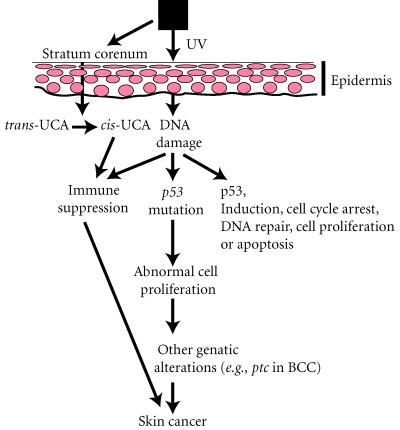
The cellular and molecular events that contribute to the development of UV-induced skin cancer is a complex process involving at least two distinct pathways that interact or converge to cause skin cancer (Figure 1). One pathway involves the action of UV on target cells (keratinocytes) for neoplastic transformation, and the other involves the effects of UV on the host's immune system [1]. There is evidence to indicate that UV-induced DNA damage plays an important role in both pathways.
Figure 1.
Pathways involved in skin cancer development.
In the normal human epidermis, cells are constantly turning over, about once a month. During this period, stem cells in the basal layer undergo cell division, and the keratinocytes differentiate into squamous cells producing keratin and other proteins, and finally desquamate. Chronic exposure to sunlight causes damage to the skin including erythema, edema, hyperplasia, formation of sunburn cells, photoaging, suppression of the immune system and skin cancer. Some of the molecular events that occur in cells following UV exposure are DNA damage, induction of p53 and p53-regulated proteins, cell cycle arrest, DNA repair, and apoptosis. UV-induced DNA damage causes an elevation of p53 expression and p53 protein is translocated to the nucleus to regulate its downstream genes. Among these genes, p21waf1/Cip1 expression is induced to mediate cell cycle arrest at G1-S phase to allow the repair of DNA damage. UV can also induce apoptosis to order cells containing damaged DNA to self-destruct. It has been shown that UV-induced apoptosis is p53-dependent [2]. Interestingly, we have recently demonstrated that p53 may up-regulate bax and down-regulate Bcl-2 protein resulting in apoptosis [3] and that Fas/Fas-ligand pathway is essential for UV-induced apoptosis [4].
Following chronic UV exposure, errors associated with DNA repair and/or replication can result into mutations in the p53 gene, especially C → T or CC → TT transitions, considered as UV-molecular signature. The p53 mutation in keratinocytes is probably an initiating event in UV skin carcinogenesis [5]. Because cells containing p53 mutations are relatively more resistant to UV-induced apoptosis, they can acquire a growth advantage. It is likely that the mutated cells expand preferentially in a clonal fashion at the expense of the normal surrounding keratinocytes, that commit massive suicide by apoptosis, leading to the appearance of p53 mutated clones in the epidermis [5, 6, 7]. Chronic exposure to sunlight may cause mutations in the other p53 allele and/or other unknown gene(s). Thus, another tumor suppressor gene known as patched (ptc) has been implicated in the development of basal cell carcinoma (BCC) [8, 9]. The human ptc gene was found to colocalize with the map location of nevoid basal cell carcinoma syndrome (NBCCS) on chromosome 9 at 9q22.3. NBCCS, also called basal cell nexus syndrome or Gorlin's syndrome, is a rare autosomal dominant disorder characterized by multiple BCC that appear at a young age on sun-exposed areas of the skin. Studies of NBCCS patients have shown that they have both genomic and sporadic mutations in the ptc gene, suggesting that these mutations are the ultimate cause of this disease. Sporadic ptc mutations have been found in BCC from otherwise normal individuals, some of which are UV-signature, either C → T or CC → TT changes. However, ptc mutations have not been reported in SCC. Nonetheless, these results suggest that genetic alterations in the ptc gene may also play a role in the development of BCC [9].
In addition, UV radiation causes immunosuppression that may contribute to the emergence of skin tumors. Immune suppression results from the induction of suppressor T cells, either by damaged Langerhans cells or inflammatory macrophages that enter the skin following UV exposure [10]. Another mechanism may be the release of cytokines such as IL-10, TNF-α, and IL-1α that can suppress the immune system and prevent T-cell mediated responses; these are known to be secreted by keratinocytes after UV damage [11]. In addition, UV irradiation can also convert normal skin chromophores into agents that are immunosuppressive, such as the conversion of trans-urocanic acid to cis-urocanic acid [12].
Thus, UV radiation plays a dual role in the development of skin cancer, by inducing genetic alterations in keratinocytes leading to their neoplastic transformation and by depressing the normal immune responses in the skin.
References
- Soehnge H, Ouhtit A, Ananthaswamy HN. Mechanisms of induction of skin cancer by UV radiation. Front Biosci. 1997;2:D538–D551. doi: 10.2741/a211. [DOI] [PubMed] [Google Scholar]
- Brash DE. Cellular proofreading. Nat Med. 1996;2:525–526. doi: 10.1038/nm0596-525. [DOI] [PubMed] [Google Scholar]
- Ouhtit A, Muller K, Davis WD, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB. Fas Ligand: A sensor for DNA damage is critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- Leffell DJ, Brash DE. Sunlight and skin cancer. Sci Am. 1996;275:52–59. doi: 10.1038/scientificamerican0796-52. [DOI] [PubMed] [Google Scholar]
- Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Invest Dermatol. 1996;1:136–142. [PubMed] [Google Scholar]
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci USA. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the Nevoid Basal Cell Carcinoma Syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Immunological effects of ultraviolet radiation. J Dermatol. 1991;18:429–433. doi: 10.1111/j.1346-8138.1991.tb03111.x. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Modulation of immunity by ultraviolet radiation: key effects on antigen presentation. J Invest Dermatol. 1995;105:30S–36S. doi: 10.1111/1523-1747.ep12315219. [DOI] [PubMed] [Google Scholar]
- Vink AA, Yarosh DB, Kripke ML. Chromophore for UV-induced immunosuppression: DNA. Photochem Photobiol. 1996;63:383–386. doi: 10.1111/j.1751-1097.1996.tb03050.x. [DOI] [PubMed] [Google Scholar]