Key Points
Question
Does supplementation with marine omega-3 fatty acids and/or vitamin D3 affect the risk of developing atrial fibrillation (AF)?
Findings
In this ancillary primary prevention AF trial that was embedded within a 2 × 2 factorial randomized clinical trial and included 25 119 participants without AF at study entry, there was no significant difference in AF incidence with marine omega-3 fatty acids vs placebo (hazard ratio, 1.09) or with vitamin D3 supplementation vs placebo (hazard ratio, 1.09) over a median 5.3 years of treatment and follow-up.
Meaning
These findings do not support the use of marine omega-3 fatty acids or vitamin D3 in adults to prevent AF.
Abstract
Importance
Atrial fibrillation (AF) is the most common heart rhythm disturbance, continues to increase in incidence, and results in significant morbidity and mortality. The marine omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and vitamin D have been reported to have both benefits and risks with respect to incident AF, but large-scale, long-term randomized trial data are lacking.
Objective
To test the effects of long-term administration of marine omega-3 fatty acids and vitamin D on incident AF.
Design, Setting, and Participants
An ancillary study of a 2 × 2 factorial randomized clinical trial involving 25 119 women and men aged 50 years or older without prior cardiovascular disease, cancer, or AF. Participants were recruited directly by mail between November 2011 and March 2014 from all 50 US states and were followed up until December 31, 2017.
Interventions
Participants were randomized to receive EPA-DHA (460 mg/d of EPA and 380 mg/d of DHA) and vitamin D3 (2000 IU/d) (n = 6272 analyzed); EPA-DHA and placebo (n = 6270 analyzed); vitamin D3 and placebo (n = 6281 analyzed); or 2 placebos (n = 6296 analyzed).
Main Outcomes and Measures
The primary outcome was incident AF confirmed by medical record review.
Results
Among the 25 119 participants who were randomized and included in the analysis (mean age, 66.7 years; 50.8% women), 24 127 (96.1%) completed the trial. Over a median 5.3 years of treatment and follow-up, the primary end point of incident AF occurred in 900 participants (3.6% of study population). For the EPA-DHA vs placebo comparison, incident AF events occurred in 469 (3.7%) vs 431 (3.4%) participants, respectively (hazard ratio, 1.09; 95% CI, 0.96-1.24; P = .19). For the vitamin D3 vs placebo comparison, incident AF events occurred in 469 (3.7%) vs 431 (3.4%) participants, respectively (hazard ratio, 1.09; 95% CI, 0.96-1.25; P = .19). There was no evidence for interaction between the 2 study agents (P = .39).
Conclusions and Relevance
Among adults aged 50 years or older, treatment with EPA-DHA or vitamin D3, compared with placebo, resulted in no significant difference in the risk of incident AF over a median follow-up of more than 5 years. The findings do not support the use of either agent for the primary prevention of incident AF.
Trial Registration
ClinicalTrials.gov Identifiers: NCT02178410; NCT01169259
This 2 × 2 factorial trial compares the effects of omega-3 fatty acids (460 mg/d of EPA and 380 mg/d of DHA) vs placebo, vitamin D₃ vs placebo, both, or neither on risk of incident atrial fibrillation (AF) among adults aged 50 years or older over a median of more than 5 years of treatment.
Introduction
Atrial fibrillation (AF) is the most common heart rhythm disturbance, with an estimated 46.3 million people affected worldwide in 2016.1 The incidence of AF increases exponentially after age 65 years, with lifetime risk estimates equal to 1 in 3 for White individuals and 1 in 5 for Black individuals.2 Once established, AF can result in thromboembolic stroke, congestive heart failure, impaired quality of life, and other adverse consequences.3 Even among populations without established cardiovascular disease (CVD), AF has been associated with elevated mortality.4 Thus, primary preventive interventions for AF that can be applied to broad populations are needed, and dietary supplements have appeal in this regard.
In observational studies, individuals with low blood levels of marine omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and vitamin D3 have higher risks of incident AF5,6,7; however, data regarding dietary or supplemental intake of these nutrients on AF risk are mixed.8,9,10 As a result, both benefits and risks have been postulated. Randomized clinical trials (RCTs) testing the effect of short-term administration of omega-3 fatty acids on recurrent AF have had disparate results,11 and recent RCTs testing longer-term treatment with omega-3 fatty acids on CVD events have reported increased risks of AF in participants randomized to active therapy vs placebo.12,13,14 Given the common use of these supplements in primary care settings, as well as recent RCT data suggesting potential harm, definitive data on potential risks and benefits on AF incidence in primary care populations are needed. To assess the role of these agents in AF primary prevention, the present study prospectively tested the effects of long-term administration of marine omega-3 fatty acids and vitamin D3 on incident AF within a large-scale RCT performed among men and women without prior vascular disease.
Methods
Trial Design and Oversight
The VITAL Rhythm Study was an ancillary trial embedded within the Vitamin D and Omega-3 (VITAL) trial. All participants were recruited and enrolled directly by mail and provided written informed consent, and approvals for the parent trial and this study were obtained from the institutional review board of Brigham and Women’s Hospital. The design of the parent trial has been previously described.15,16 In brief, the trial was a double-blind, placebo-controlled randomized trial that tested in a 2 × 2 factorial design daily supplementation with 2000 IU of vitamin D3 and/or 840 mg of marine omega-3 fatty acids, composed of 460 mg of EPA and 380 mg of DHA, in the primary prevention of CVD and cancer in 25 871 men and women in the United States. The vitamin D3 dose was designed to test the effect of high-dose supplementation on cancer and CVD in a primary prevention population,15 and the EPA-DHA dose was recommended by the American Heart Association for cardioprotection among high-risk individuals and was above the dose recommended from dietary intake for the general population.16 The placebo for vitamin D3 contained soybean oil, and the placebo for EPA-DHA contained olive oil.
For inclusion, men were required to be at least 50 years of age and women were required to be at least 55 years of age. To comply with National Institutes of Health (NIH) reporting requirements, information on self-reported race/ethnicity was collected on the screening questionnaire, which included fixed categories and an unknown category. This information was also used to oversample Black participants, who have higher rates of vitamin D deficiency than other racial groups.15 Participants were required to have no history of CVD (myocardial infarction, stroke, transient ischemic attack, angina, or revascularization) or cancer and be willing to forgo supplemental vitamin D3 intake greater than 800 IU/d, supplemental calcium intake greater than 1200 mg/d, and fish oil supplementation during the trial. Safety exclusion criteria are outlined in detail elsewhere and included kidney failure or dialysis, cirrhosis, and history of hypercalcemia.15 The study agents received Investigational New Drug approval from the US Food and Drug Administration. An independent data and safety monitoring board regularly reviewed data on end points and adverse events. The institutional review board trial protocol and the statistical analysis plan generated from the prespecified analyses in the NIH grant application are available in Supplement 1 and Supplement 2, respectively.
During a 3-month placebo run-in phase and at randomization, all participants completed questionnaires on health status, including information on preceding medical diagnoses inclusive of AF as well as cardiovascular risk factors, medications, lifestyle habits, and diet. Blood samples were requested but not required, and were collected during the run-in period. Plasma phospholipid omega-3 fatty acids (EPA plus DHA as a percentage of total plasma phospholipid fatty acids) and serum 25-hydroxyvitamin D assays were measured in stored samples using liquid chromatography–tandem mass spectrometry–mass spectrometry by Quest Diagnostics.17
After successful completion of the run-in period, participants were randomized to receive vitamin D3, EPA-DHA, both active agents, or both placebos between November 2011 and March 2014. Randomization was computer generated in block sizes of 8 within sex, race, and 5-year age groups, with an equal representation of sex and an oversampling of Black study participants. Participants enrolled in the parent trial who reported a diagnosis of AF at baseline were excluded from follow-up. At 6 months and then annually thereafter, each participant received a follow-up questionnaire that inquired about new medical diagnoses including AF, medication and supplement use, and lifestyle and clinical risk factors, as well as receiving a resupply of study agents.
Outcome Assessment
The primary outcome of the study was incident AF confirmed by medical record review, and incident paroxysmal and nonparoxysmal AF were prespecified secondary outcomes. Given the common association with AF, atrial flutter events were included in the primary and secondary AF outcomes. The outcomes were identified through 2 methods. First, participants self-reported new diagnoses of AF on annual follow-up questionnaires. Second, the VITAL population was linked to inpatient and outpatient claims data from the Centers for Medicare & Medicaid Services (CMS), and diagnosis codes for AF (International Classification of Diseases, Ninth Revision [ICD-9] diagnosis code 427.31 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] diagnosis code I48.91) and atrial flutter (ICD-9 diagnosis code 427.32 and ICD-10 diagnosis code I48.92) were ascertained. The details regarding the CMS linkage are available in the eAppendix in Supplement 3.
For all participants with an incident AF or atrial flutter diagnosis, either self-reported or identified through CMS linkage, permission was requested to obtain medical records pertaining to the diagnosis and subsequent cardiac evaluation. An end-point committee of cardiologists reviewed medical records and confirmed events according to predefined criteria.4 Electrocardiographic (ECG) evidence of AF or atrial flutter or a physician’s report outlining a diagnosis of either was required for confirmation. The date of onset was defined as the earliest documented occurrence of either arrhythmia within the medical records. If onset was prior to randomization, the participant was excluded from the statistical analysis. For the secondary outcomes, the pattern of AF at the time of diagnosis was classified in accordance with American College of Cardiology/American Heart Association/Heart Rhythm Society and European Society of Cardiology guidelines.18,19 Exploratory outcomes not reported in this article include repeated ECG characteristics collected in a subset of 911 participants, and cause-specific cardiovascular mortality, including sudden arrhythmic death.
Sample Size and Power Calculations
Based on pretrial assumptions that 23 520 participants without prevalent AF would be enrolled in VITAL and that the 5-year cumulative incidence of AF would be 3.15%, 741 primary outcome events were projected to occur during the trial, providing 90% power to detect a 22% reduction or increase in the observed hazard ratio (HR) for the primary outcome, incident AF, which was well within that observed in observational studies and in a contemporaneous meta-analysis of secondary prevention trials.5,7,8,20
Statistical Analyses
Primary Analysis
After excluding AF cases diagnosed prior to randomization (Figure 1), participants were analyzed according to randomization group. To ensure balance by randomization remained after excluding prevalent AF cases, baseline characteristics were compared for each treatment group using t tests for continuous variables and χ2 statistics for categorical variables. There was an a priori assumption of no interaction between agents, and the prespecified analyses examined the pooled main effects of EPA-DHA and vitamin D3 on incident AF within the 2 × 2 factorial design. The HR for each intervention was estimated in Cox proportional hazard models using indicators for treatment assignment, controlling for the second intervention, age, and sex. Cumulative incidence curves were estimated from the adjusted Cox model. The proportionality assumption was tested using an interaction term for treatment × log time and was met for each of the primary and secondary outcomes. Times to events were calculated as the interval between randomization and the earliest occurrence of confirmed AF event, death, or the end of randomized treatment (December 31, 2017). Participants who did not return an annual questionnaire and/or who had missing data on AF on a questionnaire were assumed to not have AF during that reporting cycle unless subsequently ascertained and confirmed to have AF during that time period.
Figure 1. Flow of Participants in the VITAL Rhythm Study.
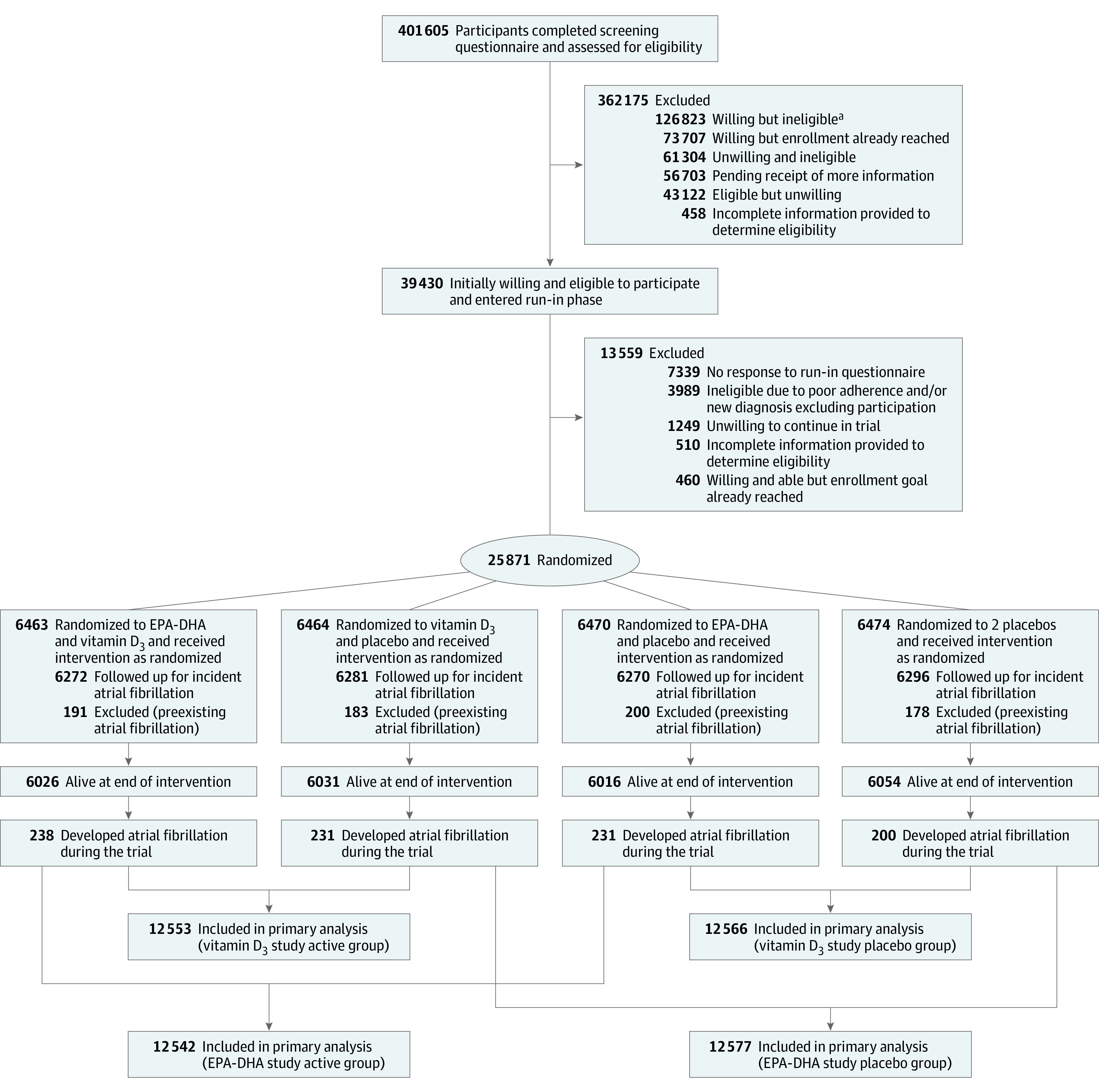
Participants were randomized to receive vitamin D3, marine omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), both active agents, or both placebos between November 2011 and March 2014. Randomization was computer generated in block sizes of 8 within sex, race, and 5-year age groups, with an equal representation of sex and an oversampling of Black/African American participants.
aWilling/ineligible category included 76 190 individuals with history of cardiovascular disease, cancer, and/or safety exclusion criteria; 32 647 individuals unwilling to forgo supplemental vitamin D3 intake greater than 800 IU/d, supplemental calcium intake greater than 1200 mg/d, or fish oil supplementation during the trial; 13 521 men younger than 50 years and women younger than 55 years; and 4465 individuals who reported another exclusion criterion (eg, participation in another trial).
Three prespecified sensitivity analyses were also performed for the primary outcome. Because determination of the initial onset of AF can be challenging, the first analysis excluded participants who had symptoms that may have been related to AF prior to randomization. Because CMS claims data are available only for select participants, primarily those aged 65 or older, the second analysis limited confirmed AF events to those identified by self-report. Third, an on-treatment, adherence-based analysis that censored follow-up data when a participant discontinued trial capsules or began taking study supplements outside of the trial was performed. In addition, 2 post hoc sensitivity analyses were performed. The first excluded events solely due to atrial flutter or postoperative AF and the second excluded events without a confirmatory ECG.
Secondary Analyses
To assess for treatment interactions in the primary outcome, multiplicative interaction terms in Cox models were used to explore interactions between vitamin D3 and EPA-DHA. Combination treatment groups (active-active, active-placebo, placebo-active) were compared with the group receiving both placebos using Cox models and cumulative incidence curves. In addition, subgroup analyses were performed that examined effect modification by a priori–selected AF risk factors, dietary fish intake, vitamin D3 intake, and blood levels of both nutrients at baseline. To test for interactions, cross-product terms were included in the full multivariable model.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc), and a 2-sided P < .05 was used to determine statistical significance. Because of the potential for type I error due to multiple comparisons, findings for secondary outcomes and analyses should be interpreted as exploratory.
Results
Among the 25 871 participants enrolled in the parent trial, 752 participants with prevalent AF at baseline were excluded from study follow-up (balanced by treatment group; P = .68). The remaining 25 119 participants were distributed across 4 randomized treatment groups as shown in Figure 1. The study treatment ended as planned on December 31, 2017, which yielded a median follow-up of 5.3 years (range, 3.8-6.1 years). Table 1 shows the characteristics of the population across the 4 treatment groups. The median age was 66.7 years (interquartile range, 62.4-71.0 years) and 50.8% were women. Participants who self-identified as Black or African American accounted for 20.1% of the population. Randomization remained balanced for CVD and AF risk factors after exclusion of prevalent AF events (Table 1).
Table 1. Baseline Participant Characteristics.
| Characteristics | EPA-DHA and vitamin D3 (n = 6272) | EPA-DHA only (n = 6270) | Vitamin D3 only (n = 6281) | Placebo only (n = 6296) |
|---|---|---|---|---|
| Age, median (IQR), y | 66.6 (62.5-71.0) | 66.7 (62.5-71.0) | 66.7 (62.4-71.1) | 66.6 (62.4-71.0) |
| Sex, No. (%) | ||||
| Female | 3192 (50.9) | 3176 (50.7) | 3192 (50.8) | 3197 (50.8) |
| Male | 3080 (49.1) | 3094 (49.3) | 3089 (49.2) | 3099 (49.2) |
| Race/ethnicity, No. (%)a | n = 6147 | n = 6130 | n = 6142 | n = 6160 |
| Non-Hispanic White | 4360 (70.9) | 4364 (71.2) | 4346 (70.8) | 4355 (70.7) |
| Black or African American | 1264 (20.6) | 1256 (20.5) | 1262 (20.5) | 1270 (20.6) |
| Hispanic | 243 (4.0) | 241 (3.9) | 266 (4.3) | 249 (4.0) |
| Asian/Pacific Islander | 99 (1.6) | 96 (1.6) | 86 (1.4) | 101 (1.6) |
| Native American/Alaska Native | 60 (1.0) | 54 (0.9) | 51 (0.8) | 50 (0.8) |
| Unknown | 121 (2.0) | 119 (1.9) | 131 (2.1) | 135 (2.2) |
| Smoking status, No. (%) | n = 6172 | n = 6182 | n = 6192 | n = 6198 |
| Never | 3196 (51.8) | 3191 (51.6) | 3179 (51.3) | 3269 (52.7) |
| Past | 2528 (41.0) | 2536 (41.0) | 2559 (41.3) | 2488 (40.1) |
| Current | 448 (7.3) | 455 (7.4) | 454 (7.3) | 441 (7.1) |
| Hypertension, No./total (%)a | 3135/6229 (50.3) | 3268/6245 (52.3) | 3272/6242 (52.4) | 3236/6267 (51.6) |
| Diabetes, No./total (%)a | 892/6257 (14.3) | 845/6261 (13.5) | 866/6270 (13.8) | 839/6286 (13.3) |
| Body mass index, median (IQR)b | 27.2 (24.4-30.9) [n = 6130] | 27.2 (24.3-30.8) [n = 6121] | 27.1 (24.3-30.7) [n = 6136] | 27.0 (24.2-30.7) [n = 6156] |
| Height, median (IQR), in | 67.0 (64.0-70.0) [n = 6160] | 67.0 (64.0-70.0) [n = 6149] | 67.0 (64.0-70.0) [n = 6158] | 67.0 (64.0-70.0) [n = 6188] |
| Cholesterol-lowering medication use, No./total (%)c | 2342/6166 (38.0) | 2292/6158 (37.2) | 2339/6171 (37.9) | 2253/6198 (36.4) |
| CHA2DS2-VASc score, No. (%)d | ||||
| 0 | 768 (12.2) | 724 (11.5) | 737 (11.7) | 751 (11.9) |
| 1 | 1504 (24.0) | 1579 (25.2) | 1522 (24.2) | 1565 (24.9) |
| ≥2 | 4000 (63.8) | 3967 (63.3) | 4022 (64.0) | 3980 (63.2) |
| Aspirin use, No./total (%) | 2757/6179 (44.6) | 2764/6180 (44.7) | 2752/6193 (44.4) | 2801/6206 (45.1) |
| Statin use, No./total (%) | 2186/6173 (35.4) | 2129/6158 (34.6) | 2186/6175 (35.4) | 2093/6206 (33.7) |
| Alcohol consumption, No. (%)e | n = 6164 | n = 6181 | n = 6168 | n = 6184 |
| <1 drink/wk | 2366 (38.4) | 2449 (39.6) | 2427 (39.3) | 2416 (39.1) |
| 1-6 drinks/wk | 2236 (36.3) | 2096 (33.9) | 2145 (34.8) | 2161 (34.9) |
| 1 drink/d | 719 (11.7) | 767 (12.4) | 705 (11.4) | 701 (11.3) |
| ≥2 drinks/d | 843 (13.7) | 869 (14.1) | 891 (14.4) | 906 (14.7) |
| Fish consumption, median (IQR), servings/wkf | 1.5 (0.9-2.5) [n = 6165] | 1.5 (0.9-2.5) [n = 6175] | 1.5 (0.9-2.5) [n = 6170] | 1.5 (0.9-2.5) [n = 6182] |
| Vitamin D consumption, No. (%)g | 2638 (42.1) | 2674 (42.6) | 2672 (42.5) | 2678 (42.5) |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range.
Race/ethnicity, hypertension, and diabetes were reported by participants.
Body mass index is calculated as weight in kilograms divided by the square of height in meters.
Cholesterol-lowering medications included both statins and nonstatins.
The CHA2DS2-VASc score is used to estimate risk of stroke in patients with atrial fibrillation and is calculated as the sum of the following risk factors: congestive heart failure (1 point); hypertension (1 point); age 65-74 years (1 point), age ≥75 years (2 points); diabetes (1 point); prior stroke, transient ischemic attack, or thromboembolism (2 points); vascular disease (myocardial infarction, peripheral arterial disease, or prior aortic plaque; 1 point); and female sex (1 point). All risk factors were ascertained via self-report at baseline. Possible scores range from 0 to 9, wherein 9 represents the highest risk category. Scores ranged from 0 to 8 in this study sample.
One drink was defined as 1 glass, bottle, or can of beer or light beer; one 5-oz glass of red or white wine; or 1 drink or shot of liquor. Data were reported by participants via food frequency questionnaire.
Data for servings of fish consumption were reported by participants via food frequency questionnaire and included canned tuna (3-4 oz); breaded fish cakes, pieces, or fish sticks (1 serving, store-bought); shrimp, lobster, or scallops as a main dish; dark-meat fish (eg, mackerel, salmon, sardines, bluefish, or swordfish; 3-5 oz); and other fish (3-5 oz). Daily servings were summed across each type of fish and multiplied by 7 to calculate weekly servings.
Restricted to ≤800 IU/d from all sources combined (individual supplements and multivitamins).
Within the study population, the rate of response to annual questionnaires averaged 93.1%, and 19 892 participants (79.2%) were successfully linked to CMS claims data (balanced by treatment group; P = .31). The majority of those linked to CMS claims data (n = 18 567 [93.3%]) were aged 65 years or older at the time of linkage. The percentage of participants who adhered to study drug and took at least two-thirds of trial capsules was similar across treatment groups (80%-82%).15,16 The prevalence of outside supplement use remained below 3.5% for fish oil and ranged from 3.8% to 10.8% for vitamin D (>800 IU/d) at 5 years of follow-up.15,16
There were 900 confirmed incident AF events identified by self-report and/or CMS claims during the trial period. The characteristics of these AF cases are shown in the eTable in Supplement 3. The majority had an ECG documenting AF (n = 656 [72.9%]), and the remainder had AF documented in a physician’s note (n = 244 [27.1%]). Five hundred twenty-six (58%) had paroxysmal AF, whereas 346 (38.4%) had persistent AF at the time of diagnosis. The remainder (n = 28 [3.1%]) could not be classified. Five hundred fifty-seven (61.9%) were documented to have symptoms at the time of diagnosis, and 58 (6.4%) had symptoms that may have preceded randomization. The remainder had either no (n = 247 [27.4%]) or unclear (n = 96 [10.7%]) symptoms at the time of diagnosis. Sixty-six (7.3%) had AF after cardiac surgery and 52 (5.8%) had only atrial flutter documented in the medical records. Echocardiograms were available in 775 cases (86.1%), and the median ejection fraction was 60% (interquartile range, 55%-63%).
Omega-3 Fatty Acid Supplementation
The primary outcome of incident AF occurred in 469 (3.7%) participants randomized to EPA-DHA vs 431 (3.4%) randomized to placebo (HR, 1.09; 95% CI, 0.96-1.24; P = .19) (Table 2). The corresponding AF incidence rates were 7.2 per 1000 person-years for EPA-DHA vs 6.6 per 1000 person-years for placebo (absolute rate difference, 0.6 [95% CI, −0.3 to 1.15] per 1000 person-years). The cumulative incidence of AF over the study follow-up by treatment group is shown in Figure 2A. Although the curves appeared to separate after the second year, there was no statistical evidence for time-dependent treatment effects (HR, 1.01; 95% CI, 0.87-1.18; P = .88 for time interaction). Randomization to EPA-DHA was also not significantly associated with incident AF in sensitivity analyses excluding AF events for which symptoms could have occurred prior to randomization (HR, 1.10; 95% CI, 0.96-1.26), analyses limited to AF events identified by self-report (HR, 1.13; 95% CI, 0.98-1.31), analyses excluding cases with a sole diagnosis of atrial flutter or postoperative AF (HR, 1.10; 95% CI, 0.96-1.27), or analyses excluding AF events without a confirmatory ECG (HR, 1.12; 95% CI, 0.96-1.30). Results remained nonsignificant when individuals were censored for nonadherence (HR, 1.13; 95% CI, 0.98-1.30).
Table 2. Incidence and Relative Hazards of AF Among Participants Randomized to EPA-DHA vs Placebo.
| End points | EPA-DHA (n = 12 542) | Placebo (n = 12 577) | EPA-DHA vs placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of events | Total person-years | Events per 1000 person-years | No. of events | Total person-years | Events per 1000 person-years | Difference in events per 1000 person-years (95% CI) | Hazard ratio (95% CI)a | P value | |
| Primary end point | |||||||||
| All incident AF | 469 | 64 942 | 7.2 | 431 | 65 242 | 6.6 | 0.6 (−0.3 to 1.5) | 1.09 (0.96-1.24) | .19 |
| Sensitivity analyses of primary end point | |||||||||
| AF events excluding those with symptoms preceding randomization | 440 | 64 840 | 6.8 | 402 | 65 154 | 6.2 | 0.6 (−0.3 to 1.5) | 1.10 (0.96-1.26) | .17 |
| AF events excluding those detected by CMS linkage data alone | 389 | 64 676 | 6.0 | 345 | 64 970 | 5.3 | 0.7 (−0.1 to 1.5) | 1.13 (0.98-1.31) | .10 |
| AF events excluding atrial flutter alone and postoperative AFb | 410 | 64 721 | 6.3 | 374 | 65 055 | 5.7 | 0.6 (−0.3 to 1.4) | 1.10 (0.96-1.27) | .18 |
| AF events excluding those without ECG confirmationb | 346 | 64 587 | 5.4 | 310 | 64 875 | 4.8 | 0.6 (−0.2 to 1.4) | 1.12 (0.96-1.30) | .15 |
| On-treatment analysisc | 403 | 51 005 | 7.9 | 358 | 51 223 | 7.0 | 0.9 (−0.1 to 2.0) | 1.13 (0.98-1.30) | .09 |
| Secondary end points | |||||||||
| Paroxysmal AF | 271 | 64 942 | 4.2 | 255 | 65 242 | 3.9 | 0.3 (−0.4 to 1.0) | 1.07 (0.90-1.27) | .46 |
| Nonparoxysmal AF | 182 | 64 942 | 2.8 | 164 | 65 242 | 2.5 | 0.3 (−0.3 to 0.8) | 1.11 (0.90-1.37) | .32 |
Abbreviations: AF, atrial fibrillation; CMS, Centers for Medicare & Medicaid Services; DHA, docosahexaenoic acid; ECG, electrocardiographic; EPA, eicosapentaenoic acid.
The hazard ratio for each intervention was estimated in Cox models using indicators for treatment group, controlling for the second intervention, age, and sex.
Post hoc analyses.
Adherence-based analyses that censored follow-up data when a participant discontinued trial capsules or began taking nonstudy fish oil supplements.
Figure 2. Cumulative Incidence of Atrial Fibrillation According to Randomized Treatment Groups.
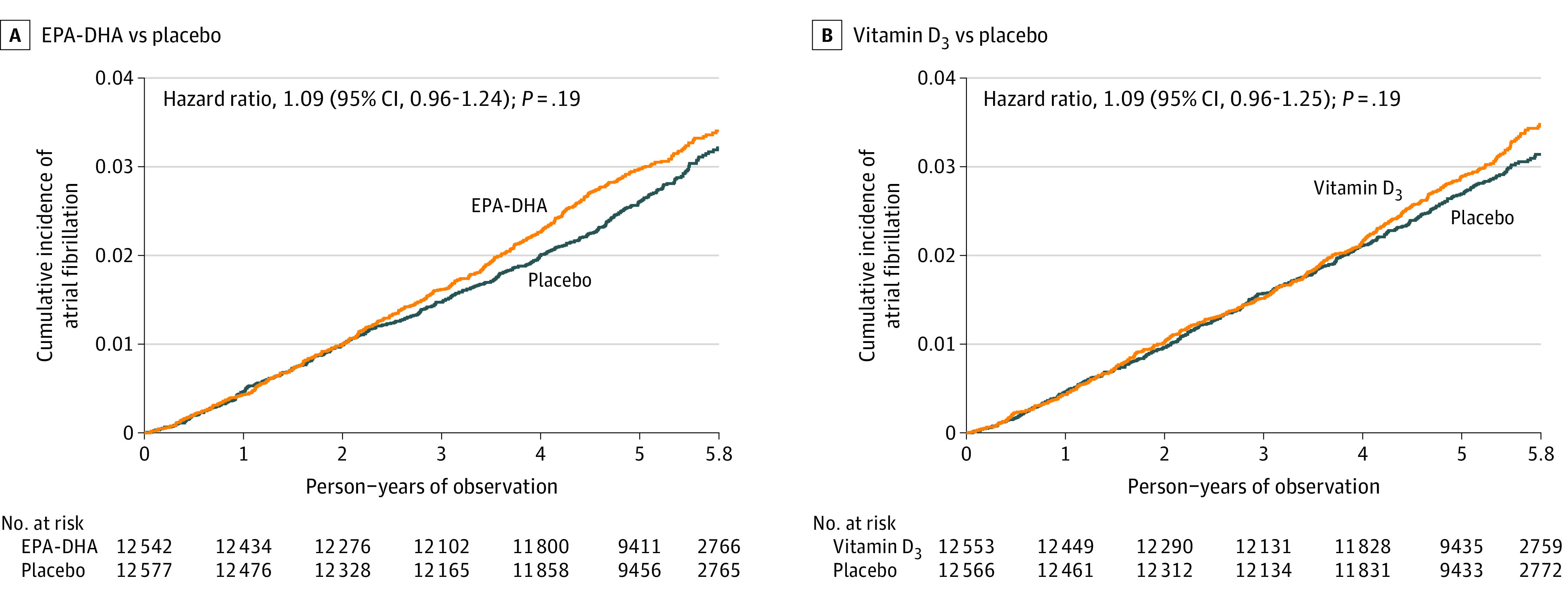
A, Cumulative incidence of the primary end point according to randomized treatment group of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) vs placebo derived from Cox regression models controlling for age, sex, and vitamin D3 randomization group. Median observation time was 5.3 (interquartile range, 5.0-5.7) person-years. B, Cumulative incidence of the primary end point according to randomized treatment group of vitamin D3 vs placebo derived from Cox regression models controlling for age, sex, and EPA-DHA randomization group. Median observation time was 5.3 (interquartile range, 5.0-5.7) person-years.
For the secondary outcomes of incident paroxysmal AF and nonparoxysmal AF, the HRs were 1.07 (95% CI, 0.90-1.27; P = .46) and 1.11 (95% CI, 0.90-1.37; P = .32), respectively (Table 2). In subgroup analyses, there was no evidence of effect modification by baseline fish intake, plasma EPA-DHA levels, or AF risk factors on the primary outcome, with the exception of height (Figure 3). Participants randomized to the EPA-DHA group who were taller than the median height of 67 in had a higher risk of incident AF (HR, 1.21; 95% CI, 1.02-1.42) compared with those randomized to placebo (HR, 0.86; 95% CI, 1.02-1.42), and there was evidence for a treatment interaction by height (nominal P = .02 for interaction).
Figure 3. Effect of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on the Primary End Point in Prespecified Subgroups.
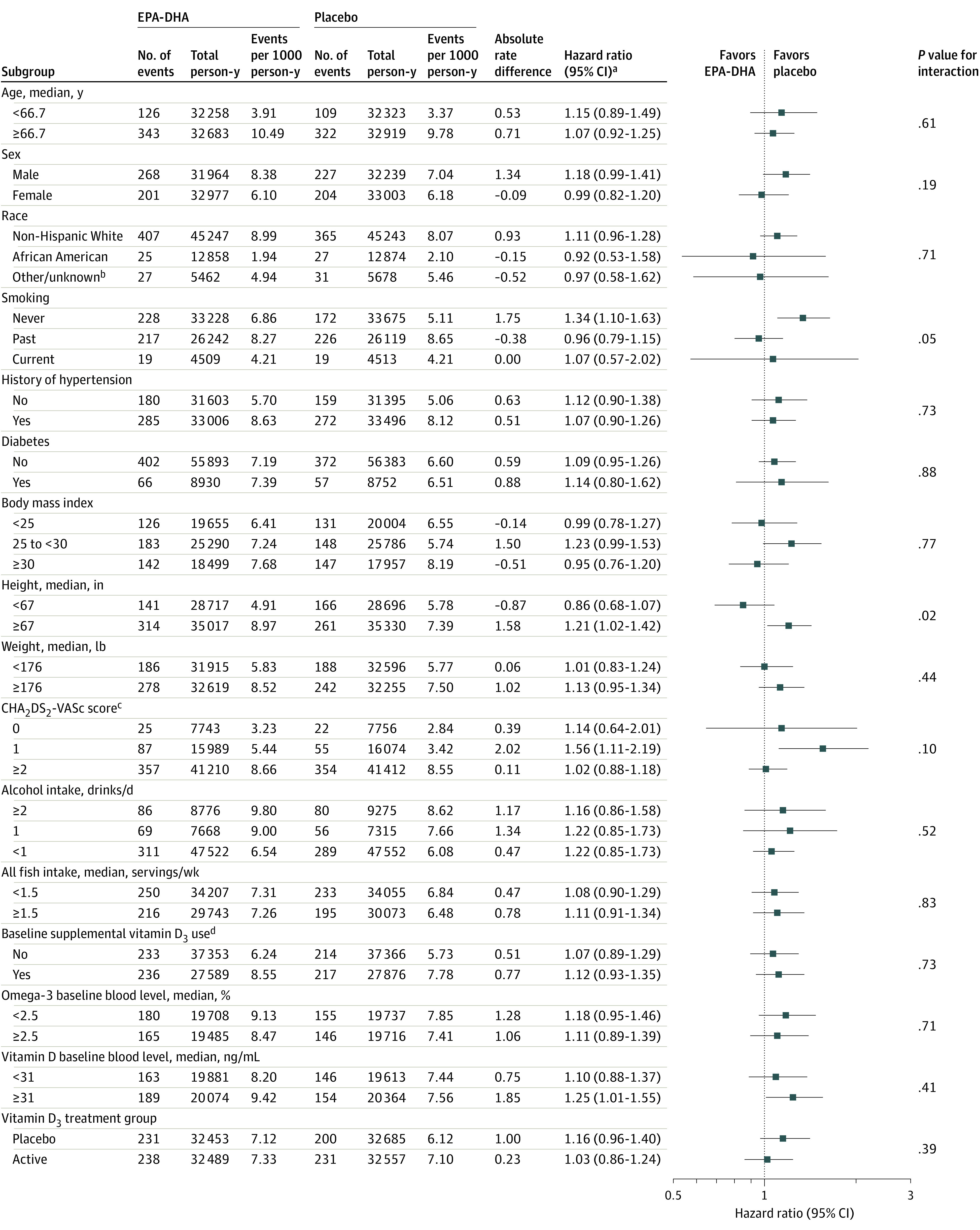
aCox regression models controlled for age, sex, and randomization group; interactions tested using multiplicative interaction terms.
bIncludes Hispanic, Asian/Pacific Islander, Native American/Alaska Native, and other, multiple, or unspecified race/ethnicity.
cCHA2DS2-VASc score estimates stroke risk in patients with atrial fibrillation (see Table 1 footnote "d" for details). Risk factors were self-reported at baseline. Scores ranged from 0 to 8 in this study.
dRestricted to ≤800 IU/d from all sources combined.
Vitamin D3 Supplementation
The primary outcome occurred in 469 (3.7%) participants randomized to vitamin D3 vs 431 (3.4%) randomized to placebo (HR, 1.09; 95% CI, 0.96-1.25; P = .19) (Table 3). The corresponding AF incidence rates were 7.2 per 1000 person-years for vitamin D3 vs 6.6 per 1000 person-years for placebo (absolute rate difference, 0.6 [95% CI, −0.3 to 1.15] per 1000 person-years). Cumulative incidence rates of AF by treatment group are shown in Figure 2B, and there was no evidence for time × treatment interaction (HR, 1.07; 95% CI, 0.92-1.25; P = .39). There remained no significant effect of vitamin D3 after censoring for nonadherence (HR, 1.09; 95% CI, 0.94-1.27) and in all the prespecified secondary analyses shown in Table 3. For the secondary outcomes of paroxysmal and nonparoxysmal AF, the HRs associated with randomization to vitamin D3 were 1.03 (95% CI, 0.87-1.23; P = .71) and 1.20 (95% CI, 0.97-1.48; P = .10), respectively (Table 3).
Table 3. Incidence and Relative Hazards of AF Among Participants Randomized to Vitamin D3 vs Placebo.
| End points | Vitamin D3 (n = 12 553) | Placebo (n = 12 566) | Vitamin D3 vs placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of events | Total person-years | Events per 1000 person-years | No. of events | Total person-years | Events per 1000 person-years | Difference in events per 1000 person-years (95% CI) | Hazard ratio (95% CI)a | P value | |
| Primary end point | |||||||||
| All incident AF | 469 | 65 046 | 7.2 | 431 | 65 137 | 6.6 | 0.6 (−0.3 to 1.5) | 1.09 (0.96-1.25) | .19 |
| Sensitivity analyses of primary end point | |||||||||
| AF events excluding those with symptoms preceding randomization | 438 | 64 948 | 6.7 | 404 | 65 046 | 6.2 | 0.5 (−0.3 to 1.4) | 1.09 (0.95-1.25) | .22 |
| AF events excluding those detected by CMS linkage data alone | 373 | 64 727 | 5.8 | 361 | 64 919 | 5.6 | 0.2 (−0.6 to 1.0) | 1.04 (0.90-1.20) | .60 |
| AF events excluding atrial flutter alone and postoperative AFb | 404 | 64 815 | 6.2 | 380 | 64 962 | 5.8 | 0.4 (−0.5 to 1.2) | 1.07 (0.93-1.23) | .36 |
| AF events excluding those confirmed by medical record aloneb | 343 | 64 671 | 5.3 | 313 | 64 790 | 4.8 | 0.5 (−0.3 to 1.2) | 1.10 (0.95-1.28) | .22 |
| On-treatment analysisc | 383 | 50 540 | 7.6 | 336 | 48 640 | 6.9 | 0.7 (−0.4 to 1.7) | 1.09 (0.94-1.27) | .24 |
| Secondary end points | |||||||||
| Paroxysmal AF | 267 | 65 046 | 4.1 | 259 | 65 137 | 4.0 | 0.1 (−0.6 to 0.8) | 1.03 (0.87-1.23) | .71 |
| Nonparoxysmal AF | 188 | 65 046 | 2.9 | 158 | 65 137 | 2.4 | 0.5 (−0.1 to 1.0) | 1.20 (0.97-1.48) | .10 |
Abbreviations: AF, atrial fibrillation; CMS, Centers for Medicare & Medicaid Services.
The hazard ratio for each intervention was estimated in Cox models using indicators for treatment group, controlling for the second intervention, age, and sex.
Post hoc analyses.
Adherence-based analyses that censored follow-up data when a participant discontinued trial capsules or began taking more than 800 IU/d of nonstudy vitamin D3.
In subgroup analyses (Figure 4), there was no statistical evidence for effect modification by vitamin D3 supplement use and 25-hydroxyvitamin D serum level on the primary outcome. There was nominal statistical evidence for effect modification by age and alcohol intake (P = .02 and P = .01 for interaction, respectively). The HR associated with vitamin D3 treatment group was significantly elevated in participants younger than the median age of 66.7 years (HR, 1.44; 95% CI, 1.11-1.86) but not in older participants (HR, 0.99; 95% CI, 0.85-1.15). For alcohol intake, the HR associated with randomization to vitamin D3 was elevated in those who consumed less than 1 drink per day (HR, 1.23; 95% CI, 1.04-1.44) but not among those who consumed 1 to 2 drinks per day (HR, 1.01; 95% CI, 0.71-1.43) or more than 2 drinks per day (HR, 0.80; 95% CI, 0.59-1.08).
Figure 4. Effect of Vitamin D3 on the Primary End Point in Prespecified Subgroups.
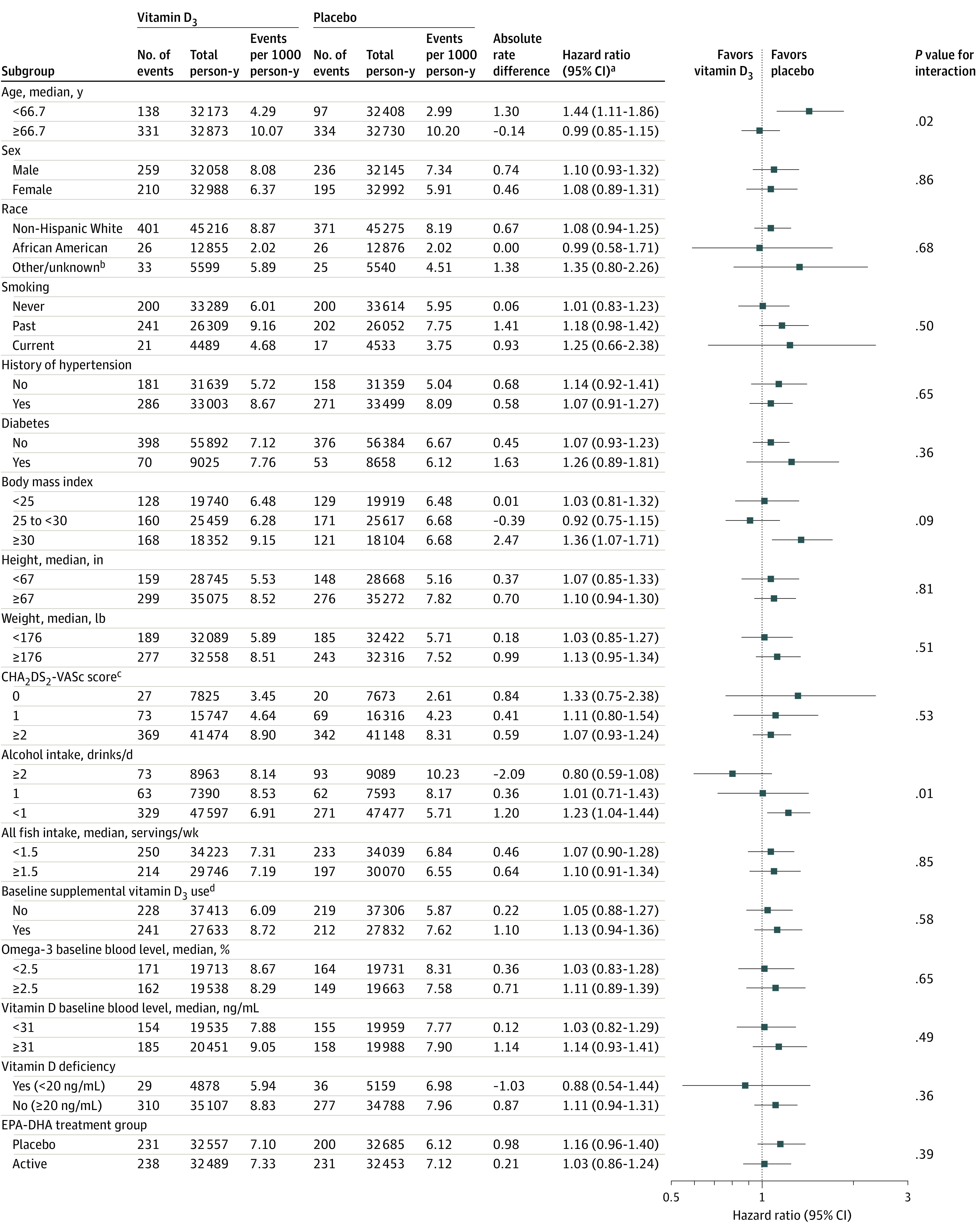
aCox regression models controlled for age, sex, and randomization group; interactions tested using multiplicative interaction terms.
bIncludes Hispanic, Asian/Pacific Islander, Native American/Alaska Native, and other, multiple, or unspecified race/ethnicity.
cCHA2DS2-VASc score estimates stroke risk in patients with atrial fibrillation (see Table 1 footnote "d" for details). Risk factors were self-reported at baseline. Scores ranged from 0 to 8 in this study.
dRestricted to ≤800 IU/d from all sources combined.
Combined Treatment Effects
There was no evidence for a treatment interaction between vitamin D3 and EPA-DHA (Figure 3 and Figure 4; P = .39 for interaction). When analyzed according to the 4 treatment groups, compared with participants randomized to both placebos, the HR for incident AF for those randomized to EPA-DHA and placebo was 1.16 (95% CI, 0.96-1.40; P = .13), for those randomized to vitamin D3 and placebo was 1.16 (95% CI, 0.96-1.40; P = .13), and for those randomized to vitamin D3 and EPA-DHA was 1.20 (95% CI, 0.99-1.45; P = .06) (eFigure in Supplement 3).
Discussion
In this large-scale, ancillary, AF primary prevention, randomized, double-blind, placebo-controlled trial, supplementation with 840 mg/d of marine omega-3 fatty acids (EPA-DHA; 1.2:1 ratio) and/or 2000 IU/d of vitamin D3 did not significantly reduce or increase the primary end point of incident AF compared with placebo over a median treatment duration of 5.3 years. There was no evidence for an interaction between the study agents or for effect modification by dietary intake or blood levels of either nutrient. Similarly, there was no statistically significant difference in incidence of secondary outcomes of paroxysmal or nonparoxysmal AF. Overall, these findings do not support the use of supplemental EPA-DHA or vitamin D3 for the primary prevention of AF and provide reassurance regarding lack of a major risk of AF incidence associated with these commonly used supplements at these doses.
In experimental models, omega-3 fatty acids have been demonstrated to have both direct electrophysiologic actions and favorable effects on biologic processes involved in atrial remodeling.21,22,23 Thus far, completed RCTs with AF as the primary outcome have examined the short-term recurrence of AF in patients with established AF or the development of AF after cardiac surgery11 in relatively small populations. While some studies demonstrated benefit,24,25,26 most did not.27,28,29 On balance, these secondary prevention trials did not support the use of omega-3 fatty acids as alternatives to antiarrhythmic drugs for the maintenance of sinus rhythm in patients with AF.11 However, given the upstream actions of omega-3 fatty acids,23,30 it remained plausible that these agents might be more effective at preventing the development of the atrial substrate leading to AF as opposed to rapidly altering it.31,32 In this large-scale AF primary prevention trial, no evidence for such a benefit was found. Although it is feasible that benefits might have accrued if treatment and follow-up were continued beyond 5 years, the observed cumulative incidence curves and HRs suggest that this would be unlikely.
In addition to hypothesized benefits on AF, there are also data in support of potential adverse effects of omega-3 fatty acids. Recently, 2 separate RCTs testing higher-dose formulations of omega-3 fatty acids performed among patients with established CVD or at high cardiovascular risk reported significantly elevated risks of AF in the treatment groups. In the REDUCE-IT trial, patients randomized to 4 g/d of highly purified EPA ethyl ester (icosapent ethyl) had a 48% higher risk of hospitalization for AF compared with placebo (3.1% vs 2.1%; P = .004) over a median 4.9 years of follow-up.12 In the STRENGTH trial, the incidence of investigator-reported new-onset AF was also greater among participants randomized to 4 g/d of omega-3 carboxylic acid, a mixture of EPA and DHA, vs placebo over a median 3.2 years of follow-up (2.2% vs 1.3%; HR, 1.69; 95% CI,1.29-2.21; P < .001).13 Another smaller trial performed in an elderly post–myocardial infarction population, the OMEMI trial, reported a nonsignificantly increased risk of incident AF among those without AF at study entry who were treated with 1.8 g/d of omega-3 fatty acids (930 mg of EPA) compared with placebo (HR, 1.84; 95% CI, 0.98-3.45; P = .06).14 These results are in contrast to the more modest, nonsignificant elevation in incident AF (3.7% vs 3.4%; HR, 1.09; 95% CI, 0.96-1.24) observed in this primary prevention trial. The large sample size and greater than anticipated number of AF events in the present study provided adequate power to detect risk elevations of the magnitude reported in these other trials, as evidenced by the upper bound of the 95% confidence interval. However, smaller elevations in AF risk up to 24%, which could be clinically meaningful, cannot be excluded. Potentially, the adverse effect on AF risk may be dose related, and the higher dosages of EPA used in these other studies might account for the significant adverse effect on AF.
To our knowledge, the present study is the only RCT to assess the effect of vitamin D3 supplementation on AF risk. Similar to omega-3 fatty acids, there are multiple upstream long-term mechanisms whereby vitamin D supplementation has been hypothesized to influence atrial structural and electrical remodeling.33,34 The data in support of supplementation are primarily based on conflicting observational studies that, when combined in meta-analysis, find a positive association between vitamin D deficiency and AF.6,35 In contrast, a recent post hoc analysis from the Women’s Health Initiative did not find a significant association between randomized treatment with 400 IU/d of vitamin D3 and 1000 mg/d of elemental calcium and incident AF based solely on Medicare claims data.36 The results of the present trial, using a higher dose of vitamin D3 and AF events prospectively ascertained by self-report and CMS linkage and confirmed by medical record review, agree with these latter null findings. In addition, subgroup analyses in patients with vitamin D levels considered deficient (<20 ng/mL) did not suggest a benefit; however, the power to detect a benefit in this much smaller subset of the population was limited.
While there were no significant differences in incident AF for either agent in the overall study population, an increased risk of incident AF associated with randomized treatment was observed in selected subgroups. For omega-3 fatty acids, AF risk was modestly increased in taller individuals, and for vitamin D3, elevations in AF risk were observed in younger individuals and participants who drank less alcohol. Although the HRs and tests for interaction were significant, the P values associated with these subgroup analyses have not been adjusted for multiple comparisons. Thus, these findings should be interpreted with caution and considered hypothesis generating.
The present study had several strengths, including the large general population sample with equal representation of women and oversampling of Black study participants, long duration of double-blind randomized treatment, high levels of treatment adherence, and prospective rigorous adjudication of AF end points. To our knowledge, this study is the first randomized, placebo-controlled trial to prospectively test the effect of any intervention on incident AF and is the only trial to test alternative upstream preventive agents for AF in a large enough population over a long enough time period to provide an assessment of the plausible benefits and risks. The study, which was performed by leveraging the infrastructure of the parent trial, illustrates how future trials might be designed to assess the effect of interventions that might jointly affect AF and CVD at marginal additional cost.
Limitations
This study has several limitations. First, despite best efforts to ascertain AF events by 2 methods (self-report and CMS linkage) and obtain records to confirm clinically manifest AF end points with a high degree of specificity within this large pragmatic trial, there is undoubtedly some underdetection of AF, particularly if asymptomatic or of short duration. However, the proportion of asymptomatic and paroxysmal AF in this study was comparable with that found in a recent trial of patients with newly diagnosed AF identified through traditional methods.37 It has also become recognized that some AF events may only be detected through screening38; however, the clinical significance of these screen-detected AF events is currently unknown.38,39 Second, the doses of the study agents were chosen based on hypothesized efficacy for prevention of CVD and cancer and not specifically for AF. However, the doses of both agents used in the trial are similar to or greater than that associated with benefit on AF in observational studies,6,8 and for EPA-DHA, recent literature suggests potential harm at greater doses.12,13 Third, these results may not be generalizable to younger populations or to patients with established CVD. Fourth, effects on atrial structural and electrical remodeling may take longer than the present study duration to affect AF events. Fifth, even with the large sample size and large number of events, the power to detect small elevations and/or reductions in AF risk was limited.
Conclusions
Among adults aged 50 years or older, treatment with EPA-DHA or vitamin D3, compared with placebo, resulted in no significant difference in the risk of incident AF over a median follow-up of more than 5 years. The findings do not support the use of either agent for the primary prevention of incident AF.
Trial Protocol
Statistical Analysis Plan
eAppendix. CMS Linkage Methods Used in the VITAL Study
eTable. Characteristics of Incident Atrial Fibrillation
eFigure. Cumulative Incidence of Atrial Fibrillation According to Four Treatment Groups
Members of the VITAL Research Group
Data Sharing Statement
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation. 2014;129(8):837-847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4-20. doi: 10.1161/CIRCRESAHA.120.316340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080-2087. doi: 10.1001/jama.2011.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation. 2009;120(23):2315-2321. doi: 10.1161/CIRCULATIONAHA.109.852657 [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Wang W, Tan Z, et al. The relationship between vitamin D and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J. 2019;18(1):73. doi: 10.1186/s12937-019-0485-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JH, Lemaitre RN, King IB, et al. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: the Cardiovascular Health Study. Circulation. 2012;125(9):1084-1093. doi: 10.1161/CIRCULATIONAHA.111.062653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110(4):368-373. doi: 10.1161/01.CIR.0000138154.00779.A5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation: the Rotterdam Study. Am Heart J. 2006;151(4):857-862. doi: 10.1016/j.ahj.2005.07.029 [DOI] [PubMed] [Google Scholar]
- 10.Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81(1):50-54. doi: 10.1093/ajcn/81.1.50 [DOI] [PubMed] [Google Scholar]
- 11.Mariani J, Doval HC, Nul D, et al. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2013;2(1):e005033. doi: 10.1161/JAHA.112.005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 13.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268-2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalstad AA, Myhre PL, Laake K, et al. ; OMEMI Investigators . Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.052209 [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luttmann-Gibson H, Mora S, Camargo CA, et al. Serum 25-hydroxyvitamin D in the Vitamin D and Omega-3 Trial (VITAL): clinical and demographic characteristics associated with baseline and change with randomized vitamin D treatment. Contemp Clin Trials. 2019;87:105854. doi: 10.1016/j.cct.2019.105854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. doi: 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Korantzopoulos P, Shehata M, Li G, Wang X, Kaul S. Prevention of atrial fibrillation with omega-3 fatty acids: a meta-analysis of randomised clinical trials. Heart. 2011;97(13):1034-1040. doi: 10.1136/hrt.2010.215350 [DOI] [PubMed] [Google Scholar]
- 21.London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office of Dietary Supplements Omega-3 Fatty Acids and Their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116(10):e320-e335. doi: 10.1161/CIRCULATIONAHA.107.712984 [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264-2274. doi: 10.1161/CIRCULATIONAHA.111.019893 [DOI] [PubMed] [Google Scholar]
- 23.Ramadeen A, Laurent G, dos Santos CC, et al. n-3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm. 2010;7(4):520-528. doi: 10.1016/j.hrthm.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 24.Calò L, Bianconi L, Colivicchi F, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45(10):1723-1728. doi: 10.1016/j.jacc.2005.02.079 [DOI] [PubMed] [Google Scholar]
- 25.Nodari S, Triggiani M, Campia U, et al. n-3 Polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation. 2011;124(10):1100-1106. doi: 10.1161/CIRCULATIONAHA.111.022194 [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Sutherland F, Morton JB, et al. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012;9(4):483-491. doi: 10.1016/j.hrthm.2011.11.034 [DOI] [PubMed] [Google Scholar]
- 27.Bianconi L, Calò L, Mennuni M, et al. n-3 Polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011;13(2):174-181. doi: 10.1093/europace/euq386 [DOI] [PubMed] [Google Scholar]
- 28.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304(21):2363-2372. doi: 10.1001/jama.2010.1735 [DOI] [PubMed] [Google Scholar]
- 29.Farquharson AL, Metcalf RG, Sanders P, et al. Effect of dietary fish oil on atrial fibrillation after cardiac surgery. Am J Cardiol. 2011;108(6):851-856. doi: 10.1016/j.amjcard.2011.04.036 [DOI] [PubMed] [Google Scholar]
- 30.Mayyas F, Sakurai S, Ram R, et al. Dietary ω3 fatty acids modulate the substrate for post-operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res. 2011;89(4):852-861. doi: 10.1093/cvr/cvq380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert CM. n-3 Polyunsaturated fatty acids for atrial fibrillation recurrence: is the horse already out of the barn? J Am Coll Cardiol. 2014;64(14):1449-1451. doi: 10.1016/j.jacc.2014.07.955 [DOI] [PubMed] [Google Scholar]
- 32.Siscovick DS, Barringer TA, Fretts AM, et al. ; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, and Council on Clinical Cardiology . Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867-e884. doi: 10.1161/CIR.0000000000000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong J, Kim GH, Wei M, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol. 2010;177(2):622-631. doi: 10.2353/ajpath.2010.091292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103(3-5):416-419. doi: 10.1016/j.jsbmb.2006.12.081 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Yang Y, Ng CY, et al. Meta-analysis of vitamin D deficiency and risk of atrial fibrillation. Clin Cardiol. 2016;39(9):537-543. doi: 10.1002/clc.22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boursiquot BC, Larson JC, Shalash OA, Vitolins MZ, Soliman EZ, Perez MV. Vitamin D with calcium supplementation and risk of atrial fibrillation in postmenopausal women. Am Heart J. 2019;209:68-78. doi: 10.1016/j.ahj.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Kirchhof P, Camm AJ, Goette A, et al. ; EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 38.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131(25):2176-2184. doi: 10.1161/CIRCULATIONAHA.114.014343 [DOI] [PubMed] [Google Scholar]
- 39.Jonas DE, Kahwati LC, Yun JDY, Middleton JC, Coker-Schwimmer M, Asher GN. Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(5):485-498. doi: 10.1001/jama.2018.4190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. CMS Linkage Methods Used in the VITAL Study
eTable. Characteristics of Incident Atrial Fibrillation
eFigure. Cumulative Incidence of Atrial Fibrillation According to Four Treatment Groups
Members of the VITAL Research Group
Data Sharing Statement


