Abstract
The main anti-diabetic effect of metformin mediated through stimulation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) is the inhibition of hepatic gluconeogenesis and triggering glucose uptake in skeletal muscles. Additionally, some new pathways, besides the AMPK activation, were discovered, that can explain wide-range properties of metformin. All these properties are now attracting the attention of researchers in the fields other than diabetes and the drug has been reported to have anti-cancer, immunoregulatory and anti-aging effects. Among others, the beneficial effects of metformin in hematological disorders like leukemias, lymphomas, and multiple myeloma were reported. Despite a great progress in therapy, these diseases are still incurable in most cases. Thus, there is an urgent need to discover novel, less toxic and more effective drugs especially for older or chemotherapy-resistant patients. In this review article, the current findings on the anti-cancer effect of metformin together with underlying possible mechanisms in blood cancers are discussed. However. to evaluate precisely these promising effects of metformin, more studies are required, because many of the published results are preclinical.
Key words: Metformin, leukemia, lymphoma, multiple myeloma, AKT/mTOR signaling pathway
Introduction
Metformin is a biguanide derivative that has been used as an anti-diabetic drug and has been known for nearly one century. Biguanides were initially derived from a plant, Galega officinalis, and were indicated to be rich in guanidine. Guanidine was shown to have hypoglycemic activity in animals, however it was toxic in clinical use.1,2 Thus, in 1929, new biguanides with ability to reduce serum glucose levels were synthesized, among them dimethyl biguanide (1,1-dimethyl biguanide hydrochloride or metformin). In contrast to sulfonylureas, metformin fails to induce insulin secretion but inhibits the release of glucose by the liver and increases muscle glucose uptake.1,2 Additionally, metformin was proved to be safe and not expensive. When it was used as a monotherapy, hypoglycemic side effects were not observed and a favorable effect on body weight was noticed.3,4 In the 1990s, the United Kingdom Prospective Diabetes Study has shown that regardless of glycemic control, metformin is able to reduce the risk of myocardial infarction and decrease all-cause mortality in diabetes patients.5-8 As a result, according to the international experts guidelines, metformin is now recommended as the firstline treatment for type 2 diabetes (T2D) and is the most frequently administered drug to treat this disease.1,3,9
Results of further research revealed the benefits of metformin in people with diabetes other than glycemic control. Interestingly, metformin was shown to reduce the risk of cancer. Many epidemiologic analyses have reported that metformin may improve prognosis of patients with different types of malignancies and even may prevent tumor initiation.1,9,10 Evans et al.8 noted as first that patients who were treated with metformin had a low risk of developing cancer. Next, a lots of studies in T2D patients have shown that metformin therapy is correlated with a reduced risk of numerous malignancies such as prostate cancer,11,12 lung cancer,13,14 head and neck cancer,15 breast cancer,16 pancreatic cancer,17 colorectal cancer,18 ovarian cancer19 and liver cancer.1,20.21
Among other things, there is also medical evidence that supports the benefits of metformin in patients with blood cancers. Unfortunately, many of the studies in this field provide only preclinical data, while heterogeneous results have been reported across different clinical trials. Therefore, more clinical and epidemiological studies are required to support the role of metformin as an anti-cancer agent. This review article presents the current findings on the anti-cancer effect of metformin in myeloproliferative and lymphoproliferative disorders. This drug may be a novel and less toxic therapeutic option for hematological malignancies and should attract the attention of researchers and physicians.
Mechanisms of metformin action
Metformin is indicated for patients with T2D, which is characterized by hyperglycemia due to tissue resistance to insulin and its low secretion. The main mechanism of metformin action is the reduction of the rate of hepatic glucose production and an increase of insulin sensitivity in liver as well as intensification of glucose absorption in muscles.22-24 Together, these actions reduce blood glucose level with very little possibility of inducing hypoglycemia. 25 An activation of anaerobic metabolism in the intestinal wall may be additional anti-glycemic effect of metformin.26-28
The most significant place of metformin action is mitochondrion. Its main function is ATP synthesis by oxidative phosphorylation. This process leads to the production of energy via oxidation of nutrients and creation of electron chemical gradient through the mitochondrial inner membrane. This gradient is used as an energy source that allows the ATP synthesis, heat production and transport of ions.29 Simultaneously, reactive oxygen species (ROS) are produced in mitochondria and may be toxic for cells and be responsible for DNA, protein and lipid damage. It may lead to oxidative stress and mitochondrial dysfunction, that has been reported to cause an insulin resistance in tissues.30-32
As far as a molecular mechanism is concerned, metformin appears to inhibit mitochondrial respiration at the level of respiratory chain complex I.33 The generally accepted mechanism of its action is stimulation of adenosine monophosphate (AMP)-activated protein kinase (AMPK). An increase in the AMP:ATP ratio in situation of metabolic stress such as hypoxia and glucose deficiency, stimulates directly the AMPK activation and can be considered as an indicator of energy level in cells.30,34,35 Metformin is accumulated within the mitochondrial matrix in hepatocytes and influences complex I of the respiratory chain [33,36]. Inhibition of this complex I results in an ATP reduction and an increase in ADP and AMP levels, and this finally leads to the AMPK stimulation [33,36,37]. AMPK blocks gluconeogenic gene transcription. Furthermore, it inhibits lipogenesis, which enhances insulin sensitivity. 23,38,39
Recently, some new pathways of metformin action, besides the AMPK activation, were discovered. The decrease in cellular energy level can directly inhibit the gluconeogenic process. An increased AMP level may inhibit the adenylate cyclase, resulting in lowering of cyclic adenosine monophosphate (cAMP) production. As a result, the activity of protein kinase A (PKA) and its targets, including cyclic AMP response element binding (CREB), are reduced. Additionally, metformin inhibits the activity of glycerol- 3-phosphate dehydrogenase (mGPD) and prevents glycerol usage in gluconeogenesis. The cytosolic redox state is increased, which diminishes the use of lactate as a gluconeogenic substrate.23,40-42 Additionally, metformin may stimulate the secretion of GLP-1 (glucagon-like peptide-1) that enhances glucose-dependent insulin release from the pancreas and leads to a glucose level reduction. 43,44
Increased risk of cancer in diabetes
The increased risk of carcinogenesis in patient with T2D was reported by many authors.45-47 There are many risk factors that are similar for T2D and cancer. These factors include obesity, hyperglycemia, hyperinsulinemia, and chronic inflammation due to overweight and body fat mass.46,47 Obesity and insulin resistance have been connected with an increased risk of several cancers, including solid tumors and hematologic disorders. There are both prospective data and meta-analyses supporting the association between an increased BMI and the increased risk of both lymphoid and myeloid neoplasms.48-51 Similarly, such a correlation was observed for T2D and the increased risk of non-Hodgkin lymphomas (NHL), leukemias and multiple myeloma (MM).52,53 Additionally, high glucose levels was reported to correlate with the increased risk of mortality in the course of MM, NHL, and leukemia.54
The important mechanisms connecting obesity and insulin resistance with cancer include the changed lipid signaling, effects of adipokine and inflammatory cytokine, as well as activation of PI3K/Akt/mTOR and RAS/RAF/MAPK/ERK pathways.45 Obesity and insulin resistance or T2D may pre-dispose to blood cancers via activation of these different pathways, and especially the aggressive and chemo-resistant types of cancers are observed in patients with underlying metabolic syndrome.45,55
Metformin and its anti-cancer activity
Indirect mechanisms of metformin effects
Metformin is able to mediate its anti-cancer effects in both in an indirect and a direct manner. The indirect mechanisms result from inhibition of tumor growth connected with high-fat diet and hyperinsulinemia that are cancer risk factors as mentioned above. It is reported that insulin and insulin-like growth factor 1 (IGF-1) can promote tumorigenesis by stimulating the cell proliferation. 23,56 Metformin, through the lowering of blood glucose and insulin levels as well as insulin receptor activation in tumor tissue, is able to influence the survival of cancer cells. This mechanism is similar to that causing tumor growth inhibition by dietary control. 57,58 Metformin is able to impair the enzymatic function of hexokinases that catalyze production of glucose-6-phosphate, as a result the use of glucose by tumor cells is inhibited.59,60 Metformin is also able to affect the inflammatory processes that are reported to be involved in tumor progression. Inhibition of a transcription factor - the nuclear factor-κ B (NF-κ B) leads to diminished secretion of pro-inflammatory cytokines.61 Moreover, metformin has been reported to stimulate the immune response to cancer cells.23,62
AMPK-dependent mechanisms of metformin effects
The direct anti-cancer activity of metformin is related to AMPK-dependent and AMPK-independent mechanisms.63 In reference to this, the key effect of AMPK activation is the inhibition of downstream AKT/mTOR signaling, that is the main regulator of cell proliferation. Such an inhibition results in the suppression of cell growth.29,64,65 Further, experimental research provides evidence that metformin, in an AMPK-dependent manner, may regulate the cell cycle through interactions with oncogenes and tumor suppressors such as c-MYC.66 AMPK has also been reported to induce the cell-cycle arrest by Ser15 phosphorylation of p53 protein. 35,67 On the other hand, it was reported that metformin treatment forces a metabolic conversion in p53-deficient cells. Thus, the drug is selectively toxic to p53-/-cells and may reduce incidence of tumors in patients being treated with metformin. Using metformin in patients with p53-deficient tumors, which are often resistant to classical forms of chemotherapy or radiotherapy may be of importance as well.35 It was also found that metformin can have an anti-folate effect that inhibits cancer cell growth, similarly to anti-folate chemotherapeutics.68,69
AMPK-independent mechanisms of metformin effects
Anti-cancer mechanism of metformin action may be also mediated by AMPK-independent mechanisms. It was reported that the drug can inhibit cell DNA damage by preventing ROS generation by complex I.70 In the absence of AMPK, metformin is also able to influence AKT/mTOR via suppression of mTORC1 signaling.71 Moreover, suppression of cyclin D1, which is an essential regulator of the cell cycle, was detected.72 Such an inhibition is reported to be connected with a p53-dependent up-regulation of REDD1 that is a result of DNA damage response. REDD1, a negative regulator of mTOR, is a new molecular target of metformin.73 Next, it was found that metformin up-regulates apoptosis and autophagy through BCL-2 pathway, which also leads to reduction of tumor growth.74 This was mediated by inactivation of the Stat3 (signal transducer and activator of transcription 3) - Bcl2 pathway. This pathway is merely marginally deteriorated by AMPK knockdown, that indicated rather reduced contribution of AMPK. It was also described that metformin reduced glucose uptake in cancer cells and such an energy source leads finally to mitochondrial depolarization and apoptosis.59,75 The possible anti-cancer effects of metformin are listed in Figure 1.
Metformin in hematological malignancies
These properties of metformin, such as anti-cancer and immunoregulatory effects, have been attracting the attention of researchers in fields other than diabetes. Among the different cancers analyzed, the beneficial effects of metformin in hematological disorders were reported. Metformin was investigated as a therapeutic agent in patients with leukemia, lymphomas, and multiple myeloma. Additionally, many studies have demonstrated the synergistic or additive effect of metformin with chemotherapeutics, which may be used in lower doses, minimizing the side effects of high doses.45,76-78
Metformin in the therapy of different types of leukemia
The AMPK/mTOR signaling pathway is involved in pathogenesis of blood neoplasms, thus metformin may be a new option for these cancers therapy. Additionally, metformin does not seem to influence the survival of normal hematopoietic stem cells.79-81 Next, some oncogenes, such as the protein kinase BCR-ABL that is a hallmark of chronic myeloid leukemia (CML) and may be present in acute leukemias as well as Tax oncoprotein, which is crucial in the pathogenesis of HTLV human retrovirus infection and adult T cell leukemia, are stimulators of the PI3K/AKT/mTOR pathway.1,81 Hence, metformin, via AMPK activation, is able to suppress the proliferation of acute and chronic leukemias cells. Many studies have been published recently confirming the beneficial effects of metformin on leukemic cells under both in vitro and in vivo conditions.
Vakana et al.82 reported that AMPK activators, such as metformin and 5-aminoimidazole-4-carboxamide ribonucleotide, were able to stimulate the mTOR signaling pathway in BCR-ABL– expressing cells. The therapy with these agents was potent to suppress of CML leukemic precursors and Ph+ ALL cells, even cells with T315I-BCR-ABL mutation. This mutation caused resistance to most of tyrosine kinase inhibitors used in the therapy of CML and Ph+ ALL. The cell lines were used in the experiments, however, the results obtained indicate that AMPK is an attractive target for the treatment of BCR-ABL–expressing blood cancers.82
Similarly to study by Vakana et al.82 Shi et al.83 analyzed the effect of metformin on immature blasts of acute lymphoblastic leukemia (ALL) with the BCR/ABL translocation. Additionally, the authors studied CML lines K562 (sensitive to imatinib) and K562R (resistant to imatinib) co-cultured with metformin. The results obtained demonstrated that metformin was able to suppress cell viability, induced apoptosis, and downregulated the mTORC1 signaling pathway. Additionally, metformin was shown to potentiate the anti-cancer efficacy of imatinib in Ph+ALL and CML cells, resensitizing the CML imatinib-resistance cells to imatinib. The experiments were performed in vitro, however the results obtained suggested that metformin might be a promising and attractive agent for the Ph+ALL or CML therapy and the continuation of the research is of importance.83
Figure 1.
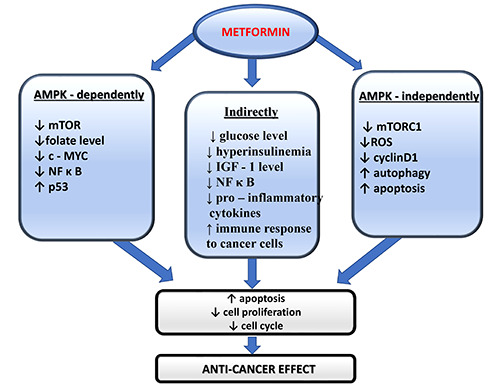
Anti-cancer effects of metformin.
Another study that proofs beneficial effects of metformin in lymphoblastic leukemia was presented by Rosilio et al.84 The authors analyzed the effect of metformin, phenformin, and an AMPK activator - AICAR, on cells of human T cell acute lympoblastic leukemia (T-ALL). The experiments were performed in an animal model, on cell lines and blood samples from patients. The results demonstrated that all of these agents were able to suppress tumor metabolism and proliferation through the prevention of mTOR activation. The drugs under study did not influence the survival rate of normal T lymphocytes, thus displaying a specific anti- cancer effect. The authors observed synergistic effects on cell death when the analyzed substances were combined with dexamethasone, but less with doxorubicin, which are the drugs used in induction therapy of ALL.84
A very interesting study performed under in vivo conditions was presented by Ramos-Panafiel et al.85 The authors determined the effect of metformin on the therapy of ALL patients with high levels of ABCB1 treatment resistance gene expression and assessed its impact on overall survival. The group of 26 patients with ALL was treated by combining standard chemotherapy with metformin and compared with patients (no=76) receiving chemotherapy without metformin. The authors observed a benefit of adding metformin in the group of patients with high ABCB1 gene expression. In the metformin group, the drug was a protective factor against early relapse and treatment failure.85 Thus, metformin may be considered as a concomitant medication in chemotherapy for ALL patients.
The recently published results by Yuang et al.86 determined the anti-leukemic activity of metformin combined with AraC in acute myeloid leukemia (AML). The experiments were done in AML cell lines and in animal xenograft tumor model to assess the effects in vivo. The authors found that metformin could synergistically sensitize AML cells to AraC by inhibiting the mTORC1/P70S6K signaling pathway.86 Such synergistic effect of AraC and metformin may be a prospective therapeutic option especially for older patients with AML who cannot be treated by intensive chemotherapy.
Effect of metformin on AML cells was also analyzed by Sabnis et al.87 They examined the combination of 6-benzylthioinosine (6- BT), a broad-spectrum metabolic inhibitor, and metformin in monocytic AML cell lines and umbilical cord blood cells. This combination resulted in high cytotoxicity of leukemic cells and was associated with inhibition of FLT3-ITD activated STAT5 and reduced c-Myc and GLUT-1 expression. The authors concluded that such combination might be a promising strategy for AML therapy [87]. These preliminary results are worth to be continued.
Anti-leukemic effects of metformin were also assessed in a specific subtype of AML - acute promyelocytic leukemia (APL). APL is characterized by the t(15;17) translocation and is treated with all-trans retinoic acid (ATRA) or arsenic trioxide (ATO) - based regimens. Huai et al.88 demonstrated that metformin synergized with trans-retinoic acid, inducing the differentiation and apoptosis in leukemic blasts of cell lines. Asik et al.89 investigated the effects of combination of a classic anti-cancer agent paclitaxel and metformin on HL-60 APL cell line. Such a combination significantly increased apoptosis of leukemic cells, so the authors suggested it could be a new option assessable for development of new treatment strategies for APL.89 These studies may be a basis for further research in APL patients.
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western countries, with a median age in the moment of diagnosis about 70 years. Approximately 20% of CLL patients have type 2 diabetes. Bruno et al.90 studied effects of metformin on CLL cells in an in vitro culture. The drug was administered to quiescent leukemic cells or during CLL cell activation, provided by co-culturing with CD40L-expressing fibroblasts. Metformin induced cell death of quiescent CLL cells and inhibition of cell cycle entry when CLL were stimulated. There was no effect on normal lymphocytes. Moreover, the drug combination experiments were performed, in which metformin accelerated the cytotoxic effects of anti-leukemic drugs - fludarabine and BCL-2 inhibitor.88 Adekola et al.91 demonstrated that treatment of CLL cells with HIV protease inhibitor ritonavir, caused toxicity comparable to that occurring upon glucose-deficiency. CLL cells resistant to ritonavir were sensitized by metformin co-therapy, possibly influencing the mitochondrial complex 1 action. The authors suggested further investigation of ritonavir and metformin for therapy of CLL patients.91 Based on these preliminary results on CLL cells, the more precise analysis in CLL patients should be performed with this therapeutic option.
Several clinical trials on metformin alone or in combination with chemotherapeutics are proposed to assess the promising effect in vivo in patients with different hematologic malignancies. Description of these clinical trials is presented in Table 1.
Metformin in therapy of lymphoproliferative diseases
Lymphomas are malignant tumors of the lymphoid system. A base of the disease is a clonal expansion of lymphocytes, which are at different stages of development. Lymphomas are frequently diagnosed blood cancers with heterogenous course and different prognosis.
Shi et al.92 were first to provide evidence on anti-tumor effects of AMPK activation in lymphomas. In their study, metformin induced a suppression of cell growth in both B-cell and T-cell lymphomas via negative control of the mTOR pathway. This suppression was a dose-dependent. Interestingly, the sensitivity of lymphoma cells to other anti-cancer agents, such as doxorubicin and temsirolimus, an mTOR Inhibitor, was enhanced by concomitant metformin treatment through the induction of autophagy.92 There was also reported that an activation of p53 by suppressing murine double minute X (MDMX) by metformin in human lymphomas was able to cause apoptosis in a mechanism additional to inhibiting the mTOR pathway.1 Quesada et al.93 described the constitutive activation of mTORC2, NF-κB, p-Akt and the concomitant expression of IGF-1R in peripheral T-cell lymphoma. Interference of this pathway may provide a clinical therapeutic benefit, therefore the use of metformin as an inhibitor of mTORC2 and NF-kB in this type of lymphoma may be worth study.93 Based on these results explaining the underlying mechanisms of metformin action in lymphomas, the further clinical trials may be undertaken.
Interestingly, it was also reported that metformin is able to reduce the risk of lymphoma incidence. Tseng et al.94 analyzed whether the metformin use might affect the risk of non-Hodgkin lymphoma (NHL). They investigated the group of 610,089 newly diagnosed T2D patients who were followed up for NHL incidence. The results obtained revealed that the metformin use was associated with a lower risk of NHL compared with non-metformin antidiabetics. 94
An analysis of patients with diffuse large B cell lymphoma (DLBCL), the most frequent type of B-lymphomas, and co-existing diabetes revealed that metformin added to first-line immunochemotherapy improved progression-free survival in comparison to other anti-diabetogenic drugs.1,95 However, the results by Smyth et al.96 published this year showed that metformin had no significant impact on survival in a retrospective population-based survey of all adults aged ≥66 years with DLBCL or transformed lymphoma treated with regimens containing rituximab. Wang et al.97 investigated if metformin influenced outcomes in newly diagnosed DLBCL and follicular lymphoma (FL) patients. However, they found no evidence that metformin use was associated with improved outcomes in newly diagnosed DLBCL and FL.97 Therefore, further studies are required to explain these inconclusive results.
Multiple myeloma (MM) is a lymphoproliferative disease of plasma cells of bone marrow that is still uncurable. Novel therapeutic options to improve outcomes in patients with MM are required. AKT involved in the PI3K/AKT/mTOR signaling path has high expression in progressive and resistant multiple myeloma. AKT contributes to many oncogenic functions in multiple myeloma which may be used in therapy. Pre-clinical data has reported the effectiveness of AKT inhibitors in MM, due to high expression of AKT in advanced and resistant MM and its contribution to multiple oncogenic functions in this disease.98
An interesting study focused on metformin in multiple myeloma was published by Zi et al.99 The authors investigated whether metformin exerts an anti-myeloma effect in in vitro and in vivo xenograft models and explored the underlying mechanism. They found that metformin could suppress MM cells proliferation by inducing apoptosis and cell cycle arrest. Metformin inhibited the expression of IGF-IR, and PI3K, PKB/AKT and mTOR. Metformin had synergistic effect with dexamethasone but not with bortezomib. The authors have concluded that metformin stops MM cell proliferation via the IGF-1R/PI3K/AKT/mTOR signaling pathway and its use with dexamethasone may be investigated as a therapeutic option for MM patients.99 However, the results provided by Jagannathan et al.100 suggested that metformin was able to influence GRP78, a key driver of bortezomib-induced autophagy, thus to enhance the anti-myeloma benefit of bortezomib, that was in contrast to results of Zi et al.99 and requires further explanation. On the other hand, Mishra et al.101 reported that metformin specifically decreased IL-6R expression which is mediated via AMPK, mTOR, and miR34a. IL-6 signaling plays a crucial role in the pathogenesis of MM and this novel finding may improve therapies targeting IL-6 signaling.100
Table 1.
Clinical trials investigating the antineoplastic effects of metformin in hematologic malignancies [based on https://clinicaltrials. gov].
| Disease | Title of the study | Metformin/other drugs | Status | Results |
|---|---|---|---|---|
| Acute lymphoblastic leukemia | Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) and Metformin for Relapsed Childhood Acute Lymphoblastic Leukemia (ALL) | Metformin Vincristine Doxorubicin Dexamethasone PEG-asparaginase | Completed | 14 patients enrolled Metformin dose 1000 mg/m2/day 9 patients evaluable for response - 5 complete remissions, 1 partial response, and 1 stable disease104 |
| Multiple myeloma Chronic lymphocytic leukemia | Metformin Hydrochloride and Ritonavir in Treating Patients With Relapsed or Refractory Multiple Myeloma or Chronic Lymphocytic Leukemia | Metformin Ritonavir | Active | |
| Acute myeloid leukemia | Metformin + Cytarabine for the Treatment of Relapsed/Refractory AML | Metformin Cytarabine | Terminated | |
| Chronic lymphocytic leukemia | Vincristine, Dexamethasone, Doxorubicin, and PEG-asparaginase (VPLD) and Metformin for Relapsed Childhood Acute Lymphoblastic Leukemia (ALL) | Metformin | Active | |
| Solid Tumors Lymphomas | Metformin and Temsirolimus in Treating Patients With Metastatic or Unresectable Solid Tumor or Lymphoma | Metformin Temsirolimus | Completed | 11 patients enrolled Metformin dose 500 mg 1,2,3 or 3 times per day 1 partial response, 5 stable disease105 |
| Diffuse large B-cell lymphoma | DA-EPOCH-Rituximab/Metformin (RM) for Double Hit Lymphoma (DLBCL) | Metformin Immune-chemotherapy | Terminated | |
| Diffuse large B-cell lymphoma | Rituximab, Cyclophosphamide, Vincristine and Prednisone (R-CHOP) Plus Metformin in Diffuse Large-B-cell Lymphoma | Metformin Immune-chemotherapy | Unknown | |
| Non-Hodgkin’s lymphomas | A Clinical Trial of Metformin in the Maintenance of Non-Hodgkin's Lymphoma Patients | Metformin | Recruiting | |
| Acute lymphoblastic leukemia | Metformin Reduce the Relapse Rate on Patients With B-cell Precursor (Ph+ Negative) Acute Lymphoblastic Leukemia | Metformin Chemotherapy | Completed | 102 ALL patients Metformin dose 850mg every 8 h Metformin was a protective factor against early relapse and treatment failure85 |
| Multiple myeloma | Clinical Activity of Metformin With High-dose of Dexamethasone in Relapse Multiple Myeloma | Metformin Dexamethasone | Unknown | |
| Multiple myeloma | Metformin and Nelfinavir in Treating Patients With Relapsed and/or Refractory Multiple Myeloma | Metformin Bortezomib Nelfinavir | Recruiting |
Wu et al.102 in a retrospective study conducted on 1240 MM patients assessed overall survival (OS) and disease status prior to death. According to the results obtained, diabetic patients had a shorter OS than non-diabetic subjects. As only the diabetic MM subjects were analyzed, Cox regression revealed that metformin predicted a longer OS, but use of insulin/analogues predicted a shorter OS [102]. Remarkable results of metformin effects in patients with monoclonal gammopathy of undetermined significance (MGUS) being a pre-myeloma stage were published by Chang et al.103 The authors performed a retrospective analysis of patients in the U.S. Veterans Health Administration database diagnosed with MGUS. Data for 3287 patients were obtained, 2003 (61%) subjects were included in the final analytical group. Metformin users were defined as subjects who had been prescribed metformin for at least 4 years. The median follow-up time was 69 months, 463 (23%) participants were metformin users and 1540 (77%) were non-users. Among the metformin users, 13 (3%) patients had progression to MM, while in the group of non-metformin users 74 (5%) of patients progressed to MM. According to the results, it may be concluded that the metformin use was associated with a reduced risk of transformation to MM.103-105
Conclusions
Among different cancers, the beneficial effects of metformin in hematological disorders seem to be hopeful. Despite a great progress in therapy, these diseases are still incurable, so new drugs should be discovered. Metformin is minimally toxic; thus it may be a good option for older or chemotherapy-resistant patients. Additionally, it could be easily combined with other chemotherapeutic agents which may be used in lower doses, reducing the side effects. However, to evaluate these encouraging effects of metformin, more studies are required, because many of the studies in this field provide only preclinical evidence There are several clinical trials ongoing now in patients with blood cancers, but heterogeneous results require to be more specified. Despite these limitations, the metformin becomes a promising anti-cancer drug and the well-established clinical research should allow its wider use in oncological practice.
References
- 1.Cunha AD, Pericole FV, Carvalheira JBC. Metformin and blood cancers. Clinics (Sao Paulo) 2018;73:e412s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CJ. Metformin: its botanical background. Pract Diab Int 2004;21:115-7. [Google Scholar]
- 3.Zhou J, Massey S, Story D, Li L. Metformin: an old drug with new applications. Int J Mol Sci 2018;19:28-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paneni F, Lüscher TF. Cardiovascular protection in the treatment of type 2 diabetes: a review of clinical trial results across drug classes. Am J Cardiol 2017;120:17-27. [DOI] [PubMed] [Google Scholar]
- 5.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. [PubMed] [Google Scholar]
- 6.UKPDS Group. Effect of intensive blood-glucose control with metiormin on complication in overweight patients with type 2 diabetes (UKPDS34). Lancet 1998;352:854-65. [PubMed] [Google Scholar]
- 7.UKPDS Group. Tight blood pressure control and risk of rnacrovascular and microvascular complications in type 2 diabetes (UKPDS 38). BMJ 1998;317:703-13. [PMC free article] [PubMed] [Google Scholar]
- 8.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Kelekar G, Shen J, et al. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Target Oncol 2016;11:447-67. [DOI] [PubMed] [Google Scholar]
- 10.Papanagnou P, Stivarou T, Tsironi M. Unexploited antineoplastic effects of commercially available anti-diabetic drugs. Pharmaceuticals (Basel) 2016;9:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng D, Yang Y, Tang X, et al. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: evidence from a meta-analysis. Diabetes Metab Res Rev 2015;31:595-602. [DOI] [PubMed] [Google Scholar]
- 12.Raval AD, Thakker D, Vyas A, et al. Impact of metformin on clinical outcomes among men with prostatecancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2015;18:110-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakoda LC, Ferrara A, Achacoso NS, et al. Metformin use and lung cancer risk in patients with diabetes. Cancer Prev Res (Phila) 2015;8:174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JJ, Gallagher EJ, Sigel K, et al. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med 2015;191:448-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen YC, Lin C, Lin SW, et al. Effect of metformin on the incidence of head and neck cancer in diabetics. Head Neck 2015;37:1268-73. [DOI] [PubMed] [Google Scholar]
- 16.Col NF, Ochs L, Springmann V, et al. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat 2012;135:639-46. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Lai ST, Xie L, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:19-26. [DOI] [PubMed] [Google Scholar]
- 18.Lee DJ, Kim B, Lee JH, et al. The effect of metformin on responses to chemotherapy and survival in stage IV colorectal cancer with diabetes. Korean J Gastroenterol 2012;60:355-61. [DOI] [PubMed] [Google Scholar]
- 19.Dilokthornsakul P, Chaiyakunapruk N, Termrungruanglert W, et al. The effects of metformin onovarian cancer: a systematic review. Int J Gynecol Cancer 2013;23:1544-51. [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZJ, Zheng ZJ, Shi R, et al. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:2347-53. [DOI] [PubMed] [Google Scholar]
- 21.Duncan BB, Schmidt MI. Metformin, cancer, alphabet soup, and the role of epidemiology in etiologic research. Diabetes Care 2009;32:1748-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hundal RS, Krssak M Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J 2015;471:307-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 2006;49:434-41. [DOI] [PubMed] [Google Scholar]
- 25.Wright AD, Cull CA, Macleod KM, Holman RR. Hypoglycemia in type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complicat 2006;20:395-401. [DOI] [PubMed] [Google Scholar]
- 26.Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs 2015;75:1071-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey CJ, Mynett KJ, Page T. Importance of the intestine as a site of metformin-stimulated glucose utilization. Br J Pharmacol 1994;112:671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol 1992;105:1009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederkehr A, Wollheim C.B. Mini review: implication of mitochondria in insulin secretion and action. Endocrinology 2006;147:2643-9. [DOI] [PubMed] [Google Scholar]
- 31.Mcbride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 2006;16:551-60. [DOI] [PubMed] [Google Scholar]
- 32.Hu F, Liu F. Mitochondrial stress: A bridge between mitochondrial dysfunction and metabolic diseases? Cell Signal 2011;23:1528-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 2007;47:185-210. [DOI] [PubMed] [Google Scholar]
- 35.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745-52. [DOI] [PubMed] [Google Scholar]
- 36.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607-14. [PMC free article] [PubMed] [Google Scholar]
- 37.Hinke SA, Martens GA, Cai Y, et al. Methyl succinate antagonises biguanide-induced AMPK-activation and death of pancreatic beta-cells through restoration of mitochondrial electron transfer. Br J Pharmacol 2007;150:1031-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foretz M, Hebrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 2013;19:1649-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess SC, He T, Yan Z, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 2007;5:313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller RA, Chu Q, Xie J, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013;494:256-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci U S A 2009;106:12121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Hong T. Combination therapy of dipeptidyl peptidase- 4 inhibitors and metformin in type 2 diabetes: rationale and evidence. Diabetes Obes Metab 2014;16:111-7. [DOI] [PubMed] [Google Scholar]
- 44.Wu T, Thazhath SS, Bound MJ, et al. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes Res Clin Pract 2014;106:e3-6. [DOI] [PubMed] [Google Scholar]
- 45.Karmali R, Dalovisio A, Borgia JA, et al. All in the family: Clueing into the link between metabolic syndrome and hematologic malignancies. Blood Rev 2015;29:71-80. [DOI] [PubMed] [Google Scholar]
- 46.Nagel G, Stocks T, Spath D, et al. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can). Ann Hematol 2012;91:1519-31. [DOI] [PubMed] [Google Scholar]
- 47.Birmann BM, Giovannucci E, Rosner B, et al. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiol Biomarkers Prev 2007;16:1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson SC, Wolk A. Obesity and risk of non-Hodgkin's lymphoma: a meta-analysis. Int J Cancer 2007;121:1564-70. [DOI] [PubMed] [Google Scholar]
- 49.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer 2008;122:1418-21. [DOI] [PubMed] [Google Scholar]
- 50.Nagel G, Stocks T, Spath D, et al. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can). Ann Hematol 2012;91:1519-31. [DOI] [PubMed] [Google Scholar]
- 51.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review andmeta-analysis of prospective observational studies. Lancet 2008;371:569-78. [DOI] [PubMed] [Google Scholar]
- 52.Castillo JJ, Mull N, Reagan JL, et al. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 2012;119:4845-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu BC, Gapstur SM, Greenland P, et al. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2348-54. [DOI] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [DOI] [PubMed] [Google Scholar]
- 55.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [DOI] [PubMed] [Google Scholar]
- 56.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008;8:915-28. [DOI] [PubMed] [Google Scholar]
- 57.Hadad SM, Baker L, Quinlan PR, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 2009;9:307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wingo SN, Gallardo TD, Akbay EA, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One 2009;4P:e5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salani B, Marini C, Rio AD, et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep 2013;3:2070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salani B, Del Rio A, Marini C, et al. Metformin, cancer and glucose metabolism. Endocr Relat Cancer 2014;21:461-71. [DOI] [PubMed] [Google Scholar]
- 61.Moiseeva O, Deschenes-Simard X, St-Germain E, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 2013;12:489-98. [DOI] [PubMed] [Google Scholar]
- 62.Pearce EL, Walsh MC, Cejas PJ, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009;460:103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sui X, Xu Y, Wang X, et al. Metformin: a novel but controversial drug in cancer prevention and treatment. Mol Pharm 2015;12:3783-91. [DOI] [PubMed] [Google Scholar]
- 64.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adeninenucleotide-independent mechanism. Diabetes 2002;51:2420-5. [DOI] [PubMed] [Google Scholar]
- 66.Blandino G, Valerio M, Cioce M. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun 2012;3:865-9. [DOI] [PubMed] [Google Scholar]
- 67.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependentmetabolic checkpoint. Mol Cell 2005;18:283-93. [DOI] [PubMed] [Google Scholar]
- 68.Cabreiro F, Au C, Leung KY, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013;153:228-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corominas-Faja B, Quirantes-Pine R, Oliveras-Ferraros C, et al. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging 2012;4:480-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Algire C, Moiseeva O, Deschenes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res 2012;5:536-43. [DOI] [PubMed] [Google Scholar]
- 71.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 2010;11:390-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metforminexerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 2008;27:3576-86. [DOI] [PubMed] [Google Scholar]
- 73.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK,induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011;71:4366-72. [DOI] [PubMed] [Google Scholar]
- 74.Marini C, Salani B, Massollo M, et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle 2013;12:3490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis 2014;5:e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: A metabolic disruptor and anti-diabetic drug to target human leukemia Cancer Letters 2014;346:188-96. [DOI] [PubMed] [Google Scholar]
- 77.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res 2011;71:3196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanna RK, Zhou C, Malloy KM, et al. Metformin potentiates the effects of paclitaxel in endometrium cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol 2012;125:458-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green AS, Chapuis N, Lacombe C, et al. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle 2011;10:2115-20. [DOI] [PubMed] [Google Scholar]
- 80.Green AS, Chapuis N, Maciel TT, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood 2010;116:4262-73. [DOI] [PubMed] [Google Scholar]
- 81.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget 2011;2:1322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vakana E, Altman JK, Glaser H, et al. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood 2011;118: 6399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi R, Lin J, Gong Y, et al. The antileukemia effect of metformin in the Philadelphia chromosome - positive leukemia cell line and patient primary leukemia cell. Anticancer Drugs 2015;29:913-22. [DOI] [PubMed] [Google Scholar]
- 84.Rosilio C, Lounnas N, Nebout M, et al. The metabolic perturbators metformin, phenformin and AICAR interfere with the growth and survival of murine PTEN-deficient T cell lymphomas and human T-ALL/T-LL cancer cells. Cancer Lett 2013;336:114-26. [DOI] [PubMed] [Google Scholar]
- 85.Ramos-Peñafiel C, Olarte-Carrillo I, Cerón-Maldonado R, et al. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J Transl Med 2018;16:245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan F, Cheng C, Xiao F, et al. Inhibition of mTORC1/P70S6K pathway by metformin synergistically sensitizes acute myeloid leukemia to Ara-C. Life Sci 2020;243:1172-6. [DOI] [PubMed] [Google Scholar]
- 87.Sabnis H, Bradley HF, Tripathi S, et al. Synergistic cell death in FLT3-ITD positive acute myeloid leukemia by combined treatment with metformin and 6-benzylthioinosine. Leuk Res 2016;50:132-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huai L, Wang C, Zhang C, et al. Metformin induces differentiation in acute promyelocytic leukemia by activating the MEK/ ERK signaling pathway. Biochem Biophys Res Commun 2012;422:398-404. [DOI] [PubMed] [Google Scholar]
- 89.Asik A, Kayabasi C, Ozmen Yelken B, et al. Antileukemic effect of paclitaxel in combination with metformin in HL-60 cell line. Gene 2018;647:213-20. [DOI] [PubMed] [Google Scholar]
- 90.Bruno S, Ledda B, Tenca C, et al. Metformin inhibits cell cycle progression of B-cell chronic lymphocytic leukemia cells. Oncotarget 2015;6:22624-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adekola KU, Dalva Aydemir S, Ma S, et al. Intigating and targeting chronic lymphocytic leukemia metabolism with the human immunodeficiency virus protease inhibitor ritonavir and metformin. Leuk Lymphoma 2015;56:450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi WY, Xiao D, Wang L, et al. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis 2012;3:e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quesada AE, Nguyen ND, Rios A, Brown RE. Morphoproteomics identifies constitutive activation of the mTORC2/Akt and NF-kB pathways and expressions of IGF- 1R, Sirt1, COX-2, and FASN in peripheral T-cell lymphomas: pathogenetic implications and therapeutic options. Int J Clin Exp Pathol 2014;7:8732-9. [PMC free article] [PubMed] [Google Scholar]
- 94.Tseng CH. Metformin is associated with a lower risk of non- Hodgkin lymphoma in patients with type 2 diabetes. Diabetes Metab 2019;45:458-64. [DOI] [PubMed] [Google Scholar]
- 95.Singh A, Gu J, Yanamadala V, et al. Metformin lowers the mitochondrial potential of lymphoma cells and its use during front- line rituximab-based chemo-immunotherapy improves the clinical outcome of diffuse Large B-cell lymphoma. Blood 2013;122:1825-9. [Google Scholar]
- 96.Smyth L, Blunt DN, Gatov E, et al. Statin and cyclooxygenase- 2 inhibitors improve survival in newly diagnosed diffuse large B-cell lymphoma: a large population-based study of 4913 subjects [published online ahead of print, 2020 Apr 17]. Br J Haematol 2020;10.1111/bjh.16635. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Maurer MJ, Larson MC, et al. Impact of metformin use on the outcomes of newly diagnosed diffuse large B-cell lymphoma and follicular lymphoma. Br J Haematol 2019;186:820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keane NA, Glavey SV, Krawczyk J, O’Dwyer M. AKT as a therapeutic target in multiple myeloma. Expert Opin Ther Targets 2014;18:897-915. [DOI] [PubMed] [Google Scholar]
- 99.Zi FM, He JS, Li Y, et al. Metformin displays antimyeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015;356:443-53. [DOI] [PubMed] [Google Scholar]
- 100.Jagannathan S, Abdel-Malek MA, Malek E, et al. Pharmacologic screens reveal metformin that suppresses GRP78- dependent autophagy to enhance the anti-myeloma effect of bortezomib. Leukemia 2015;29:2184-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mishra AK, Dingli D. Metformin inhibits IL-6 signaling by decreasing IL-6R expression on multiple myeloma cells. Leukemia 2019;33:2695-709. [DOI] [PubMed] [Google Scholar]
- 102.Wu W, Merriman K, Nabaah A, et al. The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br J Cancer 2014;111:628-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang SH, Luo S, O'Brian KK, et al. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: a population-based retrospective cohort study [published correction appears in Lancet Haematol 2015;2:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trucco M, Barredo JC, Goldberg J, et al. A phase I window, dose escalating and safety trial of metformin in combination with induction chemotherapy in relapsed refractory acute lymphoblastic leukemia: Metformin with induction chemotherapy of vincristine, dexamethasone, PEG-asparaginase, and doxorubicin. Pediatr Blood Cancer 2018;65:e27224. [DOI] [PubMed] [Google Scholar]
- 105.MacKenzie MJ, Ernst S, Johnson C, Winquist E. A phase I study of temsirolimus and metformin in advanced solid tumours. Invest New Drugs 2012;30:647-52. [DOI] [PubMed] [Google Scholar]


