Abstract
Although senescence is a defining property of euploid mammalian cells, its physiologic basis remains obscure. Previously, cell kinetics properties of normal tissue cells have not been considered in models for senescence. We now provide evidence that senescence is in fact the natural consequence of normal in vivo somatic stem cell kinetics extended in culture. This concept of senescence is based on our discovery that cells engineered to conditionally express the well-recognized tumor suppressor protein and senescence factor, p53, exhibit asymmetric cell kinetics. In vivo, asymmetric cell kinetics are essential for maintenance of somatic stem cells; ex vivo, the same cell kinetics yield senescence as a simple kinetic endpoint. This new “asymmetric cell kinetics model” for senescence suggests novel strategies for the isolation and propagation of somatic tissue stem cells in culture.
INTRODUCTION
Cellular senescence is a defining property of euploid cells in culture [1, 2]. Cultures derived from cells explanted from tissues of a variety of animal species exhibit a finite number of population doublings before undergoing an irreversible arrest of cell replication. Several genes have been implicated as effectors of senescence in culture. These include genetic components of the Rb and p53 growth regulation pathways [3, 4, 5, 6, 7, 8] and genes encoding components of telomerase [9, 10, 11]. The p53 and Rb pathways appear to effect senescence proper, being required for the initial cessation of growth by primary cell cultures [3, 8, 12, 13, 14]. In contrast, the ability of telomerase to extend culture life span is limited to cells that have first escaped senescence [8, 12, 13, 14].
It has been proposed that the requirement for p53 function in senescence reflects a p53-dependent checkpoint arrest in response to the accumulation of eroded chromosome ends due to inadequate telomerase activity in explanted somatic cells [15, 16]. This idea is now in conflict with recent demonstrations that the p53 and telomerase requirements for senescence and immortalization, respectively, are neither synonymous nor contemporaneous [8, 12, 13, 14, 17]. In this paper, we formulate an alternative explanation for the importance of p53 function in cellular senescence. This explanation is based on our discovery that immortal cells reconstituted with normal p53 function exhibit asymmetric cell kinetics (herein and [18, 19, 20]).
During asymmetric kinetics, cell divisions yield one daughter cell that is a long-lived dividing cell like its parent cell. The other daughter is the progenitor of a cell lineage whose constituents mature into differentiated cells that undergo terminal cell cycle arrest. When cycling, the cells in this lineage are called transit cells; in their mature arrested state they are called terminal cells [21, 22]. Asymmetric cell kinetics are essential for the function of somatic tissue stem cells. It allows them to maintain a relative constant fraction in their respective tissue and preserve long-term proliferative potential while simultaneously functioning to renew differentiated tissue cells [23, 24].
Based on the recognition that p53-dependent asymmetric cell kinetics pose an important barrier to the long-term propagation of primary cells in culture, we present a new “asymmetric cell kinetics model” for senescence. The new model accounts for cellular senescence in terms of natural tissue cell kinetics that continue in culture. Because of its basis in fundamental principles of somatic stem cell function, the model suggests specific strategies to achieve two elusive goals in stem cell research, identification and in vitro propagation of somatic stem cells. There is a great need for methods to enumerate somatic stem cells in vivo and expand them as pure populations in vitro for applications in medical science, cancer research, gene therapy, and tissue engineering. The presented asymmetric cell kinetics model for cellular senescence provides the conceptual basis for the development of such methods.
MATERIALS AND METHODS
Cell culture
Temperature-dependent [25] and Zn-dependent [26] p53-inducible cells were maintained as previously described. WI-38 human fibroblasts were supplied by the American Type Culture Collection. Early passage MEFs from p53 “knockout” (ko) mice and wild-type littermates were kindly provide by L. Donehower (Baylor College of Medicine, Houston, TX; 27). WI-38 cells and MEFs were maintained in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with10% fetal bovine serum (JRH Biosciences, Lenexa, KS).
Population growth analyses
Population doubling times were determined by published procedures [18, 19, 26, 27]. Fd values were calculated from population growth data as previously described [19].
Time-lapse analyses
Cells were plated at a density of 104 per 25 cm2 culture flask in 5 ml normal growth medium. After approximately 20 hours of culture, the culture medium was replaced with either fresh normal medium (WI-38 and MEFs) or fresh medium containing 65 μM ZnCl2 (p53-inducible cells and p53-null control cells) that had been equilibrated overnight in a 37°C-5% CO2 incubator. At this cell density, 65 μM ZnCl2 is sufficient to induce p53-dependent growth suppression [26]. After medium replacement, the flasks were returned to a 37°C-5% CO2 incubator for 30 minutes Thereafter, culture flasks were sealed with parafilm and analyzed by time-lapse cinematography or time-lapse digital imaging microscopy.
Time-lapse cinematography was performed in a 37°C warm room with an Olympus IMT-2 inverted light microscope integrated with a Bolex 16 mm camera and Olympus auto-control exposure and frame units. Films were analyzed using an Athena time-lapse movie projector (Carson, CA).
Time-lapse digital imaging microscopy was performed using a Quantix cooled CCD camera that was 3 × binned (from 1317 × 1035 to 439 × 345 pixels). A Nikon TE300 inverted phase-contrast microscope equipped with a temperature controlled stage incubator (accurate within 0.1°C) was used with sealed flasks. A 4 × phase objective with an NA of 0.13 was used to give a scale of 5.1 × 5.1 microns/pixel. Images were acquired by ISEE (Inovision) image acquisition and analysis software. Stored images were analyzed as animation within ISEE.
For both methods of time-lapse analysis, image collecting was begun 0.5–1.5 hours after flasks were sealed with parafilm. A single 4× magnification field of cells was photographed for 80–166 hours (average 103 ± 31 hours) at a time-lapse speed of one frame per 3 minutes. At the start of analyses, photographic fields contained 16 to 43 cells (average 25 ± 8.7).
RESULTS AND DISCUSSION
p53-dependent growth regulation is sensitive to cell density
We used an inducible expression strategy to investigate the function of p53 in cellular senescence. Immortal murine cell lines were genetically engineered to express wild-type murine p53 protein conditionally. The prototype lines were derived from C127 mammary epithelial cells by stable transfection with a gene expression system activated by reduced temperature. When grown at 37°C, their routine culture temperature, these cells express low levels of endogenous wild-type p53. In a previous report, we showed by immunoprecipitation analyses that culturing at 32.5°C induces a 2- to 3-fold increase in p53 expression [25]. A second generation of p53-inducible cell lines was derived by reconstituting spontaneously immortalized p53-null murine embryonic fibroblasts with a wild-type murine p53 cDNA controlled by a modified human metallothionein promoter. We have reported immunoprecipitation studies which show that, by increasing the Zn concentration, the p53 protein level of these cells can be increased in a graded fashion from undetectable to near normal basal levels [26, 27]. For both types of conditional p53-expressing cells, control lines were derived with expression plasmids deleted for p53 coding sequences [25, 26, 27].
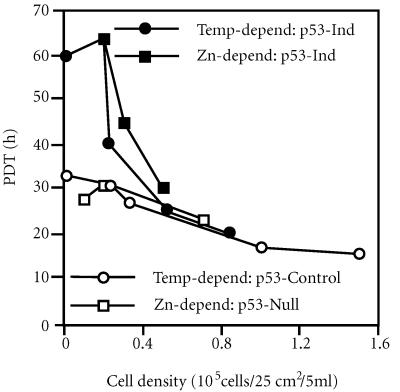
Both types of p53-inducible cells exhibit a decrease in growth rate under conditions of p53 induction [25, 26, 27]. Under the same conditions of temperature and Zn concentration, no significant growth effects occur for control cells. However, we noted that characteristically this difference in the growth between control and p53-inducible cells did not occur at high cell density (Figure 1). The population doubling time (PDT) of control and p53-induced cells is in fact quite similar until cell density falls below about 5 × 104 cells per 25 cm2. Below this density, p53-specific growth suppression is manifest. The PDT of p53-induced cells increases 3-fold over a 4-fold range of decreased cell density (Figure 1, closed symbols). Over the same range, the PDT of control cells (Figure 1, open symbols) is essentially constant. This difference increases further at colony formation densities. At a density of 100 cells per 25 cm2, p53-inducible cells form colonies at 20 to 30 percent the efficiency of control cells [25, 26]. The ability of high cell density to prevent p53-dependent growth suppression is overcome by increased levels of p53 expression. This can be seen for Zn-dependent cultures initiated at the high cell density of 1 × 105 cells per 25 cm2. Whereas 60 μM Zn does not induce p53-dependent cell cycle arrest, 75 μM Zn, which induces a higher level of p53 protein, does so [26]; see also (Figure 2D).
Figure 1.
Reconstitution of normal p53 function restores density-dependent growth to immortal cell lines. Population doubling times (PDT) were determined from growth curve analyses, performed at the indicated initial cell densities, for temperature-dependent (closed circles, lines 1h-3 and 1n-3; 25) and Zn-dependent p53-inducible cells (closed squares, lines Ind-5 and Ind-8; 26) and their respective control cells (open circles, lines 1g-1 and 1m-5, 25; open squares, lines Con-2 and Con-3, 26) grown under p53-inducing conditions (32.5°C and 60 μM Zn, respectively). Growth curves for temperature-dependent cells were performed in triplicate; growth curves for Zn-dependent cells were performed in duplicate or triplicate.
Figure 2.
Density-dependent p53 growth regulation reflects induction of linear population growth kinetics. (A), Linear growth by p53-expressing fibroblasts. Growth data for [19] as a function of the initial cell density of temperature-dependent (closed circles) and Zn-dependent times in (Figure 1). (B), Control cells. (C), p53-inducible cells. (D), Response of linear kinetics to p53 level induced by increasing Zn concentration [26]. The initial cell density ranged from 0.44 to 1.1 × 105 cells/ 25 cm2/5 ml. Two to six independent bars indicate the standard deviation.
Sensitivity to low cell density is a well-described characteristic property of presenescent cultures of primary cells of both murine and human origin [1, 28]. Earlier studies with murine embryo fibroblasts derived from transgenic p53 gene knockout mice suggested that this property might depend on wild-type p53 expression [29]. Thus, the results of the cell density studies suggested to us that cells engineered to restore wild-type p53 function might regain density-dependent cell kinetics mechanisms found in normal tissue cells.
p53 induces density-dependent linear population growth kinetics
Both temperature-dependent [18, 19, 25] and Zn-dependent p53-inducible cells (Figure 2A, closed circles) exhibit linear population growth kinetics under conditions of p53 expression. We have shown previously that nonexponential population growth by cultured cells can be due to the production of daughter cells that undergo a viable arrest at a constant rate [19]. To evaluate the effect of cell density on cell kinetics, Fd, the fraction of new daughter cells that divide, was determined for control and p53-inducible cell cultures initiated at different cell densities (Figure 2B and C).
The method of Fd determination has been described in [19]. Theoretical Fd values range from 0.0 to 1.0. Fd values greater than 0.5 indicate exponential kinetics. A value of 1.0 corresponds to ideal exponential kinetics in which all daughter cells divide with a cell cycle time (generation time, GT) that equals the population doubling time (PDT). Values less than 0.5 indicate a majority of terminal divisions that result in two nondividing cells. Since cell death and cell loss have been shown insignificant in these p53-expressing cell cultures [18, 25, 26], an Fd value near the ideal of 0.5 can indicate one of two distinct cell kinetics programs [22]. It may indicate that dividing cells and nondividing cells occur randomly at equal frequency; or that every division produces one dividing cell and one cell that produces a terminal nondividing cell lineage.
We have shown previously that the linear growth kinetics of temperature-dependent p53-induced mammary epithelial cells are due to the latter cell kinetics program [18, 19]. For these cells, the increase in population doubling time at low cell density reflects a p53-dependent switch to asymmetric cell kinetics. Consistent with this conclusion, the Fd for temperature-dependent p53-induced cells approaches 0.5 as cell density decreases (Figure 2C, closed circles). Zn-dependent p53-expressing embryonic fibroblasts yield a similar extrapolation of Fd as cell density decreases (Figure 2C, open circles). Control cells maintain an Fd near 1.0 at all tested cell densities (Figure 2B), consistent with their density-independent exponential cell kinetics.
The value of Fd depends on the level of p53 expression. p53 expression in the Zn-dependent fibroblasts increases to a maximum as Zn concentration is raised from 60 μM to 90 μM [26]. As shown in Figure 2D (open circles), at the maximum cell density used in growth experiments, Fd decreases to 0.5 as the Zn concentration is increased from 60 μM to 90 μM. In a previous report, we provided evidence that individual cell lineages exhibit either exponential kinetics or asymmetric kinetics, but not mixed patterns [18]. Therefore, Fd values between 1.0 and 0.5 indicate that whereas some cells initiate asymmetric kinetics, others continue to divide with exponential kinetics. Cell density and p53 expression appear to be interdependent determinants of the probability that a cell will adopt asymmetric cell kinetics.
p53 is a genetic determinant of asymmetric cell kinetics in cultured fibroblasts
Time-lapse cinematography and digital imaging microscopy were used to evaluate p53-dependent cell kinetics in several different types of fibroblasts. Division lineages were determined for the p53-inducible murine embryonic fibroblasts; presenescent murine embryonic fibroblasts from wild-type mice (wtMEFs) or mice with a homozygous disruption of the p53 gene (p53koMEFs; 27); and presenescent WI-38 human diploid fibroblasts. Examples of division lineages for cells expressing wild-type p53 are diagrammed in Figure 3.
Figure 3.
Division lineages for p53-expressing fibroblasts. Shown are examples of characteristic asymmetric cell kinetics lineages observed in time-lapse analyses of p53-inducible cells (line Ind-8; 26) grown under inducing conditions (A–D) and presenescent human and murine embryonic fibroblast cell strains grown under routine culture conditions. (E) and (F), WI-38 cells at passage 18 and 26, respectively; (G), wtMEFs at passage 6. Lineages are uneven at end because cell bodies do not indicate passage of time. “Fate uncertain” indicates that a cell either migrated from the field of view or was not observed for sufficient time to assign its division state with confidence.
An intriguing cell kinetics feature is evident from the lineage analyses. When symmetric divisions occur (i.e., divisions producing two dividing daughters), one of the daughters typically undergoes a terminal division (Figure 3C and D). Target gene expression analyses indicate that p53 is active in gene regulation 2 to 5 hours after induction (Y. Liu and J. L. Sherley, submitted), well before the symmetric divisions occur. This pattern is also observed in the pedigrees of WI-38 cells, which express wild-type p53 constitutively (Figure 4E and F). Therefore, it seems unlikely that sub-optimal p53 expression is responsible. In addition, symmetric divisions of this type have been observed at later times in lineages as well. These occasional symmetric divisions in otherwise simple asymmetric lineages may indicate variability in transit cell maturation mechanisms. Whereas, the predominant transit cell division number (TDN) for p53-induced “transit cells” in culture is zero (i.e., terminal arrest without division), at a low frequency, transit cells may mature to a TDN of 1 (i.e., a single division followed by terminal arrest of both daughters).
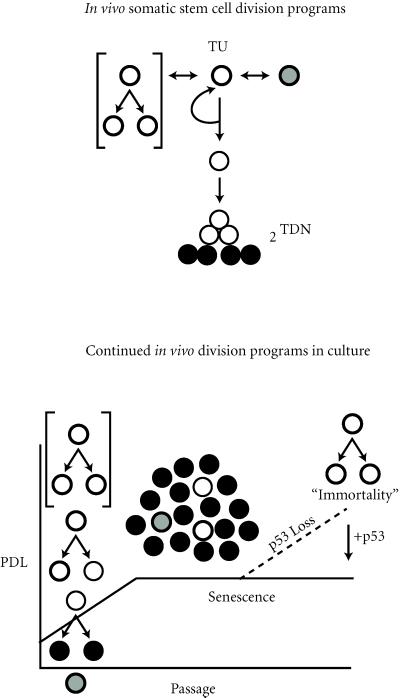
Figure 4.
Asymmetric cell kinetics model for cellular senescence. Top, somatic tissue stem cell (bold-lined circles) turnover unit (TU). In vivo there are three stem cell kinetics states: highly regulated symmetric kinetics that produce two stem cells (bracketed); dormancy (stippled circle); and asymmetric cell kinetics, the most populated stem cell kinetic state in most tissues [21, 22, 23, 24]. TDN, the number of transit cell generations before terminal arrest (closed circles), varies in different tissues from 0.0 to n. 2TDN = 0.5 × number of transit cells in the TU [24]. Bottom, the ex vivo continuation of in vivo asymmetric cell kinetics results in cellular senescence. Because symmetric stem divisions are rare and possibly proapoptotic [31], asymmetric TU kinetics predominate in culture. The dilution of asymmetric stem cells by accumulating nondividing terminal cells is responsible for the observed decline in population doubling rate. Loss of p53 function in cell culture prevents senescence by allowing asymmetric stem cells to divide symmetrically; resulting in the exponential division that characterizes immortalization. In support of this model, reconstitution of normal p53 function in immortal cells restores asymmetric cell kinetics and senescence. PDL, cumulative population doublings.
From the division lineages, we quantified the contribution of different division symmetries to the population kinetics of cells with different levels of p53 expression. We define three cell division symmetries: (1) terminal, both daughters are nondividing cells; (2) symmetric, both daughters divide; and (3) asymmetric, one daughter divides, the other does not (see Table 1A). Asymmetric divisions are rare (9%) in control cell cultures that have exponential population kinetics (i.e., p53-null control cells grown under p53-inducing conditions). In contrast, under conditions for asymmetric cell kinetics, they are 5 times more frequent than either terminal or symmetric divisions. This frequency is 2.5-fold greater than the frequency expected if daughter cells divided randomly with 50 percent efficiency. It is important to factor into this evaluation that, as noted above, symmetric divisions observed under conditions of p53 expression characteristically yield transit cells with a TDN = 1. Therefore, divisions by p53-expressing cells are effectively all asymmetric.
Table 1.
Division symmetry analyses of p53-expressing and p53-null fibroblasts
| A. Division symmetry analysis | |||||
| Symmetry | Con-3 | Ind-8 | p53koMEF | wtMEF | WI-38 |
| (n = 23) | (n = 52) | (n = 12) | (n = 5) | (n = 15) | |
| Terminal | 17% | 15% | 8% | 40% | 80% |
| Asymmetric | 9% | 72% | 0% | 40% | 13% |
| Symmetric | 74% | 13% | 92% | 20% | 7% |
| B. Cell kinetics analysis. | |||||
| Parameter | Con-3 | Ind-8 | p53koMEF | wtMEF | WI-38 |
| [Mean hours ISD (h)] | |||||
| PDT | 29 | 99 | 17 | nid | nid |
| GT | 29 ± 8 (37) | 34 ± 14 (40) | 17 ± 8 (36) | 30 ± 12 (4) | 23 ± 5 (8) |
| IDT | 3 ± 4 (19) | ≥ 23 ± 22 (22)★ | 6 ± 9 (14) | ≥ 15 ± 14 (3) | ≥ 14 ± 16 (5)★ |
The indicated cells were examined by time-lapse cinematography or digital imaging microscopy: Control p53-null, Con-3 cells, and p53-inducible, Ind-8 cells, grown under p53-inducing conditions (65 μM ZnCl2; 26); presenescent murine embryo fibroblasts from either wild-type mice (wtMEF; passage 6; 27) or mice with a homozygous disruption of the p53 gene (p53koMEF; passage 12; 27); presenescent human WI-38 diploid fibroblasts (Data from passage 18 and 26 cultures, which did not differ significantly, were combined.) All cells were plated at an initial density of 1 × 104 cells per 25 cm2 per 5 ml medium. (A) Individual divisions within pedigrees were classified as described in text. n, number of divisions classified. (B) Data from pedigree analyses were used to estimate three cell kinetics parameters. PDT, population doubling time. GT, generation time. IDT, “interdivision time” = GT of second daughter cell to divide-GT of first daughter cell to divide. Calculation of IDT requires that at least one of two new daughter cells divide during the examination period. ★In some cases, it was necessary to use the examination times of second daughter cells, that did not divide during the period of observation, to estimate a minimum value for IDT. nid, no increase in cell number detected. n, number of cells (for GT) or daughter pairs (for IDT) evaluated.
Although mortal cell strains do exhibit asymmetric divisions, they are rare compared to terminal divisions and terminal cells i.e., cells which do not divide at any time during the period of examination). Ninety-two percent (n = 38) and 63 percent (n = 27) of wtMEFs and WI-38 cells, respectively, were terminal cells. These nondividing cells were detected in earlier colony formation analyses [18]; and their contribution to the senescent growth kinetics of mortal cell strains was predicted in earlier mathematical modeling studies [19]. Asymmetric divisions have not been observed in p53koMEF cultures. Ninety-two percent of observed divisions in these cultures were symmetric. This finding is consistent with the observation that cells from both embryonic and adult tissues of p53ko mice are immortal in culture from the time of explant [29, 30].
The division lineage data provided an evaluation of three important cell kinetics parameters (see Table 1B). These are PDT, GT, and IDT, the interdivision time. PDT was calculated under the assumption of exponential growth [19] with cell count data from monitored cultures and nonmonitored cultures grown in parallel. GT was determined from the time elapsed before new daughter cells divided. GT was very similar for all cell types. Only p53koMEFs showed a statistically significant difference in GT (versus wtMEFs; p < 0.05). The GT of p53-null cells was equivalent to their PDT. IDT, the time interval between the division of two daughter cells, for p53-null cells was short compared to their GT. These two relationships define symmetric cell kinetics that produce exponential population kinetics. In contrast, despite having comparable GTs, p53-expressing cells had PDTs that were greater than or equal to 3-fold longer than their GTs. In fact, during the period of analysis, no increase in population size was detected for mortal cell strains that expressed p53. In earlier studies, the PDTs of presenescent WI-38 cells and mortal human intestinal epithelium cells were shown to be 10-fold greater than their respective GTs [18, 19]. This is not surprising, given the large fraction of terminal cells in these cultures. Importantly, the minimum estimate for the IDT of p53-expressing model cells (see ★ in Table 1B) was significantly longer than that of p53-null cells (p < 0.001). Fewer divisions have been evaluated for p53-expressing mortal strains, but the data indicate a longer IDT for these cells as well.
An asymmetric cell kinetics model for cellular senescence
Several observations implicate p53 as an important senescence determinant. p53 protein expression [32] and transcriptional regulation activity [33, 34] are elevated in senescent cells; and p53 gene mutation is frequently associated with spontaneous immortalization of cells in culture [4, 35, 36]. Several diverse proteins that inactivate wild-type p53 also immortalize primary cells in culture [3, 37]. Expression of p21waf1, the p53-induced cyclin kinase inhibitor, has been implicated as an important senescence determinant. Transgenic knockout of the p21waf1 gene is reported to extend the life span of mortal cell strains in culture [6]. More recently, it has been shown that p53-null immortal tumor cell lines undergo senescence when wild-type p53 function is restored [38].
Previously, we have shown that near-physiologic expression of the wild-type p53 protein can induce stem cell-like asymmetric kinetics in immortal epithelial cell lines [18, 19]. In this paper, we extend this seminal finding to characterization of asymmetric cell kinetics restored to immortal p53-null fibroblasts. Recognition of this cellular property of p53 brings a new view to the significance of p53 mutations in human cancers [18, 20] and explains its importance in cellular senescence. We now formulate these new ideas into a cell kinetics model for senescence in culture (Figure 4).
Many features of tissue cell maturation and differentiation are lost in culture. These processes which occur in the transit cell compartment [22] may require complex soluble tissue factor mixtures, extracellular matrix interactions, and cell-cell interactions that are disrupted or lost in culture. A critical feature of transit cell maturation in vivo is limited exponential division. However, the nature of the interactions among transit cell division, maturation, and differentiation are poorly understood. These three properties of transit cells certainly differ from tissue to tissue, and their integration is likely to differ as well. Such differences may account for idiosyncratic features of the initial growth kinetics of cell cultures derived from tissues of different type, different developmental stage, or different species. Our studies (herein; [18, 19]; and unpublished data) indicate that, despite the disruption and loss of in vivo transit cell programs, cells of diverse tissue origin maintain a rudimentary asymmetric cell kinetics program in culture.
In adult somatic tissues of vertebrates, long-lived somatic stem cells, which are responsible for tissue renewal, divide with asymmetric cell kinetics ([21, 22, 23]; see Figure 4, top). Whether asymmetric stem kinetics result from a stochastic differentiation mechanism that applies to pools of stem cells or deterministic cellular programs in individual stem cells continues as a subject of much debate [22]. In the former model, stem cells divide to produce either two stem cell daughters or two differentiating daughters. The latter model is distinguished by individual stem cells that produce both a stem cell daughter and a differentiating daughter cell from a single division. Our clonal analyses [19] and time-lapse studies (herein) demonstrate that individual mammalian cells can divide to produce daughters that are asymmetric for cell kinetics properties.
Although asymmetric cell kinetics are an essential property of somatic stem cells, these cells have a rich cell kinetics repertoire (see Figure 4, top). They can undergo highly regulated symmetric expansions in developing adult tissues and during wound repair. Some stem cells may also lie dormant for long periods before initiating division in response to specific developmental cues, as in reproductive tissues like the breast. However, the predominant kinetic state of somatic stem cells in vivo is an asymmetric division program [21, 22, 23]. After each asymmetric stem cell division, one daughter cell divides with the same kinetics as its stem cell parent, but the other daughter gives rise to a terminal differentiating lineage. The second daughter may differentiate immediately; or depending on the tissue, it may undergo a finite number of successive symmetric divisions to give rise to a larger pool of differentiating transit cells [22]. Transit cell divisions ultimately result in mature, differentiated, nondividing cells. The tissue cell renewal unit composed of an asymmetric stem cell, transit cells, and mature nondividing cells is called a turnover unit (TU; 24). A TU produces a fixed number of nondividing differentiated tissue cells per stem cell division. This regulated production of constituent cells allows vertebrates to continually renew adult tissues while maintaining relatively constant tissue cell mass [23].
Tissue explants are in effect preparations of disrupted tissue TUs. Therefore, primary cell cultures initially divide quite actively due to the division of asymmetric stem cells and symmetric transit cells. The initial cultures will also contain maturing nondividing cells. With continued passage, the greater accumulation of mature nondividing cells will lead to dilution of dividing cell types, producing an apparent cessation of growth (Figure 4, bottom). Such dilution of stem cells does not occur in vivo, because nondividing terminal cells are preferentially removed from tissues at rates that match their production. The cessation of growth in culture will develop gradually and end with an exponential increase in population doubling time that reflects dilution of TUs with linear accumulation kinetics. The exponential increase in PDT in senescing cultures has been described previously [39].
The presented asymmetric cell kinetics model leads to three predictions regarding aging and cancer. First, immortalization, the process by which rare cells in culture escape senescence, must be due to processes that inactivate the asymmetric division program of explanted TUs. Second, cancer, a disease characterized by disruption of TU asymmetric kinetics [23, 24], will be promoted by mutations in genes that control asymmetric cell kinetics. Third, the association between cellular senescence and aging is predicted to reflect loss in the number and/or function of somatic tissue TUs with increasing age. In this paper, we have shown that the first two predictions are realized by the demonstration that the p53 tumor suppressor gene, a well-known genetic factor for senescence and cancer, is a regulator of asymmetric cell kinetics (see Figure 4, bottom). The presented studies pave the way for evaluations of the final prediction.
Future applications in stem cell research
The model cells and cell kinetics principles described in this paper have potential for application to several areas of biomedicine, including stem cell research, gene therapy, and cancer drug discovery. Somatic stem cells are the ideal delivery vessel for therapeutic genes [40, 41, 42, 43]. However, the physical identification of somatic stem cells and their propagation in culture has not been accomplished [21, 22, 40, 41, 42, 43]. To the extent that asymmetric cell kinetics are unique to somatic stem cells, gene-profile investigations with the model cells may yield specific markers for stem cells.
The asymmetric cell kinetics model of senescence predicts that one obligate barrier to the propagation of somatic stem cells in culture will be their inherent asymmetric cell kinetics. As such, an essential requirement for long-term culture of somatic stem cells will be the ability to induce them to proliferate with sustained exponential kinetics. Here, we show that cell density may be an important factor that increases the frequency of symmetric stem cell divisions. The cell density required for this effect is less than 1/10 of the density of cells in confluent monolayers. Therefore, in the effective range of cell density, there is a significant opportunity for stem cell expansion.
The mechanism by which increased cell density suppresses asymmetric cell kinetics is not yet known. However, it seems unlikely that cell cycle regulatory proteins like p27Kip1 will be involved, because p27Kip1 appears to be required for cell cycle arrest when exponentially dividing cells reach confluent cell densities [44]. We speculate that the cell density effect may have an important in vivo correlate. In many tissues, stem cells are compartmentalized in a way that physically and chemically insulates them from other stem cells [23]. Under conditions of tissue damage, these barriers are breached, allowing for the possibility that stem cells then stimulate the symmetric expansion of other stem cells by paracrine mechanisms. Such a mechanism would promote tissue repair. In vitro, a similar paracrine mechanism might be responsible for the switch from asymmetric to exponential cell kinetics observed at increased cell densities.
Our earlier studies predict that agents that modulate p53 function may also have the ability to promote stem cell propagation in culture. We have shown that purine nucleotide precursors, that overcome p53-induced inhibition of guanine ribonucleotide biosynthesis, prevent p53-dependent asymmetric cell kinetics [18, 25, 26]. Given the range of tissues in which p53 appears to function, these compounds may prove effective for in vitro culture of stem cells from diverse somatic tissues. They may also have direct application to age-related tissue deficits as well. Conversely, compounds that interfere with the growth of the model cells when they divide with symmetric kinetics, but not when they divide with asymmetric kinetics, hold promise as particularly selective anticancer agents [45].
Acknowledgments
ACKNOWLEDGEMENT
We thank J. Lansita, J. Merok, and Dr. D. Lauffenburger for reviewing the manuscript and offering helpful comments. We are grateful to John Gibbas for expert flow cytometry assistance. We give special thanks to Dr. A. G. Knudson for his steadfast encouragement throughout these studies and to Dr. G. Illiakis for facilitating the initial time-lapse studies. We are indebted to L. A. Donehower for generously providing early passage wtMEFs and p53koMEFs. This work was supported by research grants from the National Institutes of Health National Cancer Institute (CA-58619, CA-56690, and CA-06927); the Pew Scholars Program in Biomedical Sciences; U. S. Healthcare, Inc.; and an appropriation from the Commonwealth of Pennsylvania.
References
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Rogan EM, Bryan TM, Hukku B, et al. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker NJ, Bryan TM, Bonnefin P, et al. Involvement of RB-1, p53, p16INK4, and telomerase in immortalisation of human cells. Oncogene. 1995;11:971–976. [PubMed] [Google Scholar]
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Xu H-J, Zhou Y, Ji W, Perng G-S, et al. Reexpression of the retinoblastoma protein in tumor cells induces senescence and telomerase inhibition. Oncogene. 1997;15:2589–2596. doi: 10.1038/sj.onc.1201446. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Gallaway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Weinrich SL, Pruzan R, Ma L, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nature Gen. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. Bumps on the road to immortality. Nature. 1997;396:23–24. doi: 10.1038/23825. [DOI] [PubMed] [Google Scholar]
- Counter CM, Hahn WC, Wei W, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1999;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen TL, Leibowitz G, Levine F. Telomerase activity is sufficient to allow transformed cells to escape from crisis. Mol Cell Biol. 1999;19:1864–1870. doi: 10.1128/mcb.19.3.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynford-Thomas D. Telomeres, p53, and cellular senescence. Oncol Res. 1996;8:387–398. [PubMed] [Google Scholar]
- Vaziri H, West MD, Allsopp RC, et al. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Sherley JL, Stadler PB, Johnson DR. Expression of the wild-type p53 antioncogene induces guanine nucleotide-dependent stem cell division kinetics. Proc Natl Acad Sci USA. 1995;92:136–140. doi: 10.1073/pnas.92.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley JL, Stadler PB, Stadler JS. A quantitative method for the analysis of mammalian cell proliferation in culture in terms of dividing and non-dividing cells. Cell Prolif. 1995;28:137–144. doi: 10.1111/j.1365-2184.1995.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Sherley JL. The p53 tumor suppressor gene as regulator of somatic stem cell renewal division. Cope. 1996;12:9–10. [Google Scholar]
- Poldosky DK. Regulation of intestinal epithelial proliferation a few answers, many questions. Am J Physiol. 1993;264:G179–G186. doi: 10.1152/ajpgi.1993.264.2.G179. [DOI] [PubMed] [Google Scholar]
- Loeffler M, Potten CS. Stem Cells. Harcourt Brace & Co.; San Diego, CA: 1997. Stem cells and cellular pedigrees—a conceptual introduction; pp. 1–28. [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Herrero-Jimenez P, Thilly G, Southam PJ, et al. Mutation, cell kinetics, and subpopulations at risk for colon cancer in the United States. Mutation Res. 1998;400:553–578. doi: 10.1016/s0027-5107(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Sherley JL. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. J Biol Chem. 1991;266:24815–24828. [PubMed] [Google Scholar]
- Liu Y, Riley LB, Bohn SA, Boice JA, Stadler PB, Sherley JL. Comparison of BAX, WAF1, and IMP dehydrogenase regulation in response to wild-type p53 expression under normal growth conditions. J Cellular Physiology. 1998;177:364–376. doi: 10.1002/(SICI)1097-4652(199811)177:2<364::AID-JCP18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bohn SA, Sherley JL. Inosine-5'-monophosphate dehydrogenase is a rate-determining factor for p53-dependent growth regulation. Mol Biol Cell. 1998;9:15–28. doi: 10.1091/mbc.9.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M, Sands AT, Weiss RS, et al. In vitro growth characterization of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993;8:2457–2467. [PubMed] [Google Scholar]
- Tsukada T, Tomooka Y, Takai S, et al. Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene. 1993;8:3313–3322. [PubMed] [Google Scholar]
- Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. British J Cancer. 1998;78:993–1003. doi: 10.1038/bjc.1998.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulju KS, Lehman JM. Increased p53 protein associated with aging in human diploid fibroblasts. Exp Cell Res. 1995;217:336–345. doi: 10.1006/excr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Atadja P, Wong H, Garkavtsev I, Veillette C, Raibowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J, Haughton M, Blaydes J, Gire V, Wynford-Thomas D, Nd Whyllie F. Evidence that transcriptional activation by p53 plays a direct role in the induction of cellular senescence. Oncogene. 1996;13:2097–2104. [PubMed] [Google Scholar]
- Harvey DM, Levine AJ. p53 alteration is a common event in the spontaneous immortalization of primary balb/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Shay JW, Tomlinson G, Piatyszek MA, Gollahon LS. Spontaneous in vitro immortalization of breast epithelial cells from a patient with Li-Fraumeni syndrome. Mol Cell Biol. 1995;15:425–432. doi: 10.1128/mcb.15.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JA, Wyllie FS, Wynford-Thomas D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene. 1994;9:1885–1889. [PubMed] [Google Scholar]
- Sugrue MM, Shin DY, Lee SW, Aaronson SA. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel LE. Aging of clones of mammalian cells. Nature. 1973;243:441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- Miller DA. Human gene therapy comes of age. Nature. 1992;357:455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Wilson JM. Vehicles for gene therapy. Nature. 1993;365:691–692. doi: 10.1038/365691a0. [DOI] [PubMed] [Google Scholar]
- Brenner MK. Gene transfer to hematopoietic cells. New Engl J Med. [DOI] [PubMed]
- Ross G, Erickson R, Knorr D, et al. Gene therapy in the United States: A five-year status report. Hum Gene Ther. 1996;7:1781–1790. doi: 10.1089/hum.1996.7.14-1781. [DOI] [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27kipl for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Sherley JL, Riley LB. Cell lines and methods for screening growth regulatory compounds. U S Patent. 1998;741(5):646. [Google Scholar]