Abstract
Objective
To determine if oxygen saturation (out‐of‐hospital SpO2), measured by New York City (NYC) 9‐1‐1 Emergency Medical Services (EMS), was an independent predictor of coronavirus disease 2019 (COVID‐19) in‐hospital mortality and length of stay, after controlling for the competing risk of death. If so, out‐of‐hospital SpO2 could be useful for initial triage.
Methods
A population‐based longitudinal study of adult patients transported by EMS to emergency departments (ED) between March 5 and April 30, 2020 (the NYC COVID‐19 peak period). Inclusion required EMS prehospital SpO2 measurement while breathing room air, transport to emergency department, and a positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) reverse transcription polymerase chain reaction test. Multivariable logistic regression modeled mortality as a function of prehospital SpO2, controlling for covariates (age, sex, race/ethnicity, and comorbidities). A competing risk model also was performed to estimate the absolute risks of out‐of‐hospital SpO2 on the cumulative incidence of being discharged from the hospital alive.
Results
In 1673 patients, out‐of‐hospital SpO2 and age were independent predictors of in‐hospital mortality and length of stay, after controlling for the competing risk of death. Among patients ≥66 years old, the probability of death was 26% with an out‐of‐hospital SpO2 >90% versus 54% with an out‐of‐hospital SpO2 ≤90%. Among patients <66 years old, the probability of death was 11.5% with an out‐of‐hospital SpO2 >90% versus 31% with an out‐of‐hospital SpO2 ≤ 90%. An out‐of‐hospital SpO2 level ≤90% was associated with over 50% decreased likelihood of being discharged alive, regardless of age.
Conclusions
Out‐of‐hospital SpO2 and age predicted in‐hospital mortality and length of stay: An out‐of‐hospital SpO2 ≤90% strongly supports a triage decision for immediate hospital admission. For out‐of‐hospital SpO2 >90%, the decision to admit depends on multiple factors, including age, resource availability (outpatient vs inpatient), and the potential impact of new treatments.
1. INTRODUCTION
1.1. Background
The 2019 novel coronavirus (COVID‐19) pandemic has had a devastating effect on the United States. By mid‐December 2020, there were over 16 million cases and over 350,000 deaths. 1 In New York City (NYC), there have been over 325,000 cases, the first diagnosed on March 1, 2020. 1 , 2 On April 6, 2020 during the peak of the crisis in NYC, the Fire Department of the City of New York 9‐1‐1 Emergency Medical Service (FDNY‐EMS) responded to 5944 9‐1‐1 calls, of which, there were 305 out‐of‐hospital cardiac arrests and hospitals reported 3327 intensive care patients of whom 2437 were intubated on mechanical ventilators—daily totals that represent substantial increases from pre‐COVID‐19 numbers. 3
1.2. Importance
The COVID‐19 pandemic has stretched hospital and ICU bed surge capacity to its limit. Given limited resources, it is important to identify those most in need of hospitalization. During the pandemic, factors independently associated with out‐of‐hospital cardiac arrest included older age, non‐white race/ethnicity, hypertension, and diabetes 3 and for in‐hospital deaths included these factors and cardiovascular disease, chronic obstructive pulmonary disease, and obesity. 4 , 5 , 6 , 7 , 8 , 9 Although these comorbid factors are useful in identifying at‐risk patients, the discovery of an easily obtainable pathophysiologic measure of the disease process may improve the accuracy of the initial triage decision as to hospital admission, close remote home monitoring, or routine follow‐up care. Recently, in 2 studies, 1 from New York University (NYU) and 1 from Wuhan, China, hypoxemia, measured as an inpatient, was found to be an independent predictor of COVID‐19 related in‐hospital mortality. 10 , 11 Hypoxemia is a common physiologic finding in severe COVID‐19 related pneumonia, sepsis, myocardial dysfunction, or embolic disease. Oxygen saturation measured by pulse oximetry (SpO2) is inexpensive and can be obtained easily and rapidly.
1.3. Goals of this investigation
The primary goal of this study was to determine if oxygen saturation, measured by SpO2 in the prehospital EMS setting, was an independent predictor of COVID‐19 in‐hospital mortality and length of stay, in which case, out‐of‐hospital SpO2 could provide added accuracy to improve patient triage decisions with respect to hospitalization.
2. METHODS
2.1. Study design and setting
This is a population‐based longitudinal study using data from adult patients (≥18 years old) transported by FDNY‐EMS to any of the 11 NYC Health + Hospitals (NYC H+H) emergency departments between March 5 and April 30, 2020, with follow‐up through June 14, 2020. March 5, 2020 was chosen as our start date as that was the first day NYC H+H began using real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) tests for COVID‐19 diagnosis confirmation. April 30, 2020 was chosen as the enrollment end date as FDNY‐EMS call volume approached its pre‐COVID‐19 baseline. The Montefiore Medical Center/Albert Einstein College of Medicine Institutional Review Board approved this study and waived the need for informed consent based on minimal risk; participants would not be adversely affected; and the research could not be practicably carried out without the waiver. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
This study is unique because it includes the largest municipal EMS agency and the largest public healthcare system in the United States. Prehospital data came from the FDNY‐EMS system, which serves a population of >8.4 million and responds to over 1.5 million medical calls annually. Hospital data came from NYC H+H, which operates 11 acute care hospitals, serving over 1.1 million patients annually.
Study inclusion required survival to ED evaluation, a positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RT‐PCR test and a prehospital SpO2 while breathing room air, obtained by FDNY‐EMS and documented in the FDNY‐EMS electronic medical record. The FDNY‐EMS electronic medical record is a commercially available product (Stryker, Kalamazoo, MI) that has been customized for NYC. FDNY‐EMS used infrared digital pulse oximetry with advanced life support units using the Phillips HealthStart MTX monitor with SpO2 sensor M1191B and basic life support units using the Curaplex Finger Tip SPO2 monitor, models MD300C28, and MD69100MS. In cases where >1 prehospital SpO2 was obtained, the lowest level recorded was included in the analysis. Prehospital SpO2 measurement is added to the medical record manually.
Between March 5 and April 30, 2020, there was a total of 1856 FDNY‐EMS transports to NYC H+H hospitals that met study inclusion criteria. Nearly all were for unique patients (n = 1852; 99.8%); an additional 2 patients had 2 EMS/H+H records each. Patients found in cardiac arrest were excluded (n = 20). Out‐of‐hospital SpO2 measures < 60% were also excluded as percentages below this level could have been due to hypoperfusion, hypothermia, hyperventilation, acid‐base disturbances, and dyshemoglobinemias—all magnified by the shape of the hemoglobin‐saturation curve during hypoxemia. 12
2.2. Outcomes
In 1673 patients, we examined the association of prehospital SpO2 obtained from the FDNY‐EMS database with in‐hospital mortality and length of stay, after controlling for the competing risk of death from the NYC H+H database. Mortality was coded as a binary variable (yes/no). Patients who died after being discharged from the hospital (n = 66) or those that remained hospitalized (n = 10) at the end of follow‐up were considered alive in our mortality analyses. Length‐of‐stay was computed as the difference in the number of hours between the date and time of discharge (from inpatient unit or ED) or death, whichever came first, and the date and time of ED arrival. Patients still in the hospital at the end of follow‐up were censored (n = 10).
2.3. Analysis
Unadjusted outcomes were compared using descriptive statistics. Continuous data (out‐of‐hospital SpO2, age, and hospital length of stay) were compared using medians and interquartile ranges (IQR) and categorical data were expressed as frequency and percentage. Multivariable logistic regression was used to model in‐hospital mortality as a function of prehospital SpO2 and included the following covariates: race/ethnicity recorded in hospital by NYC H+H; and age (in 10‐year increments), sex, and past medical history recorded on‐scene by FDNY‐EMS. A competing risk model, using the subdistribution method, was fitted to estimate the absolute effect of out‐of‐hospital SpO2, adjusted for the same covariates, on the cumulative incidence function for being discharged alive from the hospital, after controlling for the competing risk of death. 13 , 14 Cumulative incidence function plots were then fitted using the median values of out‐of‐hospital SpO2 and age (≤90% vs 91% to 100% for out‐of‐hospital SpO2 and ≤65 vs ≥66 years of age).
The Bottom Line
In this study of 1673 patients from New York City during the coronavirus disease 2019 (COVID‐19) pandemic surge, patients with confirmed COVID‐19 an out‐of‐hospital SpO2 level ≤90% was associated with over 50% decreased likelihood of being discharged alive, regardless of age. Patients over 65 years of age who had out‐of‐hospital hypoxemia (SpO2 <90%) had a 54% mortality rate compared to 26% for those with out‐of‐hospital SpO2 >90%. Patients 65 years of age or younger who had out‐of‐hospital hypoxemia (SpO2 <90%) had a 31% mortality rate compared to 11% for those with out‐of‐hospital SpO2 >90%. Overall each 1% decrease in out‐of‐hospital oxygen saturation was associated with 7% higher odds of death.
To ensure the robustness of the regression models, collinearity among variables was confirmed using a variance inflation factor of no >2, 15 and model specification was assessed by examining out‐of‐hospital SpO2 and age in a variety of ways (ie, continuous variable in linear and quadratic terms as well as a categorical variable with various breakpoints). Based on the Akaike information criterion obtained from each of the models, the best fit for our data used out‐of‐hospital SpO2 as a continuous variable in linear terms, defined as a 1 percentage point decrease, and age in 10‐year age groups. Two sensitivity analyses on these outcomes were conducted by stratifying prehospital SpO2 in 10% increments from 60% to 100% and age into quartiles (18–55, 56–65, 66–75, and ≥76). Lastly, using a subdistribution hazard model for our competing risk analyses precluded the need to meet the non‐informative censoring assumption of the Cox proportional hazards model or the assumption of independence of competing events in a cause‐specific hazard model. 13 Schoenfeld residuals were assessed to confirm the proportional hazards assumption was met in the subdistribution hazards model. We noticed a trend toward a significantly lower subdistribution hazard ratio (ie, greater in magnitude) beginning after 30 days of follow‐up. However, 96% of death events and 96% of discharge events had occurred by day‐30. A sensitivity analysis that censored all individuals at day 30 found the competing risk model results unchanged, as one would expect.
Sensitivity analyses were performed to understand the impact of missing out‐of‐hospital SpO2 data for in‐hospital mortality and discharge from hospital after controlling for the competing risk of death. We analyzed the entire group of COVID‐19 patients regardless of out‐of‐hospital SpO2 being measured by EMS (n = 3401 patients) by assigning the following values to patients missing SpO2 based on descriptive statistics from the main analysis: (1) 95%, midlevel of the upper category; (2) 75%, midlevel of lower category; (3) 90%, median out‐of‐hospital SpO2; and (4) 88%, mean out‐of‐hospital SpO2. All analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, NC) and a P value of <0.05 was considered statistically significant in all analyses.
3. RESULTS
3.1. Patient characteristics
Table 1 displays patient characteristics of COVID‐19 confirmed patients transported to NYC H+H hospitals by FDNY‐EMS. Between March 5, 2020 and April 30, 2020, there were 3584 COVID‐19 confirmed patients, not in cardiac arrest, aged ≥18 and transported to NYC‐H+H hospitals by FDNY‐EMS; of whom 1856 (51.8%) had out‐of‐hospital SpO2 measurements collected in the prehospital setting. The study cohort consisted of the 1673 with a minimum out‐of‐hospital SpO2 of at least 60%. The study cohort's median age was 66 (IQR): 55–76 years of age) and median out‐of‐hospital SpO2 was 90% (IQR: 82–96). The majority were male (59.8%) and non‐white (89.5%). The most common comorbidities were hypertension (50.3%), diabetes (35.3%), and cardiac disease (23.8%). Pneumonia was diagnosed in 73.6% of patients (based on NYC H+H assigned International Classification of Diseases, Tenth Revision codes J12‐J19). Over 90% (n = 1514) of patients were admitted to the hospital, 527 (34.5%) of patients died in the hospital and the median hospital length of stay was 6 days (IQR: 2–11 days). Patient characteristics were similar between those with an FDNY‐EMS out‐of‐hospital SpO2 and those without; however, the study cohort had a higher proportion of comorbid medical conditions than those without an out‐of‐hospital SpO2 (Table 1).
TABLE 1.
Characteristics of 3584 COVID‐19 confirmed patients transported to H+H hospitals by FDNY‐EMS a
| No out‐of‐hospital SpO2 (n = 1728) | Out‐of‐hospital SpO2 < 60 (n = 183) | Out‐of‐hospital SpO2 ≥60 (n = 1673) | |
|---|---|---|---|
| Out‐of‐hospital SpO2, median (IQR) | — | 47 (36‐53) | 90 (82‐96) |
| Out‐of‐hospital SpO2 ≤90, n(%) | — | 183 (100) | 887 (53.0) |
| Out‐of‐hospital SpO2 > 90, n(%) | — | — | 786 (47.0) |
| Age, median (IQR) | 63 (50‐75) | 69 (60‐77) | 66 (55‐76) |
| Male, n (%) | 1074 (62.2) | 115 (62.8) | 1000 (59.8) |
| Race, n(%) | |||
| White | 186 (10.8) | 10 (5.5) | 176 (10.5) |
| Asian | 97 (5.6) | 21 (11.5) | 82 (4.9) |
| Black | 552 (31.9) | 41 (22.4) | 530 (31.7) |
| Other/Hispanic b | 761 (44.0) | 91 (49.7) | 749 (44.8) |
| Unknown | 132 (7.6) | 20 (10.9) | 136 (8.1) |
| Medical history, n(%) | |||
| Cardiac disease | 323 (18.7) | 40 (21.9) | 397 (23.8) |
| Hypertension | 765 (44.3) | 94 (51.4) | 841 (50.3) |
| Diabetes | 570 (33.0) | 52 (28.4) | 590 (35.3) |
| Asthma | 189 (11.0) | 13 (7.1) | 190 (11.4) |
| Cerebrovascular accidents | 75 (4.3) | 6 (3.3) | 92 (5.5) |
| Renal disease | 75 (4.3) | 6 (3.3) | 78 (4.7) |
| Psychiatric problems | 113 (6.5) | 2 (1.1) | 102 (6.1) |
| Chronic respiratory disease | 46 (2.7) | 9 (4.9) | 54 (3.2) |
| Cancer | 69 (4.0) | 11 (6.0) | 73 (4.4) |
| Seizure disorder | 46 (2.7) | 3 (1.6) | 49 (2.9) |
| HIV/AIDS | 30 (1.7) | 2 (1.1) | 26 (1.6) |
| Admitted n(%) | 1446 (83.7) | 179 (97.8) | 1514 (90.5) |
| Death, n(%) | 588 (34.0) | 123 (67.2) | 527 (31.5) |
| Pneumonia, as final discharge diagnosis | 1053 (60.9) | 150 (82.0) | 1232 (73.6) |
| Length of stay in days, median (IQR) | |||
| Overall | 4 (1‐9) | 7 (2‐16) | 6 (2‐11) |
| In those who survived | 4 (1‐10) | 11 (1‐31) | 5 (2‐10) |
| In those who died | 5 (2‐9) | 7 (3‐12) | 6 (3‐12) |
Study population used for these estimates include non‐cardiac arrest patients aged 18 years or older who had minimum out‐of‐hospital SPO2 measures reported.
Race was obtained from H+H records, which do not include a category for Hispanics. It was assumed that because of New York City's racial makeup the majority of people in the Other group would be considered Hispanic.
COVID‐19, coronavirus disease 2019; FDNY‐EMS, Fire Department of the City of New York 9‐1‐1 Emergency Medical Service; H+H, Health + Hospitals; IQR, interquartile range.
3.2. In‐hospital mortality
Results from our multivariable model of out‐of‐hospital SpO2 and in‐hospital mortality are shown in Table 2. For every 1 percentage point decrease in out‐of‐hospital SpO2, there was a 7% increased likelihood of in‐hospital death (odds ratio [OR], 1.07; 95% CI, 1.06–1.09; P < 0.001). Increasing age in 10‐year increments was also strongly associated with in‐hospital mortality (OR, 1.45; 95% CI, 1.33–1.58; P < 0.001). No other risk factors were identified as significant (Table 2). When the analysis was stratified by age in quartiles or out‐of‐hospital SpO2 in 10% increments, no additional risk factors were statistically significant.
TABLE 2.
Multivariable logistic regression a results for risk of mortality in 1673 COVID‐19 patients with minimum out‐of‐hospital SPO2 values of ≥ 60
| Risk Factor | Adjusted OR b | 95% CI | P |
|---|---|---|---|
| Out‐of‐hospital SPO2 (per 1‐percentage point decrease) | 1.07 | 1.06, 1.09 | <.0001 |
| Age (per 10 years) | 1.45 | 1.33, 1.58 | <.0001 |
| Gender | |||
| Female | Ref | ||
| Male | 1.22 | 0.96, 1.55 | 0.102 |
| Race/Ethnicity | |||
| White | Ref | ||
| Asian | 0.68 | 0.36, 1.27 | 0.354 |
| Black | 0.79 | 0.53, 1.19 | 0.677 |
| Other/Hispanic c | 0.86 | 0.58, 1.27 | 0.769 |
| Unknown | 0.86 | 0.50, 1.48 | 0.833 |
| Past medical history | |||
| Cardiac disease | 1.33 | 1.00, 1.76 | 0.049 |
| Hypertension | 0.90 | 0.69, 1.17 | 0.437 |
| Diabetes | 1.11 | 0.86, 1.44 | 0.408 |
| Asthma | 0.91 | 0.62, 1.33 | 0.622 |
| Cerebrovascular accidents | 1.33 | 0.82, 2.17 | 0.247 |
| Renal disease | 0.98 | 0.57, 1.68 | 0.949 |
| Psychiatric problems | 1.00 | 0.61, 1.66 | 0.994 |
| Chronic respiratory disease | 1.16 | 0.62, 2.17 | 0.651 |
| Cancer | 1.26 | 0.74, 2.15 | 0.397 |
| Seizure disorders | 1.19 | 0.60, 2.34 | 0.624 |
| HIV/AIDS | 0.57 | 0.19, 1.65 | 0.298 |
Hosmer and Lemeshow Goodness‐of‐Fit Test P value = 0.440.
Odds ratios (OR), 95% confidence intervals (CI), and P values calculated using logistic regression.
Race was obtained from H+H records, which do not include a category for Hispanics. It was assumed that because of New York City's racial makeup the majority of people in the Other group would be considered Hispanic.
COVID‐19, coronavirus disease 2019; H+H, Health + Hospitals.
We found that the positive predicted values of out‐of‐hospital SpO2 level on death differed significantly by age group (Table 3). In patients with an out‐of‐hospital SpO2 ≤90%, the positive predictive value for death was high in both age groups —54.1% in patients ≥66 years and 30.7% in patients ≤65 years of age. The positive predictive value for death in patients with out‐of‐hospital SpO2 values > 90% was 25.6% and 11.4% in patients ≥66 years and ≤65 years of age, respectively. Although there were clear and statistically significant age differences, the proportion who died exceeded 30% in both age groups if their out‐of‐hospital SpO2 was ≤90% and decreased to 11.4% in younger patients ≤65 years of age if their out‐of‐hospital SpO2 was 91% to 100%.
TABLE 3.
Positive predicted values of death by out‐of‐hospital SpO2 levels, overall and by age
| Age 66 and older | Age 65 and younger | Overall | |
|---|---|---|---|
| Out‐of‐hospital SpO2 levels | |||
| ≤90% | 54.1 | 30.7 | 43.3 |
| >90% | 25.6 | 11.4 | 18.2 |
Sensitivity analyses to understand the impact for the missing out‐of‐hospital SpO2 data on in‐hospital mortality found similar measures of association to the main analysis (Table 2). However, with greater numbers of participants the comparisons for male versus female and Black versus White became significant (Appendix 1a).
3.3. Length‐of‐stay
Results from our competing risk model are shown in Table 4. Decreasing out‐of‐hospital SpO2, in 1% increments (subdistribution hazard ratio [SHR], 0.95; 95% CI, 0.94– 0.96; P < 0.001) and increasing age in 10‐year increments (SHR, 0.82.; 95% CI, 0.79–0.86; P < 0.001) were associated with a decrease in the subdistribution hazard of being discharged from the hospital on a given day, given that the patient was still in the hospital or had already died on that day. No other risk factors were identified as significant (Table 4).
TABLE 4.
Competing risk model for being discharged alive in 1673 COVID‐19 patients with minimum out‐of‐hospital SPO2 values of ≥ 60 a
| Characteristics | SHR | 95% CI | P |
|---|---|---|---|
| Out‐of‐hospital SPO2 (per 1 percentage point decrease) | 0.95 | 0.94, 0.96 | <.0001 |
| Age (per 10 years) | 0.82 | 0.79, 0.86 | <.0001 |
| Gender | |||
| Female | Ref | ||
| Male | 0.89 | 0.78, 1.01 | 0.066 |
| Race/Ethnicity | |||
| White | Ref | ||
| Asian | 1.27 | 0.92, 1.75 | 0.148 |
| Black | 1.09 | 0.88, 1.36 | 0.4338 |
| Other/Hispanic c | 1.17 | 0.95, 1.45 | 0.1444 |
| Unknown | 0.97 | 0.73, 1.27 | 0.7987 |
| Past medical history | |||
| Cardiac disease | 0.95 | 0.81, 1.12 | 0.5605 |
| Hypertension | 1.07 | 0.93, 1.24 | 0.3624 |
| Diabetes | 0.90 | 0.78, 1.03 | 0.1331 |
| Asthma | 1.21 | 0.98, 1.49 | 0.0748 |
| Cerebrovascular accidents | 0.80 | 0.61, 1.05 | 0.1015 |
| Renal disease | 0.86 | 0.63, 1.17 | 0.327 |
| Psychiatric problems | 0.91 | 0.72, 1.15 | 0.4373 |
| Chronic respiratory Disease | 0.86 | 0.62, 1.19 | 0.3481 |
| Cancer | 0.90 | 0.67‐1.20 | 0.4556 |
| Seizure disorders | 0.93 | 0.66, 1.32 | 0.6866 |
| HIV/AIDS | 1.22 | 0.78, 1.90 | 0.3951 |
Mortality was considered a competing risk.
Subdistribution hazard ratios (SHR), 95% confidence intervals (CI), and P values calculated using subdistribution hazard models.
Race was obtained from H+H records, which do not include a category for Hispanics. It was assumed that because of New York City's racial makeup the majority of people in the Other group would be considered Hispanic.
COVID‐19, coronavirus disease 2019; H+H, Health + Hospitals.
Cumulative incidence functions from our subdistribution hazard model is shown in Figure 1. An out‐of‐hospital SpO2 level ≤90% was associated with over a 50% decreased likelihood of being discharged alive (SHR, 0.48; 95% CI, 0.43–0.54; P < 0.001), regardless of age. In younger patients ≤65 years of age, an out‐of‐hospital SpO2 level above 90%, was associated with increased likelihood of survival to discharge (SHR, 1.68; 95% CI, 1.49–1.88; P < 0.001).
FIGURE 1.
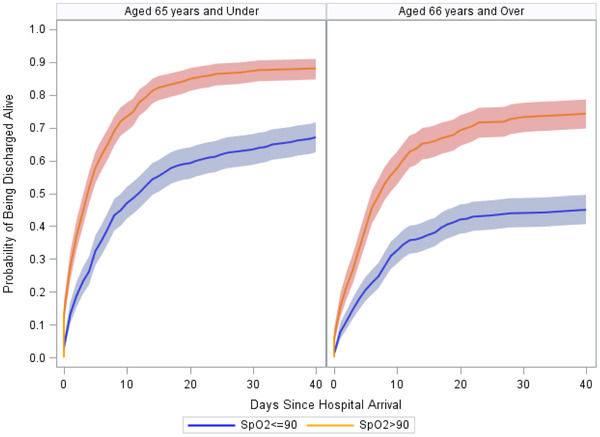
Cumulative incidence functions for the probability of being discharged from the hospital alive after accounting for death, by out‐of‐hospital SpO2 level and age. Shading represents 95% confidence intervals
Sensitivity analyses to understand the impact for the missing out‐of‐hospital SpO2 data on length of stay after controlling for the competing risk of death found similar measures of association to the main analysis (Table 4). However, comparisons for asthma versus no asthma, cerebrovascular incidents versus none, and Other/Hispanic versus White became significant for length of stay after controlling for the competing risk of death only when out‐of‐hospital SpO2 was assumed to be 75% in all missing cases (Appendix 1b).
3.4. Limitations
A limitation of our study is that it included only FDNY‐EMS patients transported for subsequent ED evaluation at 11 NYC H+H acute‐care hospitals. First, FDNY‐EMS accounts for over 60% of all NYC 9‐1‐1 ambulances. 16 There is no reason to believe patients would be treated any differently by other 9‐1‐1 ambulance emergency medical technicians and paramedics as all must follow identical medical protocols as directed by the Regional Emergency Medical Advisory Committee. Second, H+H accounts for 11 out of 62 acute‐care hospitals in NYC. We believe the use of this 1 hospital system was appropriate because FDNY ambulances are mostly located within NYC H+H hospital catchment areas; these same areas had some of the highest rates of COVID‐19 infection; and the standard of care was consistent throughout all 11 hospitals. Our results are subject to selection bias because we could not include patients presenting to ED without calling 9‐1‐1 or who after FDNY‐EMS evaluation decided to remain at home. Enrichment of risk factors for death in the FDNY‐EMS study cohort may account for failure to observe comorbid conditions as significant covariates in predicting in‐hospital mortality or length of stay after controlling for the competing risk of death. Additional limitations include that only patients with an FDNY‐EMS out‐of‐hospital SpO2 measurement (∼50%) were included. Early during the pandemic, not all FDNY‐EMS ambulances were equipped with pulse oximetry and increased call volume may have led to missing measurements or data entry. We also note that our mortality rates were higher than that seen in other parts of the country; however, this was expected as NYC was the early epicenter of COVID‐19 in the United States and knowledge on its treatment was in its infancy. Although most patient characteristics were similar between those with and without an FDNY‐EMS out‐of‐hospital SpO2 measurement a bias may have been introduced because those with measurement had a higher proportion of comorbid medical conditions (Table 1). Concerns exist regarding the accuracy of digital pulse oximetry (especially when oxygenation or perfusion is low). 12 , 17 For quality control purposes, we excluded out‐of‐hospital SpO2s < 60%. This decision may have excluded some patients with extreme hypoxemia (∼PO2 < 30 mm Hg). Out‐of‐hospital SpO2 measures can be erroneous because of skin or nail color, hypoperfusion, hypothermia, hyperventilation, acid‐base disturbances, and dyshemoglobinemias—all magnified by the shape of the hemoglobin‐saturation curve during hypoxemia. 12 By including out‐of‐hospital SpO2 < 60% without verification, our results would have demonstrated an even greater impact of hypoxia on negative patient outcomes, a bias we did not want to incur. Overall, their ease of use and low cost, combined with the large burden of illness in COVID‐19 and the risks of silent hypoxemia, make digital pulse oximetry a valuable addition for assessing and monitoring at‐risk individuals.
4. DISCUSSION
We report on 1673 COVID‐19 patients with out‐of‐hospital SpO2 measured by FDNY‐EMS in the prehospital setting who were then transported to NYC H+H ED for further evaluation and possible admission. Both age and out‐of‐hospital SpO2 were independent predictors of in‐hospital mortality and length of stay, after controlling for the competing risk of death. Among older patients ≥66 years old, the proportion who died in those with an out‐of‐hospital SpO2 > 90% was 26% compared to 54% in those with an out‐of‐hospital SpO2 ≤90%. Among younger patients ≤65 years old with an out‐of‐hospital SpO2 > 90%, 11.5% died compared to 31% in those with an out‐of‐hospital SpO2 ≤90%. After controlling for the competing risk of death, patients with an out‐of‐hospital SpO2 level ≤90% was associated with over a 50% decreased likelihood of being discharged alive (HR, 0.48; 95% CI, 0.43–0.54; P < 0.001), regardless of age. In contrast to prior reports, 8 we did not find that race/ethnicity or medical history was significant in predicting in‐hospital mortality or length of stay after controlling for the competing risk of death, but this may be lack of power because differences were found in our sensitivity analyses when including patients who did not have out‐of‐hospital SpO2 measured.
To date, several peer‐reviewed COVID‐19 studies have detailed the association between commonly accepted risk factors (ie, age, race/ethnicity, comorbidities, inflammatory biomarkers) and in‐hospital 4 , 5 , 6 , 7 , 8 and out‐of‐hospital mortality. 3 Although all studies found increasing age to be an independent predictor of mortality, we could find only 2 studies that included out‐of‐hospital SpO2 in their mortality analyses. Both found that out‐of‐hospital SpO2, measured day 1 of hospital admission while breathing supplemental oxygen, was an independent predictor of in‐hospital mortality. In the NYU study, 10 hypoxic patients (out‐of‐hospital SpO2 < 88% vs 92%) were twice as likely to die (HR, 2.00; 95% CI, 1.61–2.48; P < 0.001). In the Wuhan, China study, 11 out‐of‐hospital SpO2 was inversely related to survival (out‐of‐hospital SpO2 per 1‐unit increase; HR, 0.93, 95% CI, 0.91–0.95; P < 0.001). There were several notable differences when comparing studies. Our study required 9‐1‐1 EMS transport and used prehospital FDNY‐EMS SpO2 measurements obtained breathing room air rather than postadmission values obtained breathing supplemental oxygen. For those admitted, our length of stay, although similar to the NYU study (median 7; IQR 3–13 days), was shorter than the Wuhan study (median 14; IQR 6–26 days). Our study included 159/1,673 (9.5%) not admitted to the hospital allowing for generalizability to patients ill enough to call 9‐1‐1 but not ill enough for hospitalization. Despite including patients not admitted and without a diagnosis of pneumonia (441/1,673; 26.4%), our cohort had a higher proportion of patients with an out‐of‐hospital SpO2 ≤90%—(887/1,673; 53.0%) as compared with the NYU study (422/2729; 15.5%) or the Wuhan study (51/140; 36.4%).
Although we observed out‐of‐hospital SpO2 to be a strong predictor of in‐hospital mortality and length of stay after controlling for the competing risk of death in patients transported to hospitals by FDNY‐EMS, we cannot say that it was the determinant cause of death. Our data do not allow us to differentiate those with pneumonia from those with hypoxia owing to other COVID‐19 pathologies such as embolism, cardiomyopathy, overwhelming inflammation (cytokine storm), or viral septicemia. 18 , 19 , 20 , 21 , 22 , 23 Nor can we exclude hypoxia resulting indirectly from acute COVID‐19 infection decompensating preexisting conditions (eg, cardiopulmonary diseases or cancer). Without access to complete diagnostic studies and when available autopsy studies, all we can say is that out‐of‐hospital SpO2 is a marker of disease severity.
Mortality rates were high in our study and could justify hospital admission for all patients, regardless of age or out‐of‐hospital SpO2. During a pandemic surge, hospital resources including ICU beds become exhausted and admitting all patients is no longer possible. Triage decisions must be made. Our data inform such decisions while at the same time making a convincing argument that patients not admitted remain at risk and should be provided with close outpatient monitoring. Though we expect the association between prehospital hypoxemia and disease severity to remain a critical factor in the admission decision, the availability of new treatment options will further affect this decision.
Several algorithms exist for risk‐stratifying patients with community acquired bacterial pneumonias, of which the best validated are the CURB‐65 Scale (Confusion, Urea, Respiratory rate, Blood pressure ,and age ≥65 years) and the Pneumonia Severity Index. 24 , 25 However, there is concern that these algorithms may not be predictive for patients with COVID‐19 pneumonia as they proved inaccurate in risk‐stratifying patients with viral pneumonia during the Influenza A (H1N1) 2009 pandemic. 26 In the EMS prehospital setting, critical laboratory data required for these algorithms are not available, even if they were validated for this infection. In contrast, age and SpO2 were simple to obtain, readily available, and proved predictive of both in‐hospital mortality and length of stay after controlling for the competing risk of death.
In conclusion, age and SpO2 measured in the prehospital setting predict in‐hospital mortality and length of stay and improve our ability to risk‐stratify COVID‐19 infected patients. An out‐of‐hospital SpO2 ≤90% strongly supports a triage decision for immediate hospital admission, regardless of patient age. For out‐of‐hospital SpO2 >90%, the decision to admit depends on multiple factors including age, resource availability (outpatient vs inpatient) and the potential impact of new treatments. This study did not address alternative care situations for patients with normal out‐of‐hospital SpO2 values such as close remote home monitoring using telemedicine assisted by frequent out‐of‐hospital SpO2, temperature, and symptom checks. Further research is needed to determine if age and out‐of‐hospital SpO2 coupled with other patient characteristics (eg, sex, race/ethnicity, medical history, symptoms, temperature, and other biomarkers) could further improve our accuracy in risk‐stratifying COVID‐19 infected patients in the outpatient and prehospital settings.
AUTHOR CONTRIBUTIONS
DG and NAA conceived the study and all authors designed the trial. JB and HT managed the data. EAL and CBH provided statistical advice on study design and analyzed the data; PHL and BZ chaired the data oversight committee. DG, NAA, and DJP drafted the manuscript, and all authors contributed substantially to its revision. DJP takes responsibility for the paper as a whole.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
FUNDING INFORMATION
This research was supported by the Fire Department of the City of New York.
Biography
David Prezant,MD, is the Chief Medical Officer at the Office of Medical Affairs for the Fire Department of the City of New York (FDNY).

Lancet EA, Gonzalez D, Alexandrou NA , et al. Prehospital hypoxemia, measured by pulse oximetry, predicts hospital outcomes during the New York City COVID‐19 pandemic. JACEP Open. 2021;2:e12407. 10.1002/emp2.12407
Supervising Editor: Matthew L. Hansen, MD, MCR
Funding and support: ByJACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1. Centers for Disease Control and Prevention. United States Covid‐19 cases and deaths by state. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html. Accessed September 21, 2020.
- 2. New York City. COVID‐19: Data. 2020. https://www1.nyc.gov/site/doh/covid/covid‐19‐data.page. Accessed September 27, 2020.
- 3. Lai P, Lancet EA, Weiden MD, et al. Characteristics associated with out‐of‐hospital cardiac arrests and resuscitations during the novel Coronavirus disease 2019 pandemic in New York City. JAMA Cardiol. 2020:e202488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996‐m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metabolism. 2020;108:154262‐154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med. 2020;382(26):2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao F, Xu S, Ma X, et al. In‐hospital cardiac arrest outcomes among patients with COVID‐19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966‐m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID‐19. Mayo Clin Proc. 2020;95(6):1138‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID‐19 at home. potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17(9):1040‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statis Assoc. 1999;94(446):496‐509. [Google Scholar]
- 15. Fox J, Monette G. Generalized Collinearity Diagnostics. J Am Statis Assoc. 1992;87(417):178‐183. [Google Scholar]
- 16. Freese J, Richmond NJ, Silverman RA, Braun J, Kaufman BJ, Clair J. Impact of a citywide blackout on an urban emergency medical services system. Prehospital Disast Med. 2006;21(6):372‐378. [DOI] [PubMed] [Google Scholar]
- 17. Lipnick MS, Feiner JR, Au P, Bernstein M, Bickler PE. The accuracy of 6 inexpensive pulse oximeters not cleared by the food and drug administration: the possible global public health implications. Anesth Analg. 2016;123(2):338‐345. [DOI] [PubMed] [Google Scholar]
- 18. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost: JTH. 2020;18(7):1559‐1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with covid‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community‐acquired pneumonia. The European respiratory journal. 2006;27(1):151‐157. [DOI] [PubMed] [Google Scholar]
- 25. Niederman MS. Making sense of scoring systems in community acquired pneumonia. Respirology (Carlton, Vic). 2009;14(3):327‐335. [DOI] [PubMed] [Google Scholar]
- 26. Bjarnason A, Thorleifsdottir G, Löve A, et al. Severity of influenza A 2009 (H1N1) pneumonia is underestimated by routine prediction rules. Results from a prospective, population‐based study. PLoS One. 2012;7(10):e46816‐e46816. [DOI] [PMC free article] [PubMed] [Google Scholar]


