Abstract
Background
The spectrum of COVID-19 clinical manifestations is not yet known. In the elderly, mortality and extrapulmonary involvement appears more frequent than expected.
Methods
A multicentre-retrospective-case-series study of COVID-19 patients, aged ≥65 years, hospitalised between March 1 and June 15, 2020. Patients were classified at admission into 3 groups based on their Clinical Frailty Scale (CFS) score: 1–3 (group A), 4–6 (group B) and 7–9 (group C).
Results
Of the 206 patients in the study, 60 (29%) were assigned to group A, 60 (29%) to B and 86 (42%) to C. Significantly more frequent in group C than in B or A were: mental confusion (respectively 65%, 33%, 7%; P < 0.001), kidney failure (39%, 22%, 20%; P = 0.019), dehydration syndrome (55%, 27%, 13%; P < 0.001), electrolyte imbalance (54%, 32%, 25%; P = 0.001), and diabetic decompensation (22%, 12%, 7%; P = 0.026). Crude mortality was 27%. By multivariate logistic regression model independent predictors of death were male sex (adjusted odds ratio (aOR) = 2.87,95%CI = 1.15–7.18), CFS 7–9 (aOR = 9.97,95%CI = 1.82–52.99), dehydration at admission (aOR = 4.27,95%CI = 1.72–10.57) and non-invasive/invasive ventilation (aOR = 4.88,95%CI = 1.94–12.26).
Conclusions
Elderly patients with a high CFS showed frequent extrapulmonary signs at admission, even in the absence of lung involvement. These findings, along with a high CFS, predicted a significant risk of mortality.
Keywords: Elderly, COVID-19, SARS-CoV-2, Frailty, Extrapulmonary manifestations
Introduction
Since the end of 2019, a novel coronavirus named SARS-CoV-2 has been responsible for a dramatic outbreak of pneumonia cases rapidly spreading worldwide. COVID-19 can arise with non-specific symptoms, such as cough, fever, arthromyalgia and sore throat. The disease might then evolve into pneumonia and progress to acute respiratory distress syndrome (ARDS), a life-threatening condition (Hu et al., 2020).
During the pandemic, the elderly were particularly affected by severe forms of COVID-19, with higher reported mortality than in younger subjects (Kang and Jung, 2020, Zhou et al., 2020). Indeed, the elderly are considered a frail population, because of their increased vulnerability to endogenous and exogenous stressors (El Assar et al., 2020), due to a dysregulated innate and adaptive immune function known as immunosenescence. Moreover, “inflamm-aging”, referring to a state of chronic low-grade inflammation, is related to an imbalance of anti-inflammatory and pro-inflammatory cytokines (Pera et al., 2015).
Recent studies suggest that at diagnosis with COVID-19 older adults present more often with extrapulmonary complications, even in the absence of lung findings, than younger subjects (Gómez-Belda et al., 2021). A wide variety of manifestations were described, including acute kidney injury (Izzedine and Jhaveri, 2020), gastrointestinal symptoms (Mao et al., 2020), acute pulmonary embolism (Bavaro et al., 2020) and neurological complications (Azizi and Azizi, 2020). Although data are inconsistent, all of these factors could increase mortality in this population. Studies also demonstrate that clinicians should consider other aspects that potentially influence the overall risk of death, for example, the Clinical Frailty Scale (CFS) is a useful marker of mortality independently of comorbidities (Tehrani et al., 2021, Chinnadurai et al., 2020). This association was further confirmed by a recent meta-analysis that suggested a potential linear relationship between increasing CFS and higher mortality (Pranata et al., 2021). Other authors propose caution in placing too much emphasis on the influence of frailty alone on the risk of mortality in older COVID-19 patients (Cosco et al., 2021). We conducted a retrospective analysis of COVID-19 patients aged >65 years hospitalised in 5 large secondary and tertiary care hospitals in Italy to investigate the clinical presentation of COVID-19 and the predictors of mortality in the elderly.
Patients and methods
Study design
A case-series of all consecutive patients aged ≥65 years hospitalised between March 1 and June 15, 2020, with SARS-CoV-2 infection confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal swabs, was retrospectively analysed. Five hospitals in Southern Italy participated in the study (Infectious Diseases Unit, University of Bari, University Hospital Policlinico, Bari; Infectious Diseases Unit, Oncologic Hospital San Giuseppe Moscati, Taranto; Clinic of Infectious Disease, University of Foggia, Ospedali Riuniti, Foggia, Italy; Clinic of Infectious Disease, ASL BAT, P.O.V. Emanuele II, Bisceglie, Italy; Unit of Infectious and Tropical Diseases, St. Annunziata Hospital, Cosenza, Italy). Patients’ demographic, clinical and microbiological characteristics were retrieved from available medical records.
Laboratory diagnosis of COVID-19
Nasopharyngeal swabs were used to diagnose COVID-19 in all patients. Tests were performed at each hospital’s laboratory using RT-PCR (real-time PCR assay targeting E-gene, RdRP-gene and N-gene, performed with the protocol previously reported by the World Health Organisation [https://www.who.int/docs/default-source/coronaviruse/uscdcrt-pcr-panel-for-detection-instructions.pdf?sfvrsn=3aa07934_2]).
Clinical Frailty Scale
The Clinical Frailty Scale (CFS) is a validated scale summarising the overall level of fitness or frailty of an older adult (≥65 only) based on the assessment of an experienced clinician (Rockwood et al., 2005). The CFS is employed to predict the outcome for older people hospitalised with acute illnesses and inpatient mortality. The CFS (Supplementary Table S1) numerically ranks frailty as not frail (scores 1–3), vulnerable (score 4), mildly frail (score 5), moderately frail (score 6), or severely frail (score 7–9). A visual chart assists with the frailty classification. The patient’s level of disability heavily weights the CFS, and the degree of frailty corresponds to the degree of dementia: mild, moderate and severe dementia generally map to CFS 5, 6 and 7, respectively. This tool's accuracy rests in the clinician’s skills to evaluate the patient’s baseline status before hospitalisation. For this study, patients were grouped as follows: group A (CFS score 1– 3, not frail), group B (score 4–6, vulnerable or mild-moderate frailty) and group C (score 7–9, severe or very severe frailty).
Definitions of clinical conditions at admission
Five independent reviewers reviewed the patients’ clinical records, identified the following clinical conditions at admission and compared them to the previous clinical history of patients, identifying acute extrapulmonary manifestations related to SARS-CoV-2 infection:
i) Mental confusion: patients who presented with acute worsening of clarity and order of thought and behaviour.
ii) Acute kidney failure (Machado et al., 2014) defined as:
-
•
increase in serum creatinine by ≥0.3 mg/dL (or 26.5 μmol/L) within 48 h if compared with previous renal function; or
-
•
increase in serum creatinine to ≥1.5–2.0 times baseline, which is known or presumed to have occurred within the prior 7 days; or
-
•
urine volume of 0.5 ml/kg/h for 6 h in a patient with previously normal urine output.
iii) Electrolyte imbalance at admission: any acute abnormality of electrolyte (Na+, K+, Ca++, Cl−, Mg++) concentration at the first blood test.
iv) Dehydration at admission: significant acute loss of body water associated with skin and mucosal dryness, reduced urinary output and hypernatremia, or altered mental status.
v) Acute heart failure at admission: as defined by current European guidelines (Ponikowski et al., 2016).
vi) Diabetic decompensation: persistent blood sugar levels of >199 mg/dL despite antidiabetic therapy within the first 48 h from admission.
The above conditions were attributed to COVID-19 only if they were absent in the 2 weeks before admission. Previous comorbidities and patients' clinical history were used to decide the inclusion of these variables in the final analysis.
Data analysis
All data were anonymised and collected on an electronic database. Descriptive statistics were produced for demographic, clinical and laboratory characteristics of cases. Mean and standard deviation (SD) were obtained for normally distributed variables, median and interquartile range (IQR) for non-normally distributed variables, and number and percentages for categorical variables. The distribution between groups (according to CFS or according to age) of clinical conditions and laboratory findings was analysed by univariate parametric or nonparametric tests, Kruskal Wallis or Mann Whitney Test (where appropriate) for continuous variables and with Pearson’s χ2 test (Fisher’s exact test where appropriate) for categorical variables, according to data distribution. Survival analysis (Kaplan–Meier curves estimates) was performed to explore the impact of CFS or age on patient overall survival probability. Finally, univariate logistic regression was performed in order to assess the predictors of mortality in the elderly. A stepwise multivariate logistic regression model was applied to control for potential confounders and adjusted for variables that was significantly associated with mortality at univariate analysis (statistically significance defined as p < 0.05). Statistical analysis was performed using STATA “Special Edition” version 16.1 (STATA Corp., Lakeway Drive, Texas 77845, USA).
Ethics
The research did not require formal approval from the ethics committee according to Italian law since it was performed as an observational retrospective study in the context of normal clinical routines (art.1, leg. decree 211/2003). However, the study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. Data were previously anonymised according to the requirements set by the Italian Data Protection Code (leg. Decree 196/2003).
Results
General characteristics of the study population
A total of 206 patients aged ≥65 years, admitted to hospital between March 1 and June 15, 2020, with confirmed SARS-CoV-2 infection were included in the study. The median (IQR) age was 80 (range 72-86) years, and 48% of cases were male. Arterial hypertension was the most common comorbidity (60% of patients), followed by cardiovascular diseases (45%), diabetes (24%) and neurologic diseases (23%). Before hospitalisation, 50% of patients lived in a healthcare facility. One-third of patients suffered from severe respiratory failure: 83 (40%) required at least 10 L/min of oxygen therapy, 58 (28%) non-invasive mechanical ventilation (NIV), and 14 (9%) invasive mechanical ventilation in an Intensive Care Unit (ICU). The crude in-hospital mortality was 27%.
Comparison of clinical features according to CFS and age
Differences in patients’ clinical features according to CFS and age are shown in Table 1 . A total of 60 patients were assigned to group A (CFS 1–3), 60 to group B (CFS 4–6), and 86 to group C (CFS 7–9).
Table 1.
Comparison of clinical features according to Clinical Frailty Scale and age.
| Overall (n. 206) | Clinical Frailty Scale 1–3 (n. 60) | Clinical Frailty Scale 4–6 (n. 60) | Clinical Frailty Scale 7–9 (n. 86) | p Value | Age group 65–74 (n. 62) | Age group 75–84 (n. 72) | Age group ≥ 85 (n. 72) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| General Features | |||||||||
| Median Age (IQR), years | 80 (72 - 86) | 72 (68 - 75) | 83 (75 - 86) | 85 (80 - 91) | <.001 | \ | \ | \ | \ |
| Median (IQR) CSF score | \ | \ | \ | \ | \ | 2 (2–4) | 6 (4–7) | 7 (5–8) | <.001 |
| Male sex - n (%) | 98 (48) | 39 (65) | 31 (52) | 28 (33) | <.001 | 41 (66) | 34 (47) | 23 (32) | <.001 |
| Comorbidity - n (%) | |||||||||
| Hypertension | 122 (60) | 37 (62) | 37 (62) | 48 (56) | .757 | 35 (56) | 45 (62) | 42 (59) | .774 |
| Any Heart Disease | 92 (45) | 20 (33) | 29 (48) | 43 (51) | .098 | 20 (32) | 35 (49) | 37 (52) | .052 |
| Obesity (BMI > 30) (pts. 147) | 25 (17) | 11 (23) | 3 (7) | 11 (19) | .098 | 9 (19) | 9 (19) | 7 (13) | .655 |
| Diabetes (pts. 161) | 50 (24) | 13 (22) | 13 (22) | 24 (28) | .559 | 10 (16) | 24 (33) | 16 (23) | .062 |
| COPD/Asthma | 46 (22) | 11 (18) | 13 (22) | 22 (26) | .554 | 10 (16) | 15 (21) | 21 (30) | .165 |
| Chronic Kidney Disease (KDOQI stage III or more) | 23 (11) | 3 (5) | 5 (8) | 15 (18) | .042 | 3 (5) | 9 (12) | 11 (15) | .138 |
| Any Neurologic Disease | 48 (23) | 3 (5) | 13 (22) | 32 (38) | <.001 | 3 (5) | 23 (32) | 22 (31) | <.001 |
| Concurrent Cancer - n (%) | 27 (13) | 6 (10) | 8 (13) | 13 (15) | .649 | 7 (11) | 8 (11) | 12 (17) | .516 |
| Immunocompromised state - n (%) (pts. 203) | 7 (3) | 2 (3) | 2 (3) | 3 (4) | .994 | 4 (6) | 2 (3) | 1 (1) | .269 |
| Living in a Health Care Facility - n (%) (pts. 197) | 96 (49) | 8 (14) | 27 (47) | 61 (73) | <.001 | 8 (14) | 34 (49) | 54 (77) | <.001 |
| Respiratory Features at Admission | |||||||||
| Signs and Symptoms around the time of Hospitalization - n (%) | |||||||||
| Cough | 82 (40) | 37 (62) | 24 (40) | 21 (24) | <.001 | 37 (60) | 25 (35) | 20 (28) | <.001 |
| Dyspnea | 126 (61) | 29 (48) | 44 (73) | 53 (62) | .019 | 35 (56) | 48 (67) | 43 (60) | .458 |
| SpO2 < 92% in room air (pts. 149) | 45 (30) | 14 (30) | 7 (16) | 24 (41) | .030 | 17 (36) | 12 (26) | 16 (29) | .519 |
| Chest X-ray positive for opacities on admission - n (%) (pts. 179) | (n. 54) | (n. 52) | (n. 73) | (n. 54) | (n. 60) | (n. 65) | |||
| No opacities | 23 (13) | 5 (9) | 3 (6) | 15 (21) | .013 | 7 (13) | 2 (3) | 14 (22) | .010 |
| Monolateral opacities | 40 (22) | 11 (20) | 8 (15) | 21 (29) | 7 (13) | 17 (28) | 16 (25) | ||
| Bilateral opacities | 116 (65) | 38 (70) | 41 (79) | 37 (51) | 40 (74) | 41 (68) | 35 (54) | ||
| Chest CT-Scan positive for infiltrates/consolidations on admission - n (%) (pts. 74) | (n. 23) | (n. 25) | (n. 26) | (n. 23) | (n. 23) | (n. 28) | |||
| No infiltrates/consolidations | 13 (18) | 5 (22) | 2 (8) | 6 (23) | .378 | 5 (22) | 2 (9) | 6 (21) | .112 |
| Monolateral infiltrates/consolidations | 5 (7) | 1 (4) | 1 (4) | 3 (12) | 1 (4) | 4 (17) | 0 | ||
| Bilateral infiltrates/consolidations | 56 (76) | 17 (74) | 22 (88) | 17 (65) | 17 (74) | 17 (74) | 22 (79) | ||
| Extrapulmonary Findings at Admission | |||||||||
| Fever (> 38 °C) | 114 (55) | 39 (65) | 39 (65) | 36 (42) | .004 | 42 (68) | 39 (54) | 33 (46) | .038 |
| Confusion - n (%) - | 80 (39) | 4 (7) | 20 (33) | 56 (65) | <.001 | 8 (13) | 28 (39) | 44 (61) | <.001 |
| Acute Kidney Failure - n (%) - | 58 (28) | 12 (20) | 13 (22) | 33 (39) | .019 | 13 (21) | 27 (37) | 18 (25) | .088 |
| Dehydration - n (%) - | 71 (34) | 8 (13) | 16 (27) | 47 (55) | <.001 | 11 (18) | 31 (43) | 29 (40) | .004 |
| Electrolyte imbalance - n (%) - | 80 (39) | 15 (25) | 19 (32) | 46 (54) | .001 | 18 (29) | 29 (40) | 33 (47) | .116 |
| Heart Failure - n (%) | 12 (6) | 3 (5) | 5 (8) | 4 (5) | .613 | 2 (3) | 3 (4) | 7 (10) | .210 |
| Diabetic Decompensation - n (%) - | 30 (15) | 4 (7) | 7 (12) | 19 (22) | .026 | 7 (11) | 11 (15) | 12 (17) | .664 |
| Need of Parenteral Nutrition - n (%) - (pts. 182) | 28 (15) | 7 (12) | 3 (5) | 18 (24) | .007 | 7 (12) | 7 (10) | 14 (22) | .126 |
| Treatment Administered During Hospitalization | |||||||||
| Antiviral Treatment during hospitalization - n (%) | |||||||||
| Lopinavir/r | 93 (46) | 39 (65) | 27 (45) | 27 (33) | .001 | 46 (75) | 28 (39) | 19 (27) | <.001 |
| Hydroxychloroquine | 147 (72) | 49 (82) | 49 (82) | 49 (58) | .001 | 51 (84) | 53 (74) | 43 (61) | .012 |
| Azithromycin (pts. 163) | 37 (23) | 11 (23) | 13 (31) | 13 (18) | .269 | 12 (24) | 12 (23) | 13 (22) | .958 |
| Use of Steroid Treatment during hospitalization - n (%) | 56 (28) | 16 (27) | 15 (26) | 25 (29) | .890 | 17 (28) | 22 (31) | 17 (24) | .577 |
| Use of Tocilizumab (8 mg/Kg) during hospitalization - n (%) | 16 (8) | 7 (12) | 5 (8) | 4 (5) | .291 | 7 (11) | 5 (7) | 4 (6) | .442 |
| >10 L/min of O2 Therapy during hospitalization - n (%) | 83 (40) | 22 (37) | 29 (48) | 32 (37) | .320 | 25 (40) | 29 (40) | 29 (40) | .999 |
| Non-invasive Ventilation during hospitalization - n (%) | 58 (28) | 16 (27) | 22 (37) | 20 (23) | .198 | 18 (29) | 23 (32) | 17 (24) | .530 |
| Invasive Mechanical Ventilation during hospitalization - n (%) | 14 (9) | 6 (13) | 4 (9) | 4 (7) | .573 | 6 (13) | 5 (10) | 3 (6) | .447 |
| Outcome | |||||||||
| At least one secondary infection during hospitalization- n (%). | 52 (25) | 8 (13) | 13 (22) | 31 (36) | .006 | 12 (19) | 19 (26) | 21 (29) | .411 |
| Median Hospitalization Days (IQR), days (pts. 190) | 22 (12–39) | 22 (15–42) | 25 (14–37) | 21 (7–37) | .240 | 22 (14–35) | 21 (8–41) | 24 (10–39) | .847 |
| Survived - n (%) - | 150 (73) | 57 (95) | 44 (73) | 49 (57) | <.001 | 56 (90) | 47 (65) | 47 (65) | .001 |
Legend: COPD = chronic obstructive pulmonary disease; CT = computed tomography; boldface means statistically significant (P < 0.05).
Notably, compared to groups B and A, the patients in group C were less frequently male (33% (C), 52% (B), 65% (A); P < 0.001), and more frequently affected by neurologic diseases (38% (C), 22% (B), 5% (A); P < 0.001) and living in healthcare facilities (73% (C), 47% (B), 14% (A); P < 0.001). At admission patients in group C presented less frequently than patients in groups B and A with fever (42% (C), 65% (B), 65% (A); P = 0.004) and cough (24% (C), 40% (B), 62% (A); P < 0.001), and chest X-ray less frequently showed signs of pneumonia (P = 0.013). Secondary infections during hospitalisation were significantly associated with a higher frailty score (13% (A), 22% (B), 36% (C); P = 0.006). Survival rate stratified patients according to CFS (95% (A), 73% (B), 57% (C); P < 0.001).
Differences in the clinical features of patients were evaluated after stratification into 3 groups according to age (65–74 years, 75–84 and ≥85); older patients presented a higher median CFS score (2, 6 and 7, respectively, P < 0.001) than younger subjects. The differences between the 3 groups were roughly conserved if stratified according to age or CSF score; however, the frequency of secondary infections during hospitalisation appeared to correlate with CFS (P = 0.006) but not with age (P = 0.411).
Extrapulmonary clinical features at admission
Extrapulmonary findings at admission, and their association with either CFS or age, are presented in Table 1. Older age was only related to a higher frequency of confusion and dehydration, whereas a higher CFS score defined a more complex clinical picture at admission. Compared to patients in groups B and A, at admission patients in group C (CFS score 7-9) were more frequently confused (65% (C), 33% (B), 7% (A); P < .001), dehydrated (55% (C), 27% (B), 13% (A); P < 0.001), and more often had decompensated diabetes (22% (C), 12% (B), 7% (A); P = 0.026), acute kidney failure (39% (C), 22% (B), 20% (A); P = 0.019), and electrolyte imbalance (54% (C), 32% (B), 25% (A); P = 0.001).
Risk of in-hospital mortality
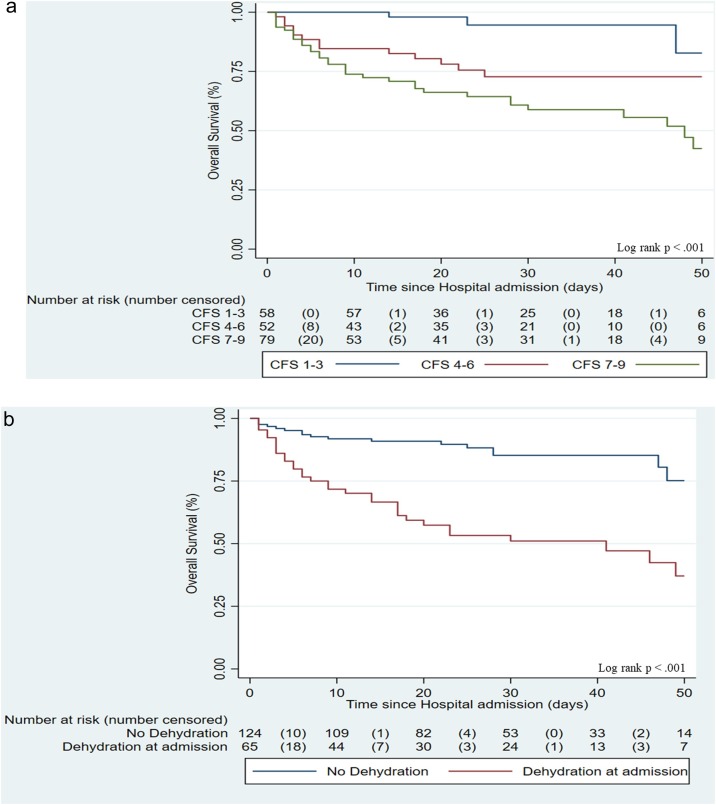
By conducting a univariate and a stepwise multivariate logistic regression model (Table 2 ), adjusted for age, sex, CFS, comorbidity, clinical picture at admission, severity of respiratory failure, secondary infections and administered treatment (steroid or Tocilizumab), only male sex (adjusted Odds Ratio [aOR] = 2.87, 95% CI = 1.15–7.18, P = 0.023), CFS score 7–9 (aOR = 9.97, 95% CI = 1.87–52.99, P = 0.007), dehydration at admission (aOR = 4.27, 95% CI = 1.72–10.57, P = 0.002), and need of non-invasive/invasive mechanical ventilation (aOR = 4.88, 95% CI = 1.94–12.26, P = 0.001) were associated with a higher risk of mortality. Finally, the Kaplan–Meier estimate curves of survival probability were also generated according to the variables of interest. A higher CFS (log-rank P < 0.001; Figure 1 a) and dehydration at admission (log-rank P < 0.001; Figure 1b) were associated with a higher risk of mortality.
Table 2.
Predictors of in-hospital mortality.
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95%C.I. | p Value | aOR | 95%C.I. | p Value | |
| Age group (years) | ||||||
| 65 to 74 | 1 | 1 | ||||
| 75 to 84 | 4.96 | 1.87–13.11 | .001 | 1.27 | 0.36–4.48 | .705 |
| 85 or more | 4.96 | 1.87–13.11 | .001 | 1.54 | 0.40–5.90 | .523 |
| Male sex | 1.03 | 0.56–1.91 | .910 | 2.87 | 1.15–7.18 | .023 |
| Clinical Frailty Scale score | ||||||
| 1-3 | 1 | 1 | ||||
| 4-6 | 6.90 | 1.89–25.20 | .003 | 4.61 | 0.93–22.68 | .060 |
| 7-9 | 14.34 | 4.16–49.42 | <.001 | 9.97 | 1.87–52.99 | .007 |
| Hypertension | 1.14 | 0.65–2.14 | .684 | \ | ||
| Diabetes Type II | 0.82 | 0.39–1.72 | .604 | \ | ||
| Chronic Kidney Disease | 2.87 | 1.18–6.97 | .019 | 1.03 | 0.29–3.68 | .953 |
| Any Neurologic Disease | 2.48 | 1.25–4.94 | .009 | 1.05 | 0.41–2.66 | .914 |
| Any Concurrent Cancer | 0.58 | 0.20–1.62 | .300 | \ | ||
| Fever higher than 38 °C at admission | 1.22 | 0.65–2.27 | .527 | \ | ||
| At least one secondary infection | 1.48 | 0.72–2.83 | .303 | \ | ||
| Acute Kidney Failure at admission | 2.27 | 1.18–4.36 | .014 | 0.78 | 0.29–3.68 | .953 |
| Dehydration at admission | 5.27 | 2.73–10.19 | <.001 | 4.27 | 1.72–10.57 | .002 |
| Electrolyte imbalance at admission | 2.06 | 1.10–3.85 | .023 | 1.12 | 0.47–2.65 | .792 |
| Confusion at admission | 4.33 | 2.26–8.30 | <.001 | 2.22 | 0.91–5.38 | .077 |
| Diabetic Decompensation at admission | 0.96 | 0.40–2.32 | .945 | \ | ||
| Need of Non-Invasive/Invasive Ventilation | 2.55 | 1.33–4.91 | .005 | 4.88 | 1.94–12.26 | .001 |
| Use of Steroid therapy during hospitalization | 2.21 | 1.14–4.29 | .018 | 1.29 | 0.54–3.07 | .552 |
| Use of Tocilizumab during hospitalization | 1.23 | 0.41–3.73 | .704 | \ | ||
| Living in Health Care Facility | 1.99 | 1.05–3.78 | .034 | 0.91 | 0.35–2.36 | .856 |
Legend: OR = odds ratio; aOR = adjusted odds ratio; boldface means statistically significant (P < 0.05).
Figure 1.
Risk of mortality according to a) Clinical Frailty Scale and b) dehydration at admission.
Discussion
The complete spectrum of extrapulmonary manifestation of COVID-19 is still debated. In particular, it is unclear which patients are at higher risk of these complications and their clinical consequences. In elderly patients, the mortality rate is relevant (Bruno et al., 2020, Balena et al., 2020a) because they are more prone to developing severe pulmonary disease (Liu et al., 2020a); however, few data are available about extrapulmonary manifestations in this population and their role in increased mortality (Neumann-Podczaska et al., 2020).
In this study of patients ≥65 years, we described the clinical manifestations of COVID-19 at hospital admission and investigated their association with the risk of in-hospital mortality. An interesting correlation between extrapulmonary manifestations and “frailty”, evaluated in terms of CFS score, was noted. Frailer patients presented more often with neurological or metabolic signs and symptoms, while fever, cough and lung infiltrates/consolidations were less frequent. Conversely, this association was not confirmed when we stratified clinical features at admission by different age groups. Hence, this study’s results, together with previous research (Gómez-Belda et al., 2021, Liu et al., 2020b, Covino et al., 2020, Balena et al., 2020b), indicate that the clinical picture of SARS-CoV-2 infection in the elderly could significantly differ from the “usual” progressive hypoxemic pneumonia described in young or middle-aged patients. Consequently, the occurrence of acute extrapulmonary symptoms, even in the absence of respiratory diseases, including non-specific findings such as general deterioration in older and frail subjects, should be actively investigated as possible COVID-19.
Theoretically, these findings might be explained by the different intensity of immune response to SARS-CoV-2 infection based on frailty level (Cunha et al., 2020); indeed, immunosenescence, a typical phenomenon of older and frail individuals, may be responsible for a milder pulmonary cytokines release, that in turn, causes reduced lung involvement and distress (Bonafè et al., 2020). Conversely, the relatively high incidence of extrapulmonary manifestations in frailer patients could be due to the deterioration caused by SARS-CoV-2 infection or pre-existing comorbidities.
Our data are in line with the current literature. In our cohort, both dehydration and a high CSF score (7–9) on admission were independent predictors of mortality, along with a severe pulmonary disease requiring non-invasive/invasive mechanical ventilation. Importantly, it should be noted that the need for non-invasive/invasive mechanical ventilation reflects a serious lung impairment and dysfunction related to COVID-19. The association with overall mortality, independent of CFS and other complications, is not surprising, particularly in our setting where no “do not treat on ICU” order was established a priori during the study period.
According to a previous study (Hewitt et al., 2020), “frailty” is associated with higher mortality rates and longer hospital stay in COVID-19 patients. Moreover, similar to a previous observation (Poloni et al., 2020), confusion and altered mental status were often observed at admission; this complication might be considered both as a direct central nervous system injury of SARS-CoV-2 or a sign of systemic impairment. Hyperglycaemia (Coppelli et al., 2020) and acute kidney injury (Batlle et al., 2020) were frequent complications of the frailest patients on presentation. These observations suggest a complex clinical picture of SARS-CoV-2 infection in the elderly, requiring a tailored diagnostic and therapeutic approach according to the level of frailty and age. Importantly, the diagnostic workup should include a complete evaluation of neurologic and metabolic conditions and the exclusion of secondary infections (recorded in one-third of our patients), which can complicate the disease's clinical course (Garcia-Vidal et al., 2020).
Physicians should be alerted to not underestimate the severity of COVID-19 in frail elderly, even in the absence of typical respiratory signs and symptoms. Moreover, the new onset of extrapulmonary signs and symptoms in frail patients should be investigated as a possible sign of COVID-19. With new waves of the pandemic, these extrapulmonary manifestations should be carefully considered by treating physicians at the time of hospitalisation to identify early subjects at risk of being infected by SARS-CoV-2. Early recognition of “atypical” COVID-19 could be pivotal in healthcare facilities, where the spread of infections might be rapid and burdened by dramatic epidemiologic consequences.
This study’s strengths are the multicentre cohort including multiple large hospitals in Southern Italy, the detailed information regarding the clinical picture at admission, and the well-balanced number of subjects included in the different CFS and age groups, making comparative analysis possible. This study’s main limitations are its retrospective nature, which potentially implies incomplete or missing data and the relatively limited number of subjects involved.
In conclusion, this study suggests that elderly COVID-19 patients with a high CFS showed frequent extrapulmonary signs at admission, even in the absence of lung involvement. These findings, along with a high CFS, predicted a significant risk of mortality.
Contributions
Study design: BDF, FC, MA, BGB, CS, LCS, ST, ML, AG, SA.
Data collection: DL, SR, BIF, CA, SCR, BG, BG.
Data analysis: BDF.
Writing and reviewing: all authors.
Funding
None.
Conflict of interests
No author has any conflict of interest to declare.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.03.021.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Azizi S.A., Azizi S.A. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J Neurovirol. 2020;26(October (5)):631–641. doi: 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balena F., Bavaro D.F., Fabrizio, Bottalico I.F., Calamo A., Santoro C.R. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. J Gerontol Geriatr. 2020;68(December (04 Special)):197–203. doi: 10.36150/2499-6564-283. [DOI] [Google Scholar]
- Balena F., Bavaro D.F., Fabrizio C., Bottalico I.F., Calamo A., Santoro C.R. Tocilizumab and corticosteroids for COVID-19 treatment in elderly patients. J Gerontol Geriatr. 2020;68:197–203. doi: 10.36150/2499-6564-283. [DOI] [Google Scholar]
- Batlle D., Soler M.J., Sparks M.A., Hiremath S., South A.M., Welling P.A., Swaminathan S., COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaro D.F., Poliseno M., Scardapane A., Belati A., De Gennaro N., Stabile Ianota A.A. Occurrence of acute pulmonary embolism in COVID-19 — a case series. Int J Infect Dis. 2020;98:225–226. doi: 10.1016/j.ijid.2020.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafè M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno G., Perelli S., Fabrizio C. Short-term outcomes in individuals aged 75 or older with severe coronavirus disease (COVID-19): First observations from an infectious diseases unit in Southern Italy. J Infect. 2020;81(August (2)):e86–e88. doi: 10.1016/j.jinf.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai R., Ogedengbe O., Agarwal P., Money-Coomes S., Abdurrahman A.Z., Mohammed S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting — a cohort study. BMC Geriatr. 2020;20(October (1)):409. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppelli A., Giannarelli R., Aragona M., Penno G., Falcone M., Tiseo G. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–2348. doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- Cosco T.D., Best J., Davis D., Bryden D., Arkill S., van Oppen J. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing. 2021;(January) doi: 10.1093/ageing/afab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covino M., De Matteis G., Santoro M., Sabia L., Simeoni B., Candelli M., Ojetti V. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatr Gerontol Int. 2020;20:704–708. doi: 10.1111/ggi.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha L.L., Perrazio S.F., Azzi J. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol. 2020;(August) doi: 10.3389/fimmu.2020.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Assar M., Angulo J., Rodríguez-Mañas L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic Biol Med. 2020;149(March):72–77. doi: 10.1016/j.freeradbiomed.2019.08.011. Epub 15 August 2019. PMID: 31422077. [DOI] [PubMed] [Google Scholar]
- Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2020;(July) doi: 10.1016/j.cmi.2020.07.041. S1198-1743X(20)30450-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Belda A.B., Fernández-Garcés M., Mateo-Sanchis E., Madrazo M., Carmona M., Piles-Roger L. COVID-19 in older adults: what are the differences with younger patients? Geriatr Gerontol Int. 2021;21(January (1)):60–65. doi: 10.1111/ggi.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A. The effect of frailty on survival in patients with COVID-19(COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127(June) doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzedine H., Jhaveri K.D. Acute kidney injury in patients with COVID-19: an update on the pathophysiology. Nephrol Dial Transplant. 2020;(September) doi: 10.1093/ndt/gfaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.J., Jung S.I. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020;52(June (2)):154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M.N., Nakazone M.A., Maia L.N. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc. 2014;29(3):299–307. doi: 10.5935/1678-9741.20140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(June (6)):e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical feature of COVID‐19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(July (7)):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Podczaska A., Al-Saad S.R., Karbowski L.M., Chojnicki M., Tobis S., Wieczorowska-Tobis K. COVID 19 - clinical picture in the elderly population: a qualitative systematic review. Aging Dis. 2020;11(4):988–1008. doi: 10.14336/AD.2020.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera A., Campos C., López N., Hassouneh F., Alonso C., Tarazona R. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82(September (1)):50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Poloni T.E., Carlos A.F., Cairati M., Cutaia C., Medici V., Marelli E. Prevalence and prognostic value of Delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. Clin Med. 2020;26 doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016 Dec 30] Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021;93(March–April) doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(August (5)):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani S., Killander A., Åstrand P., Jakobsson J., Gille-Johnson P. Risk factors for death in adult COVID-19 patients: frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–421. doi: 10.1016/j.ijid.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(March (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.