Abstract
Aims
Although severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is mainly a respiratory system disease, recent studies reported that cardiac injury is associated with poor outcomes in this population. There are few studies which assessed standard electrocardiogram (ECG) as a prognostic tool during the course of SARS-CoV-2 infection. The aim of this study is to identify the relationship between of ECG parameters and prognosis of patients infected with SARS-CoV-2.
Method and results
A total of 114 consecutive patients with a confirmed diagnosis of SARS-CoV-2 infection between March 2020 and May 2020 were included in the study. Standard 12‑lead surface ECG was reviewed for presence of fragmented QRS (fQRS), abnormal Q wave, T wave inversion, and duration of QRS. fQRS was observed in 36.8% (n = 42) of the patients who had SARS-CoV-2. Patient groups with and without fQRS did not differ in terms of age, gender, the presence of comorbid diseases and medical treatment. Hospitalization duration, intensive care unit(ICU) requirement, all-cause mortality, and cardiac mortality were found to be higher in patients with fQRS (all p values <0.05). There was a positive correlation between QRS duration and duration of hospital stay (p < 0.001, r = 0.421). QRS duration was also found to be associated with intensive care need, all-cause mortality, and cardiac mortality.
Conclusion
Our data shows that QRS duration and the presence of fQRS on standard ECG can help to identify patients with worse clinical outcome admitted for SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2 infection, Electrocardiography, Fragmented QRS, Cardiac mortality
Introduction
The novel coronavirus infection, which was first reported in China in December 2019, affected the whole world in a short time and it was declared as pandemic by the World Health Organization. COVID-19, which mainly involves the respiratory tract, has also been reported to have negative effects on the cardiovascular system [1]. Previous reports showed that myocardial damage can occur either directly by the virus itself or as a result of systemic inflammation [2]. It is also known that people who had a history of cardiovascular disease are more likely to be infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and tend to have a more severe course. Many studies have shown that COVID-19 is more fatal in those who have developed cardiac damage during the course of infection or previously had a history of cardiac disease [3]. Therefore, prediction of the negative effects of SARS-CoV-2 infection on cardiovascular system and early detection of myocardial damage is crucial.
The presence of the fragmented QRS complex (fQRS) on the standard electrocardiogram (ECG) is an indicator of ventricular conduction disorder which was shown to be a marker of myocardial damage resulting from various diseases [4,5]. In several studies, the relationship between myocardial scar due to coronary artery disease (CAD) and the presence of fQRS has been shown [6]. The localization of the fQRS on the ECG was also found to have a good correlation with the localization of the scar in the left ventricle. [7]. Compared to the other ECG markers of ischemia, fQRS is more sensitive in detecting myocardial fibrosis detected by SPECT imaging in patients with CAD [8].
ECG is a cheap and easy-to-apply test which carries lower risk of infection spread among health care workers during the pandemic, especially compared to other diagnostic tools such as echocardiography. The detection of fQRS on the ECG can help early, practical and safe detection of the myocardial damage during the course of COVID-19. The main purpose of this study is to investigate the relationship between the ECG features and cardiac outcomes in patients hospitalized with SARS-CoV-2 infection.
Methods
Patient population
This retrospective study included consecutive patients hospitalized with SARS-CoV-2 infection in a university hospital between March 2020 and May 2020. All cases of COVID-19 were confirmed by polymerase chain reaction (PCR) on nasopharyngeal swabs (Bioeksen R&D Technologies Co Ltd).
Inclusion and exclusion criteria
Patients who were followed at least 24 h in the hospital with an ECG obtained at the initial presentation or during the follow up were included in the study. Hospitalized patients, either in the regular inpatient ward or in the intensive care unit were analyzed. Patients who had complete ventricular pacing were excluded from the study.
Data collection
Baseline demographical characteristics, hospitalization duration, need for intensive care unit (ICU) treatment and mortality data were obtained from medical records. Demographics, pre-existing comorbid conditions and serum cardiac troponin I (cTnI) levels (AQT90 FLEX TnI test) were recorded manually.
A standard 12 lead surface ECG (0.5–150 Hz, 25 mm/s, 10 mm/mV) which was recorded at patient's initial presentation or during follow up at the hospital was personally reviewed and interpreted by two cardiology specialists who were blinded to the clinical status of the patients. Rhythm, QRS duration, presence of fQRS, negative T waves and abnormal Q waves were assessed.
Definitions
The fQRS was defined as notching in the R or S wave, RSR' pattern or multiple R' in at least two consecutive leads corresponding to the areas supplied by the major coronary arteries [6]. Patients were divided into two groups according to the presence or absence of fragmentation on the ECG as fQRS (+) and fQRS (−). The criteria of T-wave inversion was ≥0.1 mV in at least two contiguous leads [9] and abnormal Q wave was defined as the presence of Q wave at least ≥30 msec in duration and ≥ 0.1 mV in depth in at least two leads except aVR [10]. QRS duration was measured from the beginning of the earliest to the end of the last QRS deflection.
Arrhythmia was defined as documented atrial fibrillation, ventricular tachycardia or ventricular fibrillation, second or third degree atrio-ventricular block. Pulmonary disease refers to chronic obstructive pulmonary disease or asthma, whereas renal disease refers to chronic renal insufficiency (GFR < 30).
Ethics
This study was approved by national ministry of health (reference number: 202005-04T23_28_26) and the protocol was approved by the local ethics committee of the university. Informed consent was obtained from all patients to participate in the study in accordance with the Declaration of Helsinki.
Clinical outcomes
The primary end-point of the study was cardiac mortality which was defined as death attributable to myocardial ischemia or infarction, heart failure, cardiac arrest because of other or unknown causes. Secondary end-points were defined as hospitalization duration, ICU requirement and all-cause mortality. The survival status of patients was ascertained by reviewing medical records.
Statistical analyses
Statistical analyses were conducted using SPSS (version 25.0, SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation for continuous variables and percentage for categorical variables. Statistical significance was defined as a p value <0.05 for all comparisons. Shapiro-Wilk test was used to test for normal distribution. Continuous variables were compared using Student's t-test for independent samples that showed normal distribution, while the Mann-Whitney U test was used for non-normally distributed samples based on the presence of fQRS on the surface ECG. Chi-square test was used to analyze the associations of the categorical variables between groups. The association of QRS duration with both cardiac and all-cause mortality was demonstrated with separate box-plot graphics. Pearson's correlation analysis was used to test the relationship between the QRS duration and the total days of hospitalization. The results of the correlations were shown on a scatted-dot graph with the corresponding r and p values. ROC analysis was performed to obtain a cut-off QRS duration to predict mortality, and the results were shown on a ROC curve with the accompanying AUC, CI and p values.
Results
A total of 114 patients were included in the study. The mean age was 57.1 ± 15.1 years and 54% were men. Patients were divided into 2 groups according to the presence of fQRS (fQRS+, n = 42, 36.8%) or absence of fQRS (fQRS-, n = 72, 63.2%) on the surface ECG. Basal characteristics and the clinical features of the study population are summarized in Table 1 . There was no significant difference between the two groups in terms of age, gender, comorbid diseases and medical treatment (p > 0.05 for all comparisons). Hypertension (HT) and diabetes (DM) were the most common cardiovascular conditions among both groups (38% and 26%, respectively).
Table 1.
Baseline characteristics of the study population according to the presence of fragmentation on the surface electrocardiograms in patients with fQRS(+) and fQRS(−).
| Study population (n = 114) |
fQRS(+) (n = 42) |
fQRS(−) (n = 72) |
p value | |
|---|---|---|---|---|
| Age (years) | 57.1 ± 15.1 | 58.2 ± 13.5 | 54.5 ± 14.8 | 0.52 |
| Gender (male), n (%) | 62 (54) | 23 (55) | 39 (54) | 0.91 |
| HTN, n (%) | 43 (38) | 20 (48) | 23 (32) | 0.09 |
| CAD, n (%) | 15 (13) | 8 (19) | 7 (10) | 0.16 |
| DM, n (%) | 30 (26) | 14 (33) | 16 (22) | 0.19 |
| Arrhythmia, n (%)* | 12 (11) | 5 (12) | 7 (10) | 0.48 |
| Pulmonary disease, n (%)+ | 7 (6) | 3 (7) | 4 (5) | 0.14 |
| Malignancy, n (%) | 3 (3) | 1 (2) | 2 (3) | 0.98 |
| Renal disease, n (%)‡ | 12 (11) | 4 (10) | 8 (11) | 0.42 |
| Medications, n (%) | ||||
| Plaquenil | 114 (100) | 42 (100) | 72 (100) | 1.0 |
| Azytromycin | 49 (43) | 16 (38) | 33 (46) | 0.42 |
| Oseltamavir | 46 (40) | 17 (41) | 29 (40) | 0.98 |
| Favipiravir | 46 (40) | 19 (45) | 27 (38) | 0.45 |
fQRS: fragmented QRS; HTN: hypertension; CAD: coronary artery disease; DM: diabetes mellitus. * Atrial fibrillation, ventricular tachycardia or ventricular fibrilllation, second or third degree atrio-ventricular block. + Chronic obstructive pulmonary disease or asthma. ‡ Chronic renal insufficiency (GFR < 30).
Study patients were subdivided into groups based on the ECG features such as fQRS, negative T waves (negT) and pathological Q waves (patQ) and compared in terms of duration of hospital stay, intensive care requirement, all-cause mortality, and cardiac mortality as shown in Table 2 . Presence of negT and patQ did not seem to have any impact on clinical outcome variables (all p values >0.05). However, patients with fQRS were found to have prolonged duration of hospital stay (14.0 ± 8.3 days vs. 7.5 ± 4.0 days), higher need for intensive care unit (ICU) treatment (35.7% vs. 5.5%), higher rates of all-cause mortality (33.3% vs. 8.3%, p < 0.05%), and higher rates of cardiac mortality (14.2% vs. 4.1%) compared to patients without fQRS on the ECG (all p values <0.05).
Table 2.
Comparison of the study population in terms of prognostic end-points according to the presence of ECG findings such as fragmented QRS (fQRS), T wave inversion (negT), and pathologic Q waves (patQ).
| fQRS(+) (n:42) |
fQRS(−) (n:72) |
p value | negT (+) (n:31) |
negT (−) (n:83) |
p value | patQ (+) (n:13) |
patQ (−) (n:101) |
p value | |
|---|---|---|---|---|---|---|---|---|---|
| Duration of hospital stay, (days) | 14.0 ± 8.3 | 7.5 ± 4.0 | <0.01 | 9.1 ± 8.8 | 10.3 ± 5.0 | 0,13 | 11.4 ± 6.4 | 9.7 ± 4.3 | 0.08 |
| Need for ICU treatment, n (%) | 15 (35.7) | 4 (5.5) | <0.01 | 5 (16.1) | 14 (16.8) | 0,71 | 2 (15.3) | 17 (16.8) | 0.16 |
| All-cause mortality, n (%) | 14 (33.3) | 6 (8.3) | <0.01 | 6 (19.3) | 14 (16.8) | 0.19 | 2 (15.3) | 18 (17.8) | 0.14 |
| Cardiac mortality, n (%) | 6 (14.2) | 3 (4.1) | <0.01 | 3 (9.6) | 6 (7.2) | 0.12 | 1 (7.6) | 8 (7.9) | 0.90 |
ICU: intensive care unit; fQRS: fragmented QRS; negT: T wave inversion; patQ: pathologic Q waves.
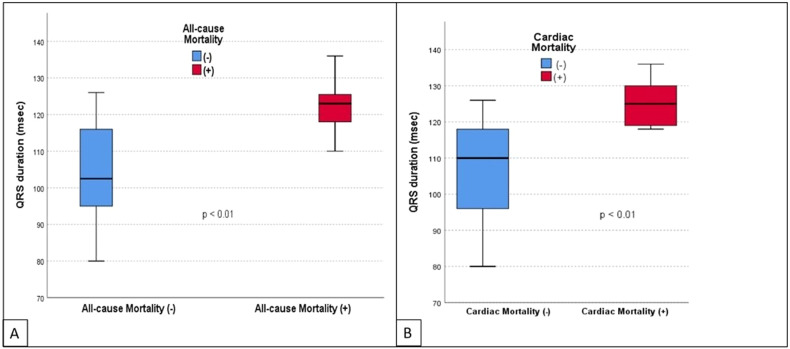
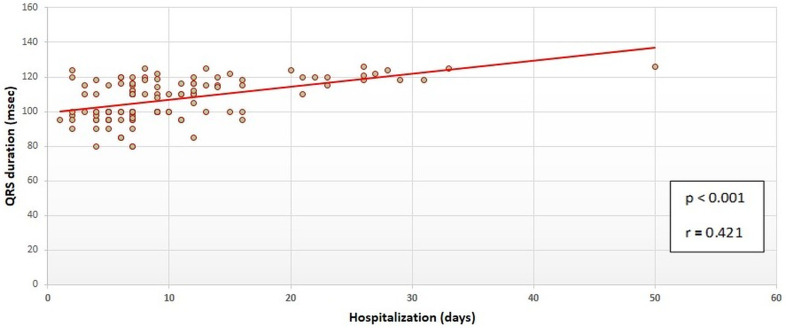
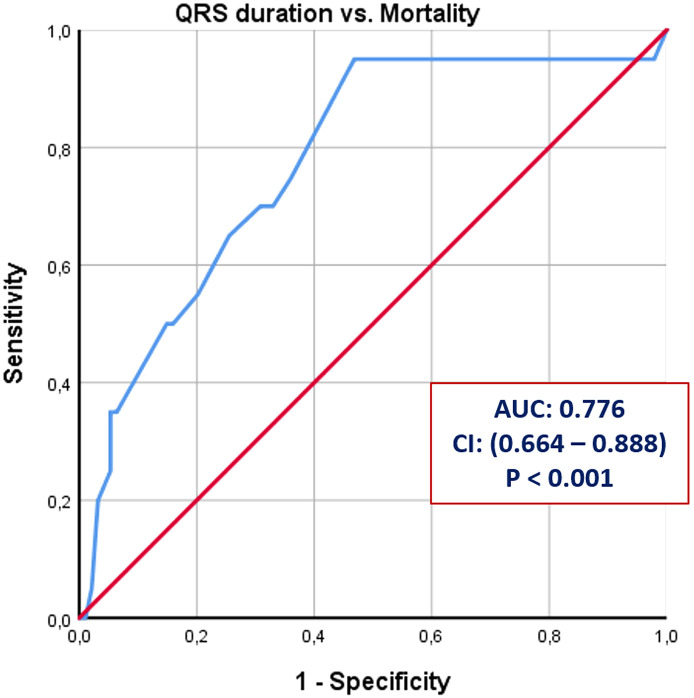
During the study, a total of 20 deaths was observed among the total number of 114 patients (17.5%), and 12 of the deaths were cardiac in origin (11%). The impact of age, comorbidities (such as hypertension, diabetes, and coronary artery disease), QRS duration and cTnI levels on all-cause and cardiac mortality was also investigated (Table 3 ). Mean age of the patients with all-cause mortality was significantly higher than the survivors of the COVID-19 (65.9 ± 10.4 vs. 55.6 ± 17.6, p = 0.01). However, we did not observe the same relationship for cardiac mortality. Similarly, none of the comorbidities was found to be associated with all-cause or cardiac mortality. Mean QRS duration was observed to be related to both all-cause and cardiac mortality. Compared to patients with the survivors, the mean QRS duration was higher both in patients with all-cause mortality (122.4 ± 6.1 msec vs. 104.6 ± 12.9 msec, p < 0.01) and those with cardiac mortality (125.2 ± 5.5 msec vs. 110.3 ± 7.1 msec, p < 0.01) as shown in Fig. 1 . As a continuous variable, QRS duration was also positively and significantly correlated with the duration of hospital stay (p < 0.001, r = 0.421) as shown in a scatted-dot graph in Fig. 2 . ROC analysis revealed that a cut-off QRS duration of 115 milliseconds had 80% sensitivity and 70% specificity for prediction of all-cause mortality (AUC: 0.776, CI: 0.664–0.888, p < 0.001) as shown in Fig. 3 .
Table 3.
Analysis of the study population in terms of all-cause and cardiac mortality according to the clinical features, QRS duration and Troponin levels.
| All-cause Mortality (+) (n = 20) |
All-cause Mortality (−) (n = 94) |
p value | Cardiac Mortality (+) (n = 12) |
Cardiac Mortality (−) (n = 102) |
p value | |
|---|---|---|---|---|---|---|
| Age (years) | 65.9 ± 10.4 | 55.6 ± 17.6 | 0.01 | 61.1 ± 9.3 | 56.9 ± 17.8 | 0.43 |
| HTN, n (%) | 9 (45) | 34 (36) | 0.46 | 4 (33) | 39 (38) | 0.74 |
| DM, n (%) | 6 (30) | 24 (26) | 0.68 | 3 (25) | 27 (27) | 0.91 |
| CAD, n (%) | 3 (15) | 12 (13) | 0.79 | 2 (17) | 13 (13) | 0.71 |
| QRS duration (msec) | 122.4 ± 6.1 | 104.6 ± 12.9 | <0.01 | 125.2 ± 5.5 | 110.3 ± 7.1 | <0.01 |
| TnI level (ug/mL) | 1.03 ± 2.01 | 0.04 ± 0.10 | <0.01 | 0.75 ± 0.83 | 0.22 ± 0.96 | 0.07 |
HTN: hypertension; DM: diabetes mellitus; CAD: coronary artery disease; TnI: troponin I.
Fig. 1.
The box-plot graphs that show the association of QRS duration with both all-cause mortality (A) and cardiac mortality (B).
Fig. 2.
The scatted-dot graph showing the correlation of QRS duration and hospital stay.
Fig. 3.
ROC curve analysis for QRS duration to predict all-cause mortality with 80% sensitivity and 70% specificity.
Cardiac troponin I levels were also investigated for the association with all-cause and cardiac mortality. Compared to the survivors of the COVID-19, mean cTnI levels were statistically higher in patients with all-cause mortality (1.03 ± 2.01 μg/L vs. 0.04 ± 0.10 μg/L, p < 0.01), and numerically higher in patients with cardiac mortality (0.75 ± 0.83 μg/L vs. 0.22 ± 0.96 μg/L, p = 0.07).
Discussion
In this study, the relationship between the presence of fQRS on ECG and the prognosis of patients diagnosed with COVID-19 was investigated. The main findings of the study can be listed as follows; hospitalization duration of patients with fQRS on surface ECG was longer. Moreover, intensive care need, all-cause mortality and cardiac mortality were also more frequent in patients with fQRS.
As it is well known, COVID-19 is not just a respiratory system disease. It is also associated with systematic inflammation, cytokine storm and sepsis. As a result, it leads to multi-organ failure and death [11]. Although, exact mechanism for the effects of SARS-CoV-2 infection on the cardiovascular system is not fully known, various mechanisms have been suggested. It has been reported that the virus may have a cardiovascular effect due to indirect systemic inflammation, as well as it may directly damage the myocardial tissue [12]. Several studies have shown that prognosis is worse in patients with cardiovascular system involvement due to COVID-19 [13]. Therefore, early detection of cardiac involvement is crucial for improvement of prognosis during the course of the disease.
Several studies have investigated several imaging and biochemical tests which may be indicative of prognosis in COVID-19. One of these diagnostic tests is the surface ECG. In a previous study, McCullough and his colleagues examined ECG's of 750 COVID-19 patients and they revealed that atrial premature contractions (APCs), right bundle branch block/intraventricular conduction block (RBBB/IVB), localized T-wave inversion and nonspecific repolarization abnormalities were associated with an increased odds of death [14]. However, the relationship between ECG parameters and cardiac mortality of COVID-19 patients has not been investigated.
fQRS is a depolarization disorder that can be easily detected from a routine ECG recording. It represents the conduction delay caused by fibrotic tissue in the myocardium [6]. This conduction delay results in notching of the QRS complex in ECG [15]. It has been shown that fQRS detected on surface ECGs is associated with myocardial scar in coronary artery disease patients [8]. A recent study showed that fQRS was useful in predicting scar areas detected by magnetic resonance imaging (MRI) [16]. It has been proven that fQRS can be the predictor of non-ischemic myocardial scar, too. Schuller et al. showed that in patients with pulmonary sarcoidosis, fQRS complex was associated with cardiac involvement [17]. These results are supported by another small study on cardiac sarcoidosis which showed a close relation of late gadolinium enhancement on cardiac MRI with fQRS [18]. Similarly, in another study, healthy subjects with fQRS presented regional left ventricular (LV) systolic dysfunction, assessed by global longitudinal strain (GLS) on echocardiography, in the presence of a normal ejection fraction. These data suggest that fQRS may be a promising tool to identify apparently healthy subjects with regional LV systolic dysfunction [19].
In our study, the relationship between the presence of fQRS and the prognosis of patients who were admitted for COVID-19 was investigated. Prognostic outcome variables were designated as hospitalization duration, need for ICU treatment, all-cause and cardiac mortality. Patients with fQRS on surface ECGs were observed to have worse prognosis as well as higher mortality rates. Our findings suggest that worse clinical course may be linked to subclinical cardiac involvement in these patients. Few studies indicate that cardiac involvement, especially myocarditis, is a potential result of SARS-CoV-2 infection. In addition, several pathways associated with the systemic involvement of the viral disease may contribute to destabilize coronary plaques in COVID-19 patients [20]. Myocyte necrosis and mononuclear cell infiltrates that are reported in cardiac muscle autopsy specimens of COVID-19 patients [21] can be linked to both these ischemic and non-ischemic effects of SARS-CoV-2 infection. Cardiomyocyte damage by the virus may be reflected as fQRS which is a proven predictor of myocardial scar.
Apart from fQRS, there are other ECG features that may be markers of myocardial scar. These are pathological Q wave presence, negative T wave presence and prolonged QRS duration [22,23]. However, in the current study we could not establish a relationship between the presence of pathological Q waves or negative T waves and clinical outcome of COVID-19 patients. On the other hand, our study showed a positive correlation between QRS duration and hospitalization duration, intensive care need, all-cause mortality and cardiac mortality. These findings are in line with the results of previous studies that showed the relationship between QRS duration and both myocardial dysfunction and sudden cardiac death [22,24].
In various studies, several echocardiographic and radiological methods to detect myocardial fibrosis were addressed similar to the data obtained from ECG [25]. However, these modalities are often more expensive and sophisticated. fQRS can be easily detected by routine ECG recordings and does not require any special equipment. Therefore, it seems judicious to choose simple diagnostic methods which will minimize contact time when there is an infection with high contagiousness such as COVID-19.
Several studies have shown the relationship between high level of serum cardiac troponin I and poor prognosis in COVID-19 patients. In a recently published study, Lippi et all. Reported that the levels of cardiac troponin were significantly increased in severely affected COVID-19 cases [26]. In our study, serum troponin I level was shown to be significantly related to hospitalization duration, intensive care need and all-cause mortality but it was not found to be associated with cardiac mortality. The possible explanation for the fact that increased troponin I levels did not reflect cardiac mortality is that high levels of troponin I may be associated with the severity of the disease, but not directly related to the cardiac status of COVID-19 patients.
Conclusion
fQRS on surface ECG can be used as a marker of cardiac prognosis in hospitalized patients with SARS-CoV-2 infection. ECG being a simple, cheap and safe investigation modality may help in identifying patients at increased risk of cardiovascular involvement. Therefore, it can be used as an initial screening tool in COVID-19 patients to recognize severely affected individuals with possible cardiovascular mortality.
Limitations
The major limitation of our study was the small sample size requiring that the results should be validated in a larger population. Another drawback was that echocardiographic data was not recorded to be compared with the ECG findings due to the design of the study. In addition, it is not clear whether medications applied prior to the obtainment of the ECG had an effect on ECG findings, since the ECG was recorded at the initial presentation in some patients and during the course of the treatment in the remaining. But we did not observe any statistical difference between the study groups according to the medications used for treatment of COVID-19.
Funding
None.
Declaration of competing interest
The author(s) declare(s) that there is no conflict of interest.
Acknowledgements
None.
Footnotes
The study was conducted in Cardiology Department of Medipol University Hospital.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45(3):230–232. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel CPERE The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41(2):145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Michael M.A., El Masry H., Khan B.R., Das M.K. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis. 2007;50(3):198–208. doi: 10.1016/j.pcad.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Pietrasik G., Goldenberg I., Zdzienicka J., Moss A.J., Zareba W. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q-wave myocardial infarction. Am J Cardiol. 2007;100(4):583–586. doi: 10.1016/j.amjcard.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 6.Das M.K., Saha C., El Masry H., Peng J., Dandamudi G., Mahenthiran J., et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4(11):1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Ratheendran A.C., Subramanian M., Bhanu D.K., Prabhu M.A., Kannan R., Natarajan K., et al. Fragmented QRS on electrocardiography as a predictor of myocardial scar in patients with hypertrophic cardiomyopathy. Acta Cardiol. 2019 doi: 10.1080/00015385.2018.1547355. [DOI] [PubMed] [Google Scholar]
- 8.Das M.K., Khan B., Jacob S., Kumar A., Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113(21):2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 9.De Lazzari M., Zorzi A., Cipriani A., Susana A., Mastella G., Rizzo A., et al. Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J Am Heart Assoc. 2018;7(22) doi: 10.1161/JAHA.118.009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., White H.D., TASK FORCE MEMBERS: Chairpersons: Kristian Thygesen JSA, Harvey D. White *, Biomarker Group, Allan S., Jaffe C., et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 11.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93(6) doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough S.A., Goyal P., Krishnan U., Choi J.J., Safford M.M., Okin P.M. Electrocardiographic findings in COVID-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020 doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner P., Ursell P., Fenoglio J., Jr., Wit A. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72(3):596–611. doi: 10.1161/01.cir.72.3.596. [DOI] [PubMed] [Google Scholar]
- 16.Homsi M., Alsayed L., Safadi A., Das M.K., Mahenthiran J. Fragmented QRS complexes on a 12-lead ECG as a marker of non-coronary artery disease related myocardial disease by gadolinium delayed enhancement cardiac magnetic resonance imaging. J Cardiovasc Magn Reson. 2009;11(S1) [Google Scholar]
- 17.Schuller J.L., Olson M.D., Zipse M.M., Schneider P.M., Aleong R.G., Wienberger H.D., et al. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22(11):1243–1248. doi: 10.1111/j.1540-8167.2011.02099.x. [DOI] [PubMed] [Google Scholar]
- 18.Homsi M., Alsayed L., Safadi B., Mahenthiran J., Das M.K. Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 2009;14(4):319–326. doi: 10.1111/j.1542-474X.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikoo M.H., Jamali Z., Razeghian-Jahromi I., Sayadi M., Verdecchia P., Abtahi F. Fragmented QRS as an early predictor of left ventricular systolic dysfunction in healthy individuals: a nested case-control study in the era of speckle tracking echocardiography. Cardiovasc Ultrasound. 2020;18(1):1–6. doi: 10.1186/s12947-020-00216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380(2):171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 21.Commission CNH . 2020. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment. [Google Scholar]
- 22.Laukkanen J.A., Di Angelantonio E., Khan H., Kurl S., Ronkainen K., Rautaharju P. T-wave inversion, QRS duration, and QRS/T angle as electrocardiographic predictors of the risk for sudden cardiac death. Am J Cardiol. 2014;113(7):1178–1183. doi: 10.1016/j.amjcard.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaum Y., Sclarovsky S., Zlotikamien B., Herz I., Chetrit A., Olmer L., et al. Abnormal Q waves on the admission electrocardiogram of patients with first acute myocardial infarction: prognostic implications. Clin Cardiol. 1997;20(5):477–481. doi: 10.1002/clc.4960200515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panicker G.K., Narula D.D., Albert C.M., Lee D.C., Kothari S., Goldberger J.J., et al. Validation of electrocardiographic criteria for identifying left ventricular dysfunction in patients with previous myocardial infarction. Ann Noninvasive Electrocardiol. 2020 doi: 10.1111/anec.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellis C., Martin J., Narula J., Marwick T.H. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol. 2010;56(2):89–97. doi: 10.1016/j.jacc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 26.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]