This case report discusses progressive multifocal leukoencephalopathy occurring with ocrelizumab monotherapy in a patient with progressive multiple sclerosis without prior immunomodulation.
Key Points
Question
Can ocrelizumab contribute to the development of progressive multifocal leukoencephalopathy?
Findings
In this case report, progressive multifocal leukoencephalopathy was observed in a 78-year-old patient with progressive multiple sclerosis who had been treated with ocrelizumab for 2 years without prior immunotherapy. The progressive multifocal leukoencephalopathy occurrence was a result of the immunomodulatory function of ocrelizumab as well as age-related immunosenescence.
Meaning
This case report emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections such as elderly patients.
Abstract
Importance
Progressive multifocal leukoencephalopathy (PML) is an opportunistic infection caused by the JC virus that has no proven effective treatment. Although rare cases of PML have occurred with other anti-CD20 therapies, there had been no prior cases associated with ocrelizumab.
Objective
To report the first ever case of PML occurring with ocrelizumab monotherapy in a patient with progressive multiple sclerosis without prior immunomodulation.
Design, Setting, and Participant
This case was reported from an academic medical center. The patient had multiple sclerosis while receiving ocrelizumab monotherapy.
Exposures
Ocrelizumab monotherapy.
Results
A 78-year-old man with progressive multiple sclerosis treated with ocrelizumab monotherapy for 2 years presented with 2 weeks of progressive visual disturbance and confusion. Examination demonstrated a right homonymous hemianopia, and magnetic resonance imaging revealed an enlarging nonenhancing left parietal lesion without mass effect. Cerebrospinal fluid revealed 1000 copies/mL of JC virus, confirming the diagnosis of PML. Blood work on diagnosis revealed grade 2 lymphopenia, with absolute lymphocyte count of 710/μL, CD4 of 294/μL (reference range, 325-1251/μL), CD8 of 85/μL (reference range, 90-775/μL), CD19 of 1/μL, preserved CD4/CD8 ratio (3.45), and negative HIV serology. Retrospective absolute lymphocyte count revealed intermittent grade 1 lymphopenia that preceded ocrelizumab (absolute lymphocyte count range, 800-1200/μL). The patient’s symptoms progressed over weeks to involve bilateral visual loss, right-sided facial droop, and dysphasia. Ocrelizumab was discontinued and off-label pembrolizumab treatment was initiated. The patient nevertheless declined rapidly and ultimately died. PML was confirmed at autopsy.
Conclusions and Relevance
In this case report, PML occurrence was likely a result of the immunomodulatory function of ocrelizumab as well as age-related immunosenescence. This case report emphasizes the importance of a thorough discussion of the risks and benefits of ocrelizumab, especially in patients at higher risk for infections such as elderly patients.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a life-threatening opportunistic disease caused by JC virus (JCV) reactivation, characterized by oligodendrocyte infection and central nervous system demyelination. PML typically occurs in individuals with severe impairment in cell-mediated immunity, although rare cases of PML have occurred with only mild or occult immunosuppression.1 Hematological malignancies, HIV, and natalizumab account for more than 95% of PML cases worldwide.2
While a rare occurrence, anti-CD20 therapy has also been associated with PML. PML has been reported with use of rituximab and ofatumumab for treatment of lymphoproliferative disorders or in combination with other immunosuppressive agents for several systemic autoimmune disorders.3 This is difficult to interpret because patients with lymphoproliferative disorders are inherently immunocompromised, and patients with rheumatologic conditions have almost always been exposed to other agents that affect cellular immunity and predispose to PML. Hence, because anti-CD20 therapy is not the sole factor leading to immunosuppression in such cases, the extent of each factor’s contribution to the development of PML remains unclear. Notably, no cases had been previously reported with rituximab or ocrelizumab monotherapy for multiple sclerosis (MS).3
Methods
This case report did not necessitate institutional review board review per the Northwell Health policy. Serum antibodies to JCV were determined using the Stratify JCV antibody (with index) assay (Quest Diagnostics; Clinical Laboratory Improvement Amendments 49D-0221801; test code 91665; enzyme-linked immunosorbent assay). JCV copy number in the cerebrospinal fluid was determined using quantitative real-time polymerase chain reaction (Viracor Eurofins; Clinical Laboratory Improvement Amendments 26D-0983643; test code 3500). Immunostaining was performed for SV40 viral inclusions using SV40 antibody (clone MRQ-4; Cell Marque; catalog 351M-18-ASR; dilution: ready to use). Detection was achieved using Polymer Refine DAB (diaminobenzidine) kit (Leica Biosystems).
Case
A 78-year-old man with progressive MS treated with ocrelizumab monotherapy for 2 years presented with 2 weeks of progressive visual disturbance and confusion. The patient had been diagnosed with MS almost 3 decades prior after developing gradual gait dysfunction and bilateral asymmetric leg weakness throughout several years. Despite continued slow gait worsening over years, he had not been treated with immunotherapy until ocrelizumab was approved by the US Food and Drug Administration for primary progressive MS in 2017. He began taking ocrelizumab at age 76 years soon after Food and Drug Administration approval. The patient’s JCV index was 2.48 prior to initiation, and the theoretical risk of PML was discussed prior to starting. He tolerated the treatments well but continued to exhibit worsening gait dysfunction.
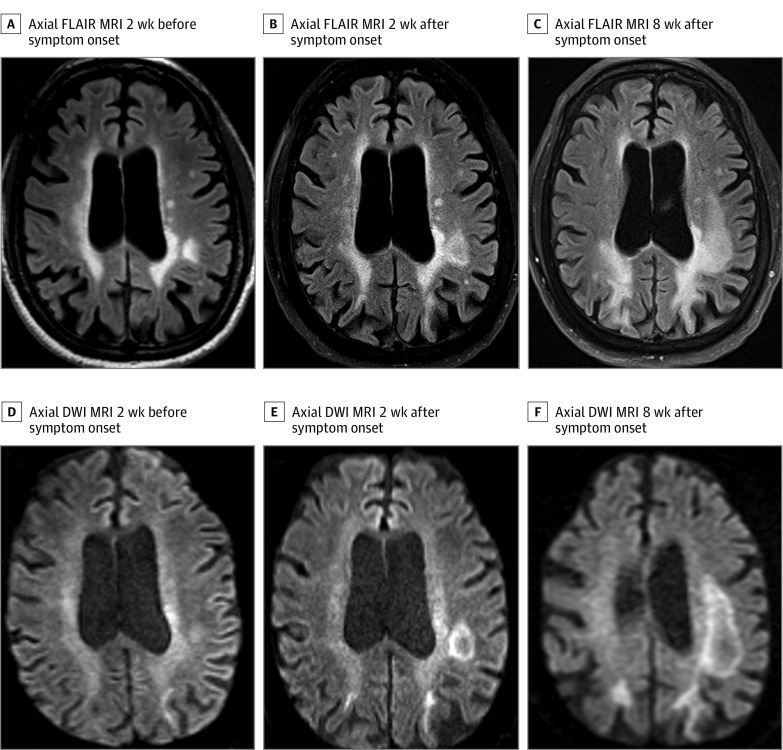
Two years after beginning the medication and 6 months after the last ocrelizumab infusion, he developed subacute visual disturbance and confusion. Initial neurological examination demonstrated a right homonymous hemianopia, and brain magnetic resonance imaging revealed an enlarging nonenhancing left parietal lesion without mass effect and with rim diffusion restriction (Figure 1). Cerebrospinal fluid analysis revealed no pleocytosis, present oligoclonal bands, protein level of 207 mg/dL, and 1000 JCV copies per mL, confirming a clinical diagnosis of PML. Blood work on diagnosis revealed grade 2 lymphopenia, with absolute lymphocyte count (ALC) of 710/μL (to convert to ×109/L, multiply by 0.001), CD4 count of 294/μL (reference range, 325-1251/μL), CD8 count of 85/μL (reference range, 90-775/μL), CD19 count of 1/μL, preserved CD4/CD8 ratio (3.45), and negative HIV serology results. Retrospective analysis revealed prior intermittent grade 1 lymphopenia that preceded ocrelizumab therapy (ALC range, 800-1200/μL). See Figure 2 for cell count timeline.
Figure 1. Neuroimaging.
Axial fluid-attenuated inversion recovery (FLAIR) sequences from 2 weeks prior (A), 2 weeks after (B), and 8 weeks after (C) symptom onset. There is progressive enlargement of a nonenhancing parietal lesion without mass effect or surrounding edema. There is also progressive extension of bilateral confluent parieto-occipital white matter T2 hyperintensity over time. Axial diffusion-weighted imaging (DWI) (D-F) sequences from the same time points demonstrate typical rim diffusion restriction. MRI indicates magnetic resonance imaging.
Figure 2. Absolute Lymphocyte Count and Lymphocyte Subset Timelines.
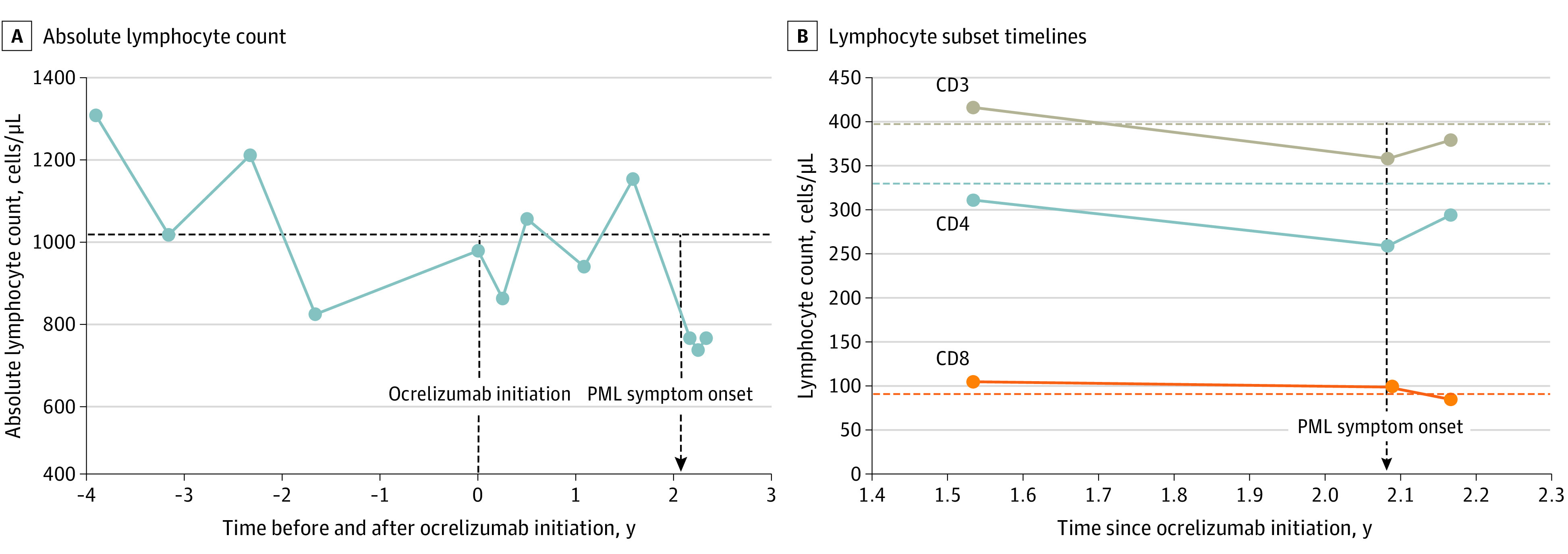
A, Absolute lymphocyte count values from 4 years prior to ocrelizumab initiation until shortly after progressive multifocal leukoencephalopathy (PML) onset. Prior to the development of PML, the patient exhibited intermittent mild (grade 1) lymphopenia, with absolute lymphocyte count values ranging from 800 to 1200 cells/μL (to convert to ×109/L, multiply by 0.001). B, Lymphocyte subsets at several times points after ocrelizumab initiation. No prior lymphocyte subset panel was obtained. CD19 cell counts (not shown) remained near 0 at all time points. Horizontal dashed lines correspond with lower limit of normal of each y-axis value. Vertical dashed line corresponds with ocrelizumab initiation, and vertical dashed arrow corresponds with PML symptom onset.
The patient’s symptoms progressed over weeks to involve bilateral visual loss, right-sided facial droop, and dysphasia. Ocrelizumab was held and off-label pembrolizumab (200 mg, intravenous infusion) was initiated based on literature suggesting its benefit.4 The patient also began mefloquine (250 mg daily for 3 days, then 250 mg weekly), mirtazapine (30 mg daily), and maraviroc (300 mg twice daily).
However, he exhibited rapid deterioration in visual, motor, and language function. Repeated brain magnetic resonance imaging revealed extension of PML without signs of immune reconstitution inflammatory syndrome (Figure 1). The patient ultimately decided on palliative measures 2 weeks after receiving the first dose of pembrolizumab, and he died 1 month after the initial diagnosis of PML. Autopsy revealed attenuated lymphoid tissue in the spleen and nodes, deemed attributable to immunotherapy, but there was no evidence of leukemia or other neoplasm. Myeloid immune cells showed hyperplasia, likely representing compensation for attenuated lymphoid elements. Neuropathology demonstrated extensive demyelination and JCV inclusions within infected oligodendrocytes, confirming the diagnosis of PML (Figure 3). Lymphocytic infiltrate was sparse, demonstrating the absence of substantial cellular immune response.
Figure 3. Histopathology (Paraffin Sections) .

A, Occipital white matter: JC virus inclusions (arrowheads) in the infected oligodendrocytes (immunohistochemistry with immunomarker for SV40; hematoxylin counterstain; bar, 50 μm). B, Discrete demyelination patches (arrowheads) in occipital lobe white matter (low-power magnification; myelin stain with Luxol fast blue and periodic acid–Schiff counterstain; bar, 800 μm). C and D, Cerebral white matter showing patches of demyelination where myelin is replaced by mild inflammatory reaction (arrowheads; medium to high power; hematoxylin-eosin stain; bar, 400 μm).
Discussion
We present, to our knowledge, the first case of PML associated with ocrelizumab monotherapy. Notably, the patient had never been treated with another disease-modifying therapy and did not carry any known predisposing diagnoses. While there have been other PML cases diagnosed in patients after ocrelizumab initiation, all others to date have been cases related to prior natalizumab or fingolimod use, and ocrelizumab has been deemed noncontributory.5
While we believe that ocrelizumab was associated with PML in the present case, it is important to note other potential factors. Older age may have also played a role in the development of PML in the patient, as it has emerged as a risk factor for immunotherapy-related PML, although the risk varies widely with different disease-modifying therapies.6,7,8 Furthermore, there exist reports of PML in otherwise immunocompetent elderly individuals, although this remains exceedingly rare.9 While the precise mechanism that brings about PML is not entirely understood, it is likely facilitated by the decrease in lymphocyte production and potency associated with age-related immunosenescence.10 While the OPERA I and II studies11 and the ORATORIO study12 restricted recruitment to patients aged 18 to 55 years,5 the Food and Drug Administration granted ocrelizumab the designation of being a breakthrough therapy and approved it as the first and only treatment for primary progressive MS without restriction on age. Given the lack of other treatment options, this led many older patients to opt to start the medication shortly after its approval, despite there not being specific safety data for this population. This issue is of paramount importance as it is estimated that a quarter of people with MS are older than 65 years, a number that will increase with time.13 Patients with primary progressive MS tend to be older at diagnosis, and they can continue to accrue disability over decades.14 In a 2019 longitudinal study of patients with MS 65 years or older, most patients were receiving a disease-modifying therapy, while the proportion of untreated patients decreased over the study period.15 Notably, the use of anti-CD20 therapy increased dramatically throughout the course of the study. This underscores the need for safety and efficacy research aimed at the needs of the aging MS population, with several efforts underway.
The patient exhibited an intermittent idiopathic grade 1 lymphopenia (ALC range, 800-1200/μL) with preserved CD4/CD8 ratio that preceded ocrelizumab initiation. Autopsy was unrevealing and a precise cause was not found, although age-related changes may have contributed.10 Notably, while anti-CD20 therapies can lead to mild decreases in both CD4 and CD8 levels,16 no such pattern was seen with the patient’s ALC level, although lymphocyte subsets had been checked only after treatment initiation (Figure 2). While rare PML cases have been described in patients with mild or occult immunosuppression, it remains exceedingly rare for PML to occur in the setting of only mild lymphopenia.1 Hence, while one can theoretically postulate that ocrelizumab use was not associated and was only coincidental, we believe this is unlikely. In fact, the onset of PML 2 years after ocrelizumab initiation is similar to the median onset seen with natalizumab,6 a temporal association that is consistent with the possibility of medication effect.
Although there are no known effective treatments for PML and prevention is paramount, certain agents with potential efficacy are often used based on in vitro studies and case reports. Mirtazapine, mefloquine, and maraviroc have all been suggested for use based on antiviral properties, whereas pembrolizumab, nivolumab, and interleukin 7 have been used in hopes of improving cellular immune function.1,3 The patient was treated with mirtazapine, mefloquine, maraviroc, and pembrolizumab. However, he continued a precipitous decline despite these agents. Given such rapid change, there was initial concern for the development of immune reconstitution inflammatory syndrome, but there was no evidence of a substantial immune response on magnetic resonance imaging or autopsy.
Limitations
It is important to note that all case reports are inherently limited in that they cannot establish definitive links or cause-effect relationships.
Conclusions
This report details the first and only case, to our knowledge, of pathologically proven PML occurring with ocrelizumab monotherapy in a patient without prior immunomodulation. Ultimately, while age-related immunosenescence and mild lymphopenia may have predisposed the patient to the condition, we believe that ocrelizumab was associated with the development of PML. Although PML will continue to be rare with anti-CD20 therapy, this case emphasizes the importance of a thorough discussion of the potential risks and benefits of ocrelizumab in older patients with primary progressive MS and underscores the need for further research aimed at the needs of the growing elderly MS population.
References
- 1.Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81(3):247-254. doi: 10.1136/jnnp.2009.187666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M; Progressive Multifocal Leukeoncephalopathy Consortium . Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255-273. doi: 10.1177/1756285615602832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger JR, Malik V, Lacey S, Brunetta P, Lehane PB. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J Neurovirol. 2018;24(3):323-331. doi: 10.1007/s13365-018-0615-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380(17):1597-1605. doi: 10.1056/NEJMoa1815039 [DOI] [PubMed] [Google Scholar]
- 5.Genentech. Ocrelizumab & PML: prescribing information. Accessed August 2, 2020. https://www.ocrelizumabinfo.com/content/dam/gene/ocrelizumabinfo/pdfs/progressive-multifocal-leukoencephalopathy.pdf
- 6.Prosperini L, Scarpazza C, Imberti L, Cordioli C, De Rossi N, Capra R. Age as a risk factor for early onset of natalizumab-related progressive multifocal leukoencephalopathy. J Neurovirol. 2017;23(5):742-749. doi: 10.1007/s13365-017-0561-9 [DOI] [PubMed] [Google Scholar]
- 7.Berger JR, Cree BA, Greenberg B, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology. 2018;90(20):e1815-e1821. doi: 10.1212/WNL.0000000000005529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan AL, Yang J, Fisher CJ, Racke MK, Mao-Draayer Y. Progressive multifocal leukoencephalopathy in dimethyl fumarate-treated multiple sclerosis patients. Mult Scler. 2020;1352458520949158. doi: 10.1177/1352458520949158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch J, Sumalla J, Mauleón A, Rovira A, Molins M, Acarín N. Progressive multifocal leukoencephalopathy in elderly immunocompetent patients: report of 2 cases [in Spanish]. Rev Neurol. 1999;29(2):133-137. [PubMed] [Google Scholar]
- 10.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179-189. doi: 10.1111/j.1399-0039.2007.00891.x [DOI] [PubMed] [Google Scholar]
- 11.Hauser SL, Bar-Or A, Comi G, et al. ; OPERA I and OPERA II Clinical Investigators . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. [DOI] [PubMed] [Google Scholar]
- 12.Montalban X, Hauser SL, Kappos L, et al. ; ORATORIO Clinical Investigators . Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209-220. [DOI] [PubMed] [Google Scholar]
- 13.Buhse M. The elderly person with multiple sclerosis: clinical implications for the increasing life-span. J Neurosci Nurs. 2015;47(6):333-339. doi: 10.1097/JNN.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 14.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology. 2009;73(23):1996-2002. doi: 10.1212/WNL.0b013e3181c5b47f [DOI] [PubMed] [Google Scholar]
- 15.Rosso M, Gonzalez CT, Manieri M, Healy BC, Weiner HL, Chitnis T. Longitudinal study of disease burden and complications in a cohort of multiple sclerosis patients over the age of 65. Presented at: America’s Committee For Treatment and Research in Multiple Sclerosis Forum 2019; March 1, 2019; Dallas, TX. https://actrims.confex.com/actrims/2019/meetingapp.cgi/Paper/4153 [Google Scholar]
- 16.Gingele S, Jacobus TL, Konen FF, et al. Ocrelizumab depletes CD20+ T cells in multiple sclerosis patients. Cells. 2018;8(1):8. doi: 10.3390/cells8010012 [DOI] [PMC free article] [PubMed] [Google Scholar]