Abstract
(1) Background: Organophosphorus pesticides (OPPs) are major chemicals used in agriculture for eradication of insecticides/pesticides. Unfortunately, the longtime exposure of human beings to OPPs could lead to metabolic disorder such as high blood pressure, hyperglycemia, overweight or dyslipidemia. The aim of this research is to evaluate the possible metabolic dysregulations as a consequence of chronic OPPs exposure to individuals in Cameroon and Pakistan. (2) Methods: Blood samples were collected from 300 participants in each country, into ethylenediaminetetraacetic acid (EDTA) tubes. The samples were extracted with solid phase extraction (methanol/water) for analysis of OPPs with gas chromatography mass spectrometry. The spectrophotometry and Enzyme Linked ImmunoSorbent Assay (ELISA) were used to measure the hepatic, renal, pancreatic and cardiovascular functions. The atherogenic index (AI) was also determined in OPPs exposed and nonexposed cohorts. (3) Results: The results showed the presence of malathion, parathion and chlorpyrifos OPPs residues in Cameroonians, and malathion and chlorpyrifos in Pakistani samples, respectively. Elevated Body Mass Index (BMI), insulin, blood glucose, dyslipidemia and hypertension were noted in OPPs chronic exposed groups. In addition, dysregulated liver and kidney function profiles were observed in all participants regardless of gender and age groups. (4) Conclusions: The study concludes that both the study cohorts showed several metabolic dysregulations attributable to chronic exposure to a mixture of OPPs which may provide precursors for establishment of metabolic syndrome and other chronic diseases. Further different extended population-based studies are suggested to understand the differential metabolic dysfunctions caused by structurally different OPPs mixtures exposure.
Keywords: metabolic disorder, pesticides, organophosphorus, dyslipidemia, liver malfunctioning
1. Introduction
Development of metabolic diseases as a consequence of exposure to pesticides has been extensively reported in the literature [1]. Pesticides are hazardous for human health because they can impair metabolic homeostasis [2]. Epidemiological studies have provided evidence for linkage of pesticide exposure with endocrine disruption, insulin resistance, nonalcoholic fatty liver disease, obesity, diabetes and so forth [3,4]. Of these metabolic diseases, metabolic syndrome (MetS) refers to a combination of interdependent metabolic abnormalities whose clinical significance and exact etiology remains elusive. This syndrome is clinically diagnosed when the subject has at least three of the following symptoms: high blood pressure, high blood sugar, overweight or dyslipidemia. The combination of these symptoms can lead to complications such as heart disease, stroke, and liver and/or kidney disease [5]. The prevalence of MetS has been significantly increasing worldwide over the past decades [5]. For example, in the United States, the prevalence of MetS has increased from 32.9% in 2004 to 34.7% in 2015; in Korea from 24.9% in 1998 to 31.3% in 2007; and in Malaysia, where the last recorded prevalence of metabolic syndrome was 42.5% in 2013 [6]. Similarly, in Pakistan, more than 18% of the population suffers from metabolic diseases [7]. In sub-Saharan Africa, it was projected that MetS affected around 4.5% of the population in 2011 and that is projected to increase to 19% by 2035 [8]. Multiple factors have been shown to be responsible for the appearance and progression of these pathologies, which can be either of endogenous origin such as susceptibility genes, or exogenous, that is, a set of environmental factors. Among the environmental factors, pesticides represent significant environmental contaminants and are the cause of a significant amount of mortality and morbidity in areas of high exposure [9]. Epidemiological studies also show that the prevalence of cancer, birth defects, infertility problems, neurological problems or a weakened immune system are significantly increasing in pesticide-exposed individuals [1]. Organophosphorus compounds are heavily used as pesticides (about 50 percent of the global use) and are expected to increase considerably by 2030, which in turn will pose a risk of direct health consequences [10,11]. The harmful effects of several organophosphorus pesticides on the etiology of metabolic diseases have been demonstrated by a growing number of studies involving both humans and laboratory animals [12,13]. The results of those studies have often come up with controversial results; for example, Li et al. [1] showed that exposure to OPPs (organophosphate pesticides) increases several biochemical parameters including glucose, LDL cholesterol, insulin, HbA1c and HOMA-IR, predisposing individuals to serious metabolic diseases such as diabetes, cardiovascular disease, kidney disease, stroke, liver disease and atherosclerosis. However, other studies showed that despite OPPs exposure, the biochemical parameters remained unchanged (normoglycemia [14], normo-insulinemia [15]) or decreased (hypoglycemia [16], hypoinsulinemia [14]).
It is noteworthy that most of the studies on metabolic diseases are in context with acute exposure, mostly to the organochlorine group of pesticides. Almost negligible reports may be traced to the incidence of metabolic diseases in the context of chronic exposure of the mixture of organophosphates. The present work has studied the possible metabolic dysregulation due to chronic exposure of two or more OPPs pesticides on distinct cohorts of Pakistani and Cameroonian origin. To the best of our knowledge, this is the first such study with geographically and genetically distinct populations in connection with chronic exposure to OPPs and development of metabolic disorder.
2. Materials and Methods
2.1. Study Design and Sampling
A cross-sectional study with random sampling from known pesticide-exposed agriculture areas was selected. Across the board, the study participants were recruited from cotton farming areas where organophosphates are mainly applied. In Cameroon, the agricultural towns located in the North (Mora and Figuil) and in the South (Njobe and Sa’a) were selected on this basis. In Pakistan, the cities of Depalpur and Multan were selected. Ethical approvals, CIIT/BIO/ERB/19/90 and No. 488/CE/CNERSH were obtained from the ethical review board of CUI, Islamabad and Cameroon, respectively, for the study. The study followed the principles of the Declaration of Helsinki on human trials. Research participants were informed about the goal of the study and permissions were taken in writing before sample collections. The age group of recruited volunteers was 18 to 40 years. In addition, they were inhabitants of the pesticide-sprayed areas for at least six months and were exposed to OPPs directly (farmers and sprayers) or indirectly (people living in the spraying areas) during the period. Subject volunteers using products other than OPPs and those suffering from diseases such as diabetes, neurological diseases, cancers and all chronic pathologies were excluded from the study.
The participants whose blood tests showed the presence of pesticides other than OPPs were also excluded from the study. Samples from unexposed subjects were collected in nonagricultural areas with less risk of exposure to pesticides (thus limiting indirect exposure).
Sociodemographic characteristics and parameters such as age, sex, height, weight, tobacco/narcotic consumption, history of exposure, use of protective equipment, duration of activity, and professional data were collected using a questionnaire. MetS has been diagnosed when the subject exhibits at least three of the following symptoms: high blood pressure, high blood sugar, overweight or dyslipidemia, as previously reported [5].
Using convenient nonprobability sampling, 300 blood samples were collected from exposed persons in each country. The sample size was calculated using the Lorenz formula for a cross-sectional study according to the prevalence of metabolic disease in the Cameroonian and Pakistani populations.
Blood samples were taken once from all participants by venipuncture and transported to the laboratory using an icebox. In the laboratory, the blood was centrifuged at 3500 rpm for 10 min at 25 °C, and the resulting plasma was then stored at −80 °C until further analysis. The samples were analyzed at the IMPM laboratory, Yaoundé, Cameroon, and in the laboratories of the Department of Biosciences of COMSATS Islamabad University, Pakistan.
2.2. Assesment of Pesticide Residues
Pesticides were screened in plasma by gas chromatography coupled with high resolution mass spectrometry (GC-MS System 5975C Agilent, Milton Keynes U.K.) as described in [17]. The types of pesticides were further confirmed from farmers (Cotton), the pesticides in stock, and after the inspection of the fields and dumping points of abandoned pesticide packages in villages. The column was calibrated at 120 °C for 1 min, then set to increase its temperature up to 290 °C. The temperatures of the injection orifice and the interface were stabilized at 250 °C, and the temperature of the detector at 290 °C. The injection mode without division and helium with a flow rate of 1.0 mL/min were used as carrier gas. Calibration assay and internal quality control were performed by addition of known concentrations of commercial solutions of chlorpyrifos, malaoxon, parathion, and internal standards (azobenzene) to drug-free whole blood. The six concentrations used for calibration curves of chlorpyrifos were 0.15, 0.5, 1.0, 1.25, 2.5 and 5.0 mg/L. In the case of malaoxon and parathion, these were 0.17, 0.5, 1.0, 1.25, 2.5, 5.00 mg/L and 0.13, 0.5, 1.0, 1.25, 2.5, 5.00 mg/L, respectively. The concentration of internal standard was 0.25 mg/L. Electron impact (EI) mass spectra of pesticides and internal standard were recorded by total ion monitoring. For quantification, the surface/peak ratios of the target ion of different insecticides and azobenzene (m/z 182) were calculated as a function of the concentration of the substance. The data were recorded only when the peak was clearly visible, and the signal-to-noise ratio was greater than three [18]. Limit of detection (LOD) and limit of quantification (LOQ) were estimated for the validation of this method. LOD and LOQ were determined by analyzing drug-free blood samples fortified with known drug concentrations. Each concentration was measured in five replicates. LOD was defined as the lowest concentration giving a response of three times the average baseline noise defined from five unfortified samples. LOQ was defined as the lowest observed concentration within 10% of the theoretical concentration with acceptable qualifier ion ratios for all five replicates. Ten blank blood samples were analyzed for chromatographic interference with each analyte.
2.3. Cholinesterase Activity
Organophosphates exposure was also assessed by measuring cholinesterases, that is red blood cell-acetylcholinesterase (RBC-AChE) and butyrylcholinesterase (BChE). RBC-AChE and BChE activity were determined using Ellamn’s method modified by Worek et al. [19] using Specord 50 plus spectrophotometer Number 233H1280C (Analytic Jena, Thuringia, Germany). RBC-AChE was measured from whole blood, and plasma was taken for BChE. Blood and plasma dilutions were prepared according to the method described by Worek et al. [19]. Spectrophotometric measurement was taken at 436 nm (ε = 10.6 × 103 M−1 cm−1) at 37 °C for AChE and BChE. For hemoglobin, absorption was noted at 546 nm (ε = 10.8 × 103 M−1 cm−1).
2.4. Biochemical Assesment
Plasma glucose was determined by an enzymatic and colorimetric method using glucose oxidase (GOD) according to the manufacturer’s protocols (Biolabo Kit 80009, from BIOLABO SA, Les Hautes Rives, 02160, Maizy, France). Creatinin (using Biolabo kit 80107), Blood urea nitrogen (using Biolabo kit 80221), Aspartate transaminase (using Biolabo kit 80025), Alanine transaminase (using Biolabo kit 80027), Gamma-glutamyl-transpeptidase (γGT) (using Biolabo kit 81110), Alkaline phosphatase (using Biolabo kit 92214), Lactate dehydrogenase (using Biolabo kit 92111), Bilirubin total and direct (using Biolabo kit 80403), and HDL (using Biolabo Kit 90206) were determined by enzymatic assays according to the manufacturer’s protocols provided by the kits of BIOLABO SA. The triglycerides were also determined in plasma by an enzymatic and colorimetric method using the Biolabo kit from Biolabo.
LDL Cholesterol and the atherogenic index (AI) were determined according to the formula provided by HDL kit:
LDL = Total cholesterol – TG/5 – HDL.
AI = LDL Cholesterol/HDL Cholesterol [20].
The Lipoprotein a Lp (a) was determined using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (IBL-RE59011, IBL, Hamburg, Germany) according to the manufacturer’s protocol. Insulin was also determined by the ELISA assay (IBL-Kit RE 53171. Paraoxonase 1 (PON1; kit E-EL, Houston, TX, USA) was determined by the ELISA technique according to the manufacturer’s protocol.
2.5. Statistical Analysis
Statistical analysis of the data was performed using Stata 15 and R 3.2.0 software. Regression and correlation models were used to establish a linear relationship between dependent and independent variables. The Shapiro–Wilk test was used to check the normality of the distribution of variables. A one-way ANOVA test and post hoc tests were performed where needed. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated to establish the margins of significance. The differences were considered statistically significant if p value < 0.05.
3. Results
3.1. Socio-Demographic and Clinical Characteristics of OPPss Exposed Study Subjects
The distribution of the population by socio-demographic characteristics shows that the average age was 26.57 years in Cameroon against 28.22 years in Pakistan. The sex ratio (Male/Female) was 2.16 in favor of men in the Pakistani cohort against 0.90 in the Cameroonian group. The most represented level of education was the primary level among Pakistanis and the secondary/university level among Cameroonians. The variation of anthropometric and clinical parameters as a function of exposure (Table 1) reveals that in the exposed group, 40.45% of Pakistan’s population were overweight (BMI ≥ 25), 39.09% were obese, 62.27% had high blood pressure, and 12.73% take tobacco at a regular frequency. In the exposed population of Cameroon, 38.64% were overweight and 40.91% were obese, 76.36% had high blood pressure, and 4.54% had a high frequency of smoking.
Table 1.
Socio-demographic and Anthropometric data of our study population.
| Groups | Pakistan | Cameroon | ||
|---|---|---|---|---|
| Exposed | Non-Exposed | Exposed | Non-Exposed | |
| Age (Mean ± SD) | 28.22 ± 10.12 | 27.80 ± 10.40 | 26.57 ± 8.74 | 26.31 ± 9.07 |
| N (%) | N (%) | N (%) | N (%) | |
| Gender | ||||
| Male | 154 (70) | 58 (64.44) | 104 (47.27) | 45 (47.37) |
| Female | 66 (30) | 32 (35.56) | 116 (52.73) | 50 (52.63) |
| BMI, frequency | ||||
| Underweight | 10 (4.55) | 4 (4.44) | 8 (3.63) | 5 (5.26) |
| Normal range | 35 (15.91) | 11 (12.22) | 37 (16.91) | 15 (15.79) |
| Overweight | 89 (40.45) | 45 (50) | 85 (38.64) | 41 (43.16) |
| Obese | 86 (39.09) | 30 (33.34) | 90 (40.91) | 34 (35.79) |
| High Blood Pressure: mm Hg | ||||
| Yes | 137 (62.27) | 56 (62.22) | 168 (76.36) | 65 (68.42) |
| No | 83 (37.73) | 34 (37.78) | 52 (23.64) | 30 (31.58) |
| Smoking | ||||
| Yes | 48 (21.82) | 11 (12.22) | 10 (4.54) | 4 (4.21) |
| No | 172 (78.18) | 79 (87.78) | 210 (95.46) | 91 (95.79) |
N = total number of samples, Results are in mean ± SD.
3.2. OPPs Exposure Markers
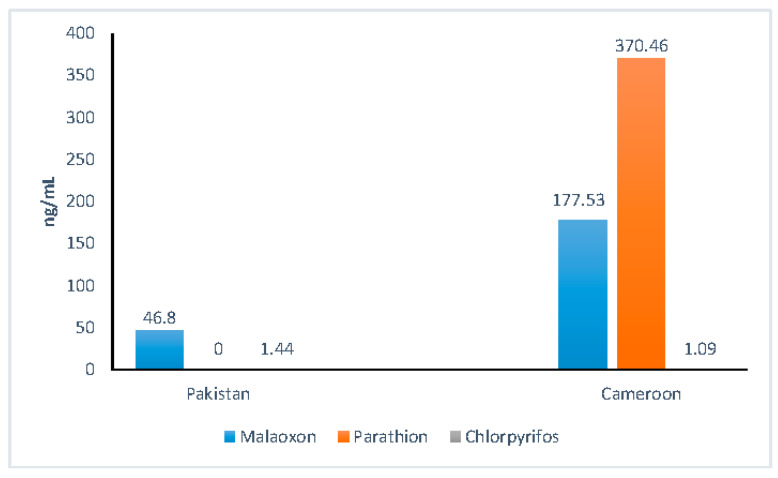
OPPs exposure markers, that is AChE and BChE, were decreased in exposed individuals in both countries with inhibition of 62.5% for Pakistanis and 54.54% for Cameroonians. Inhibition was more pronounced in the Pakistanis group. Table 2 shows the variation of AChE, BChE and paraoxonase (PON-1) in the two population groups. A significant decrease of PON-1 enzyme was observed in both populations groups. The determination of OPPs residues by GC-MS (Figure 1) revealed the presence of three OPPs: malathion (177.53 ± 99.74 ng/mL), parathion (370.46 ± 129.81 ng/mL) and chlorpyrifos (1.09 ± 0.44 ng/mL) in Cameroonian OPPs-exposed individuals. In the Pakistani exposed population, only malathion and chlorpyrifos were detected with values of 48.80 ± 30.12 ng/mL and 1.44 ± 0.98 ng/mL, respectively. Apart from biochemical confirmation from blood samples of the participants, agriculture areas were visited, and the empty containers of pesticides used in the area were inspected and verbally confirmed by the spraying personnel.
Table 2.
Variation of Cholinergic/PON1 enzymes of OPPs exposed and nonexposed groups in two populations.
| Parameters | Pakistan | Cameroon | ||||
|---|---|---|---|---|---|---|
| Exposed N = 220 |
Unexposed N = 90 |
p-Value | Exposed N = 220 |
Unexposed N = 95 |
p-Value | |
| RBC-AChE mU/µmol Hb |
0.13 ± 0.04 b | 0.30 ± 0.07 d | ˂0.01 | 0.10 ± 0.06 a | 0.23 ± 0.10 c | ˂0.01 |
| BChE µmol/l/min |
0.021 ± 0.010 b | 0.031 ± 0.007 c | ˂0.05 | 0.018 ± 0.011 a | 0.023 ± 0.010 bc | ˂0.05 |
| PON1 ng/mL |
5.49 ± 1.01 b | 15.11 ± 4.20 d | ˂0.01 | 2.86 ± 1.47 a | 11.83 ± 2.17 c | ˂0.01 |
a,b,c,d = statistical difference of post-hoc test (Anova one way). Results are in mean ± SD; the result are statistically significant when p ≤ 0.05).
Figure 1.
Distribution of blood pesticides levels in Pakistani and Cameroonian groups.
3.3. Biochemical Tests: Lipid Profile
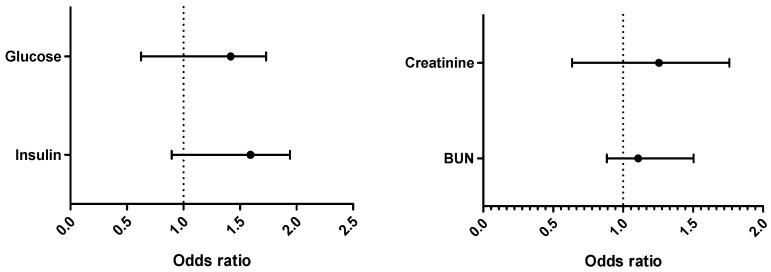
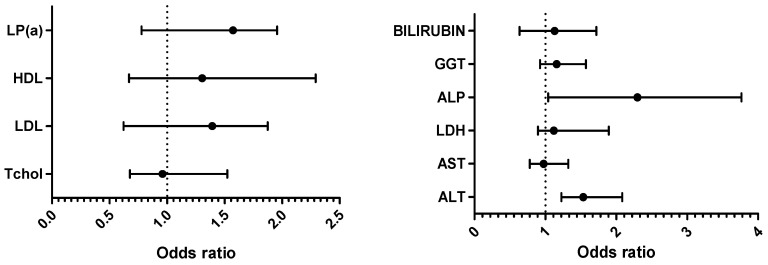
Variations in the biochemical parameters are shown in Table 3. All the tested lipid profile parameters were significantly affected in OPPs-exposed groups with metabolic syndrome risks. However, HDL was significantly different in the Cameroonian group. Logistic regression of biochemical parameters in exposed populations (Figure 2) shows that in Pakistani exposed individuals, the values of total cholesterol were 3.14 times higher compared to Cameroonian exposed individuals. The same observation was made for LDL (Pakistani values were 3.23 times higher).
Table 3.
Lipid profile of the OPPs exposed and nonexposed groups in two populations.
| Pakistan | Cameroon | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposed and MetS N = 100 |
Non-Exposed and MetS N = 90 |
Exposed but No MetS N = 120 |
p Value | Exposed and MetS N = 108 |
Non-Exposed and MetS N = 95 |
Exposed but No MetS N = 112 |
p Value | |
| Tchosl * (mg/dL) | 260 ± 60 b | 200 ± 51 a | 200 ± 54 a | <0.001 | 300 ± 58 b | 251 ± 62 a | 263 ± 80 a | <0.001 |
| HDL *# (mg/dL) |
46 ± 6 a | 64 ± 9 c | 58 ± 12 a | <0.001 | 39 ± 4 a | 48 ± 11 a | 47 ± 17 a | >0.05 |
| LDL * (mg/dL) |
170 ± 7 b | 130 ± 10 a | 136 ± 8 a | <0.001 | 201 ± 64 b | 184 ± 39 a | 180 ± 54 a | <0.05 |
| Lp(a) * (mg/dL) |
30.12 ± 2.30 c | 15.31 ± 2.49 a | 22.22 ± 5.23 b | <0.01 | 31.25 ± 7.20 b | 10.05 ± 6.30 a | 30.04 ± 6.31 b | <0.001 |
N = total number of samples, Tchosl = total cholesterol; HDL = high density lipoprotein; LDL = low density lipoprotein; Lp(a) = Lipoparticule a. a,b,c = statistical difference of post hoc test (Anova one way). Results are in mean ± SD. * statistically significant (p ≤ 0.05), # only Cameroonian group is statistically significant (p ≤ 0.05).
Figure 2.
Binomial logistic regression (BLR) of all the biochemical parameters: Exposed Pakistan vs. exposed Cameroon (Tchosl = total cholesterol; HDL = high density lipoprotein; LDL = low density lipoprotein; Lp(a) = Lipoparticule a; AI = Arterogenic Index; BUN = Blood urea nitrogen; AST = aspartate transaminase; ALT = alanine transaminase; LDH = lactate deshydrogenase; GGT = gamma-glutamyl transferase; ALP = alkalin phosphatase; DBil = direct bilirubin; TBil = total bilirubin).
3.4. Biochemical Tests: Hepatic Function
Table 4 depicts the hepatic function in terms of biochemical tests. AST, ALT and LDH were noticeably raised (p < 0.05) in both population groups, while Gamma glutamyl transferase (γGT) was statistically different in the Cameroonian group only. Logistic regression (Figure 2) shows that ALP was 4 times and bilirubin was 3.06 times higher in the Pakistani than in the Cameroonian group.
Table 4.
Hepatic function variables in OPPs exposed and nonexposed groups in two populations.
| Pakistan | Cameroon | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposed and MetS N = 100 |
Non-Exposed and MetS N = 90 |
Exposed but No MetS N = 120 |
p Value | Exposed and MetS N = 108 |
Non-Exposedand MetS N = 95 |
Exposed but No MetS N = 112 |
p Value | |
| AST * (IU/L) |
45.52 ± 30.24 b | 26.09 ± 7.90 a | 44.80 ± 31.37 b | <0.01 | 47.20 ± 16.10 b | 26.50 ± 9.72 a | 50.61 ± 20.01 b | <0.01 |
| ALT * (IU/L) |
50.44 ± 33.45 b | 26.55 ± 5.50 a | 50.17 ± 30.06 b | <0.01 | 52.02 ± 10.28 b | 30.30 ± 13.56 a | 55.46 ± 14.43 b | <0.01 |
| LDH * (IU/L) |
280.34 ± 60.80 b | 208.61 ± 59.14 a | 264.19 ± 75.50 b | <0.01 | 300.82 ± 40.20 b | 220.54 ± 24.61 a | 262.40 ± 38.17 ab | <0.01 |
| γGT # (IU/L) |
27.70 ± 9.84 a | 27.69 ± 10.10 a | 29.79 ± 10.87 a | >0.05 | 28.91 ± 10.40 a | 30.50 ± 16.90 a | 35.80 ± 17.27 b | <0.05 |
| ALP (IU/L) |
70.14 ± 23.14 a | 68.79 ± 16.20 a | 67.02 ± 20.20 a | >0.05 | 64.23 ± 13.20 a | 60.82 ± 14.78 a | 60.52 ± 14.12 a | >0.05 |
| D Bil (mg/L) |
4.19 ± 2.11 a | 3.61 ± 0.56 a | 4.19 ± 2.30 a | >0.05 | 4.38 ± 1.49 a | 4.57 ± 1.70 a | 4.27 ± 1.81 a | >0.05 |
| T Bil (mg/L) |
10.09 ± 4.90 a | 10.40 ± 1.92 a | 9.59 ± 4.90 a | >0.05 | 10.83 ± 3.68 a | 11.07 ± 2.83 a | 11.44 ± 3.27 a | >0.05 |
N = total number of samples, AST = aspartate transaminase; ALT = alanine transaminase; LDH = lactate deshydrogenase; GGT = gamma-glutamyl transferase; ALP = alkalin phosphatase; DBil = direct bilirubin; TBil = total bilirubin. Results are mean ± SD. * statistically significant (p ≤ 0.05). # only Cameroonian group is statistically significant (p ≤ 0.05). a,b = statistical difference of post hoc test (Anova one way).
3.5. Biochemical Tests: Pancreatic and Renal Function
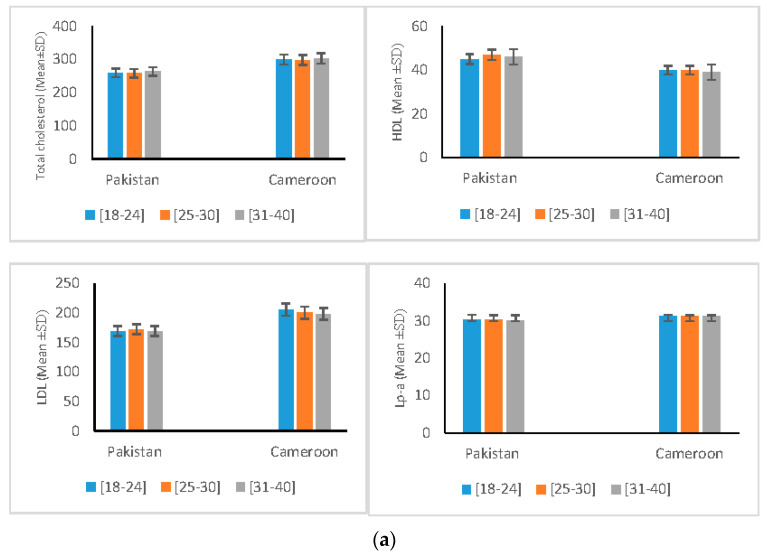
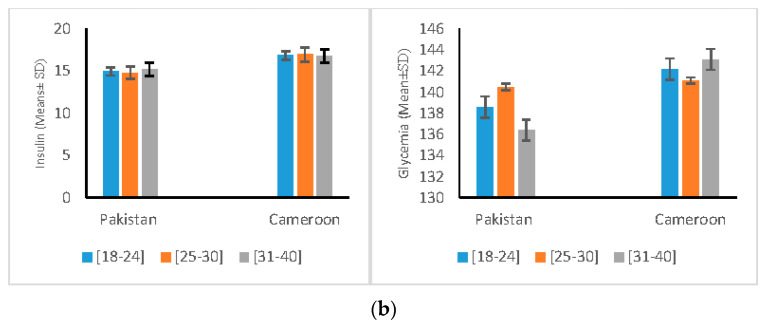
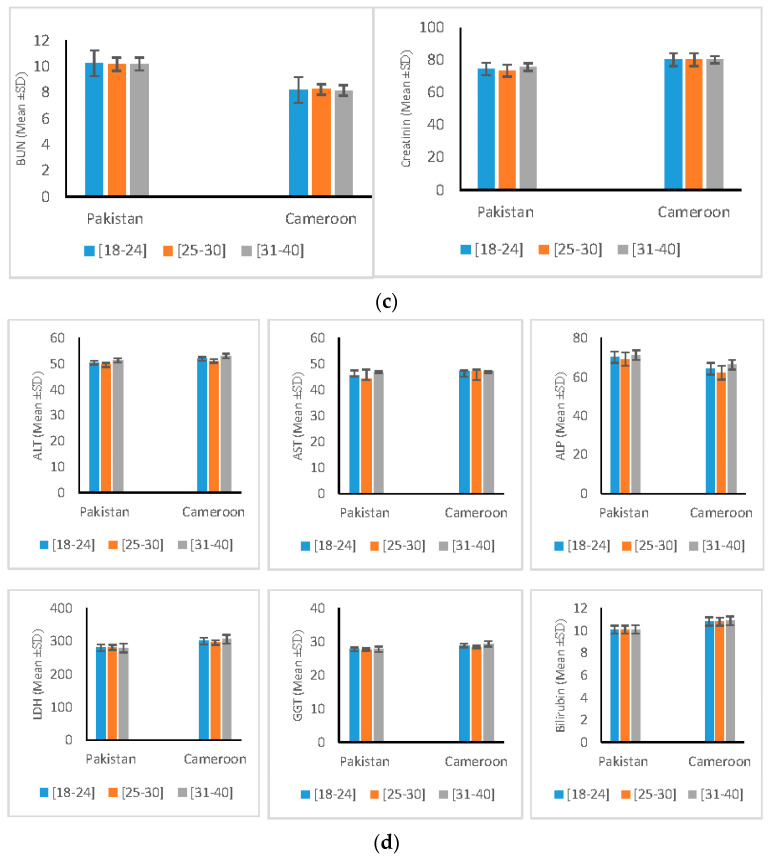
All the studied parameters for pancreatic and renal function (Table 5) show the predisposition and risk of metabolic dysregulation in OPPs exposed groups. No differences in the extent of metabolic dysregulation were observed with regard to gender (in male and female). Additionally, no significant differences in the metabolic dysregulation were noted in any age groups of the two populations (Figure 3).
Table 5.
Pancreatic and renal function related variables in two population groups.
| Pakistan | Cameroon | |||||||
|---|---|---|---|---|---|---|---|---|
| Exposed and MetS N = 100 |
Non-Exposed and MetS N = 90 |
Exposed but No MetS N = 120 |
p Value | Exposed and MetS N = 108 |
Non-Exposed and MetS N = 95 |
Exposed but No MetS N = 112 |
p Value | |
| Glucose * (mg/dL) |
138.50 ± 12.22 b | 125.25 ± 10.50 a | 123.56 ± 8.81 a | <0.05 | 142.12 ± 1.51 a | 135.02 ± 1.18 a | 131.01 ± 2.12 a | >0.05 |
| Insulin * (µIU/mL) |
14.97 ± 2.71 a | 12.46 ± 1.50 a | 11.57 ± 3.64 a | <0.05 | 16.91 ± 0.70 a | 17.35 ± 0.73 a | 17.10 ± 0.66 a | >0.05 |
| BUN *# (mmol/L) |
10.20 ± 3.90 b | 6.14 ± 1.3 a | 9.12 ± 4.18 b | <0.01 | 8.20 ± 2.70 a | 9.06 ± 1.20 a | 8.15 ± 1.99 a | >0.05 |
| Creatinine * (µmol/L) |
74.49 ± 20.14 b | 60.40 ± 14.70 a | 70.28 ± 20.33 b | <0.05 | 80.20 ± 20.01 b | 65.23 ± 12.02 a | 78.15 ± 18.80 b | <0.05 |
BUN = Blood urea nitrogen, N = total number of samples, a,b = statistical difference of post hoc test (Anova one way). * statistically significant at p ≤ 0.05. # not significant in Cameroon group.
Figure 3.
Variation of Biochemical Profile according to age groups, (a) Variation of Lipid Profile Parameters according to age group, (b) Variation of Diabetic Related Variables Stratified according to age group, (c) Variation of Kidney Function Parameters according to age group, (d) Variation of Hepatic Function Profile p according to age group.
4. Discussion
Use of pesticides has emerged as a major a public health problem globally. Mortality and diseases due to pesticide toxicity have been overwhelmingly documented in the literature [21,22,23,24,25]. More than 80% of insecticides/pesticides used worldwide are organophosphates compounds [26]. In agricultural practice, pesticides are usually applied in combinations and not singly. Organophosphates pesticides are usually used in combination with different OPPs for better pesticide effect. An accumulating number of studies have implicated mixtures of organophosphates in development of chronic ailments as a result of acute or prolonged exposure [27,28,29,30,31]. Metabolic dysregulation has been reported earlier for OPPs as a toxic manifestation but mainly for acute OPPs toxicity. Albeit, long-term OPPs exposure and its possible effects on metabolic homeostasis has been scarcely explored. The aim of the current study was therefore to assess the association of long-term human exposure to OPPs and metabolic disorder in the two population cohorts.
The present study showed the presence of different OPPs in the studied populations, three in Cameroon (Malaoxon, Parathion, and Chlorpyrifos) and two in Pakistan (Malaoxon and Chlorpyrifos). An overt inhibition of RBC-AChE (54.54% in Cameroon and 62.50% in Pakistan) was found, which is considered evidence for OPPs exposure. Decrease of AChE activity is a main characteristic feature and an essential biological marker of OPPs exposure as noted by previous studies [10,32,33]. In fact, organophosphorus compounds can potentially bind to cholinesterase and block their hydrolysis, leading to their rapid accumulation resulting in neurological symptoms (loss of coordination, convulsions, paralysis and possibly death) [34].
Concerning metabolic-related biochemical variables in exposed individuals (with MetS and without MetS), our study revealed a statistically significant increase of LDL, lipoparticles, Creatinine, AST and ALT, and decreased HDL compared to unexposed participants in both countries. The study also observed that exposed people had higher levels of insulin and blood sugar in the Pakistani population. The metabolic dysregulations were more evident in the exposed group with MetS. These metabolic imbalances may be due to the fact that chronic exposure to OPPs may lead to endocrine disruptions, which in turn deregulate enzymes of several metabolic pathways [14,35]. An increase in all these metabolic-related variables (LDL, Lpa, glucose, BMI and blood pressure) has been linked with heightened risk of cardiovascular disease (CVD) and metabolic diseases such as type 2 diabetes. These results support a growing number of studies that have reported that exposure to pesticides such as OPPs would result in the increased blood glucose levels, insulin resistance, and dyslipidemias that are precursors of cardio–metabolic disorders, albeit with conflicting findings [15,36,37,38,39,40,41,42,43,44]. No effect on glucose metabolism after OPPs exposure has also been reported [45]. In the present study, a significant increase in parameters of renal function (creatinine and BUN) and liver function (ALT, AST, LDH, ALP and Bilirubin) was observed in OPPs-exposed subjects in both countries. This increase may be explained by one of two phenomena: the first is the direct toxic effect due to the accumulation of low-dose OPPs over a long period of exposure; the second is that induced type 2 diabetes (T2D) and/or the direct consequences of T2D may lead to the progressive onset of MetS, nonalcoholic fatty liver disease and renal failure [46,47]. The logistic regression model applied to the Pakistani and Cameroonian groups revealed that Pakistani exposed subjects were more likely to develop metabolic diseases compared to other groups. This difference may be due to the fact that the two populations were subject to different environmental conditions (types of pesticides, exposure level, exposure time); the tolerance threshold for toxicity is also different due to genetic differences in the two populations [48,49,50,51].
Although the use and presence of OPPs have been confirmed in the studied groups, certain potential confounding factors such as diet could not be addressed and may constitute a limiting factor of the study.
5. Conclusions
This study showed that exposure to a mixture of OPPs significantly affected the metabolic system and may have led to metabolic syndrome in both populations. It is suggested that more randomized controlled studies using larger sample sizes, genetically distinct different cohorts and better exposure monitoring are still needed to ascertain the role of OPPs exposure in initiating metabolic disorders.
Author Contributions
M.N.L.J. and S.M.N. designed the study, collected the samples, performed experiments. N.J.L., R.H. and S.T.A.S. reviewed and provided necessary expertise for analysis of biochemical parameters. K.K. and M.V. provided material support and critical review of the manuscript. S.M.N. supervised the study. All authors have revised the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge COMSATS University Islamabad, Islamabad Campus and The World Academy of Sciences (TWAS) for providing the opportunity and resources to complete the research work, which is part of the Ph.D. dissertation of Mbah Ntepe Leonel Javeres. It was supported by UHK project VT2019-2021. This study was also partially supported by grants from the Ministry of Health of the Czech Republic (FN HK 00179906) and the Charles University in Prague, Czech Republic (PROGRES Q40/15).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of CUI, Islamabad (CIIT/BIO/ERB/19/90) and Cameroon (No. 488/CE/CNERSH) respectively.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li J., Ren F., Li Y., Luo J., Pang G. Chlorpyrifos Induces Metabolic Disruption by Altering Levels of Reproductive Hormones. J. Agric. Food Chem. 2019;67:10553–10562. doi: 10.1021/acs.jafc.9b03602. [DOI] [PubMed] [Google Scholar]
- 2.Peris-Sampedro F., Blanco J., Cabré M., Basaure P., Guardia-Escote L., Domingo J.L., Sánchez D.J., Colomina M.T. New mechanistic insights on the metabolic-disruptor role of chlorpyrifos in apoE mice: A focus on insulin- and leptin-signalling pathways. Arch. Toxicol. 2018;92:1717–1728. doi: 10.1007/s00204-018-2174-3. [DOI] [PubMed] [Google Scholar]
- 3.Al-Eryani L., Wahlang B., Falkner K.C., Guardiola J.J., Clair H.B., Prough R.A., Cave M. Identification of Environmental Chemicals Associated with the Development of Toxicant-associated Fatty Liver Disease in Rodents. Toxicol. Pathol. 2015;43:482–497. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahlang B., Falkner K.C., Gregory B., Ansert D., Young D., Conklin D.J., Bhatnagar A., McClain C.J., Cave M. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J. Nutr. Biochem. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baygi F., Jensen O.C., Qorbani M., Farshad A., Salehi S.A., Mohammadi-Nasrabadi F., Asayesh H., Shidfar F. Prevalence and associated factors of cardio-metabolic risk factors in Iranian seafarers. Int. Marit Health. 2016;67:59–65. doi: 10.5603/IMH.2016.0013. [DOI] [PubMed] [Google Scholar]
- 6.Ching Y.K., Chin Y.S., Appukutty M., Gan W.Y., Ramanchadran V., Chan Y.M. Prevalence of Metabolic Syndrome and Its Associated Factors among Vegetarians in Malaysia. Int. J. Environ. Res. Public Health. 2018;15:2031. doi: 10.3390/ijerph15092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal Hydrie M.Z., Shera A.S., Fawwad A., Basit A., Hussain A. Prevalence of Metabolic Syndrome in Urban Pakistan (Karachi): Comparison of Newly Proposed International Diabetes Federation and Modified Adult Treatment Panel III Criteria. Metab. Syndr. Relat. Disord. 2009;7:119–124. doi: 10.1089/met.2008.0055. [DOI] [PubMed] [Google Scholar]
- 8.Ewane M.E., Mandengue S.-H., Ahmadou G., Tamba S.M., Dzudie A., Luma H.-N. Dépistage des maladies cardiovasculaires et des facteurs de risque dans une cohorte de 270 Camerounais: Effets des activités physiques et sportives: Screening for cardiovascular diseases and risk factors in a cohort of 270 Cameroon inhabitants: Effect of physical and sport activities. Méd. Mal. Métab. 2011;5:655–658. [Google Scholar]
- 9.Bettiche F., Grunberge O., Belhamra M. Contamination of Water by Pesticides under Intensive Production System. [(accessed on 27 October 2019)];2017 Available online: http://revues.univ-biskra.dz/index.php/cds/article/view/2189.
- 10.Czajka M., Matysiak-Kucharek M., Jodłowska-Jędrych B., Sawicki K., Fal B., Drop B., Kruszewski M., Kapka-Skrzypczak L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ. Res. 2019;178:108685. doi: 10.1016/j.envres.2019.108685. [DOI] [PubMed] [Google Scholar]
- 11.Nurulain S., Szegi P., Tekes K., Nh Naqvi S. Antioxidants in Organophosphorus Compounds Poisoning. Arch. Ind. Hyg. Toxicol. 2013;64:169–177. doi: 10.2478/10004-1254-64-2013-2294. [DOI] [PubMed] [Google Scholar]
- 12.Rathish D., Agampodi S.B., Jayasumana M.A.C.S., Siribaddana S.H. From organophosphate poisoning to diabetes mellitus: The incretin effect. Med. Hypotheses. 2016;91:53–55. doi: 10.1016/j.mehy.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Santed F., Colomina M.T., Herrero Hernández E. Organophosphate pesticide exposure and neurodegeneration. Cortex. 2016;74:417–426. doi: 10.1016/j.cortex.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Pakzad M., Fouladdel S., Nili-Ahmadabadi A., Pourkhalili N., Baeeri M., Azizi E., Sabzevari O., Ostad S.N., Abdollahi M. Sublethal exposures of diazinon alters glucose homostasis in Wistar rats: Biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic Biochem. Physiol. 2013;105:57–61. doi: 10.1016/j.pestbp.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Navarro G., Lopez-Aceves T., Rojas-Ochoa A., Fernandez Mejia C. Effect of dichlorvos on hepatic and pancreatic glucokinase activity and gene expression, and on insulin mRNA levels. Life Sci. 2006;78:1015–1020. doi: 10.1016/j.lfs.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Arsenault A.L., Gibson M.A., Mader M.E. Hypoglycemia in Malathion-treated chick embryos. Can. J. Zool. 1975;53:1055–1057. doi: 10.1139/z75-122. [DOI] [PubMed] [Google Scholar]
- 17.Pérez J.J., Williams M.K., Weerasekera G., Smith K., Whyatt R.M., Needham L.L., Barr D.B. Measurement of pyrethroid, organophosphorus, and carbamate insecticides in human plasma using isotope dilution gas chromatography–high resolution mass spectrometry. J. Chromatogr. B. 2010;878:2554–2562. doi: 10.1016/j.jchromb.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armbruster D.A., Pry T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008;29:S49. [PMC free article] [PubMed] [Google Scholar]
- 19.Worek F., Mast U., Kiderlen D., Diepold C., Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin. Chim. Acta. 1999;288:73–90. doi: 10.1016/S0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- 20.Youmbissi T.J., Djoumessi S., Nouedoui C., Ndobo P., Meli J. Profil lipidique d’un groupe d’hypertendus camerounais noirs africains. Méd. d’Afrique Noire. 2001;48:305–314. [Google Scholar]
- 21.Amir A., Haleem F., Mahesar G., Abdul Sattar R., Qureshi T., Syed J.G., Ali Khan M. Epidemiological, Poisoning Characteristics and Treatment Outcomes of Patients Admitted to the National Poisoning Control Centre at Karachi, Pakistan: A Six Month Analysis. [(accessed on 31 January 2020)];Cureus. 2019 doi: 10.7759/cureus.6229. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6929263/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang C.-S., Yang K.-W., Yen C.-M., Lin C.-L., Kao C.-H. Risk of Seizures in Patients with Organophosphate Poisoning: A Nationwide Population-Based Study. Int. J. Environ. Res. Public Health. 2019;16:3147. doi: 10.3390/ijerph16173147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwesiga B., Ario A.R., Bulage L., Harris J., Zhu B.-P. Fatal cases associated with eating chapatti contaminated with organophosphate in Tororo District, Eastern Uganda, 2015, case series. BMC Public Health. 2019;19:767. doi: 10.1186/s12889-019-7143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul K.C., Ling C., Lee A., To T.M., Cockburn M., Haan M., Ritz B. Cognitive decline, mortality, and organophosphorus exposure in aging Mexican Americans. Environ. Res. 2018;160:132–139. doi: 10.1016/j.envres.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyasu M., Dida T., Worku Y., Worku S., Shafie M. Acute poisonings during pregnancy and in other non-pregnant women in emergency departments of four government hospitals, Addis Ababa, Ethiopia: 2010–2015. Trop. Med. Int. Health. 2017;22:1350–1360. doi: 10.1111/tmi.12940. [DOI] [PubMed] [Google Scholar]
- 26.Karunarathne A., Gunnell D., Konradsen F., Eddleston M. How many premature deaths from pesticide suicide have occurred since the agricultural Green Revolution? Clin. Toxicol. 2019;58:227–232. doi: 10.1080/15563650.2019.1662433. [DOI] [PubMed] [Google Scholar]
- 27.Casida J.E., Baron R.L., Eto M., Engel J.L. Potentiation and neurotoxicity induced by certain organophosphates. Biochem. Pharmacol. 1963;12:73–83. doi: 10.1016/0006-2952(63)90011-X. [DOI] [PubMed] [Google Scholar]
- 28.Frawley J.P., Fuyat H.N., Hagan E.C., Blake J.R., Fitzhugh O.G. Marked Potentiation in Mammalian Toxicity from Simultaneous Administration of Twoanticholinesterase Compounds. J. Pharmacol. Exp. Ther. 1957;121:96–106. [PubMed] [Google Scholar]
- 29.Mbah J., Raza S., Judith N., Anwar F., Habib R., Batool S., Nurulain S.M. Mixture of Organophosphates Chronic Exposure and Pancreatic Dysregulations in Two Different Population Samples. [(accessed on 2 January 2021)];Front. Public Health. 2020 doi: 10.3389/fpubh.2020.534902. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7655777/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbah L.J., Habib R., Judith Laure N., Raza S., Nepovimova E., Kuca K., Batool S., Muhammad Nurulain S. Oxidative Stress and Analysis of Selected SNPs of ACHE (rs 2571598), BCHE (rs 3495), CAT (rs 7943316), SIRT1 (rs 10823108), GSTP1 (rs 1695), and Gene GSTM1, GSTT1 in Chronic Organophosphates Exposed Groups from Cameroon and Pakistan. Int. J. Mol. Sci. 2020;21:6432. doi: 10.3390/ijms21176432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mbah N.L.J., Habib R., Judith N., Iqbal M., Nepovimova E., Kuca K., Batool S., Nurulain S.M. Analysis of PON1 gene polymorphisms (rs662 and rs854560) and inflammatory markers in organophosphate pesticides exposed cohorts from two distinct populations. Environ. Res. 2020;191:110210. doi: 10.1016/j.envres.2020.110210. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz-Quezada M.T., Lucero B.A., Iglesias V.P., Muñoz M.P., Cornejo C.A., Achu E., Baumert B., Hanchey A., Concha C., Brito A.M., et al. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: A review. Int. J. Occup. Environ. Health. 2016;22:68–79. doi: 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vidyasagar J., Karunakar N., Reddy M.S., Rajnarayana K., Surender T., Krishna D.R. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J. Pharmacol. 2004;36:76. [Google Scholar]
- 34.Nurulain S.M., Ojha S., Tekes K., Shafiullah M., Kalasz H., Adem A. Efficacy of N-Acetylcysteine, Glutathione, and Ascorbic Acid in Acute Toxicity of Paraoxon to Wistar Rats: Survival Study. [(accessed on 21 October 2018)];Oxid. Med. Cell Longev. 2015 doi: 10.1155/2015/329306. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4488549/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pournourmohammadi S., Ostad S.N., Azizi E., Ghahremani M.H., Farzami B., Minaie B., Larijani B., Abdollahi M. Induction of insulin resistance by malathion: Evidence for disrupted islets cells metabolism and mitochondrial dysfunction. Pestic Biochem. Physiol. 2007;88:346–352. doi: 10.1016/j.pestbp.2007.02.001. [DOI] [Google Scholar]
- 36.Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A.M., Wilks M.F., Spandidos D.A., et al. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review) Int. J. Mol. Med. 2016;38:1012–1020. doi: 10.3892/ijmm.2016.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamath V., Rajini P. Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicology. 2007;231:137–146. doi: 10.1016/j.tox.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 38.Lee D.-H., Steffes M.W., Sjödin A., Jones R.S., Needham L.L., Jacobs D.R., Jr. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS ONE. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mostafalou S., Abdollahi M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017;91:549–599. doi: 10.1007/s00204-016-1849-x. [DOI] [PubMed] [Google Scholar]
- 40.Panahi P., Vosough-Ghanbari S., Pournourmohammadi S., Ostad S.N., Nikfar S., Minaie B., Abdollahi M. Stimulatory Effects of Malathion on the Key Enzymes Activities of Insulin Secretion in Langerhans Islets, Glutamate Dehydrogenase and Glucokinase. Toxicol. Mech. Methods. 2006;16:161–167. doi: 10.1080/15376520500191623. [DOI] [PubMed] [Google Scholar]
- 41.Rattner B.A., Franson J.C. Methyl parathion and fenvalerate toxicity in American kestrels: Acute physiological responses and effects of cold. Can. J. Physiol. Pharmacol. 1984;62:787–792. doi: 10.1139/y84-129. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues M.A.L.R., Puga F.R., Chenker E., Mazanti M.T. Short-term effect of malathion on rats’ blood glucose and on glucose utilization by mammalian cells in vitro. Ecotoxicol. Environ. Saf. 1986;12:110–113. doi: 10.1016/0147-6513(86)90046-1. [DOI] [PubMed] [Google Scholar]
- 43.Vadhana M.S.D., Carloni M., Nasuti C., Fedeli D., Gabbianelli R. Early life permethrin insecticide treatment leads to heart damage in adult rats. Exp. Gerontol. 2011;46:731–738. doi: 10.1016/j.exger.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Sharaf A.A., Mohaned A.M., Abu M.E.-G., Mousa A.H. Control of snail hosts of bilharziasis in Egypt. 3. Effect of the organophosphorous insecticide, dursban, on carbohydrate metabolism of the snails Biomphalaria alexandria and Bulinus truncatus. Egypt J. Bilharz. 1975;2:49–61. [PubMed] [Google Scholar]
- 45.Rezg R., Mornagui B., El-Arbi M., Kamoun A., El-Fazaa S., Gharbi N. Effect of subchronic exposure to malathion on glycogen phosphorylase and hexokinase activities in rat liver using native PAGE. Toxicology. 2006;223:9–14. doi: 10.1016/j.tox.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 46.Brůha R. Non-Alcoholic Fatty Liver Disease. Vnitr Lek. 2019;65:571–575. [PubMed] [Google Scholar]
- 47.Tong J., Guo J.-J. Key molecular pathways in the progression of non-alcoholic steatohepatitis. Eur. Rev. Med. Pharmacol. Sci. 2019;23:8515–8522. doi: 10.26355/eurrev_201910_19165. [DOI] [PubMed] [Google Scholar]
- 48.Mossa A., Refaie A., Ramadan A. Effect of Exposure to Mixture of Four Organophosphate Insecticides at No Observed Adverse Effect Level Dose on Rat Liver: The Protective Role of Vitamin C-SciAlert Responsive Version. [(accessed on 31 October 2019)];2011 Available online: https://scialert.net/fulltextmobile/?doi=rjet.2011.323.335.
- 49.Castorina R., Bradman A., McKone T.E., Barr D.B., Harnly M.E., Eskenazi B. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: A case study from the CHAMACOS cohort. Environ. Health Perspect. 2003;111:1640–1648. doi: 10.1289/ehp.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández A.F., Parrón T., Tsatsakis A.M., Requena M., Alarcón R., López-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Rizzati V., Briand O., Guillou H., Gamet-Payrastre L. Effects of pesticide mixtures in human and animal models: An update of the recent literature. Chem. Biol. Interact. 2016;254:231–246. doi: 10.1016/j.cbi.2016.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.