Abstract
Purpose of review:
Pericytes are essential components of capillaries in many tissues and organs, contributing to vessel stability and integrity, with additional contributions to microvascular function still being discovered. We review current and foundational studies identifying pericyte differentiation mechanics and their roles in the earliest stages of vessel formation.
Recent findings:
Recent advances in pericyte-focused tools and models have illuminated critical aspects of pericyte biology including their roles in vascular development.
Pericytes likely collaborate with endothelial cells undergoing vasculogenesis, initiating direct interactions during sprouting and intussusceptive angiogenesis. Pericytes also provide important regulation of vascular growth including mechanisms underlying vessel pruning, rarefaction, and subsequent regrowth.
Keywords: pericyte, endothelial cell, vascular development, angiogenesis, vasculogenesis, mural cell differentiation
Summary:
A phenotypic transition likely occurs as pericytes shift from roles in vascular development to supporting vessel maturation, homeostasis, and physiology. We provide a forward-looking perspective on pericyte-focused studies of vascular development and applications aimed at basic and clinically relevant insights into pericyte biology.
Introduction
Vascular pericytes have recently attracted significant attention as therapeutic targets in a wide range of pathologies including Alzheimer’s Disease, cancer, and tissue fibrosis [1]. Our understanding of their roles in these clinically relevant scenarios will be strengthened by a deeper knowledge of their embryonic origins and their fundamental contributions to vascular development processes under normal, physiological conditions [2]. For instance, treatment for diseases that arise from compromised vascular barrier function will likely benefit from uncovering the roles of pericytes in maintaining vessel wall integrity during angiogenic remodeling as well as in quiescence [3]. Moreover, clinical management of vascular occlusive diseases such as coronary and peripheral vascular diseases will move forward with increased insight into the transcriptional programming of pericytes and how they might be induced to contribute to vascular smooth muscle cell (vSMC) expansion during arteriogenesis and collateralization.
Until recently, vascular development studies have focused almost exclusively on endothelial cells and their roles in blood vessel formation, logically following their central role as the “basic building blocks” for the vascular system [4]. Pericytes, although known to intimately associate with quiescent microvasculature, have remained relatively under-studied in establishing their unique contributions to building nascent vascular networks. The field of vascular biology has likely only just begun to identify how pericytes may directly affect developmental processes that have previously been attributed only to the endothelium such as vasculogenesis and angiogenesis. Pericytes may also be influenced in specific ways by neighboring endothelial cells during vessel formation. It is therefore critical to understand the dynamic interactions between pericytes and endothelial cells at multiple levels during the earliest developmental stages to delineate their individual contributions to vascular development [5]. A key aspect of this interplay is identifying the earliest time point at which both cell types emerge from their respective precursors during embryonic cell differentiation. This timing is especially relevant to pericyte biology, as they potentially share developmental origins with other cell types that may inform our understanding of their emerging roles in vascular physiology (i.e. contractility and relationship with vSMCs) and disease, specifically in vessel “dropout” and potential for fibrosis or fibroblast conversion [6].
With this rationale for understanding pericytes in vascular development in view, here we present recent advances in illuminating pericyte biology in the context of previous studies that have laid the foundation for these discoveries. Specifically, we discuss the cellular origins of pericytes as they differentiate from more primitive, intermediate cell types (Figure 1). We further consider that, as pericytes emerge from these precursors, they begin contributing to the earliest stages of vessel formation, perhaps as early as vasculogenesis, but certainly in angiogenic remodeling and vessel maturation. Lastly, we present recent studies that suggest pericytes may transition developmentally from being directly involved in structurally forming new vessels to distinct functional roles underlying vessel homeostasis and physiology.
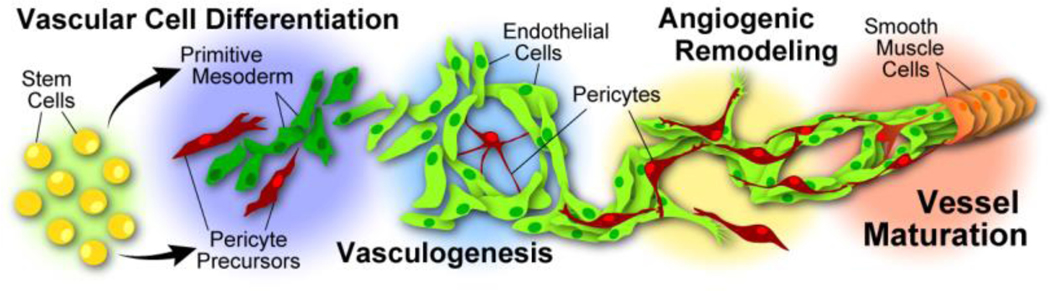
Figure 1.
Schematic illustrating a simplified progression for blood vessel development from vascular cell differentiation to vasculogenesis, subsequent angiogenic remodeling, and lastly vessel maturation and homeostasis. During the early stages of vascular formation, primitive mesoderm (dark green) and pericyte precursors (red) arise from pluripotent embryonic stem cells (yellow). Endothelial cells (green) emerge from mesodermal angioblasts and begin constructing basic vascular structures during vasculogenesis, perhaps with the involvement of mural cells including pericytes. These primitive blood vessels undergo angiogenic remodeling, recruiting pericytes to actively growing vessel networks. A subset of pericytes may further differentiate (red to orange) to contribute to arteriolar vascular smooth muscle cells (orange), as vessel maturation nears completion, and the microvasculature reaches quiescence and homeostasis.
Pericyte Differentiation
The neural crest (i.e. embryonic ectoderm germ layer) has been consistently described as a source for vascular pericytes [7], especially for those that contribute to the vasculature in the head region and specifically in the central nervous system (CNS). In fact, pre-migratory neural crest cells labeled via a Sox10-Cre construct were fate-mapped and found to yield pericytes tightly associated with vessels in the adult thymus [8], a consistent finding in parallel studies [9]. Pericytes have also been assigned a mesoderm/mesenchymal origin, but they have further been suggested to transition into mesenchyme from epithelial precursors (i.e. via epithelial-to-mesenchymal transition, EMT) [2]. Tissue-specific mesothelium for instance has been implicated in giving rise to vascular mural cells including pericytes. More specifically, this single-layer of epithelium has been observed to generate vascular mural cells in the lung [10], gastrointestinal system [11], and in the heart [12–15]. In the liver, the septum transversum mesenchyme (STM) has been shown to yield hepatic stellate cells (HSCs) and perivascular mesenchymal cells (PMCs) located adjacent to the vasculature [16]. While these populations include vSMCs and portal/central vein fibroblasts, it is unclear (i) if vascular pericytes in the liver are generated unilaterally by these cells (i.e. HSCs and PMCs), and (ii) the relative pericyte abundance in the morphologically-heterogeneous microcirculation of the liver (e.g. on bile duct microvessels vs. sinusoidal capillaries, which are known to be fenestrated to facilitate filtration, among other functions). Another unique source of pericytes was recently identified in the skin vasculature where tissue myeloid progenitor cells were found to differentiate into pericytes [17]. Endothelial cells have also been described as a source for cardiac pericytes and vSMCs [18], though other double-labeled animal models [19] will be useful in further corroborating these findings.
This potential overlap between pericyte and vSMC origins has often suggested that pericytes lineages might be extrapolated from tracing vSMC differentiation. Pericytes and vSMCs eventually associate with distinct locations in a vascular network, and they are believed to be functionally and morphologically dissimilar, though their origins and differentiation programs may overlap to a certain extent [18,20]. An example of this shared lineage yielding divergent populations was recently identified in the development of coronary artery SMCs [21]**. Specifically, pericyte recruitment into the mouse heart vasculature occurred around embryonic day 11.5 (E11.5) via the platelet-derived growth factor-BB (PDGF-BB) pathway. Notch signaling from the neighboring endothelium then promoted vSMC differentiation [22–25] and development from a subset of these pericytes, while another pericyte sub-population remained associated with the coronary microcirculation [21]**. This paradigm may exist in other tissues where pericytes have been identified at early developmental time points. For instance, Jung et al. recently observed pericytes (and/or their precursors) invested in the capillary wall of blood vessels in the E10.5 mouse brain, and these cells expanded their coverage within the CNS microcirculation through the rest of embryonic and postnatal development [19]. Similarly, in extra-embryonic tissues such as the developing mouse yolk sac, PDGF receptor-β (PDGFRβ)-expressing pericytes (and/or pericyte precursors) were found alongside Tie2-positive capillary endothelium as early as E8.5 [26].
These and other vascular development studies highlight a challenge present across many lineage tracing studies and that is the positive identification of the terminally differentiated cell type of interest, here being pericytes. As stated above, PDGFR β is a well-accepted marker of pericytes [2], but this cell surface receptor is also present on vSMCs and brain glia and can therefore present challenges in confident identification of pericytes in many tissues [27]. Vascular pericytes are also known to express neural glial antigen-2 (NG2, gene name: Cspg4, Chondroitin Sulfate Proteoglycan-4), which can be found on oligodendrocyte precursors (OPCs) in the brain, again confounding interpretation of cell identity [28]. An additional obstacle in pericyte identification is the presence of numerous cell types in peri-endothelial/capillary locations throughout the microcirculation including fibroblasts, macrophages, and epithelial cells [29–31]. One helpful distinction between pericytes and these cells is that non-vascular cell types rarely, if ever, integrate themselves within the vascular basement membrane (vBM). Thus, this unique configuration of ECM proteins (e.g. Type IV Collagen (Col-IV), laminins, perlecan, etc.) surrounding microvascular endothelia and pericytes can offer another criteria for their identification [32]. Ultimately, pericytes are best identified through a combination of pericyte and endothelial markers, rigorous assessment of their morphology (which may include some slight heterogeneity [33,34]**), and their location within microvascular networks. Coupling these benchmarks with next generation sequencing approaches and single-cell analysis will sharpen existing pericyte-specific tools, and allow greater insight into critical questions such as when terminally differentiated pericytes first emerge during embryonic development and how they expand their coverage along the rapidly developing vasculature.
Wide-ranging signaling mechanisms have been implicated in orchestrating pericyte differentiation during embryogenesis. Cell surface PDGFR β conveys intracellular signals after binding PDGF-BB ligands that are secreted by angiogenic endothelium [35], and these signals are believed to play an instrumental role in the differentiation, proliferation, and recruitment of both pericytes and vSMCs [36]. Similarly, transforming growth factor- β (TGF β) is also important for mural cell differentiation from primitive mesenchyme, requiring the conversion of latent TGF β to an active form via a dynamic interplay between Connexin43 (Cx43) gap junctions at the mural cell-endothelial interface, integrins, and matrix metalloproteinases (MMPs) [37]**. Angiopoietin-Tie signaling has been described as affecting pericyte differentiation and recruitment, though the exact role of this pathway in driving the pericyte lineage remains unclear [4,38]. Genetic knockout studies such as those for Tie2 and Ang1 have suggested that pericytes emerge and home to nascent vessels despite developmental abnormalities in other tissue compartments [39,40]. Recent observations involving the reprogramming of human induced pluripotent stem cells (iPSCs) into pericyte-like cells have suggested the importance of serum-containing media [41,42] and perhaps the presence of basic fibroblast growth factor (bFGF or FGF2) [20]. However, a study by Guimarães-Camboa et al. raised an important caution when interpreting pericyte differentiation and identity in experiments involving cell manipulations ex vivo [43]. This message is reinforced by data indicating that initiation and maintenance of the vascular pericyte identity in a variety of contexts may be tightly linked to a sustained physical association with the microvascular endothelium [44].
Pericytes in the Earliest Stages of Vessel Formation
As discussed above, pericyte differentiation has been linked to direct contact with terminally differentiated endothelium as well as to endothelial cell-derived cues such as PDGF-BB. These observations suggest a developmental time-line in which endothelial cells differentiate first and likely commence the de novo formation of blood vessels (i.e. vasculogenesis) prior to the initiation of pericyte differentiation and subsequent association with the endothelium [45,46]. This working model will likely require further validation, as it is currently unclear whether vascular pericytes (and/or their precursors) may actually: (i) differentiate concurrently with the emergence of endothelial cells, and (ii) participate in the earliest stages of blood vessel formation including vasculogenesis and subsequent developmental processes. Pericytes may temper endothelial cell proliferation for example [47,48], promoting endothelial survival independent of vascular endothelial growth factor-A (VEGF-A) as vessels transition to a more quiescent state [49,50].
Pericyte Involvement in Vasculogenesis
As differentiating angioblasts within the primitive mesoderm give rise to endothelial cells, these newly derived cells begin to organize and coalesce into primitive vascular structures during the vasculogenic phase of blood vessel development. Before these primordial vessels expand into an interconnected network, they contain hematopoietic cells and/or their precursors, inspiring their classification as “blood islands,” such as observed in developing mouse yolk sac [51–54]. As stated earlier, PDGFRβ+ pericytes have been observed alongside the developing mouse yolk sac vasculature [26], but it is unclear how involved these pericytes are in early vessel development, or if they are recruited to vessels after these primitive “tubes” have completed an initial phase of assembly and lumenization, which has been documented within in vitro co-culture studies [55]. Given that pericytes establish gap junctions with endothelial cells during early development and beyond [37,56–60]**, it is intriguing to speculate that, even at the earliest stages of vessel formation, pericytes may provide initiating and/or reinforcing cues for endothelial cell polarity [61], that is, in establishing apical-basolateral surfaces as well as lateral edges. This potential role for pericytes is consistent with recent work implicating pericytes in maintaining vessel barrier function [62] by promoting synthesis and localization of junctional proteins along these endothelial lateral edges (i.e. tight junctions: ZO-1, claudin-5, occludin) and modulating vesicular transport, most notably at the blood-brain barrier (BBB) [63].
Pericytes appear to be present along the endothelium at the earliest stages of vessel formation. For example, in chick-quail graft experiments, mesenchymal cells (neural crest-derived) organized as mural cells around the mesoderm-derived, endothelial cell-lined tube of the aortic arch primordium as well as adjacent primitive capillaries [7]. Yet we are still clarifying the precise time-line for, and mechanisms by which, pericytes engage with developing vascular networks. Pericytes may increase their numbers during embryonic development largely independent of vessel-association, and then subsequently home to vessel networks that are actively expanding via sprouting angiogenesis and “splitting” intussusception. Alternatively, or perhaps concurrently, mural cells including pericytes may first invest within the walls both major vessels [64,65] and microvessels during vasculogenesis and then proliferate based on direct chemical and mechanical signals from the endothelium. Addressing these fundamental questions regarding early vessel formation and pericyte biology will likely have important therapeutic consequences, as a deeper understanding of the mechanisms underlying pericyte proliferation for example will be important in targeting pericyte expansion in disorders associated with their dysfunction or “dropout” from the vessel wall such as in Alzheimer’s disease [66–69] and diabetic retinopathy [70–73]. Thus, incorporating recent pericyte-targeted tools into developmental models will usher in new insights into pericyte dynamics during vascular development and their roles in blood vessel physiology and pathology.
Pericyte-Endothelial Cell Interactions during Angiogenesis
Across numerous developing tissues and organs, the growth of new blood vessels from pre-existing microvasculature, or angiogenesis, occurs primarily, if not exclusively, on the capillary level where pericytes are most abundant. In particular, microvascular endothelial cells that sprout and initiate outward migration from a parent vessel secrete PDGF-BB [74,75], presumably to stimulate neighboring pericytes into synchronized expansion and chemotaxis [76,77]. The PDGF-BB ligand can anchor in the neighboring extracellular matrix (ECM) via binding by heparin sulfate proteoglycan-2 (Hspg2, or perlecan) [78]. A recent study by Dubrac and colleagues further demonstrated that PDGF-BB-induced pericyte migration is also dependent on NCK1 and NCK2 adaptor proteins, specifically in retinal neovascularization [79]. Heparin-binding EGF-like growth factor (HB-EGF) is another endothelial-derived signal that can promote pericyte recruitment to the vessel wall [80], though this cue may be more prevalent during vasculogenic tube formation. As highlighted by the ECM association of several pericyte-targeted signals, the specialized ECM known as the vascular basement membrane (vBM), and the individual components therein, may be as important in mediating pericyte-endothelial cell crosstalk during angiogenesis as soluble growth factors and other cytokines [34]. Endothelial cells deposit Col-IV, for instance, which acts as a scaffold for the incorporation of other vBM constituents such as fibronectin, laminin isoforms, perlecan, and nidogen-1 [55]. Collectively, these endothelial-derived cues create a local microenvironment that actively enhances pericyte migration along extending vessel sprouts as well as promotes the recruitment, investment, and retention of pericytes along these newly forming vessel branches [81]. Nevertheless, more work will be necessary to establish exactly how pericytes migrate and invest systemically throughout the microcirculation, that is, via migration to specific vessel locations and then undergoing investment, or by attaching to the nearest blood vessel, propagating and investing along the microvasculature as they expand in number.
While pericyte elaboration along new vessels may be largely governed by the signals released from sprouting endothelial cells, pericytes may reciprocally influence angiogenic remodeling. For example, among other vBM components [82], pericytes deposit vitronectin within the vessel wall of the CNS microcirculation [83], which may be involved in clinical conditions such as cerebral autosomal dominant arteriopathy with sub-cortical infarcts and leukoencephalopathy (CADASIL) by accelerating white matter lesions during aging [84]. Furthermore, for pericytes to actively participate in sprouting angiogenesis, they would likely need to present alongside endothelial cells in the act of outward migration from an existing vessel, which is observed in the developing mouse brain (Figure 2, [34]) and retina [85], as well as in an aortic ring explant model [86].
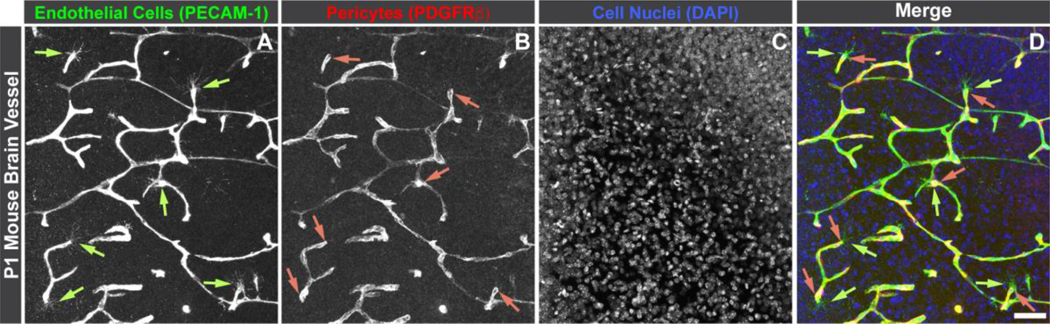
Figure 2.
Confocal image of sprouting endothelial cells labeled for platelet-endothelial cell adhesion molecule-1 (PECAM-1: A and green in D, indicated by light green arrows) with associated pericytes labeled for platelet-derived growth factor receptor-β (PDGFRβ: B and red in D, indicated by pink arrows) in a postnatal day 1 (P1) mouse brain. Cell nuclei are labeled with DAPI (C, and blue in D). All of the denoted pericytes appear to be positioned along emerging endothelial “tip” cells from the base of the filopodia, along the sprout, to the parent vessel. Scale bar in D, 50 μm.
Sprout-associated pericytes have also been suggested to influence the levels and distribution of pro-angiogenic cues including VEGF-A [87,88] and certain microRNAs [89]. However, it is not clear if this is true in other organs/tissues or across all developmental windows, as there may be important time-delays between endothelial cell sprouting and pericyte recruitment to allow for greater plasticity in angiogenic remodeling [50]. Pericytes have also been observed bridging gaps between endothelial cells, both at the sprout “tip” as well as between endothelial cells of an incomplete vessel branch [90,91]. These observations, alongside many others [82,92–100], have further suggested that pericytes may also lead endothelial sprouting in various healthy and pathological conditions. A recent study from Errede et al. has further suggested that brain pericytes construct Col-IV-based tunneling nanotubes to guide endothelial cell filopodial extensions and perhaps even facilitate long-range communication between endothelial cells and pericytes on discrete vessel segments [101]. This study, as well as those described above, highlights the fact that we have much more to discover in establishing the dynamic interplay between vascular pericytes and the remodeling endothelium, as well as other neighboring cell types [102], during sprouting angiogenesis.
New vessel branches can arise from existing vasculature via another mode of structural remodeling known as intussusceptive, or “splitting,” angiogenesis [103]. Introducing a bifurcation into a single vessel, thereby creating two parallel channels, involves the protrusion and subsequent expansion of a tissue pillar into the lumen of an existing vessel. Relative to sprouting angiogenesis, the mechanisms underlying intussusception remain understudied, as detecting these events by light microscopy can be challenging. Karthik and colleagues recently applied serial block face-scanning electron microscopy (SBF-SEM) to the zebrafish caudal vein plexus and found complex intussusceptive remodeling to refine the hierarchical nature of the developing vascular network [104]. While this group did not focus on the involvement of mural cells, and specifically of pericytes, previous observations have suggested that pericytes extend cytoplasmic processes along inter-endothelial cell junctions as trans-capillary pillars form [105,106]. The molecular mechanisms governing pericyte interactions with intussusceptive angiogenic endothelium are largely inferred from the sprouting angiogenesis context [107], but as pericyte-focused tools continue to expand, models such as the zebrafish caudal vein plexus can be revisited to refine current models of pericyte involvement during intussusception.
Pericyte Contributions to Vessel Pruning and Regrowth
Pericytes have been implicated not only in vessel formation, but also in the selective pruning of vasculature that may have been produced in excess or is no longer required for tissue perfusion [108]. Pericyte-mediated vessel regression, for example, has been shown to occur via calpain activation by CXCR3, which causes an involution of human dermal microvascular endothelial cells in an in vitro Matrigel assay [109]. Pericyte deposition of endosialin within the vBM can also promote vessel rarefaction via endothelial cell apoptosis, in part by limiting endothelial cell adhesion to the ECM [110]. In contrast, pericytes can affect vBM remodeling to limit vessel regression by blocking matrix metalloproteinases (MMPs). Pericytes secrete tissue inhibitor of metalloproteinase-3 (TIMP-3), for example, to block the activity of MMP-1 and MMP-10 and sustain nascent and established vascular structures [111–113]. Thus, remodeling of the local capillary microenvironment, especially targeting the vessel wall ECM, is a mode by which pericytes regulate vascular growth and maintenance of new microvessel branches. The physical association of pericytes with the endothelium has also been identified as an important modulator of capillary stability [114] and in turn vessel regression. In a model of hyperoxia, disrupted pericyte-endothelial cell associations contributed to retinal vessel regression and abnormal microvascular remodeling [50]. Pericyte detachment or absence has also been associated with the rarefaction of kidney capillaries [115] and with the malformation of retinal vasculature akin to defects observed in diabetic retinopathy [70] and in tumors [116]. This role for direct pericyte-endothelial contact in vessel stability may involve intercellular communication mechanisms such as those mediated by Cx43-based gap junctions [117], but the specific molecular cues regulating these interactions are still being established.
While pericyte loss has been associated with vessel pruning and rarefaction, pericytes have also been observed to persist in locations where capillary regression has occurred. These surviving pericytes appear to provide a scaffold for rapid revascularization of tissues that are transiently exposed to anti-angiogenic agents [118]. Interestingly, these pericytes also attach to and maintain vBM components such as Col-IV [119–121], perhaps so that these Col-IV “sleeves” may be re-used by endothelial cells in the event that vessel re-growth is stimulated. Not all of these pericytes remain in avascular locations after vessel regression, however, as some have been reported to migrate to surviving vessels, specifically in a model of VEGF receptor inhibition in the mouse retina [122].
Pericytes in Vessel Maturation, Homeostasis, and Physiology
As the active formation of blood vessels transitions to establishing mature, quiescent microvascular networks, pericytes also shift from structural remodeling and expansion roles to supporting vessel physiology and homeostasis. After pericytes divide and migrate to establish sufficient coverage within the microcirculation [123], their proliferative capacity appears to diminish, as recent work by Berthiaume and colleagues suggests that selective ablation of quiescent brain capillary pericytes induces neighboring pericytes to extend cellular processes and re-establish vessel coverage without notable changes in pericyte proliferation [124]. These data suggest that established microvascular pericytes might be a post-mitotic cell type as seen with cortical neurons [125] and certain muscle cell types, though vessel remodeling stimuli re-engage mechanisms capable of inducing pericyte division [86], suggesting a level of retained plasticity. As pericytes establish defined locations within the microvasculature, they likely refine the vBM surrounding themselves and the endothelium to transform the provisional ECM constructed during vessel remodeling to a more mature basement membrane [83]. The vBM is a critical structure for the barrier function of the microcirculation in many tissues, seen most clearly in the BBB and associated vBM defects contributing to specific neurovascular pathologies [126–128]. Along with fine-tuning the vBM and reducing systemic proliferation, pericytes promote vessel maturation in part by becoming more invested in the vessel wall and down-regulating mechanisms facilitating their migration [79,129]. Reduced migration might also be essential for establishing more permanent cell-cell interaction with the endothelium, as ultra-structural observations have identified “peg-and-socket” contact points [130–133]; interestingly, the exact time course for these structures is not well-established, and they may actually be present early in vascular development, aligning with pericyte differentiation via endothelial gap junction formation [58–60,117,133,134]. Pericytes are capable of deconstructing “peg-and-socket” junctions and restarting active migration along and outward from the vessel wall in certain scenarios such as during inflammation [135,136] and in disease settings like diabetic retinopathy [72,73,137]. Thus, hallmarks of pericytes that are transitioning to quiescence while concurrently promoting vessel maturation and stability include a reduction in proliferation and migration while increasing deposition of vBM components that reinforce the microvessel wall.
In addition to mechanisms governing the phenotypic shift in pericytes from remodeling to more stable, pericyte location within a microvascular network also appears to be tightly regulated. For example, pericytes appear to establish non-overlapping domains such that no two pericytes cover the same “territory” along the microvessel wall [124], though the mechanisms underlying this coordinated coverage remain to be fully established. Pericytes at different locations throughout the microcirculation also appear to have distinct morphological characteristics [33]**, with recent classifications including: (1) ensheathing pericytes that contain α-smooth muscle actin (αSMA) and wrap fully around pre-capillary arterioles, (2) mesh pericytes lacking αSMA and begin coverage of capillary-sized vessels, and (3) thin-strand pericytes also without αSMA but morphologically distinct with single cellular extensions along segments of the microvasculature [33]**. This topology and morphological heterogeneity suggests that, just as endothelial cells can acquire arterio-venous identity based on their location within the vasculature [138,139], pericytes might also integrate chemical and mechanical cues to assume discrete phenotypic roles within the microcirculation. Consistent with this idea are observations of pericytes giving rise to vSMCs [21]** and perhaps contributing to arteriogenic remodeling [140] and collateral artery formation. Pericytes have also been reported to respond to vasoactive cues [141], propagate electrical signals within the microcirculation (e.g. via gap junction-mediated conducted vasomotion) [142–144], and perform vasomotor functions similar to vSMCs [145–150], though passive regulation of vessel diameter by pericytes has also been described [124,151,152]. These studies highlight the need for more innovative models [153,154] and techniques [66,155] to resolve this potential role for capillary pericytes across various tissues and organs.
Perspectives: Looking Ahead
Recognition of pericytes and their vital roles in forming and maintaining the microcirculation is continuing to grow. A notable example of this increased appreciation can be seen with the recent interest in establishing in vitro platforms to model pericyte-mediated vessel integrity and especially the BBB [156,157]. Many of these models seek to differentiate human induced pluripotent stem cells (iPSCs) into various vascular cell types including pericytes [41,42]. It will therefore be critical to build a more solid understanding of pericyte differentiation programs and the transcriptional and behavioral hallmarks of their progression into a mature, terminally differentiated cell type. Validating these and other pericyte cell lines will further require a more comprehensive understanding of the phenotypic heterogeneity within pericyte populations as they differentiate and contribute to the discrete stages within vascular development (Figure 1). Lastly, greater insight into the various roles that pericytes acquire during blood vessel formation will be essential for “benchmarking” pericytes used in cell-based assays as well as for potentially targeting pericyte functions in certain disease conditions. Coupling recent advances in imaging and transcriptional profiling modalities with pericyte-focused tools and models will ensure that realizing many, if not all, of these goals is just on the horizon and very much within reach.
Acknowledgements:
This work was supported in part by funding from the National Institutes of Health (R01-HL146596 to J.C.C.), the National Science Foundation (CAREER Award 1752339 to J.C.C.), and the American Heart Association (19TPA34910121 to J.C.C. and 19POST34380560 to L.B.P.).
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest to disclose. This work was supported in part by funding from the National Institutes of Health (R01-HL146596 to J.C.C.), the National Science Foundation (CAREER Award 1752339 to J.C.C.), and the American Heart Association (19TPA34910121 to J.C.C. and 19POST34380560 to L.B.P.).
This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA (2019) Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front Cell Neurosci 13:282. doi: 10.3389/fncel.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21 (2):193–215. doi:S1534–5807(11)00269–3 [pii] 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 3.Geevarghese A, Herman IM (2014) Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 163 (4):296–306. doi: 10.1016/j.trsl.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97 (6):512–523. doi:97/6/512 [pii] 10.1161/01.RES.0000182903.16652.d7 [DOI] [PubMed] [Google Scholar]
- 5.Sinha S, Santoro MM (2018) New models to study vascular mural cell embryonic origin: implications in vascular diseases. Cardiovasc Res 114 (4):481–491. doi: 10.1093/cvr/cvy005 [DOI] [PubMed] [Google Scholar]
- 6.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Volarevic V (2018) Molecular mechanisms underlying therapeutic potential of pericytes. J Biomed Sci 25 (1):21. doi: 10.1186/s12929-018-0423-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Lièvre CS, Le Douarin NM (1975) Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. Journal of Embryology and Experimental Morphology 34 (1):125–154 [PubMed] [Google Scholar]
- 8.Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR (2008) Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol 180 (8):5344–5351. doi: 10.4049/jimmunol.180.8.5344 [DOI] [PubMed] [Google Scholar]
- 9.Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M (2008) Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol 180 (5):3183–3189. doi: 10.4049/jimmunol.180.5.3183 [DOI] [PubMed] [Google Scholar]
- 10.Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL (2008) Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A 105 (43):16626–16630. doi: 10.1073/pnas.0808649105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM (2005) The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132 (23):5317–5328. doi: 10.1242/dev.02141 [DOI] [PubMed] [Google Scholar]
- 12.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454 (7200):104–108. doi: 10.1038/nature06969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikawa T, Gourdie RG (1996) Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 174 (2):221–232. doi: 10.1006/dbio.1996.0068 [DOI] [PubMed] [Google Scholar]
- 14.Wessels A, Perez-Pomares JM (2004) The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol 276 (1):43–57. doi: 10.1002/ar.a.10129 [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454 (7200):109–113. doi: 10.1038/nature07060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asahina K, Zhou B, Pu WT, Tsukamoto H (2011) Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53 (3):983–995. doi: 10.1002/hep.24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS (2017) Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell reports 18 (12):2991–3004. doi: 10.1016/j.celrep.2017.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH (2016) Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nature communications 7:12422. doi: 10.1038/ncomms12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung B, Arnold TD, Raschperger E, Gaengel K, Betsholtz C (2018) Visualization of vascular mural cells in developing brain using genetically labeled transgenic reporter mice. J Cereb Blood Flow Metab 38 (3):456–468. doi: 10.1177/0271678X17697720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II (2017) Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell reports 19 (9):1902–1916. doi: 10.1016/j.celrep.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I, Red-Horse K (2015) Pericytes are progenitors for coronary artery smooth muscle. Elife 4. doi: 10.7554/eLife.10036** This study provides key insights into the lineage relationships shared by microvascular pericytes and vascular smooth muscle cells.
- 22.Henshall TL, Keller A, He L, Johansson BR, Wallgard E, Raschperger E, Mae MA, Jin S, Betsholtz C, Lendahl U (2015) Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler Thromb Vasc Biol 35 (2):409–420. doi: 10.1161/ATVBAHA.114.304849 [DOI] [PubMed] [Google Scholar]
- 23.Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U (2008) Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res 102 (12):1483–1491. doi: 10.1161/CIRCRESAHA.107.167965 [DOI] [PubMed] [Google Scholar]
- 24.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A (2004) Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18 (22):2730–2735. doi:18/22/2730 [pii] 10.1101/gad.308904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, Joutel A (2003) Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol 162 (1):329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French WJ, Creemers EE, Tallquist MD (2008) Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol Cell Biol 28 (18):5646–5657. doi: 10.1128/MCB.00441-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortuna V, Pardanaud L, Brunet I, Ola R, Ristori E, Santoro MM, Nicoli S, Eichmann A (2015) Vascular Mural Cells Promote Noradrenergic Differentiation of Embryonic Sympathetic Neurons. Cell reports 11 (11):1786–1796. doi: 10.1016/j.celrep.2015.05.028 [DOI] [PubMed] [Google Scholar]
- 28.Trotter J, Karram K, Nishiyama A (2010) NG2 cells: Properties, progeny and origin. Brain Res Rev 63 (1–2):72–82. doi: 10.1016/j.brainresrev.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He H, Mack JJ, Guc E, Warren CM, Squadrito ML, Kilarski WW, Baer C, Freshman RD, McDonald AI, Ziyad S, Swartz MA, De Palma M, Iruela-Arispe ML (2016) Perivascular Macrophages Limit Permeability. Arterioscler Thromb Vasc Biol 36 (11):2203–2212. doi: 10.1161/ATVBAHA.116.307592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, Racchumi G, Murphy M, Van Rooijen N, Anrather J, Iadecola C (2016) Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. doi: 10.1172/JCI86950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neng L, Zhang W, Hassan A, Zemla M, Kachelmeier A, Fridberger A, Auer M, Shi X (2013) Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat Protoc 8 (4):709–720. doi: 10.1038/nprot.2013.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sava P, Cook IO, Mahal RS, Gonzalez AL (2015) Human microvascular pericyte basement membrane remodeling regulates neutrophil recruitment. Microcirculation 22 (1):54–67. doi: 10.1111/micc.12173 [DOI] [PubMed] [Google Scholar]
- 33.Grant RI, Hartmann DA, Underly RG, Berthiaume AA, Bhat NR, Shih AY (2019) Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 39 (3):411–425. doi: 10.1177/0271678X17732229** This study provides a framework for classifying and understanding phenotypic heterogeneity of pericytes within the brain microcirculation.
- 34.Payne LB, Zhao H, James CC, Darden J, McGuire D, Taylor S, Smyth JW, Chappell JC (2019) The pericyte microenvironment during vascular development. Microcirculation 26 (8):e12554. doi: 10.1111/micc.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C (2003) Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17 (15):1835–1840. doi: 10.1101/gad.26680317/15/1835 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA (1999) Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res 84 (3):298–305 [DOI] [PubMed] [Google Scholar]
- 37.Hirschi KK, Burt JM, Hirschi KD, Dai C (2003) Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93 (5):429–437. doi: 10.1161/01.RES.0000091259.84556.D5** This study provided one of the first key insights into pericyte-endothelial cell contact and gap junction formation as key components underlying pericyte differentiation.
- 38.Gaengel K, Genove G, Armulik A, Betsholtz C (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29 (5):630–638. 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- 39.Patan S (1998) TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res 56 (1):1–21. doi: 10.1006/mvre.1998.2081 [DOI] [PubMed] [Google Scholar]
- 40.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87 (7):1171–1180. doi: 10.1016/s0092-8674(00)81813-9 [DOI] [PubMed] [Google Scholar]
- 41.Faal T, Phan DTT, Davtyan H, Scarfone VM, Varady E, Blurton-Jones M, Hughes CCW, Inlay MA (2019) Induction of Mesoderm and Neural Crest-Derived Pericytes from Human Pluripotent Stem Cells to Study Blood-Brain Barrier Interactions. Stem Cell Reports 12 (3):451–460. doi: 10.1016/j.stemcr.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stebbins MJ, Gastfriend BD, Canfield SG, Lee MS, Richards D, Faubion MG, Li WJ, Daneman R, Palecek SP, Shusta EV (2019) Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties. Sci Adv 5 (3):eaau7375. doi: 10.1126/sciadv.aau7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM (2017) Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 20 (3):345–359 e345. doi: 10.1016/j.stem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt MM, van Dijk CGM, Maringanti R, Chrifi I, Kramann R, Verhaar MC, Duncker DJ, Mokry M, Cheng C (2019) Transcriptome analysis reveals microvascular endothelial cell-dependent pericyte differentiation. Sci Rep 9 (1):15586. doi: 10.1038/s41598-019-51838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagedorn M, Balke M, Schmidt A, Bloch W, Kurz H, Javerzat S, Rousseau B, Wilting J, Bikfalvi A (2004) VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dyn 230 (1):23–33. doi: 10.1002/dvdy.20020 [DOI] [PubMed] [Google Scholar]
- 46.Fruttiger M (2002) Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43 (2):522–527 [PubMed] [Google Scholar]
- 47.Orlidge A, D’Amore PA (1987) Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 105 (3):1455–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato Y, Rifkin DB (1989) Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol 109 (1):309–315. doi: 10.1083/jcb.109.1.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E (1999) Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103 (2):159–165. doi: 10.1172/JCI5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamin LE, Hemo I, Keshet E (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125 (9):1591–1598 [DOI] [PubMed] [Google Scholar]
- 51.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376 (6535):62–66. doi: 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- 52.Wang R, Clark R, Bautch VL (1992) Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development 114 (2):303–316 [DOI] [PubMed] [Google Scholar]
- 53.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T (1988) Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102 (3):471–478 [DOI] [PubMed] [Google Scholar]
- 54.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45 [PubMed] [Google Scholar]
- 55.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE (2009) Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114 (24):5091–5101. doi:blood-2009–05-222364 [pii] 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durham JT, Dulmovits BM, Cronk SM, Sheets AR, Herman IM (2015) Pericyte chemomechanics and the angiogenic switch: insights into the pathogenesis of proliferative diabetic retinopathy? Invest Ophthalmol Vis Sci 56 (6):3441–3459. doi: 10.1167/iovs.14-13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tien T, Muto T, Barrette K, Challyandra L, Roy S (2014) Downregulation of Connexin 43 promotes vascular cell loss and excess permeability associated with the development of vascular lesions in the diabetic retina. Molecular vision 20:732–741 [PMC free article] [PubMed] [Google Scholar]
- 58.Fang JS, Dai C, Kurjiaka DT, Burt JM, Hirschi KK (2013) Connexin45 regulates endothelial-induced mesenchymal cell differentiation toward a mural cell phenotype. Arterioscler Thromb Vasc Biol 33 (2):362–368. doi: 10.1161/ATVBAHA.112.255950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li AF, Sato T, Haimovici R, Okamoto T, Roy S (2003) High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci 44 (12):5376–5382 [DOI] [PubMed] [Google Scholar]
- 60.Cuevas P, Gutierrez-Diaz JA, Reimers D, Dujovny M, Diaz FG, Ausman JI (1984) Pericyte endothelial gap junctions in human cerebral capillaries. Anat Embryol (Berl) 170 (2):155–159 [DOI] [PubMed] [Google Scholar]
- 61.Iruela-Arispe ML, Davis GE (2009) Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16 (2):222–231. doi:S1534–5807(09)00041–0 [pii] 10.1016/j.devcel.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468 (7323):562–566. doi:nature09513 [pii] 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468 (7323):557–561. doi:nature09522 [pii] 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 64.Hungerford JE, Owens GK, Argraves WS, Little CD (1996) Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol 178 (2):375–392. doi: 10.1006/dbio.1996.0225 [DOI] [PubMed] [Google Scholar]
- 65.Nakamura H (1988) Electron microscopic study of the prenatal development of the thoracic aorta in the rat. Am J Anat 181 (4):406–418. doi: 10.1002/aja.1001810409 [DOI] [PubMed] [Google Scholar]
- 66.Kisler K, Nelson AR, Rege SV, Ramanathan A, Wang Y, Ahuja A, Lazic D, Tsai PS, Zhao Z, Zhou Y, Boas DA, Sakadzic S, Zlokovic BV (2017) Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nature neuroscience. doi: 10.1038/nn.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163 (5):1064–1078. doi: 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV (2013) Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nature communications 4:2932. doi: 10.1038/ncomms3932 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Hamilton NB, Attwell D, Hall CN (2010) Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2. doi: 10.3389/fnene.2010.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park DY, Lee J, Kim J, Kim K, Hong S, Han S, Kubota Y, Augustin HG, Ding L, Kim JW, Kim H, He Y, Adams RH, Koh GY (2017) Plastic roles of pericytes in the blood-retinal barrier. Nature communications 8:15296. doi: 10.1038/ncomms15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL (2014) Targeting pericytes for angiogenic therapies. Microcirculation 21 (4):345–357. doi: 10.1111/micc.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendel TA, Clabough EB, Kao DS, Demidova-Rice TN, Durham JT, Zotter BC, Seaman SA, Cronk SM, Rakoczy EP, Katz AJ, Herman IM, Peirce SM, Yates PA (2013) Pericytes derived from adipose-derived stem cells protect against retinal vasculopathy. PLoS One 8 (5):e65691. doi: 10.1371/journal.pone.0065691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP (2008) Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57 (9):2495–2502. doi: 10.2337/db08-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445 (7129):776–780. doi:nature05571 [pii], or 10.1038/nature05571 [DOI] [PubMed] [Google Scholar]
- 75.Rolny C, Nilsson I, Magnusson P, Armulik A, Jakobsson L, Wentzel P, Lindblom P, Norlin J, Betsholtz C, Heuchel R, Welsh M, Claesson-Welsh L (2006) Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood 108 (6):1877–1886. doi:blood-2006–04-014894 [pii] 10.1182/blood-2006-04-014894 [DOI] [PubMed] [Google Scholar]
- 76.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C (1998) Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125 (17):3313–3322 [DOI] [PubMed] [Google Scholar]
- 77.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126 (14):3047–3055 [DOI] [PubMed] [Google Scholar]
- 78.Abramsson A, Kurup S, Busse M, Yamada S, Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J, Ringvall M, Landegren U, Kjellen L, Bondjers G, Li JP, Lindahl U, Spillmann D, Betsholtz C, Gerhardt H (2007) Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev 21 (3):316–331. doi:21/3/316 [pii] 10.1101/gad.398207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dubrac A, Kunzel SE, Kunzel SH, Li J, Chandran RR, Martin K, Greif DM, Adams RH, Eichmann A (2018) NCK-dependent pericyte migration promotes pathological neovascularization in ischemic retinopathy. Nature communications 9 (1):3463. doi: 10.1038/s41467-018-05926-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stratman AN, Schwindt AE, Malotte KM, Davis GE (2010) Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116 (22):4720–4730. doi:blood-2010–05-286872 [pii] 10.1182/blood-2010-05-286872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Darden J, Payne LB, Zhao H, Chappell JC (2018) Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation. Angiogenesis. doi: 10.1007/s10456-018-9648-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L (2007) An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 10 (1):35–45. doi: 10.1007/s10456-006-9061-x [DOI] [PubMed] [Google Scholar]
- 83.He L, Vanlandewijck M, Raschperger E, Andaloussi Mae M, Jung B, Lebouvier T, Ando K, Hofmann J, Keller A, Betsholtz C (2016) Analysis of the brain mural cell transcriptome. Sci Rep 6:35108. doi: 10.1038/srep35108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capone C, Cognat E, Ghezali L, Baron-Menguy C, Aubin D, Mesnard L, Stohr H, Domenga-Denier V, Nelson MT, Joutel A (2016) Reducing Timp3 or vitronectin ameliorates disease manifestations in CADASIL mice. Ann Neurol 79 (3):387–403. doi: 10.1002/ana.24573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walpole J, Gabhann FM, Peirce SM, Chappell JC (2017) Agent-based Computational Model of Retinal Angiogenesis Simulates Microvascular Network Morphology as a Function of Pericyte Coverage. Microcirculation. doi: 10.1111/micc.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiaverina G, di Blasio L, Monica V, Accardo M, Palmiero M, Peracino B, Vara-Messler M, Puliafito A, Primo L (2019) Dynamic Interplay between Pericytes and Endothelial Cells during Sprouting Angiogenesis. Cells 8 (9). doi: 10.3390/cells8091109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao R, Xue Y, Hedlund EM, Zhong Z, Tritsaris K, Tondelli B, Lucchini F, Zhu Z, Dissing S, Cao Y (2010) VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci U S A 107 (2):856–861. 10.1073/pnas.0911661107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eilken HM, Dieguez-Hurtado R, Schmidt I, Nakayama M, Jeong HW, Arf H, Adams S, Ferrara N, Adams RH (2017) Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nature communications 8 (1):1574. doi: 10.1038/s41467-017-01738-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y, Fortunato O, Avolio E, Cesselli D, Beltrami AP, Angelini G, Emanueli C, Madeddu P (2011) Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res 109 (8):894–906. doi: 10.1161/CIRCRESAHA.111.251546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ozerdem U, Charbono WL, Stallcup WB (2002) Plastic casting of embryonic, placental, and tumor vasculature in the mouse. Microvasc Res 64 (3):486–490. doi: 10.1006/mvre.2002.2447 [DOI] [PubMed] [Google Scholar]
- 91.Ozerdem U, Monosov E, Stallcup WB (2002) NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res 63 (1):129–134. doi: 10.1006/mvre.2001.2376 [DOI] [PubMed] [Google Scholar]
- 92.Archer DB, Gardiner TA (1981) Electron microscopic features of experimental choroidal neovascularization. Am J Ophthalmol 91 (4):433–457. doi: 10.1016/0002-9394(81)90230-0 [DOI] [PubMed] [Google Scholar]
- 93.Verhoeven D, Buyssens N (1988) Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system. Virchows Arch B Cell Pathol Incl Mol Pathol 54 (5):263–272. doi: 10.1007/bf02899222 [DOI] [PubMed] [Google Scholar]
- 94.Schlingemann RO, Rietveld FJ, Kwaspen F, van de Kerkhof PC, de Waal RM, Ruiter DJ (1991) Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol 138 (6):1335–1347 [PMC free article] [PubMed] [Google Scholar]
- 95.Nehls V, Denzer K, Drenckhahn D (1992) Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res 270 (3):469–474. doi: 10.1007/bf00645048 [DOI] [PubMed] [Google Scholar]
- 96.Tsuzuki H, Sasa S (1994) Ultrastructural observation of capillary sprouts in the dental organs of rat molars. Kaibogaku Zasshi 69 (5):684–696 [PubMed] [Google Scholar]
- 97.Wesseling P, Schlingemann RO, Rietveld FJ, Link M, Burger PC, Ruiter DJ (1995) Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol 54 (3):304–310. doi: 10.1097/00005072-199505000-00003 [DOI] [PubMed] [Google Scholar]
- 98.Amselgruber WM, Schafer M, Sinowatz F (1999) Angiogenesis in the bovine corpus luteum: an immunocytochemical and ultrastructural study. Anat Histol Embryol 28 (3):157–166. doi: 10.1046/j.1439-0264.1999.00195.x [DOI] [PubMed] [Google Scholar]
- 99.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160 (3):985–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ponce AM, Price RJ (2003) Angiogenic stimulus determines the positioning of pericytes within capillary sprouts in vivo. Microvasc Res 65 (1):45–48. doi: 10.1016/s0026286202000146 [DOI] [PubMed] [Google Scholar]
- 101.Errede M, Mangieri D, Longo G, Girolamo F, de Trizio I, Vimercati A, Serio G, Frei K, Perris R, Virgintino D (2018) Tunneling nanotubes evoke pericyte/endothelial communication during normal and tumoral angiogenesis. Fluids Barriers CNS 15 (1):28. doi: 10.1186/s12987-018-0114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P (2011) The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8 (1):8. doi: 10.1186/2045-8118-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mentzer SJ, Konerding MA (2014) Intussusceptive angiogenesis: expansion and remodeling of microvascular networks. Angiogenesis 17 (3):499–509. doi: 10.1007/s10456-014-9428-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karthik S, Djukic T, Kim JD, Zuber B, Makanya A, Odriozola A, Hlushchuk R, Filipovic N, Jin SW, Djonov V (2018) Synergistic interaction of sprouting and intussusceptive angiogenesis during zebrafish caudal vein plexus development. Sci Rep 8 (1):9840. doi: 10.1038/s41598-018-27791-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burri PH, Djonov V (2002) Intussusceptive angiogenesis--the alternative to capillary sprouting. Mol Aspects Med 23 (6S):S1–27. doi: 10.1016/s0098-2997(02)00096-1 [DOI] [PubMed] [Google Scholar]
- 106.Djonov V, Baum O, Burri PH (2003) Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res 314 (1):107–117. doi: 10.1007/s00441-003-0784-3 [DOI] [PubMed] [Google Scholar]
- 107.De Spiegelaere W, Casteleyn C, Van den Broeck W, Plendl J, Bahramsoltani M, Simoens P, Djonov V, Cornillie P (2012) Intussusceptive angiogenesis: a biologically relevant form of angiogenesis. J Vasc Res 49 (5):390–404. doi: 10.1159/000338278 [DOI] [PubMed] [Google Scholar]
- 108.Korn C, Augustin HG (2015) Mechanisms of Vessel Pruning and Regression. Dev Cell 34 (1):5–17. doi: 10.1016/j.devcel.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 109.Bodnar RJ, Rodgers ME, Chen WC, Wells A (2013) Pericyte regulation of vascular remodeling through the CXC receptor 3. Arterioscler Thromb Vasc Biol 33 (12):2818–2829. doi: 10.1161/ATVBAHA.113.302012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simonavicius N, Ashenden M, van Weverwijk A, Lax S, Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM (2012) Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120 (7):1516–1527. doi: 10.1182/blood-2011-01-332338 [DOI] [PubMed] [Google Scholar]
- 111.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE (2006) Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175 (1):179–191. doi: 10.1083/jcb.200603176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis GE, Saunders WB (2006) Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11 (1):44–56. doi: 10.1038/sj.jidsymp.5650008 [DOI] [PubMed] [Google Scholar]
- 113.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS (2012) Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23 (5):868–883. doi: 10.1681/ASN.2011080851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chatterjee S, Naik UP (2012) Pericyte-endothelial cell interaction: a survival mechanism for the tumor vasculature. Cell Adh Migr 6 (3):157–159. doi: 10.4161/cam.20252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS (2014) The role of pericyte detachment in vascular rarefaction. J Vasc Res 51 (4):247–258. doi: 10.1159/000365149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caporarello N, D’Angeli F, Cambria MT, Candido S, Giallongo C, Salmeri M, Lombardo C, Longo A, Giurdanella G, Anfuso CD, Lupo G (2019) Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int J Mol Sci 20 (24). doi: 10.3390/ijms20246351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivanova E, Kovacs-Oller T, Sagdullaev BT (2017) Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J Neurosci 37 (32):7580–7594. doi: 10.1523/JNEUROSCI.0187-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116 (10):2610–2621. doi: 10.1172/JCI24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Runge A, Hu J, Wieland M, Bergeest JP, Mogler C, Neumann A, Geraud C, Arnold B, Rohr K, Komljenovic D, Schirmacher P, Goerdt S, Augustin HG (2014) An inducible hepatocellular carcinoma model for preclinical evaluation of antiangiogenic therapy in adult mice. Cancer Res 74 (15):4157–4169. doi: 10.1158/0008-5472.CAN-13-2311 [DOI] [PubMed] [Google Scholar]
- 120.Franco CA, Jones ML, Bernabeu MO, Geudens I, Mathivet T, Rosa A, Lopes FM, Lima AP, Ragab A, Collins RT, Phng LK, Coveney PV, Gerhardt H (2015) Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol 13 (4):e1002125. doi: 10.1371/journal.pbio.1002125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Korn C, Scholz B, Hu J, Srivastava K, Wojtarowicz J, Arnsperger T, Adams RH, Boutros M, Augustin HG, Augustin I (2014) Endothelial cell-derived non-canonical Wnt ligands control vascular pruning in angiogenesis. Development 141 (8):1757–1766. doi: 10.1242/dev.104422 [DOI] [PubMed] [Google Scholar]
- 122.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM (2006) Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290 (2):H547–559. doi: 10.1152/ajpheart.00616.2005 [DOI] [PubMed] [Google Scholar]
- 123.McDougall SR, Chaplain MAJ, Stéphanou A, Anderson ARA (2010) Modelling the Impact of Pericyte Migration and Coverage of Vessels on the Efficacy of Vascular Disrupting Agents. Math Model Nat Phenom 5 (1):163–202 [Google Scholar]
- 124.Berthiaume AA, Grant RI, McDowell KP, Underly RG, Hartmann DA, Levy M, Bhat NR, Shih AY (2018) Dynamic Remodeling of Pericytes In Vivo Maintains Capillary Coverage in the Adult Mouse Brain. Cell reports 22 (1):8–16. doi: 10.1016/j.celrep.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anda FC, Madabhushi R, Rei D, Meng J, Graff J, Durak O, Meletis K, Richter M, Schwanke B, Mungenast A, Tsai LH (2016) Cortical neurons gradually attain a post-mitotic state. Cell Res 26 (9):1033–1047. doi: 10.1038/cr.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomsen MS, Routhe LJ, Moos T (2017) The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37 (10):3300–3317. doi: 10.1177/0271678X17722436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71 (11):1018–1039. doi: 10.1002/dneu.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamazaki Y, Kanekiyo T (2017) Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. Int J Mol Sci 18 (9). doi: 10.3390/ijms18091965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aguilera KY, Brekken RA (2014) Recruitment and retention: factors that affect pericyte migration. Cell Mol Life Sci 71 (2):299–309. doi: 10.1007/s00018-013-1432-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allsopp G, Gamble HJ (1979) An electron microscopic study of the pericytes of the developing capillaries in human fetal brain and muscle. J Anat 128 (Pt 1):155–168 [PMC free article] [PubMed] [Google Scholar]
- 131.Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL (1996) In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res 51 (2):213–228. doi: 10.1006/mvre.1996.0022 [DOI] [PubMed] [Google Scholar]
- 132.Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A (2009) Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res 29 (1):449–453 [PubMed] [Google Scholar]
- 133.Fujimoto K (1995) Pericyte-endothelial gap junctions in developing rat cerebral capillaries: a fine structural study. Anat Rec 242 (4):562–565. doi: 10.1002/ar.1092420412 [DOI] [PubMed] [Google Scholar]
- 134.Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F (1991) Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 6 (2):269–286 [PubMed] [Google Scholar]
- 135.Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, Harada E, Miyaji H, Koga M, Nishioku T, Yamauchi A, Kataoka Y (2011) Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation 8:106. doi: 10.1186/1742-2094-8-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tigges U, Boroujerdi A, Welser-Alves JV, Milner R (2013) TNF-alpha promotes cerebral pericyte remodeling in vitro, via a switch from alpha1 to alpha2 integrins. J Neuroinflammation 10:33. doi: 10.1186/1742-2094-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME (2008) The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci 49 (5):2163–2171. doi: 10.1167/iovs.07-1206 [DOI] [PubMed] [Google Scholar]
- 138.Castillo M, Alvarez H (2011) Artery or vein: to be or not to be? AJNR Am J Neuroradiol 32 (5):791–793. doi: 10.3174/ajnr.A2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gridley T (2010) Notch signaling in the vasculature. Curr Top Dev Biol 92:277–309. doi:S0070–2153(10)92009–7 [pii] 10.1016/S0070-2153(10)92009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arreola A, Payne LB, Julian MH, de Cubas AA, Daniels AB, Taylor S, Zhao H, Darden J, Bautch VL, Rathmell WK, Chappell JC (2018) Von Hippel-Lindau mutations disrupt vascular patterning and maturation via Notch. JCI Insight 3 (4). doi: 10.1172/jci.insight.92193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG (2003) ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol 551 (Pt 3):787–799. doi: 10.1113/jphysiol.2003.047977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hillman EM (2014) Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37:161–181. doi: 10.1146/annurev-neuro-071013-014111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Segal SS (2005) Regulation of blood flow in the microcirculation. Microcirculation 12 (1):33–45. doi: 10.1080/10739680590895028 [DOI] [PubMed] [Google Scholar]
- 144.Bagher P, Segal SS (2011) Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202 (3):271–284. doi: 10.1111/j.1748-1716.2010.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443 (7112):700–704. doi: 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508 (7494):55–60. doi: 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O’Farrell FM, Mastitskaya S, Hammond-Haley M, Freitas F, Wah WR, Attwell D (2017) Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife 6. doi: 10.7554/eLife.29280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neuhaus AA, Couch Y, Sutherland BA, Buchan AM (2017) Novel method to study pericyte contractility and responses to ischaemia in vitro using electrical impedance. J Cereb Blood Flow Metab 37 (6):2013–2024. doi: 10.1177/0271678X16659495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ (2017) Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 23 (6):733–741. doi: 10.1038/nm.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crawford C, Wildman SS, Kelly MC, Kennedy-Lydon TM, Peppiatt-Wildman CM (2013) Sympathetic nerve-derived ATP regulates renal medullary vasa recta diameter via pericyte cells: a role for regulating medullary blood flow? Front Physiol 4:307. doi: 10.3389/fphys.2013.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology 7 (4):452–464. doi: 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J (2015) Regional Blood Flow in the Normal and Ischemic Brain Is Controlled by Arteriolar Smooth Muscle Cell Contractility and Not by Capillary Pericytes. Neuron 87 (1):95–110. doi: 10.1016/j.neuron.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao H, Chappell JC (2019) Microvascular bioengineering: a focus on pericytes. J Biol Eng 13:26. doi: 10.1186/s13036-019-0158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao H, Darden J, Chappell JC (2018) Establishment and Characterization of an Embryonic Pericyte Cell Line. Microcirculation:e12461. doi: 10.1111/micc.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bahrami N, Childs SJ (2019) Development of vascular myogenic responses in zebrafish. bioRxiv:713248. doi: 10.1101/713248 [DOI] [Google Scholar]
- 156.Campisi M, Shin Y, Osaki T, Hajal C, Chiono V, Kamm RD (2018) 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180:117–129. doi: 10.1016/j.biomaterials.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gastfriend BD, Palecek SP, Shusta EV (2018) Modeling the blood-brain barrier: Beyond the endothelial cells. Curr Opin Biomed Eng 5:6–12. doi: 10.1016/j.cobme.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]