Abstract
This study aimed to investigate type of loco-regional treatment received, associated treatment factors and mortality outcomes in New Zealand women with early-stage breast cancer who were eligible for breast conserving surgery (BCS). This is a retrospective analysis of prospectively collected data from the Auckland and Waikato Breast Cancer Registers and involves 6972 women who were diagnosed with early-stage primary breast cancer (I-IIIa) between 1 January 2000 and 31 July 2015, were eligible for BCS and had received one of four loco-regional treatments: breast conserving surgery (BCS), BCS followed by radiotherapy (BCS + RT), mastectomy (MTX) or MTX followed by radiotherapy (MTX + RT), as their primary cancer treatment. About 66.1% of women received BCS + RT, 8.4% received BCS only, 21.6% received MTX alone and 3.9% received MTX + RT. Logistic regression analysis was used to identify demographic and clinical factors associated with the receipt of the BCS + RT (standard treatment). Differences in the uptake of BCS + RT were present across patient demographic and clinical factors. BCS + RT was less likely amongst patients who were older (75+ years old), were of Asian ethnicity, resided in impoverished areas or areas within the Auckland region and were treated in a public healthcare facility. Additionally, BCS + RT was less likely among patients diagnosed symptomatically, diagnosed during 2000–2004, had an unknown tumour grade, negative/unknown oestrogen and progesterone receptor status or tumour sizes ≥ 20 mm, ≤50 mm and had nodal involvement. Competing risk regression analysis was undertaken to estimate the breast cancer-specific mortality associated with each of the four loco-regional treatments received. Over a median follow-up of 8.8 years, women who received MTX alone had a higher risk of breast cancer-specific mortality (adjusted hazard ratio: 1.38, 95% confidence interval (CI): 1.05–1.82) compared to women who received BCS + RT. MTX + RT and BCS alone did not have any statistically different risk of mortality when compared to BCS + RT. Further inquiry is needed as to any advantages BCS + RT may have over MTX alternatives.
Keywords: breast conserving therapy, mastectomy, survival, associated factors
1. Introduction
Breast cancer poses a serious public health issue globally. Worldwide, an estimated 2,088,849 women were diagnosed (46.3/100,000—age standardised rate (*)) and 626,697 deaths (13.0/100,000*) were estimated in 2018 [1]. Comparatively, New Zealand has one of the highest incidences of breast cancer (3504 women diagnosed in 2018, (92.6/100,000*)) yet one of the lowest worldwide mortality (632 deaths in 2018, (10.9/100,000*)) [1].
Surgery is the mainstay treatment for women with early-stage breast cancer. In New Zealand [2], and similarly in other countries [3,4,5], women with stage I–IIIa breast cancer are offered the choice of breast conserving surgery plus radiotherapy (BCS + RT) or mastectomy (MTX). These recommendations were partly based on earlier randomised controlled trials (RCTs) illustrating equivalent survival outcomes between BCS + RT and MTX [6,7]. However, more recent observational studies suggest BCS + RT may offer greater survival advantages compared to MTX [8,9].
A woman’s choice to receive a particular treatment may be influenced by access barriers and inconvenience, such as travel distance to treatment centres, as well as the quantity, duration and costs involved in receiving a particular treatment. Previous research has found factors such as education level, area deprivation, tumour stage, age and ethnicity to be associated with differences in the uptake of loco-regional treatment of breast cancer patients. More specifically, residing in impoverished areas, having a later tumour stage, being older (greater than 49 years) and of Asian/Pacific ethnicity have been associated with a decreased likelihood in BCS + RT uptake [10,11].
This study aims to investigate type of loco-regional treatment received by New Zealand women with early-stage breast cancer, factors associated with the receipt of BCS + RT (standard treatment) and compares breast cancer-specific mortality across BCS, BCS + RT, MTX and MTX + RT surgical treatments.
2. Materials and Methods
2.1. Study Population
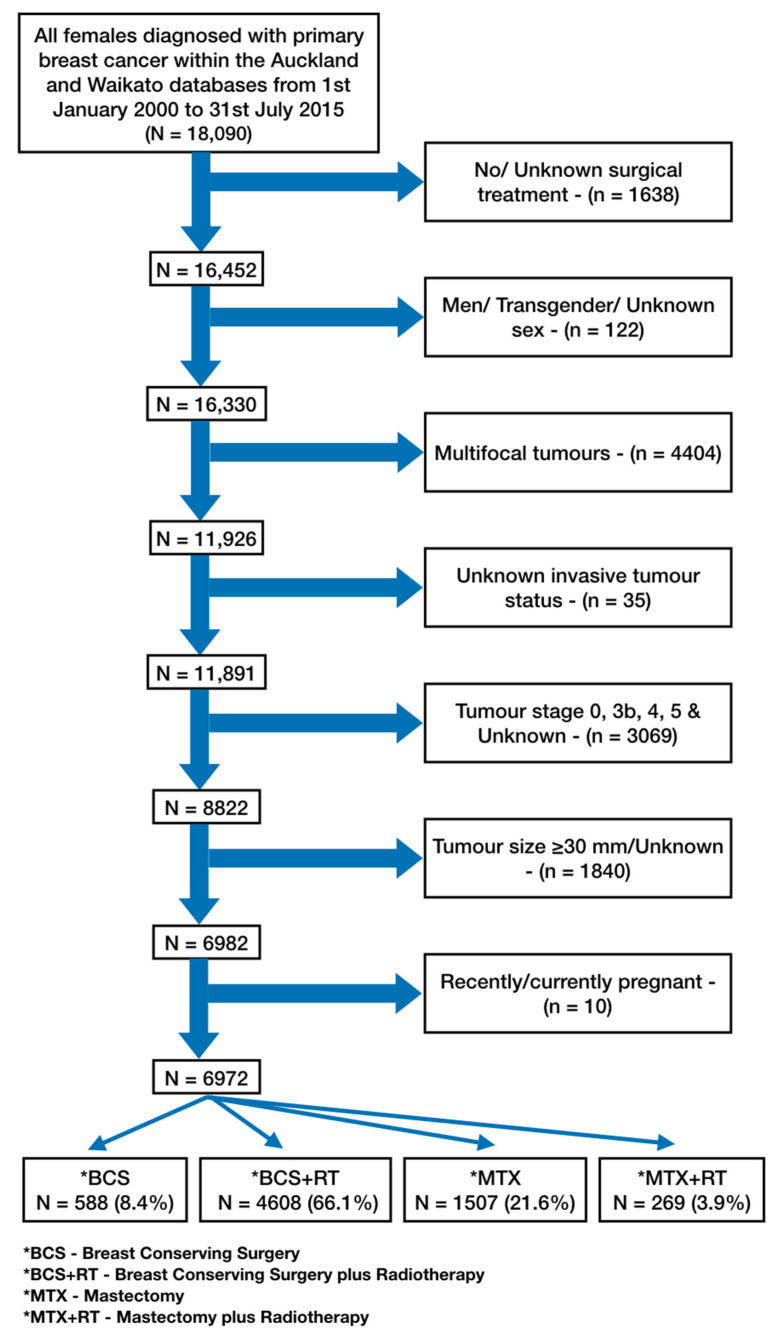
Our sample consisted of 6972 women diagnosed with early-stage (stage I–IIIA) primary breast cancer between 1 January 2000 and 31 July 2015 who were eligible for BCS and underwent one of the four loco-regional treatments: BCS, BCS + RT, MTX or MTX + RT (Figure 1). Women were identified from the Auckland and Waikato breasts cancer registers which encompass four health regions (Waitemata, Auckland, Counties Manukau and Waikato district health boards) with over 2 million residents.
Figure 1.
Sample restriction flowchart.
2.2. Data Sources
The Auckland and Waikato breast cancer registers are prospectively maintained, opt-out, population-based registers with detailed demographic and clinical data [12]. Information on women identified with breast cancer are extracted in a structured format by trained data entry personnel from all clinical and pathological reports [13]. Record data is extracted from clinical reports, operations records, multi-disciplinary meetings records, oncology reports, palliative care records as well as private and public hospital records [13]. Extracted data is entered into both Auckland and Waikato registers depending on the patient’s area of residence and is cross referenced with the New Zealand Cancer Register (NZCR) and Mortality Collection annually. Under the Cancer Registry Act 1993, all malignant tumours first diagnosed in New Zealand are legally required to be registered in the NZCR, with the exception of basal cell and squamous cell skin tumours [14]. Additionally, all deaths are legally required to be registered in the Mortality Collection [15]. Both the NZCR and Mortality Collection are run and organised by the Ministry of Health [13]. Since 2000, the Auckland and Waikato registers have enrolled virtually all newly diagnosed breast cancer cases within their respective health regions. The Waikato breast cancer register was found to be 99 percent complete when cross referenced with the National Cancer Registry, and the Auckland breast cancer register only had a one percent loss to follow-up [13,16,17].
Ethical approval for the use of anonymised patient data in this study was obtained from the University of Auckland Human Participants Ethics Committee (Ref. No. 21851).
2.3. Variables of Interest
The main exposure of interest is the type of loco-regional treatment received after diagnosis of breast cancer, i.e., BCS, BCS + RT, MTX or MTX + RT.
Potential confounders were identified from previous studies as well as the possible confounding effect on the exposure (treatment)–outcome (breast cancer-specific mortality) association [18,19]. Patient demographic factors included age, ethnicity, New Zealand deprivation Index 2013, urban/rural residence, region of residence and whether treatment was undertaken in a public or private facility. Clinical variables included were year of diagnosis, mode of detection (screen-detected or symptomatic), tumour stage, grade, hormone receptor status, histological type, tumour size, lymph node involvement and lympho-vascular invasion (LVI).
The New Zealand deprivation index 2013 is a unique measure of deprivation used in New Zealand to assess the level of deprivation present in a particular area. Here, the New Zealand deprivation score represents the likely deprivation of a given patient based on the area they reside in. The index provides a score out of ten, with one representing the least deprived area and ten representing the most deprived area. The New Zealand deprivation index 2013 is based on nine variables collected at the time of New Zealand’s 2013 census [20].
2.4. Statistical Analysis
Logistic regression models were used to assess factors associated with the receipt of BCS + RT (Table 1). Cox models were used in the competing risk analyses to estimate the probability of breast cancer-specific mortality, with breast cancer-specific death being the main failure event of interest and death from other causes being the competing event. Survival time was calculated from date of cancer diagnosis until failure (breast cancer-specific death), a competing event or censorship (Table 2 and Table 3) [21,22]. Patients were censored on the 31 July 2018 if they did not experience failure or a competing event.
Table 1.
Odds ratios (OR) from logistic regression across BCS + RT 1 vs. BCS 2/MTX 3/MTX + RT 4.
| Covariates | Crude OR | Adjusted OR |
|---|---|---|
| DEMOGRAPHIC VARIABLES | ||
| Age | ||
| <45 | 1.00 | 1.00 |
| 45–59 | 1.59 (1.35–1.87) | 1.14 (0.96–1.36) |
| 60–74 | 1.52 (1.28–1.79) | 1.01 (0.84–1.22) |
| 75+ | 0.40 (0.33–0.50) | 0.34 (0.27–0.43) |
| Ethnicity | ||
| European | 1.00 | 1.00 |
| Maori | 0.91 (0.77–1.08) | 0.91 (0.75–1.10) |
| Pacific | 0.79 (0.60–1.03) | 0.94 (0.70–1.27) |
| Asian | 0.58 (0.49–0.69) | 0.58 (0.48–0.69) |
| Other | 1.12 (0.76–0.65) | 1.02 (0.68–1.53) |
| Unknown | 1.06 (0.78–1.44) | 1.02 (0.74–1.41) |
| NZ Deprivation | ||
| 1–2 | 1.00 | 1.00 |
| 3–4 | 1.09 (0.93–1.27) | 1.16 (0.99–1.37) |
| 5–6 | 0.99 (0.85–1.16) | 1.00 (0.85–1.18) |
| 7–8 | 1.06 (0.90–1.25) | 1.04 (0.87–1.25) |
| 9–10 | 0.80 (0.69–0.93) | 0.76 (0.64–0.91) |
| Unknown | 0.91 (0.68–1.22) | 1.10 (0.38–3.20) |
| Urban Rural | ||
| Urban | 1.00 | 1.00 |
| Rural | 1.28 (1.05–1.57) | 0.97 (0.78–1.20) |
| Unknown | 0.92 (0.70–1.21) | 0.94 (0.33–2.70) |
| Region | ||
| Auckland | 1.00 | 1.00 |
| Waikato | 1.46 (1.29–1.65) | 1.97 (1.70–2.29) |
| Public/Private Treatment | ||
| Private | 1.00 | 1.00 |
| Public | 0.76 (0.69–0.85) | 0.79 (0.70–0.89) |
| CLINICOPATHOLOGICAL VARIABLES | ||
| Year of Diagnosis | ||
| 2000–2004 | 1.00 | 1.00 |
| 2005–2009 | 1.26 (1.11–1.44) | 1.24 (1.08–1.42) |
| 2010–2015 | 1.21 (1.07–1.37) | 1.18 (1.03–1.35) |
| Screened/Symptomatic | ||
| Screened | 1.00 | 1.00 |
| Symptomatic | 0.47 (0.42–0.52) | 0.60 (0.54–0.68) |
| Grade | ||
| 1 | 1.00 | 1.00 |
| 2 | 0.71 (0.63–0.80) | 0.90 (0.78–1.02) |
| 3 | 0.65 (0.56–0.74) | 1.09 (0.91–1.31) |
| Unknown | 0.37 (0.21–0.63) | 0.45 (0.25–0.81) |
| Hormone Receptor Status | ||
| ER 5- and PR 6-positive | 1.00 | 1.00 |
| ER 5- and PR 6-negative | 1.49 (1.30–1.71) | 0.68 (0.57–0.81) |
| ER 5- or PR 6-positive | 1.03 (1.08–1.55) | 0.89 (0.76–1.03) |
| Unknown | 0.89 (0.62–1.28) | 0.53 (0.36–0.78) |
| Histology | ||
| Ductal | 1.00 | 1.00 |
| Lobular | 0.89 (0.75–1.07) | 0.89 (0.73–1.09) |
| Others | 1.06 (0.87–1.29) | 1.04 (0.84–1.29) |
| Tumour Size | ||
| <20 mm | 1.00 | 1.00 |
| ≥20–≤50 mm | 0.48 (0.43–0.54) | 0.63 (0.56–0.71) |
| Positive Lymph Node Status | ||
| 0 | 1.00 | 1.00 |
| 1–3 | 0.71 (0.62–0.80) | 0.83 (0.72–0.95) |
| 4+ | 0.47 (0.36–0.61) | 0.60 (0.44–0.80) |
| Lymphovascular Invasion | ||
| No | 1.00 | 1.00 |
| Yes | 0.79 (0.70–0.90) | 1.01 (0.87–1.17) |
1 Breast Conserving Surgery plus Radiotherapy (BCS + RT); 2 Breast Conserving Surgery (BCS); 3 Mastectomy (MTX); 4 Mastectomy plus Radiotherapy (MTX + RT); 5 Oestrogen Receptor (ER); 6 Progesterone Receptor (PR).
Table 2.
Baseline characteristics of surgical treatment groups by demographic, clinical and systemic treatment variables (See Appendix A, Table A1 for table with row percentages).
| Factors | Total | BCS 1 | BCS + RT 2 | MTX 3 | MTX + RT 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Col% | No. | Col% | No. | Col% | No. | Col% | ||
| Total No. | 6972 | 588 | 100.0 | 4608 | 100.0 | 1507 | 100.0 | 269 | 100.0 |
| DEMOGRAPHIC VARIABLES | |||||||||
| Median Age | 58 (50–66) 5 | 58 (49–68) 5 | 58 (50–65) 5 | 61 (51–71) 5 | 53 (45–63) 5 | ||||
| Age Group | |||||||||
| <45 | 789 | 78 | 13.3 | 475 | 10.3 | 172 | 11.4 | 64 | 23.8 |
| 45–59 | 3006 | 236 | 40.1 | 2124 | 46.1 | 531 | 35.2 | 115 | 42.8 |
| 60–74 | 2538 | 184 | 31.3 | 1767 | 38.3 | 523 | 34.7 | 64 | 23.8 |
| 75+ | 639 | 90 | 15.3 | 242 | 5.3 | 281 | 18.6 | 26 | 9.7 |
| Ethnicity | |||||||||
| European | 5180 | 429 | 73.0 | 3495 | 75.8 | 1063 | 70.5 | 193 | 71.7 |
| Māori | 653 | 50 | 8.5 | 427 | 9.3 | 139 | 9.2 | 37 | 13.8 |
| Pacific | 229 | 29 | 4.9 | 142 | 3.1 | 53 | 3.5 | 5 | 1.9 |
| Other | 715 | 58 | 9.9 | 410 | 8.9 | 218 | 14.5 | 29 | 10.8 |
| Unknown | 195 | 22 | 3.7 | 134 | 2.9 | 34 | 2.3 | 5 | 1.9 |
| NZ Deprivation | |||||||||
| 1–2 | 1534 | 140 | 23.8 | 1021 | 22.2 | 307 | 20.4 | 66 | 24.5 |
| 3–4 | 1394 | 108 | 18.4 | 954 | 20.7 | 296 | 19.6 | 36 | 13.4 |
| 5–6 | 1415 | 118 | 20.1 | 940 | 20.4 | 298 | 19.8 | 59 | 21.9 |
| 7–8 | 1121 | 80 | 13.6 | 760 | 16.5 | 233 | 15.5 | 48 | 17.8 |
| 9–10 | 1289 | 117 | 19.9 | 792 | 17.2 | 326 | 21.6 | 54 | 20.1 |
| Unknown | 219 | 25 | 4.3 | 141 | 3.1 | 47 | 3.1 | 6 | 2.2 |
| Main Urban Areas | |||||||||
| Urban | 6252 | 515 | 87.6 | 4112 | 89.2 | 1377 | 91.4 | 248 | 92.2 |
| Rural | 496 | 46 | 7.8 | 353 | 7.7 | 81 | 5.4 | 16 | 5.9 |
| Unknown | 224 | 27 | 4.6 | 143 | 3.1 | 49 | 3.3 | 5 | 1.9 |
| Region | |||||||||
| Auckland | 5479 | 468 | 79.6 | 3526 | 76.5 | 1287 | 85.4 | 198 | 73.6 |
| Waikato | 1493 | 120 | 20.4 | 1082 | 23.5 | 220 | 14.6 | 71 | 26.4 |
| Public/Private | |||||||||
| Public | 2245 | 170 | 28.9 | 1574 | 34.2 | 416 | 27.6 | 85 | 31.6 |
| Private | 4727 | 418 | 71.1 | 3034 | 65.8 | 1091 | 72.4 | 184 | 68.4 |
| CLINICAL VARIABLES | |||||||||
| Year of Diagnosis | |||||||||
| 2000–2004 | 1861 | 143 | 24.3 | 1165 | 25.3 | 456 | 30.3 | 97 | 36.1 |
| 2005–2009 | 2240 | 148 | 25.2 | 1520 | 33.0 | 489 | 32.4 | 83 | 30.9 |
| 2010–2015 | 2871 | 297 | 50.5 | 1923 | 41.7 | 562 | 37.3 | 89 | 33.1 |
| Screen-Detected/Symptomatic | |||||||||
| Screen-Detected | 3605 | 283 | 48.1 | 2675 | 58.1 | 587 | 39.0 | 60 | 22.3 |
| Symptomatic | 3367 | 305 | 51.9 | 1933 | 41.9 | 920 | 61.0 | 209 | 77.7 |
| Stage | |||||||||
| IA | 4521 | 434 | 73.8 | 3192 | 69.3 | 854 | 56.7 | 41 | 15.2 |
| IB | 193 | 17 | 2.9 | 133 | 2.9 | 37 | 2.5 | 6 | 2.2 |
| IIA | 1587 | 104 | 17.7 | 963 | 20.9 | 452 | 30.0 | 68 | 25.3 |
| IIB | 446 | 27 | 4.6 | 208 | 4.5 | 149 | 9.9 | 62 | 23.0 |
| IIIA | 225 | 6 | 1.0 | 112 | 2.4 | 15 | 1.0 | 92 | 34.2 |
| Grade | |||||||||
| 1 | 2191 | 218 | 37.1 | 1572 | 34.1 | 373 | 24.8 | 28 | 10.4 |
| 2 | 3182 | 251 | 42.7 | 2050 | 44.5 | 756 | 50.2 | 125 | 46.5 |
| 3 | 1545 | 109 | 18.5 | 960 | 20.8 | 362 | 24.0 | 114 | 42.4 |
| Unknown | 54 | 10 | 1.7 | 26 | 0.6 | 16 | 1.1 | 2 | 0.7 |
| Hormone Receptor Status | |||||||||
| ER 6 and PR 7 negative | 1018 | 72 | 12.2 | 600 | 13.0 | 264 | 17.5 | 82 | 30.5 |
| ER 6 and PR 7 positive | 4801 | 413 | 70.2 | 3270 | 71.0 | 975 | 64.7 | 143 | 53.2 |
| ER 6 or PR 7 positive | 1016 | 90 | 15.3 | 661 | 14.3 | 227 | 15.1 | 38 | 14.1 |
| Unknown | 137 | 13 | 2.2 | 77 | 1.7 | 41 | 2.7 | 6 | 2.2 |
| Histology | |||||||||
| Ductal | 5909 | 472 | 80.3 | 3912 | 84.9 | 1286 | 85.3 | 239 | 88.8 |
| Lobular | 567 | 45 | 7.7 | 361 | 7.8 | 140 | 9.3 | 21 | 7.8 |
| Other | 496 | 71 | 12.1 | 335 | 7.3 | 81 | 5.4 | 9 | 3.3 |
| Tumour Size (mm) | |||||||||
| <20 | 4847 | 445 | 75.7 | 3452 | 74.9 | 859 | 57.0 | 91 | 33.8 |
| ≥20–≤50 | 2125 | 143 | 24.3 | 1156 | 25.1 | 648 | 43.0 | 178 | 66.2 |
| Positive Lymph Node | |||||||||
| 0 | 5453 | 498 | 84.7 | 3717 | 80.7 | 1169 | 77.6 | 69 | 25.7 |
| 1–3 | 1295 | 85 | 14.5 | 779 | 16.9 | 323 | 21.4 | 108 | 40.1 |
| 4+ | 224 | 5 | 0.9 | 112 | 2.4 | 15 | 1.0 | 92 | 34.2 |
| Lympho-vascular Invasion | |||||||||
| Yes | 5799 | 508 | 86.4 | 3885 | 84.3 | 1254 | 83.2 | 152 | 56.5 |
| No | 1173 | 80 | 13.6 | 723 | 15.7 | 253 | 16.8 | 117 | 43.5 |
| SYSTEMIC TREATMENT VARIABLES | |||||||||
| Systemic Treatment | |||||||||
| None | 2303 | 275 | 46.8 | 1464 | 31.8 | 545 | 36.2 | 19 | 7.1 |
| Both | 932 | 44 | 7.5 | 602 | 13.1 | 180 | 11.9 | 106 | 39.4 |
| Chemotherapy | 711 | 47 | 8.0 | 449 | 9.7 | 141 | 9.4 | 74 | 27.5 |
| Hormonal therapy | 2961 | 204 | 34.7 | 2064 | 44.8 | 624 | 41.4 | 69 | 25.7 |
| Unknown | 65 | 18 | 3.1 | 29 | 0.6 | 17 | 1.1 | 1 | 0.4 |
1 Breast Conserving Surgery (BCS); 2 Breast Conserving Surgery plus Radiotherapy (BCS + RT); 3 Mastectomy (MTX); 4 Mastectomy plus Radiotherapy (MTX + RT); 5 Interquartile Range; 6 Oestrogen Receptor (ER); 7 Progesterone Receptor (PR).
Table 3.
Hazard ratios (HR) for breast cancer-specific mortality from competing risks analysis.
| Type of Loco-Regional Treatment | Crude HR (95% CI) | Adjusted HR (95% CI) 1 | Adjusted HR (95% CI) 2 | Adjusted HR (95% CI) 3 |
|---|---|---|---|---|
| BCS + RT 4 | 1.00 | 1.00 | 1.00 | 1.00 |
| BCS 5 | 1.16 (0.75–1.78) | 1.13 (0.73–1.76) | 1.21 (0.77–1.88) | 1.11 (0.71–1.75) |
| MTX 6 | 1.73 (1.35–2.22) | 1.78 (1.37–2.30) | 1.45 (1.10–1.90) | 1.38 1.05–1.82) |
| MTX + RT 7 | 2.87 (1.93–4.27) | 2.73 (1.82–4.09) | 1.02 (0.65–1.60) | 1.05 (0.66–1.67) |
1 Adjusted for demographic factors: age, ethnicity, NZ deprivation, urban status, region, public/private; 2 Adjusted as above and for clinic-pathological factors: screen-detected/symptomatic, grade, hormone receptor status, histology, tumour size, lymph node status, lympho-vascular invasion (LVI); 3 Adjusted as above and for systemic treatment factors: chemo and hormonal therapies; 4 Breast Conserving Surgery plus Radiotherapy (BCS + RT); 5 Breast Conserving Surgery (BCS); 6 Mastectomy (MTX); 7 Mastectomy plus Radiotherapy (MTX + RT).
Logistic regression analyses controlled for demographic and clinicopathological factors with exception to the competing risk analyses, which also controlled for systemic treatment factors. All statistical analyses were carried out using STATA MP version 16.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient Characteristics
Of the 6972 women included in this analysis, 588 (8.4%) received BCS alone, 4608 (66.1%) received BCS + RT, 1507 (21.6%) received MTX alone and 269 (3.9%) received MTX + RT. A total of 320 breast cancer-specific deaths occurred: BCS—n = 23 (7.2%), BCS + RT—n = 170 (53.1%), MTX—n = 98 (30.6%) and MTX + RT—n = 29 (9.1%). The median age was 58 (IQR: 50–66) years for the whole sample, 58 (IQR: 49–68) years for those who received BCS only, 58 (IQR: 50–65) years for those who received BCS + RT, 61 (IQR: 51–71) years for those who received MTX alone and 53 (IQR: 45–63) years for those who received MTX + RT (Table 2). Table 2 shows the distribution of demographic, clinical and systemic treatment variables within our sample population across BCS, BCS + RT, MTX and MTX + RT surgical treatment groups. The majority of women in our sample were: aged between 45 and 59, European, resided in urban areas, resided within the Auckland region and treated publicly. Clinically, women in our study were diagnosed between 2010 and 2015, and were most likely to have tumours of stage IA, grade 2, be oestrogen receptor- (ER) and progesterone receptor (PR)-positive, ductal tumours, <20 mm in size, with no nodal involvement, no lymphovascular invasion and likely to receive only hormone therapy.
3.2. Factors Associated with the Receipt of BCS + RT
Demographic factors associated with the receipt of BCS + RT were age, ethnicity, deprivation, public/private facility type and region. Older women (75+ years old) were less likely (odds ratio (OR): 0.34, 95% confidence interval (CI): 0.27–0.43) to receive BCS + RT compared to their younger counterparts (<45 years old). Relative to European women, Asian women were less likely to receive BCS + RT (OR: 0.58, 95% CI: 0.48–0.69). Patients residing in deprived areas (deprivation levels 9–10) were less likely to receive BCS + RT (OR: 0.76, 95% CI: 0.64–0.91) compared to those residing in more affluent areas (deprivation level 1–2). Similarly, patients treated through the public system (OR: 0.79, 95% CI: 0.70–0.89) were less likely to receive BCS + RT compared to their privately treated counterparts. Lastly, patients treated in Waikato were more likely to receive BCS + RT (OR: 1.97, 95% CI: 1.70–2.29) compared to Auckland patients.
Clinical factors associated with the receipt of BCS + RT included: year of diagnosis, detection method, hormone receptor status, tumour size and lymph node involvement. Compared to women diagnosed during the 2000–2004 period, those diagnosed between 2005–2009 (OR: 1.24, 95% CI: 1.08–1.42) and 2010–2015 (OR: 1.18, 95% CI: 1.03–1.35) periods were more likely to receive BCS + RT. Compared to women detected through screening, symptomatic women were less likely to receive BCS + RT (OR: 0.60, 95% CI: 0.54–0.68). Patients who were oestrogen (ER) and progesterone (PR) receptor-negative (OR: 0.68, 95% CI: 0.57–0.81) or had an unknown status (OR:0.53, 95% CI:0.36–0.78) were less likely to receive BCS + RT compared to their ER- and PR-positive receptor counterparts. Women with a tumour size ≥ 2–≤50 mm were less likely (OR: 0.63, 95% CI: 0.56–0.71) to receive BCS + RT compared to women with tumour(s) < 20 mm. Lastly, the likelihood of BCS + RT decreased as lymph node involvement increased (0 positive nodes OR: 1.00; 1–3 positive nodes OR: 0.83, 95% CI: 0.72–0.95; 4+ positive nodes OR: 0.60, 95% CI: 0.44–0.80).
3.3. Breast Cancer-Specific Death
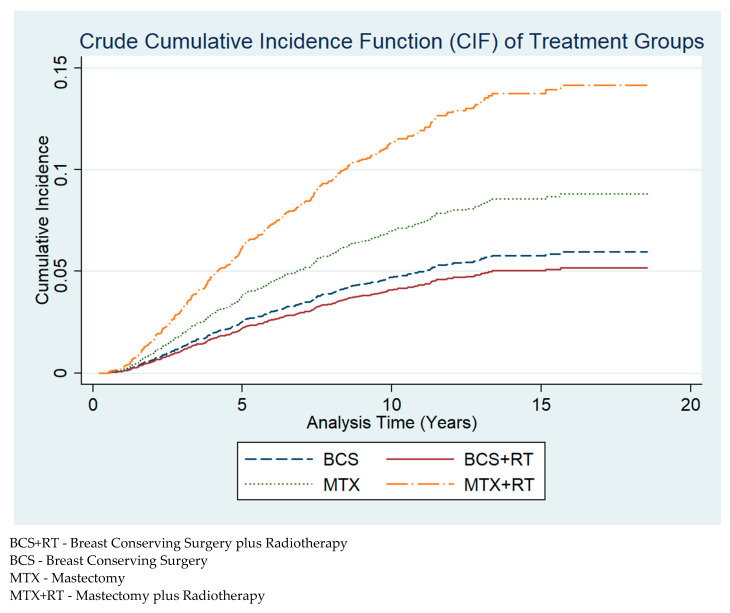
There were 320 (4.6%) deaths from breast cancer over a median follow-up period of 8.8 years. The crude cumulative incidence function (CIF) graph (Figure 2) demonstrates that breast cancer-specific mortality risk was highest in women who received MTX + RT, followed by MTX, which has roughly half the risk as that of MTX + RT, and subsequently BCS, closely followed by BCS + RT. After adjustment for demographic, clinical and systemic treatment factors (Table 3), MTX had a higher risk of breast cancer-specific mortality (HR 3: 1.38, 95% CI: 1.05–1.82) relative to BCS + RT (Table 3).
Figure 2.
Crude cumulative incidence function (CIF) plot of breast cancer-specific mortality across BCS, BCS + RT, MTX and MTX + RT surgical treatment groups.
4. Discussion
4.1. Main Findings
In this population-based study involving over 6900 women with early-stage breast cancer, BCS + RT was the most commonly used loco-regional treatment, but its uptake differed significantly by a number of demographic and clinicopathological factors. Compared to women who received BCS + RT, those who received MTX had a higher risk of breast cancer-specific mortality over the median follow-up period of 8.8 years. Patients receiving MTX + RT or BCS alone did not have any statistically different risk of mortality when compared to BCS + RT.
4.2. Interpretation
Amongst our cohort of New Zealand women with early-stage (stage I–IIIA) breast cancer, a number of patterns emerged. Those aged 75+ were less likely to receive BCS + RT compared to younger women aged < 45. Younger women may opt for the least invasive of the two surgical procedures due to self-image concerns, whereas elderly women are less likely to be as concerned and opt for the most convenient option [23].
Women residing in more deprived areas were less likely to receive BCS + RT compared to those living in more affluent areas. Deprived neighbourhoods tend to be less likely equipped with radiotherapy treatment facilities and hence women residing in low-deprivation areas would likely face a greater travel distance [24,25,26], cost and inconvenience in receiving BCS + RT treatment compared to women residing in more affluent neighbourhoods [25,27].
Patients residing within the Auckland region were less likely to receive BCS + RT compared to their counterparts in Waikato. Given that Auckland is a more urban area than Waikato and there are less access barriers in urban areas, differences in surgical treatments here may be due to physician influence between the two regions. It could be likely that physicians practising within the Waikato region are more comfortable utilising breast conserving surgical techniques as opposed to mastectomy surgeries and hence their preferences/skills may be indicative of the patient’s final choice. Furthermore, areas with a low ratio of medical oncologists to patients have been shown to result in a greater uptake of BCS + RT, which may be the case in Waikato when compared to Auckland [28].
Patients treated in a public facility were less likely to receive BCS + RT compared to their privately treated counterparts. Private health facilities likely have more resources available relative to demand, translating to less wait times for elective surgeries/treatment [29]. Compared to MTX alone, BCS + RT is more resource-intensive, requiring a multi-faceted team approach and numerous subsequent treatments. Churilla and colleagues have found the likelihood of BCS + RT decreases with a decrease in the density of radiation oncologists present [30]. Thus, the lack of resources and delay in accessing breast cancer treatment within the public sectors could explain the decreased likelihood of BCS + RT uptake relative to privately treated patients.
The likelihood of BCS + RT decreased if patients were diagnosed during 2000–2004, detected symptomatically, had tumour sizes ≥ 20–≤50 mm, negative/unknown oestrogen and progesterone receptor status, unknown grade and with increasing lymph node involvement.
Patients receiving BCS + RT had the lowest risk of breast cancer-specific mortality over a median follow-up period of 8.8 years, with patients treated with MTX alone having a 38% (95% CI: 5–82%) increased risk of mortality comparatively. One reason for these differences may be due to the administration of radiotherapy in BCS, which helps further suppress/kill smaller cancerous cells that may have been missed during surgery, whereas for patients undergoing MTX alone, they rely solely on tumorous growth being detected by their surgeon. Overall, our results are consistent with recent observational studies [8,9,18,31,32,33].
4.3. Strengths
To our knowledge, this is the first observational study in New Zealand that identified factors associated with differences in the uptake of surgical treatment for women with early-stage breast cancer and investigated differences in breast cancer mortality across four surgical treatment groups (BCS, BCS + RT, MTX and MTX + RT). We used two opt-out prospectively maintained population-based databases which contained comprehensive and near complete data with respect to patients diagnosed with primary breast cancer [12,13,17]. Linkage to national databases also allowed for the extraction of data pertaining to cause of death and patient comorbidities with minimal loss to follow-up [13,17]. We undertook a competing risks analysis which aims to more accurately reflect risk of breast cancer-specific mortality and has not been undertaken in previous observational studies [19].
4.4. Limitations
Despite efforts to control for confounding, residual confounding may still be present as some key variables that could influence choice of surgery and/or mortality were not controlled for due to lack of data availability, e.g., antigen Ki-67 [34], body mass index (BMI), alcohol intake [35], co-morbidities and presence of Breast Cancer Gene 1 (BRCA1) and Breast Cancer Gene 2 (BRCA2) gene mutations. Lack of routine Human Epidermal Growth Factor Receptor 2 (HER2) testing prior to 2006 in our database resulted in a significant number of patients with an unknown HER2 status (n = 2147—20.9% unknown). Socioeconomic status (NZDep2013) was based on area level deprivation rather than individual level deprivation.
4.5. Implications
Demographic differences in loco-regional treatment of women with early-stage breast cancer highlight some of the largely modifiable inequities currently present in our healthcare system, providing room for gaining more equitable outcomes. Our findings underscore more efforts to identify and alleviate the access barriers the disadvantaged populations are faced with.
BCS + RT has certain advantages over MTX alone. BCS + RT is a minimally invasive treatment with reduced post-operative complications, a faster recovery time and patients generally report a better overall self-image [23,36]. Where appropriate, BCS + RT should be recommended rather than MTX. It is important to note that MTX should still remain the treatment of choice for certain groups of women, such as those who have a contraindication to radiation therapy, e.g., women with certain connective tissue disorders, pregnancy, certain hereditary gene mutations such as BRCA1 and BRCA2 or where large tumour to breast ratio makes BCS + RT impractical [2,37].
Currently, in New Zealand, women with early-stage breast cancer are treated based on the guidelines set out by the New Zealand Guidelines Group [2]. The option between BCS + RT and MTX exists for women [2], implying survival outcomes for both treatments are the same if not very similar. It is worth inquiring further as to the survival advantages of BCS + RT and MTX treatments given the findings from this study and a growing body of international population-based literature.
5. Conclusions
Overall, our study shows that older women, Asians, those residing in impoverished areas or in the Auckland region and those who were diagnosed with symptomatic cancer were less likely to receive BCS + RT even after taking clinical factors into account. We also found that compared to women who received BCS + RT, women who received MTX alone had a greater risk of breast cancer-specific mortality. Our findings underscore the need for more efforts needed to ensure equitable cancer care in New Zealand.
Acknowledgments
We thank all the patients who contributed data to these registries and all the physicians and other healthcare staff who have maintained the registers.
Appendix A
Table A1.
Baseline tables by row percentage.
| Factors | Total | BCS 1 | BCS + RT 2 | MTX 3 | MTX + RT 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Row% | No. | Row% | No. | Row% | No. | Row% | ||
| Total No. | 6972 | 588 | 8.4 | 4608 | 66.1 | 1507 | 21.6 | 269 | 3.9 |
| DEMOGRAPHIC VARIABLES | |||||||||
| Median Age | 58 (50–66) 5 | 58 (49–68) 5 | 58 (50–65) 5 | 61 (51–71) 5 | 53 (45–63) 5 | ||||
| Age Group | |||||||||
| <45 | 789 | 78 | 9.9 | 475 | 60.2 | 172 | 21.8 | 64 | 8.1 |
| 45–59 | 3006 | 236 | 7.9 | 2124 | 70.7 | 531 | 17.7 | 115 | 3.8 |
| 60–74 | 2538 | 184 | 7.2 | 1767 | 69.6 | 523 | 20.6 | 64 | 2.5 |
| 75+ | 639 | 90 | 14.1 | 242 | 37.9 | 281 | 44.0 | 26 | 4.1 |
| Ethnicity | |||||||||
| European | 5180 | 429 | 8.3 | 3495 | 67.5 | 1063 | 20.5 | 193 | 3.7 |
| Māori | 653 | 50 | 7.7 | 427 | 65.4 | 139 | 21.3 | 37 | 5.7 |
| Pacific | 229 | 29 | 12.7 | 142 | 62.0 | 53 | 23.1 | 5 | 2.2 |
| Other | 715 | 58 | 8.1 | 410 | 57.3 | 218 | 30.5 | 29 | 4.1 |
| Unknown | 195 | 22 | 11.3 | 134 | 68.7 | 34 | 17.4 | 5 | 2.6 |
| NZ Deprivation | |||||||||
| 1–2 | 1534 | 140 | 9.1 | 1021 | 66.6 | 307 | 20.0 | 66 | 4.3 |
| 3–4 | 1394 | 108 | 7.7 | 954 | 68.4 | 296 | 21.2 | 36 | 2.6 |
| 5–6 | 1415 | 118 | 8.3 | 940 | 66.4 | 298 | 21.1 | 59 | 4.2 |
| 7–8 | 1121 | 80 | 7.1 | 760 | 67.8 | 233 | 20.8 | 48 | 4.3 |
| 9–10 | 1289 | 117 | 9.1 | 792 | 61.4 | 326 | 25.3 | 54 | 4.2 |
| Unknown | 219 | 25 | 11.4 | 141 | 64.4 | 47 | 21.5 | 6 | 2.7 |
| Main Urban Areas | |||||||||
| Urban | 6252 | 515 | 8.2 | 4112 | 65.8 | 1377 | 22.0 | 248 | 4.0 |
| Rural | 496 | 46 | 9.3 | 353 | 71.2 | 81 | 16.3 | 16 | 3.2 |
| Unknown | 224 | 27 | 12.1 | 143 | 63.8 | 49 | 21.9 | 5 | 2.2 |
| Region | |||||||||
| Auckland | 5479 | 468 | 8.5 | 3526 | 64.4 | 1287 | 23.5 | 198 | 3.6 |
| Waikato | 1493 | 120 | 8.0 | 1082 | 72.5 | 220 | 14.7 | 71 | 4.8 |
| Public/Private | |||||||||
| Public | 2245 | 170 | 7.6 | 1574 | 70.1 | 416 | 18.5 | 85 | 3.8 |
| Private | 4727 | 418 | 8.8 | 3034 | 64.2 | 1091 | 23.1 | 184 | 3.9 |
| CLINICAL VARIABLES | |||||||||
| Year of Diagnosis | |||||||||
| 2000–2004 | 1861 | 143 | 7.7 | 1165 | 62.6 | 456 | 24.5 | 97 | 5.2 |
| 2005–2009 | 2240 | 148 | 6.6 | 1520 | 67.9 | 489 | 21.8 | 83 | 3.7 |
| 2010–2015 | 2871 | 297 | 10.3 | 1923 | 67.0 | 562 | 19.6 | 89 | 3.1 |
| Screen-Detected/Symptomatic | |||||||||
| Screen-Detected | 3605 | 283 | 7.9 | 2675 | 74.2 | 587 | 16.3 | 60 | 1.7 |
| Symptomatic | 3367 | 305 | 9.1 | 1933 | 57.4 | 920 | 27.3 | 209 | 6.2 |
| Stage | |||||||||
| IA | 4521 | 434 | 9.6 | 3192 | 70.6 | 854 | 18.9 | 41 | 0.9 |
| IB | 193 | 17 | 8.8 | 133 | 68.9 | 37 | 19.2 | 6 | 3.1 |
| IIA | 1587 | 104 | 6.6 | 963 | 60.7 | 452 | 28.5 | 68 | 4.3 |
| IIB | 446 | 27 | 6.1 | 208 | 46.6 | 149 | 33.4 | 62 | 13.9 |
| IIIA | 225 | 6 | 2.7 | 112 | 49.8 | 15 | 6.7 | 92 | 40.9 |
| Grade | |||||||||
| 1 | 2191 | 218 | 9.9 | 1572 | 71.7 | 373 | 17.0 | 28 | 1.3 |
| 2 | 3182 | 251 | 7.9 | 2050 | 64.4 | 756 | 23.8 | 125 | 3.9 |
| 3 | 1545 | 109 | 7.1 | 960 | 62.1 | 362 | 23.4 | 114 | 7.4 |
| Unknown | 54 | 10 | 18.5 | 26 | 48.1 | 16 | 29.6 | 2 | 3.7 |
| Hormone Receptor Status | |||||||||
| ER 6 and PR 7 negative | 1018 | 72 | 7.1 | 600 | 58.9 | 264 | 25.9 | 82 | 8.1 |
| ER 6 and PR 7 positive | 4801 | 413 | 8.6 | 3270 | 68.1 | 975 | 20.3 | 143 | 3.0 |
| ER 6 or PR 7 positive | 1016 | 90 | 8.9 | 661 | 65.1 | 227 | 22.3 | 38 | 3.7 |
| Unknown | 137 | 13 | 9.5 | 77 | 56.2 | 41 | 29.9 | 6 | 4.4 |
| Histology | |||||||||
| Ductal | 5909 | 472 | 8.0 | 3912 | 66.2 | 1286 | 21.8 | 239 | 4.0 |
| Lobular | 567 | 45 | 7.9 | 361 | 63.7 | 140 | 24.7 | 21 | 3.7 |
| Other | 496 | 71 | 14.3 | 335 | 67.5 | 81 | 16.3 | 9 | 1.8 |
| Tumour Size (mm) | |||||||||
| <20 | 4847 | 445 | 9.2 | 3452 | 71.2 | 859 | 17.7 | 91 | 1.9 |
| ≥20–≤50 | 2125 | 143 | 6.7 | 1156 | 54.4 | 648 | 30.5 | 178 | 8.4 |
| Positive Lymph Node | |||||||||
| 0 | 5453 | 498 | 9.1 | 3717 | 68.2 | 1169 | 21.4 | 69 | 1.3 |
| 1–3 | 1295 | 85 | 6.6 | 779 | 60.2 | 323 | 24.9 | 108 | 8.3 |
| 4+ | 224 | 5 | 2.2 | 112 | 50.0 | 15 | 6.7 | 92 | 41.1 |
| Lympho-vascular Invasion | |||||||||
| Yes | 5799 | 508 | 8.8 | 3885 | 67.0 | 1254 | 21.6 | 152 | 2.6 |
| No | 1173 | 80 | 6.8 | 723 | 61.6 | 253 | 21.6 | 117 | 10.0 |
| SYSTEMIC TREATMENT VARIABLES | |||||||||
| Systemic Treatment | |||||||||
| None | 2303 | 275 | 11.9 | 1464 | 63.6 | 545 | 23.7 | 19 | 0.8 |
| Both | 932 | 44 | 4.7 | 602 | 64.6 | 180 | 19.3 | 106 | 11.4 |
| Chemotherapy | 711 | 47 | 6.6 | 449 | 63.2 | 141 | 19.8 | 74 | 10.4 |
| Hormonal therapy | 2961 | 204 | 6.9 | 2064 | 69.7 | 624 | 21.1 | 69 | 2.3 |
| Unknown | 65 | 18 | 27.7 | 29 | 44.6 | 17 | 26.2 | 1 | 1.5 |
1 Breast Conserving Surgery (BCS); 2 Breast Conserving Surgery plus Radiotherapy (BCS + RT); 3 Mastectomy (MTX); 4 Mastectomy plus Radiotherapy (MTX + RT); 5 Interquartile Range; 6 Oestrogen Receptor (ER); 7 Progesterone Receptor (PR).
Author Contributions
Conceptualization, M.S.A., M.E., and S.T.T.; methodology, M.S.A., M.E. and S.T.T.; software, M.S.A.; validation, M.S.A., S.T.T., M.E., R.L., and I.C.; formal analysis, M.S.A. and S.T.T.; investigation, M.S.A. and S.T.T.; resources, M.S.A., M.E., S.T.T.; data curation, M.S.A.; writing—original draft preparation, M.S.A.; writing—review and editing, M.S.A., S.T.T., M.E., R.L., and I.C.; visualization, M.S.A.; supervision, S.T.T. and M.E.; project administration, M.S.A., M.E., S.T.T.; funding acquisition, S.T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Research Council of New Zealand, grant number 18/764.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Auckland, Human Participants Ethics Committee (Ref. No. 21851). Written informed consent was not sought as it was not feasible to trace all patients, some of whom are deceased.
Informed Consent Statement
This study was based on anonymised data on patients recorded over many years in an active cancer registry. Ethical review confirmed that individual patient consent was not required as patients did not need to be individually contacted.
Data Availability Statement
No new data were created or analysed in this study. Ethical approval is restricted to the use of the data by the named investigators. Access by others will require application to the cancer registers used and to the ethical committees.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer Estimated Number of Deaths in 2018, Worldwide, Females, All Ages. In Estim. Number Deaths 2018 Worldw. Females Ages. [(accessed on 26 June 2020)];2018 Available online: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_population=countries&population=900&populations=&key=asr&sex=2&cancer=39&type=1&statistic=5&prevalence=0&population_group=18&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1#collapse-group-0-5.
- 2.Ministry of Health . Management of Early Breast Cancer—Evidence-based Best Practice Guideline. Ministry of Health; Wellington, New Zealand: 2009. [Google Scholar]
- 3.Nationaal Borstkanker Overleg Nederland (NBON) Dutch Breast Cancer Guidelines. NABON; Utrecht, The Netherlands: 2012. [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN Guidelines for Patients Invasive Breast Cancer. Harbourside Press; Huntington, NY, USA: 2020. [Google Scholar]
- 5.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version) Chin. J. Cancer Res. 2019;31:259–277. doi: 10.21147/j.issn.1000-9604.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litière S., Werutsky G., Fentiman I.S., Rutgers E., Christiaens M.-R., Van Limbergen E., Baaijens M.H.A., Bogaerts J., Bartelink H. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13:412–419. doi: 10.1016/S1470-2045(12)70042-6. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A., Aguilar M., Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S., Pappas L., Neumayer L., Kokeny K., Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–274. doi: 10.1001/jamasurg.2013.3049. [DOI] [PubMed] [Google Scholar]
- 9.Onitilo A.A., Engel J.M., Stankowski R.V., Doi S.A. Survival comparisons for breast conserving surgery and mastectomy revisited: Community experience and the role of radiation therapy. Clin. Med. Res. 2015;13:65–73. doi: 10.3121/cmr.2014.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris C.R., Cohen R., Schlag R., Wright W.E. Increasing trends in the use of breast-conserving surgery in California. Am. J. Public Health. 2000;90:281–284. doi: 10.2105/ajph.90.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albain K.S., Green S.R., Lichter A.S., Hutchins L.F., Wood W.C., Henderson I.C., Ingle J.N., O’Sullivan J., Osborne C.K., Martino S. Influence of patient characteristics, socioeconomic factors, geography, and systemic risk on the use of breast-sparing treatment in women enrolled in adjuvant breast cancer studies: An analysis of two intergroup trials. J. Clin. Oncol. 1996;14:3009–3017. doi: 10.1200/JCO.1996.14.11.3009. [DOI] [PubMed] [Google Scholar]
- 12.Elwood J.M., Marshall R.J., Tin Tin S., Barrios M.E., Harvey V.J. Bias in survival estimates created by a requirement for consent to enter a clinical breast cancer registry. Cancer Epidemiol. 2019;58:178–183. doi: 10.1016/j.canep.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Seneviratne S., Campbell I., Scott N., Shirley R., Peni T., Lawrenson R. Accuracy and completeness of the New Zealand Cancer Registry for staging of invasive breast cancer. Cancer Epidemiol. 2014;38:638–644. doi: 10.1016/j.canep.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health . New Zealand Cancer Registry. Ministry of Health; Wellington, New Zealand: 2014. [Google Scholar]
- 15.Ministry of Health . Mortality Collection Data Dictionary Version 1.7. Ministry of Health; Wellington, New Zealand: 2017. [Google Scholar]
- 16.Gurney J., Sarfati D., Dennett E., Koea J. The completeness of cancer treatment data on the National Health Collections. N. Z. Med. J. 2013;126:69–74. [PubMed] [Google Scholar]
- 17.Neave L., Harvey V., Benjamin C., Thompson P., Pellett O., Whitlock J., Jones W., Poole G. The Auckland breast cancer register: A special project of the Auckland breast cancer study group. N. Z. Med. J. 2003;116:1–12. [PubMed] [Google Scholar]
- 18.Chen K., Liu J., Zhu L., Su F., Song E., Jacobs L.K. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: A NCDB analysis. Oncotarget. 2015;6:40127–40140. doi: 10.18632/oncotarget.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Maaren M.C., de Munck L., de Bock G.H., Jobsen J.J., van Dalen T., Linn S.C., Poortmans P., Strobbe L.J.A., Siesling S. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol. 2016;17:1158–1170. doi: 10.1016/S1470-2045(16)30067-5. [DOI] [PubMed] [Google Scholar]
- 20.Exeter D.J., Shackleton N., Browne M., Zhao J., Lee A., Crengle S. Different domains of deprivation and their relationship with obesity in New Zealand 4-year-old children. Pediatr. Obes. 2019;14:e12520. doi: 10.1111/ijpo.12520. [DOI] [PubMed] [Google Scholar]
- 21.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donoghoe M.W., Gebski V. The importance of censoring in competing risks analysis of the subdistribution hazard. BMC Med. Res. Methodol. 2017;17:52. doi: 10.1186/s12874-017-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasry J.-C.M., Margolese R.G., Poisson R., Shibata H., Fleischer D., LaFleur D., Legault S., Taillefer S. Depression and body image following mastectomy and lumpectomy. J. Chronic Dis. 1987;40:529–534. doi: 10.1016/0021-9681(87)90010-5. [DOI] [PubMed] [Google Scholar]
- 24.Bigby J., Holmes M.D. Disparities across the breast cancer continuum. Cancer Causes Control. 2005;16:35–44. doi: 10.1007/s10552-004-1263-1. [DOI] [PubMed] [Google Scholar]
- 25.Ambroggi M., Biasini C., Del Giovane C., Fornari F., Cavanna L. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist. 2015;20:1378–1385. doi: 10.1634/theoncologist.2015-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel V., Olivotto I., Hislop T.G., Sawka C., Coldman A., Holowaty E.J. Patterns of initial management of node-negative breast cancer in two Canadian provinces. Can. Med Assoc. J. 1997;156:25–35. [PMC free article] [PubMed] [Google Scholar]
- 27.Gu J., Groot G., Boden C., Busch A., Holtslander L., Lim H. Review of Factors Influencing Women’s Choice of Mastectomy Versus Breast Conserving Therapy in Early Stage Breast Cancer: A Systematic Review. Clin. Breast Cancer. 2018;18:e539–e554. doi: 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Samet J.M., Hunt W.C., Farrow D.C. Determinants of receiving breast-conserving surgery. The surveillance, epidemiology, and end results program, 1983–1986. Cancer. 1994;73:2344–2351. doi: 10.1002/1097-0142(19940501)73:9<2344::AID-CNCR2820730917>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Tin Tin S., Elwood J.M., Lawrenson R., Campbell I., Harvey V., Seneviratne S. Differences in breast cancer survival between public and private care in New Zealand: Which factors contribute? PLoS ONE. 2016;11:e0153206. doi: 10.1371/journal.pone.0153206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Churilla T.M., Donnelly P.E., Leatherman E.R., Adonizio C.S., Peters C.A. Total Mastectomy or Breast Conservation Therapy? How Radiation Oncologist Accessibility Determines Treatment Choice and Quality: A SEER Data-base Analysis. Breast J. 2015;21:473–480. doi: 10.1111/tbj.12449. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann-Johnsen O.J., Kåresen R., Schlichting E., Nygård J.F. Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: A registry-based follow-up study of Norwegian women primary operated between 1998 and 2008. Ann. Surg. Oncol. 2015;22:3836–3845. doi: 10.1245/s10434-015-4441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang E.S., Lichtensztajn D.Y., Gomez S.L., Fowble B., Clarke C.A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: The effect of age and hormone receptor status. Cancer. 2013;119:1402–1411. doi: 10.1002/cncr.27795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofvind S., Holen A., Aas T., Roman M., Sebuødegård S., Akslen L.A. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur. J. Surg. Oncol. EJSO. 2015;41:1417–1422. doi: 10.1016/j.ejso.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Urruticoechea A., Smith I.E., Dowsett M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 35.Connor J., Kydd R., MacLennan B., Shield K., Rehm J. Alcohol-attributable cancer deaths under 80 years of age in New Zealand. Drug Alcohol. Rev. 2016;36:415–423. doi: 10.1111/dar.12443. [DOI] [PubMed] [Google Scholar]
- 36.Fang S.-Y., Shu B.-C., Chang Y.-J. The effect of breast reconstruction surgery on body image among women after mastectomy: A meta-analysis. Breast Cancer Res. Treat. 2012;137:13–21. doi: 10.1007/s10549-012-2349-1. [DOI] [PubMed] [Google Scholar]
- 37.Faermann R., Sperber F., Schneebaum S., Barsuk D. Tumor-to-breast volume ratio as measured on MRI: A possible predictor of breast-conserving surgery versus mastectomy. Sat. 2014;30:20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analysed in this study. Ethical approval is restricted to the use of the data by the named investigators. Access by others will require application to the cancer registers used and to the ethical committees.