–10025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G, Wiking S, Dahl M (1998) Judging words at face value: interference in a word processing task reveals automatic processing of affective facial expressions. Cogn Emot 12(6):755–782 [Google Scholar]
- Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18(6):643 [Google Scholar]
- Swick D, Ashley V, Turken U (2008) Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci 9(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT (1998) Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci 95(26):15855–15860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Quesada M, Korb FM, Funes MJ, Lupianez J, Egner T (2014) Comparing neural substrates of emotional vs. non-emotional conflict modulation by global control context. Front Hum Neurosci 8:66. 10.3389/fnhum.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002) Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16(3):765–780 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp 33(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Laird AR (2012) The cognitive paradigm ontology: design and application. Neuroinform 10(1):57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt M-A, Baeken C, Van Schuerbeek P, Luypaert R, De Mey J, De Raedt R (2013) How brooding minds inhibit negative material: an event-related fMRI study. Brain Cogn 81(3):352–359 [DOI] [PubMed] [Google Scholar]
- Vartanian O, Skov M (2014) Neural correlates of viewing paintings: evidence from a quantitative meta-analysis of functional magnetic resonance imaging data. Brain Cogn 87:52–56 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100(6):3328–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P, Lordier L, Bediou B, Sander D (2014) Human amygdala response to dynamic facial expressions of positive and negative surprise. Emotion 14(1):161–169 [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L (2007) Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci 2(2):150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008) Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59(6):1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G (2008) Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res Neuroimaging 163(2):143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yahata N, Kawakubo Y, Inoue H, Takano Y, Iwashiro N, Natsubori T, Takao H, Sasaki H, Gonoi W (2013) Network structure underlying resolution of conflicting non-verbal and verbal social information. Soc Cogn Affect Neurosci 9(6):767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R, Latinus M, Noguchi T, Garrod O, Crabbe F, Belin P (2013) Dissociating task difficulty from incongruence in face-voice emotion integration. Front Hum Neurosci 7:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’sullivan J, Lambon Ralph MA, Jefferies E (2010) The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex 21(5):1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittfoth M, Schröder C, Schardt DM, Dengler R, Heinze H-J, Kotz SA (2010) On emotional conflict: interference resolution of happy and angry prosody reveals valence-specific effects. Cereb Cortex 20(2):383–392. 10.1093/cercor/bhp106 [DOI] [PubMed] [Google Scholar]
- Wu H, Luo Y, Feng C (2016) Neural signatures of social conformity: a coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev 71:101–111 [DOI] [PubMed] [Google Scholar]
- Xu M, Xu G, Yang Y (2016) Neural systems underlying emotional and non-emotional interference processing: an ALE meta-analysis of functional neuroimaging studies. Front Behav Neurosci 10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Wang X, Chang J, Liu J, Qiu J (2016) Amplitude of low-frequency oscillations associated with emotional conflict control. Exp Brain Res 234(9):2561–2566 [DOI] [PubMed] [Google Scholar]
- Yantis S, Serences JT (2003) Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol 13(2):187–193 [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM (2002) Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5(10):995–1002 [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106(3):1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Hennigan K, Weber J, Ochsner KN (2010) Social cognitive conflict resolution: contributions of domain-general and domain-specific neural systems. J Neurosci 30(25):8481–8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Geng X, Lee TM (2017) Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct Funct 222(9):1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


